Abstract
Our aim was to evaluate the protective and antioxidant effects of ginger extract against cadmium-induced renal toxicity in animal models and to support the use of ginger as anti-renal failure natural remedy. Seventy rats were examined in a 4-week experiment to evaluate the effect of Ginger (Zingiber officinale) at doses of 100 and 200 mg/kg body weight on molecular DNA content, antioxidant status, and renal function in rats intoxicated with cadmium at dose of (5 mg/kg) using biochemical and histological analysis. Renal dysfunction, kidney tissue damage, and oxidative effect were evident in cadmium intoxicated rats as estimated by significant increase in (creatinine, urea), decrease in (creatinine clearance and reabsorption rate of urine albumin), increase in MDA, decrease in total antioxidant status (TAC), reduction in DNA content, and histopathological changes of kidneys’ tissues compared to control rats. Treatment with ginger resulted in significant restoring of renal function biomarkers, TAC, molecular DNA, and histological improvements which occurs via free radical scavenging and regenerative mechanisms. The activity of ginger was supported by estimation of bioactive phenolic and falvinods constituents. Twenty-eight polyphenolic compounds were estimated in ginger extract; [6]-gingerol, [6]-shogaol, citral and pyrogallol were the highest amounts in ginger, and supposed to be responsible for its major antioxidant and free radical scavenging activity as shown by In vitro DPPH/β-carotene-linolic acid assay tests. Consequently, ginger extracts could have a potent protective effects against nephrotoxicity induced by various toxicants.
Keywords: Antioxidant, Nephrotoxicity, Lipid peroxidation, CdCl2–toxicity, Biological activity, Ginger extract
1. Introduction
Cadmium (Cd) is the most disseminated contaminant prevails in all environmental resources including food and cigarette smoke (Sullivan and Krieger, 2001).
The exposure of human to both acute and chronic Cd toxicity occurs through natural contaminated sources such as food, air, water, and industrial products (Duruibe et al., 2007), however the most drastic biological defects results from chronic Cd exposure (Satarug et al., 2004), this is largely relates to its accumulation in most important biological tissues such as liver, lungs, kidneys, bones, and reproductive organs (Alvarez et al., 2007), and ultimately produces several clinical problems such as renal dysfunction, bone diseases, and hepatic dysfunction (Järup et al., 2000), depending on dose, duration and route of exposure (Obianime and Roberts, 2009).
Previous studies, reported that Cd toxicity promotes the induction of various pathological events among humans and animals via free radical initiation mechanisms which produces active oxidative free radicals from biological components of the cell such as lipids, proteins and DNA (Liu et al., 2008).
The released lipid peroxidation (LPO), thiobarbituric acid reactive substances (TBARS), and hydroperoxides were the major indicators of oxidative tissue damage caused by Cd-toxicity (Swarup, et al., 2007).
Most studies concluded that, liver and kidney were the most of sensitive biological organs easily affected by Cd toxicity (Ognjanović et al., 2010a, Ognjanović et al., 2010b). The induction of Cd nephrotoxicity for example produced via many mechanisms including frees radicals, cell necrosis, and apoptosis (El-Sharaky et al., 2007).
Previously, some research works focused on using metals such as zinc, copper, germanium, and selenium as antagonists or antioxidant agents for protection against cadmium toxicity. These metals were shown to effect on the absorption and transmission of cadmium compounds within biological systems through masking and displacing mechanisms (Boscolo et al., 2005, González-Trujano and Navarrete, 2011). However, the use of these metals in high doses may have serious biological effects (Minta et al., 1995).
In recent years, extensive research work performed on herbal plants to produce new sources of alternative and safe antioxidant remedies with minimal side effects against many diseases (Lopez, et al., 2007). Many herbal extracts of natural origin such as green tea, curcumin and black cumin were used as protective agents against Cd toxicity (Hamden et al., 2009, Deevika et al., 2012).
Ginger (Zingiber officinale) is one of the most popular food spices known among Asian and African people for long periods (El-Sharaky et al., 2009). It includes versatile range of active constituents such as volatile oils, phenolic, proteolytic enzymes, vitamins, and trace elements (Onwuka et al., 2011). These constituents gave ginger the priority to be used in many diseases as anti-inflammatory and antioxidant modulator (Khaki and Khaki, 2010).
The strong antioxidant and protection abilities of ginger to biological tissues and cell membrane lipids against toxicity occurs via free radical scavenging activity in a dose dependent manner (Farag et al., 2010, Stoilova et al., 2007). So, this increases its tendency to be used in various experimental models as protective agent against various toxicants (Johari et al., 2013, Haniadka et al., 2013a, Haniadka et al., 2013b).
In this regard, the present study conducted to evaluate the protective and antioxidant effects of ginger extract against cadmium-induced renal toxicity in animal models and to support the use ginger as anti-renal failure natural remedy by determining the total polyphenols and free radical scavenging activity of ginger extract in vitro.
2. Materials and methods
2.1. Experimental animals
In this study 70 male adult wistar albino rats, weighing (150–200 g) were recruited in this study. The animals were housed and subjected to normal feeding, drinking, and health care mechanisms according to the guidelines of the experimental animal care center, college of applied medical sciences, King Saud Univ., Riyadh, Saudi Arabia. The experiment and the procedures were approved by the Ethics Committee of the Experimental Animal Care Society, Rehabilitation Research Chair (RRC), College of Applied Medical Sciences, King Saud Univ., Riyadh, Saudi Arabia, under file number (RRC-2014-013).
2.2. Preparation of ginger ethanolic extract
Two kilograms of fresh ginger rhizomes were purchased from a convenience store (Othaim Markets) in Riyadh, KSA. The fresh ginger rhizomes were extracted in 95% ethanol and a dose of 100 mg/b.w. and 200 mg/b.w. of ginger extract were prepared as previously reported in literature (Bhandari et al., 2005).
2.3. Experimental design
Both ginger extract and cadmium chloride (CdCl2) were administrated orally according to body weight of experimental animals. The animals were classified into seven groups (10 rats/group). These groups arranged as follows: Group 1 (Control), rats fed on normal diet plus a solution of 2%, Tween-80; Group 2 (Cd-treated), rats treated with 5 mg/kg cadmium chloride for 30 days); Group 3 (Ginger Treated), rats treated with ethanolic-extract of ginger (100 mg/kg body weight for 30 days); Group 4 (Ginger Treated), rats treated with ethanolic-extract of ginger (200 mg/kg body weight for 30 days); Group 5 (Cd plus Ginger), rats treated with (5 mg/kg cadmium chloride plus100 mg/kg ginger extract for 30 days); Group 6 (Cd plus Ginger), rats treated with (5 mg/kg cadmium chloride + 200 mg/kg ginger extract for 30 days); group 7 (treated with gradual concentrations of ginger; 50 mg to 200 mg/rat). Kidney tissues and blood samples were collected for subsequent histological and biochemical analysis.
2.4. Analysis of phenolic compounds in ginger extract
Total phenolic compounds of 100 mg of ginger extract were analyzed at Bio-technology Lab., Plant Pathology Institute, Agricultural Research Center, Giza, Egypt. Analysis was performed with a liquid chromatography “HP1050” equipped with a 4.6 mm × 150 mm ODS C18 column with UV detector and the injection volume was 5 µl. The mobile phase yielded results of 40% methanol: 60% distilled water. The wave length in the UV detector was 230 nm; total run time for the separation was 15 min at a flow rate of 0.60 ml/min according to the proposed method previously reported (Waskmundzka et al., 2007).
2.5. Estimation of total phenolic and flavonoid content of ginger extract
Total polyphenols were determined by Folin-Ciocalteu method as described previously (Hamed et al., 2012). The results were obtained from gallic acid calibrated curve and expressed as mg of Gallic Acid Equivalents (GAE)/100 mg of fractions. The total flavonoids were estimated according to the Dowd method as adapted by Lamien-Meda et al. (2008). The amounts of flavonoids in plant fractions were expressed as mg of Quercetin Equivalents (QE)/100 mg of fractions from the calibrated curve.
2.6. Estimation of antioxidant activity of ginger extract
-
–
DPPH free radical scavenging assay
Spectrophotometric analysis was performed to evaluate the radical scavenging ability of the ginger extract against DPPH as described previously by Brand et al. (1995). Various concentrations from the extracted ginger were prepared in methanol. The concentrations were subjected to the inhibition rate of DPPH radical and the decrease in absorbance was measured at λ = 517 nm. The radical scavenging activity was calculated from the equation:
-
–
Β-Carotene-linoleic acid assay
The antioxidant activity of the ginger extract was measured spectrophotometrically as previously reported (Mothana, 2011). The activity of ginger extracts was recommended by measuring the inhibition rate of peroxidation in linoleic acid system. The antioxidant activity was calculated using the equation:
where Abs0 and Abs∗ 0 are the absorbance values measured at 0 time of incubation for sample extract and control, respectively. Abst and Abs∗ t are the absorbance values for sample extract and control, respectively, at t = 120 min.
-
–
Total Antioxidant Capacity (TAC)
Serum total antioxidant capacity (TAC) was measured Colorimetric Assay Kit (Catalog #K274-100; BioVision Incorporated; CA 95035 USA). The antioxidant equivalent concentrations were measured at 570 nm as a function of Trolox concentration according to the manufacturer's instructions.
where Sa is the sample amount (in nmol) read from the standard curve; Sv is the undiluted sample volume added to the wells.
2.7. Kidney function tests
Kidney function tests were performed according routine lab methodology previously reported in the literature (Fawcett and Scott, 1960, Teitz, 1987). Urinary albumin was quantified by immunoassay technique using ELISA kit (Cat no., STA-383, Cell Biolabs, Inc., USA). Creatinine clearance as an index of glomerular filtration rate was calculated from serum creatinine and a 24-h urine sample creatinine levels.
2.8. Acute toxicity test
Ginger extract was investigated for toxicity studies as previously reported (Organization for Economic Cooperation and Development (OECD), 1999). The extract was supplemented to healthy group of rats (10 rats) orally in drinking water with gradual concentrations (50 mg to 200 mg/rat). Toxic symptoms were observed on the animals directly after the first 4 h of dosing. After 24 h, the survived animals were maintained under daily observations for two weeks.
2.9. Histopathological evaluation
Histological and pathological examinations of kidney tissue sample were performed according to recommended routine methods previously reported in literature (Bancroft and Gamble, 2002).
2.10. Determination of DNA content in kidney
DNA was estimated in kidney tissue samples by using diphenylamine as organic solvent for extraction and the amount of DNA of each sample was measured colorimetrically (Dische and Schwartez, 1937).
2.11. Determination of malondialdehyde (MDA)
Malondialdehyde (MDA) was estimated in homogenized kidney tissue samples according a colorimetric standard method previously reported (Utley et al., 1967). The concentration of MDA (nmol/g wet tissue) per tissue samples was measured via absorbance rate of 535 run. The data were then calculated via standard calibration curve.
2.12. Statistical analysis
The data of this study were performed using SPSS version 17. All data were tabulated as mean ± SD. The statistical correlation among the studied parameters in treated and control groups was performed by Student’s t-test. P < 0.05 was considered statistically significant.
3. Results
Total phenolic and flavonoids constituents comprise 26.3 mg and 9.13 mg/100 g of ginger extract respectively (Table 1). The active biological constituents of ginger extract were estimated using liquid chromatography. The data showed that [6]-gingerol was the highest amount (24.8 mg/g) followed by [6]-shogaol (19.4 mg/g), citral (16.2 mg/g), and pyrogallol (15.2 mg/g). This was in addition to small amounts of other phenolic compounds as shown in Table 2.
Table 1.
Biological activities, total phenolic and flavonoids constituents of ginger extract (mg/100 gm).
| Contents/biological activity | Ginger Extract (100 mg) |
|---|---|
| Phenolic content (mg/100 gm) | 26.3 |
| Flavonoids content (mg/100 gm) | 9.13 |
| Radical scavenging activity (ΒCLA; %) | |
| At cons. of 500 μg/mL | 86.7 |
| At cons. 1000 μg/mL | 92.3 |
| Total antioxidant activity (DPPH; %) | 89.55 |
Table 2.
Phenolic compounds content (mg/g) of ginger ethanol extract using liquid chromatography analysis (HPLC).
| Component | Phenolic content (mg/100 g) | Component | Phenolic content (mg/100 g) |
|---|---|---|---|
| Gallic Acid | 0.250 | Ferulic | 0.743 |
| Pyrogallol | 15.2 | Iso-ferulic | 0.08 |
| 4-amino-benzoic | 0.159 | Reversetrol | 0.16 |
| 3-OH-Tyrosol | 1.677 | Ellagic | 0.12 |
| protocatchuic | 0.2461 | E- vanillic | 0.233 |
| Catechein | 0.799 | Alpha-coumaric | 0.0264 |
| Chlorogenic | 2.544 | Benzoic | 4.95 |
| Catechol | 0.766 | 3,4,5-methoxy-cinnamic | 0.34 |
| Epicatechein | 0.978 | Coumarin | 0.592 |
| Caffeine | 0.263 | Salycilic | 1.193 |
| p-OH benzoic | 1.22 | p-coumaric | 0.12 |
| Caffeic | 0.7951 | Cinnamic | 3.5 |
| vanillic | 0.34 | [6]-shogaol | 19.4 |
| [6]-gingerol | 24.8 | Citral | 16.2 |
The biological antioxidant activity of ginger was measured in vitro and calculated in relation to the inhibition of linoleic acid oxidation and of DPPH radical scavenging activity. Ginger extract recorded radical scavenging activity (86.7; 92.3 %) at concentrations 500 and 1000 μg/mL respectively. Whereas the same extract reported antioxidant activity with mean of (89.55%), according to the β-carotene bleaching rate of ginger extract as shown in Table 1.
The tested animal administrated varies doses of ginger extract (50–200 mg/kg). The data of acute toxicity test showed no toxicity and lethality (LD50 value = 0) observed up to 200 mg/kg of ginger extract in the animals.
Table 3 shows the effect of cadmium and ginger administration on relative body and kidney weights of studied animal groups. In cadmium treated group, there was significant (P < 0.01) decrease in body weight and increase (P < 0.01) in kidney weight of rats compared with normal control group (Table 3). However, treatment of Cd-treated rats with different doses (100 mg/kg and 200 mg/kg) of ginger extract significantly (P < 0.01) restored the body and kidney weight towards normal ranges as compared to Cd treated group. Whereas, normal rats treated with ginger extracts (100 mg/kg; 200 mg/kg) showed significant (P < 0.01) increase in body and kidney weight compared to control group.
Table 3.
Effect of ginger extract on body weight, kidney weight, and the levels of kidney Function biomarkers in cadmium chloride intoxicated experimental rats.
| Group | Parameters |
|||||
|---|---|---|---|---|---|---|
| Final weight (g) |
Kidney function |
|||||
| Body | Kidney | Creatinie (mg/dl) | Urea (mg/dl) | Urine albumin (mg/24 h | Creatinine Clearance | |
| Control (normal diet) | 185 ± 2.21 | 0.49 ± 0.016 | 0.48 ± 0.08 | 22.45 ± 1.54 | 0.13 ± 0.03 | 0.6 ± 0.04 |
| Cd (5 mg/kg) | 153 ± 2.7 ** | 0.78 ± 0.02** | 1.35 ± 0.12** | 89.6 ± 6.7** | 1.63 ± 0.13** | 0.21 ± 0.02** |
| Ginger (100 mg/kg) | 189 ± 1.9** | 0.46 ± 0.12** | 0.46 ± 0.1** | 21.9 ± 1.23** | 0.12 ± 0.06** | 0.56 ± 0.05** |
| Ginger (200 mg/kg) | 195 ± 1.3** | 0.42 ± 0.16** | 0.43 ± 0.12** | 20.8 ± 1.3** | 0.11 ± 0.02** | 0.58 ± 0.03** |
| Cd (5 mg/kg) + Ginger (100 mg/kg) | 168 ± 2.1** | 0.73 ± 0.06** | 1.0 ± 0.10** | 28.3 ± 2.3** | 0.63 ± 0.03** | 0.32 ± 0.03** |
| Cd (5 mg/kg) + Ginger (200 mg/kg) | 175 ± 1.8** | 0.68 ± 0.04** | 0.68 ± 0.14** | 25.6 ± 1.4** | 0.25 ± 0.04** | 0.39 ± 0.04** |
All values represent mean ± SD. * P < 0.05. *** P < 0.001. Student’s t-test.
P < 0.01.
Also, Table 3 showed that CdCl2 administration produced significant (P < 0.01) increase in the levels of serum creatinine, urea, and decrease in creatinine clearance with excessive excretion of albumin through urine of cadmium treated group compared to normal rats. Whereas, in groups administrated ginger extract at doses of 100 and 200 mg/kg either alone or with cadmium treatment showed significant reduction (P < 0.01) in the levels of serum creatinine, urea, and decrease in the excretion rate of albumin through urine of treated rats along with an increase in creatinine clearance. However, the improvement was significantly (P < 0.01) higher in rats treated with 200 mg/kg ginger compared with 100 mg/kg ginger treated and cadmium intoxicated groups (Table 3).
Also, in normal rats treated with the same ginger doses showed significant (P < 0.01) improvement in biomarkers of kidney function compared to control non-treated rats (Cd-treated group).
The oxidative free radical initiation activity of cadmium induced renal toxicity was represented with MDA and total antioxidant capacity (TAC) as oxidative stress markers (Table 4). The comparison between these parameters is conversely related. There was significant increase (P < 0.01) in MDA and decrease (P < 0.01) in TAC of cadmium treated rats compared with control group. However, these oxidative damage effects of cadmium on kidney tissues were restored with ginger administration at doses of 100 and 200 mg/kg. The protective action of ginger towards oxidative damage induced as result of cadmium toxicity was supported by significant increase (P < 0.001) in TAC and decrease (P < 0.001) in MDA levels in kidney of ginger treated rats compared to CdCl2 intoxicated group (Table 4).
Table 4.
Effect of ginger extract on renal lipid peroxidation, Total antioxidant capacity, and DNA content as kidney fibrotic marker in CdCl2 –intoxicated experimental rats.
| Group | MDA (nmol/dl) | TAC (nmol/mM Trolox eq.) | DNA content (μg/106 cells) |
|---|---|---|---|
| Control (normal diet) | 10.7 ± 2.3 | 8.9 ± 2.8 | 0.075 ± 0.1 |
| Cd (5 mg/kg) | 45.7 ± 6.9** | 3.7 ± 1.2** | 0.047 ± 0.6** |
| Ginger (100 mg/kg) | 10.2 ± 2.0** | 9.1 ± 2.75** | 0.076 ± 0.2** |
| Ginger (200 mg/kg) | 9.7 ± 2.3** | 9.5 ± 2.9** | 0.077 ± 0.2** |
| Cd (5 mg/kg) + Ginger (100 mg/kg) | 35.8 ± 3.7*** | 6.5 ± 2.8*** | 0.058 ± 0.7*** |
| Cd (5 mg/kg) + Ginger (200 mg/kg) | 26.4 ± 2.5*** | 9.2 ± 1.8*** | 0.069 ± 0.5*** |
All values represent mean ± SD. * P < 0.05.
P < 0.01.
P < 0.001. Student’s t-test.
Also, DNA content of renal cells was estimated as molecular marker of oxidative renal tissue damage induced by cadmium toxicity on molecular level (Table 4). There was significant decrease (P < 0.01) in DNA content in cadmium treated rats compared to control group. However, supplementation of ginger extract at doses of 100 and 200 mg/kg to cadmium intoxicated rats caused significant improvement of renal tissue cells via increasing DNA content (P < 0.001) as compared with intoxicated group. Similarly, when the same ginger doses applied in non toxicated normal rats, the data showed significant improvement in DNA content of renal cells compared with control group (Table 4), confirming the preventive action of ginger in normal cases.
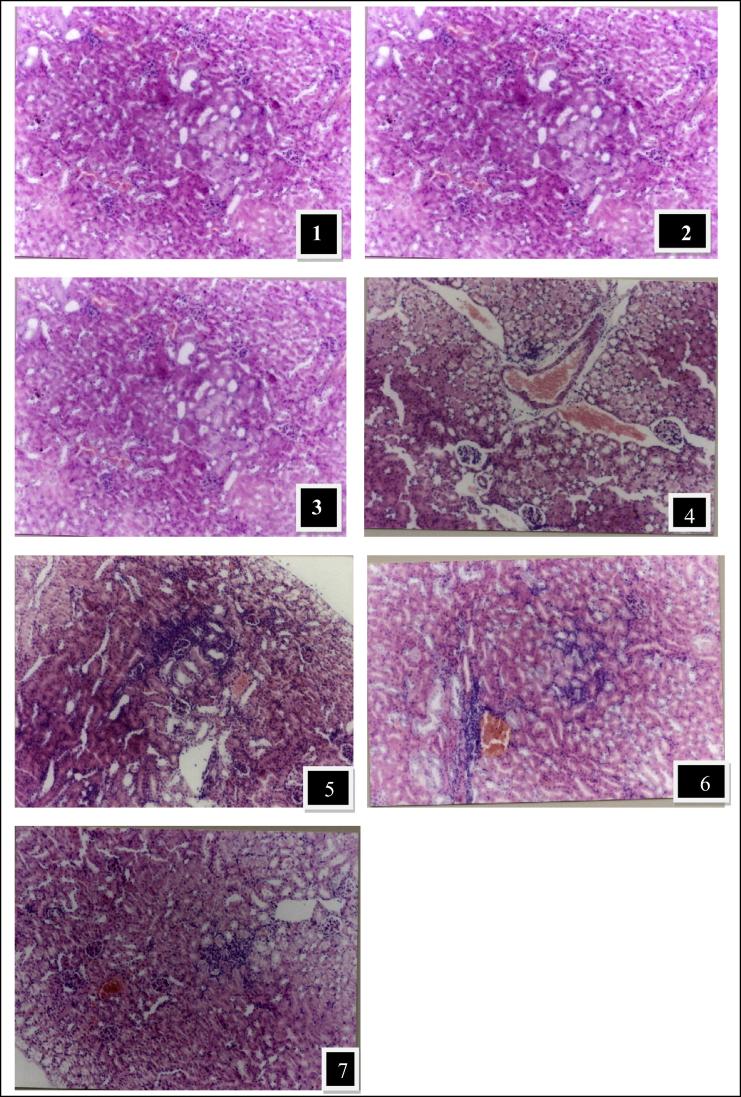
The protective effect of ginger against cadmium toxicity was evaluated in kidney tissue using histological examinations as shown in Fig. 1–7. Normal renal cortex, tubules, parenchyma, and glomeruli medulla were observed in the kidney of control rats (Fig. 1) and ginger extract alone at doses of 100 and 200 mg/kg (Figs. 2 and 3). In animals treated with cadmium showed congestion of the cortical blood vessels, focal replacement of the renal parenchyma with numerous lymphocytes infiltrates, and dilation of glomeruli (Figs. 4 and 5). In groups treated with Cd along with ginger extracts at doses of 100 mg/kg (Fig. 6) and 200 mg/kg (Fig. 7) showed regeneration and restore of glomeruli; renal tubules, and cells.
Fig. 1–7.
photos of hematoxylin and eosin stained sections of kidney tissues showing the protective effect of ginger extract against cadmium toxicity. (1) Normal control; (2) Ginger treated (100 mg/kg); (3) Ginger treated (200 mg/kg); both group 1,2, and 3 showing normal renal cortex, tubules, parenchyma, and glomeruli; (4 and 5) Cadmium treated group showing congestion of the cortical blood vessels and focal replacement of the renal parenchyma with numerous lymphocytes infeltertes, and dilation of glomeruli. (6) Cadmium + Ginger treated (100 mg/kg); (7) Cadmium + Ginger treated (200 mg/kg). Ginger treated groups (6 and 7) showed regeneration and restore of glomeruli; renal tubules, and cells [H & E 120x].
4. Discussion
Cadmium and its derivatives considered one of the most dangerous toxic compounds adversely effect on the health of living organisms (Patra et al., 2011). It was reported that exposure to cadmium compounds increase the generation of free radicals which produces oxidative damage of many biological tissues (Liu et al., 2008). It was reported that liver and kidney are the most sensitive organs towards cadmium toxicity (Liu et al., 2010).
The accumulation of cadmium in human organs contributes with many pathological diseases including renal dysfunction (Arisawa et al., 2007).
So, studying the mechanism of toxicity induced by toxins and drugs encourage more scientists to search for new biologically active constituents from herbal plants which could have intrinsic antioxidant activity to protect biological systems from free radical oxidative damage (Sarwat et al., 2011).
Our study aimed to evaluate the potency of ginger extract at different doses against cadmium induced renal toxicity, through estimation of renal function biomarkers, histological and oxidant- antioxidant status of kidney tissues along with DNA content of renal cells as markers for oxidative tissue damage.
The current study showed high levels of polyphenolic (26.3 mg) and flavonoid (9.33 mg) compounds with high antioxidant and anti-free radical scavenging activity for ginger. It was reported that food products of plant origin are enriched with higher amounts of phenolic and polyphenolic compounds. These compounds supposed to have remarkable antioxidant and nephroprotective activities (Shan et al., 2005). Also, another research work supported that food containing higher amounts of flavonoids reduce susceptibility to certain human diseases (Policegoudra et al., 2011).
In the present study, the active biological constituents and free radical scavenging activity of ethanolic ginger extract were estimated using liquid chromatography, DPPH, and β-carotene tests. From the studied sample of ginger extract, only 28 of active phenolic constituents were identified. 6-gingerol were the highest amount (24.8 mg/g) followed by 6-shogaol (19.4 mg/g), citral (16.2 mg/g), and pyrogallol (15.2 mg/g). This was in addition to small amounts of other phenolic compounds.
Most recent phytochemical studies reported the presence of zingerone, shogaols, gingerols in addition to numerous other active compounds in ginger (Haniadka et al., 2013a, Haniadka et al., 2013b, Palatty et al., 2013). Also, gingerol (polyphenol) was identified as the major active component of fresh ginger rhizome in addition to volatile oils such as mono sesquiterpenes; camphene, beta-phellandrene and curcumin (Policegoudra et al., 2011). These compounds support the ginger as natural antioxidants to play a pivotal role in the chemoprevention of diseases resulting from free radical oxidative stress.
In the present study, ethanolic ginger extract showed remarkable antioxidant activity against both DPPH and β-carotene tests (89.55 % and 92.3 %) respectively. The obtained results suggested that the phenolic constituents of ginger have the capability to suppress the chain reactions of lipid peroxidation via free radical scavenging mechanism. The data of our study were in line with others who reported that ginger extracts have free radical scavenging effects against superoxide, hydroxyl, nitric oxide in vitro (Höferl, et al., 2015). Similarly, most phytochemicals of ginger like zingerone and [6]-Gingerol are shown to scavenge various free radicals such as superoxide, peroxyl radicals, and inhibition the production of nitric oxide (NO) and generation of inducible nitric oxide synthase (iNOS) in all biological systems (Shin et al., 2005, Ippoushi et al., 2003).
Previous study reported that exposure to Cd was responsible for many specific clinical disorders in renal function (Jin et al., 2004).
In the present study, there was significant decrease in body weight gain and increase in the weight of kidney following four weeks of Cd exposure. The data obtained matched with other reports which suggested inhibition of growth following cadmium toxicity (El-Demerdash et al., 2004). The loss of weight may be related to significant decrease in protein content and severe diarrhea. Whereas, hypertrophy and nephrotoxicity caused by CdCl2 accumulation in the kidney were the most causes of the increase in the weight of kidney along with decrease in total body weight (Chieko et al., 1981, Karmakar and Chatterjee, 1998).
However, in current study, treatment with ginger extracts at doses of 100 and 200 mg/kg increased the body weight and restored kidney weight to normal range compared to Cd induced rats. In our experimental model the data also reported that ginger extract at doses 200 mg/kg is not toxic (LD50 = 0) and proved to be very safe to humans as expected. The improvement in body and kidney weights may be related to the antioxidant and free radical scavenging ability of ginger. According to the presence of oxygen groups in ginger constituents, it behaves like a ligand and prevents cadmium accumulation within kidney via complexation mechanism (Telisman, et al., 2007). Also, it was proposed that vitamin content of ginger especially vitamin A, B6, and retinoids, play a vital role in body growth, fat reserves, and protein synthesis (Ali et al., 2008, Goyall and Kadnur, 2006). Similarly, phenolic compounds of ginger may increase appetite and stimulate the feeding control in the central nervous system followed by body weight gain (Goyall, and Kadnur, 2006).
In the present study, serum creatinine, creatinine clearance, urine albumin, and urea were estimated to assess renal glomerular function. The data obtained showed significant increase in renal markers; creatinine, urea, urine albumin along with decrease in creatinine clearance following four weeks of Cd exposure. The change in the levels of serum renal biomarkers represents renal damage caused by Cd as previously reported in literature (Bekheet et al., 2011). These data were supported with others who reported that cadmium toxicity induced tubular necrosis or loss of the brush border and damage in the small tubules of kidney (Wang et al., 2009). In the same manner, the increase in excretion of albumin supports that cadmium toxicity induced dysregulation of renal proximal tubule which prevents reabsorption of glomerular-filtered albumin (Gena et al., 2010). In addition to that, the elevated blood urea and lower creatinine clearance caused by cadmium toxicity may be related to disorder in protein catabolism as result of increase in the synthesis of arginase enzyme involved in urea production (Tormanen, 2006), and glomerular functional disturbances such as lower filtration rate which results in poor creatinine clearance in cadmium intoxicated rats (L'Azou et al., 2002).
In this study, the administration of ethanolic ginger extract at doses of 100 and 200 mg/kg showed significant improvement in renal function by decrease in the levels of urea and creatinine along with increase in creatinine clearance and reabsorption of glomerular-filtered albumin. The data obtained suggest a pivotal anti oxidative and chelating effect of ginger against Cd nephrotoxicity, and that the administration of this plant extract alone has no effect on the levels of renal function markers proving the safe use of this plant on kidneys.
The potential effect of ginger extract was related to the antioxidant and nephroprotective activities of its phenolic and flavonoid compounds (Shan et al., 2005, Policegoudra et al., 2011). Our data matched with others who reported significant decrease in BUN among experimental animals following administration of ginger extract for 20 day (Masuda et al., 2004). These support the importance of ginger as therapeutic herbal remedy to manage renal function in patients with uremia as previously reported in literature (Maghsoudi et al., 2011).
Previous reported concluded that the release of cadmium within cells is responsible for production of oxidative free radicals, lipid peroxidation, reduction of antioxidant enzymes, protein cross-linking, and DNA damage, collectively -induced cell death or apoptosis (Brennan, 1996). It was proposed that renal toxicity is attributed with abnormal production of oxidative free radicals which in turn activates renal cell damage (Assadi, 2012).
Our results of the present study showed significant increase in kidney MDA as lipid peroxide marker and decrease in total antioxidant capacity (TAC) as marker of antioxidant status following four weeks of cadmium treatment. The data were in line with others who reported the decrease in antioxidant status such as SOD and CAT in kidney and increase in oxidative free radicals following cadmium toxicity (Ognjanović et al., 2010a, Ognjanović et al., 2010b, Renugadevi and Prabu, 2010), the increase in oxidative free radicals of lipid peroxidation (LPO) produces significant changes in antioxidant enzymatic proteins and lipids of biological membranes which in turn produce tissue cell damage and nephrotoxicity (Messaoudi, et al., 2009).
The potential protective effect of ginger extract at doses 100 and 200 mglkg against kidney dysfunction and change in haemostatic balance of oxidant-antioxidant status induced by cadmium intoxication in this study may be related to active biological constituents of ginger which possess higher antioxidant and free radical scavenging efficiency (Hamed et al., 2012). The improvement in antioxidant status in kidney tissues following ginger administration which noticed by the increase in TAC as collective marker of antioxidant profile may be due to the active phytochemicals present in ginger such as gingerols, shogaols and other related bioactive phenolic compounds as shown previously (Haniadka et al., 2013a, Haniadka et al., 2013b, Palatty et al., 2013).
The production of reactive oxidative radicals promotes damage of cells and release of LPO radicals, alteration in biological proteins and nucleic acid structure (DNA). The imbalance between oxidant -antioxidant status increased the oxidative damage of DNA (Cooke et al., 2003).
In the present, DNA content of renal cells was estimated as a marker of oxidative renal tissue damage induced by cadmium toxicity on molecular level. There was significant decrease in DNA of renal cell treated with cadmium compared to control group. It was reported previously that cadmium can interfere with gene expression, signal transduction, and DNA repair processes (Bertin and Averbeck, 2006, Schwerdtle et al., 2010), via generation of reactive oxidative radicals which promote inhibition of Na, K-ATPase, and activate cellular DNA fragmentation which leading to the induction of necrosis and apoptosis (Mao et al., 2011, Valverde et al., 2001).
The results of this study revealed a protective effect of ginger against free radical oxidative damage caused by cadmium intoxication. There was improvement in DNA content in ginger treated rats at doses of 100 and 200 mg/kg following exposure to cadmium toxicity for four weeks. This may be due to higher antioxidant, free radical scavenging, and chelating activity of polyphenolic and flavonoid constituents of ginger. The data matched with others who reported a protective effect of [6]-Gingerol, the major bioactive constituent of ginger against DNA damage via (•OH) radical scavenging through hydrogen atom (H•) transfer mechanism (Lin et al., 2014). Also, it was reported that ginger oil showed a promising protective effect against oxidative DNA damage induced by H2O2, and concluded that ginger acts a scavenger of oxygen radical (Lu et al., 2003). In addition, ginger constituents have been observed to attenuate oxidative cell death and prevent genotoxicity induced by toxicants (Lee et al., 2011, Yang et al., 2011).
In relation to histological modifications observed in the kidneys, we can conclude that cadmium toxicity cause tissue damage via progressive tubular damage whereas the proximal tubules were sensitive to cadmium toxicity as a result of their high re-absorptive activity (Rodrigo and Bosco, 2006).
The generated oxidative free radicals in kidney tissue especially the glomerulus and tubular epithelial cells stimulate NFkB which triggers the release of cytokines, chemokines, adhesion molecules leading to inflammation of renal tissues which in turn resulting in fibrosis, glomerular damage, and tubule interstitial damage (Ohno, 2011). Our histological observations supported both the biochemical and DNA analysis, and confirmed that ginger (Zingiber officinale) has a protective and curative activity against Cd-nephrotoxicity. Whereas, the improvement in kidney tissues as reported by histological examinations were in agreement with the improvement in kidney functional markers, and significantly supports the potential protective role of ginger against cadmium induced renal toxicity.
Finally, the results of our study clearly indicate that, ginger treatments at doses of 100 and 200 mg/kg significantly hamper Cd- nephrotoxicity and prevent renal tissue and DNA damage. This significantly related to the free radical scavenging and chelating activity of ginger bioactive constituents such as phenolic, flavonoid, and vitamin A.
In conclusion, the data concluded that ginger significantly protects renal cell dysfunction; decrease the severity of tubular cell, and DNA damage caused by cadmium intoxication via antioxidant and free radical scavenging activity. Thus, ginger ethanolic extract is implied and proposed to be useful in reducing nephrotoxicity induced by various toxicants. But more research studies are necessary to establish its clinical uses.
Acknowledgements
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Acknowledgments
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ali B.H., Blunden G., Tanira M.O., Nemmar A. Some phytochemical pharmacological and toxicological properties of ginger (Zinger officinale Roscoe): a review of recent research. Food Chem. Toxicol. 2008;46(2):409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Alvarez S.M., Gomez N.N., Scardapane L., Fornes M.W., Gimenez M.S. Effects of chronic exposure to cadmium on prostate lipids and morphology. Biometals. 2007;20:727–1241. doi: 10.1007/s10534-006-9036-9. [DOI] [PubMed] [Google Scholar]
- Arisawa K., Uemura H., Hiyoshi M., Dakeshita S., Kitayama A., Saito H., Soda M. Cause-specific mortality and cancer incidence rates in relation to urinary beta2-microglobulin: 23-year follow-up study in a cadmium-polluted area. Toxicol. Lett. 2007;28:168–174. doi: 10.1016/j.toxlet.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Assadi F. The epidemic of pediatric chronic kidney disease: the danger of skepticism. J. Nephropathol. 2012;1:61–64. doi: 10.5812/nephropathol.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J.D., Gamble M. 5th ed. London, New York and Philadelphia; Churchill Livingstone: 2002. Theory and Practice of Histological Techniques. [Google Scholar]
- Bekheet S.H., Awadalla E.A., Salman M.M., Hassan M.K. Bradykinin potentiating factor isolated from Buthus occitanus venom has a protective effect against cadmium induced rat liver and kidney damage. Tissue Cell. 2011;43:337–343. doi: 10.1016/j.tice.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Bertin G., Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88:1549–1559. doi: 10.1016/j.biochi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bhandari U., Kanojia R., Pillai K.K. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J. Ethnopharmacol. 2005;97:227–230. doi: 10.1016/j.jep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Boscolo P., Di Giampaolo L., Qiao N., Reale M., Castellani M.L., Lucci I., Travaglini P., Kouri M., Verna N., Volpe A.R., Carmignani M., Paganelli R., Di Gioacchino M. Inhibitory effects of cadmium on peripheral blood mononuclear cell proliferation and cytokine release are reversed by zinc and selenium salts. Ann. Clin. Lab. Sci. 2005;35:115–129. [PubMed] [Google Scholar]
- Brand W.W., Cuvelier H.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995;82:25. [Google Scholar]
- Brennan R.J. Cadmium is an inducer of oxidative stress in yeast. Mutat. Res. 1996;356:171–178. doi: 10.1016/0027-5107(96)00051-6. [DOI] [PubMed] [Google Scholar]
- Chieko S., Naoki S., Hirotsugu M. Decrease of plasma vitamin A, albumin and zinc in cadmium treated rats. Toxicol. Lett. 1981;8(6):323–329. doi: 10.1016/0378-4274(81)90121-1. [DOI] [PubMed] [Google Scholar]
- Cooke M.S., Evans M.D., Dizdraoglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Deevika B., Asha S., Taju G., Nalini T. Cadmium acetate induced nephrotoxicity and protective role of curcumin in rats. Asian J. Pharm. Clin. Res. 2012;5(3):186–188. [Google Scholar]
- Dische Z., Schwartez K. Determination of pentoses and hexoses. Mikrochim. Acta. 1937;2:13. [Google Scholar]
- Duruibe J.O., Ogwuegbu M.O.C., Egwurugwu J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007;2(5):112–118. [Google Scholar]
- El-Demerdash F.M., Yousef M.I., Kedwany F.S., Baghdadi H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β- carotene. Food Chem. Toxicol. 2004;42(10):1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- El-Sharaky A.S., Newairy A.A., Badreldeen M.M., Eweda S.M., Sheweita S.A. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology. 2007;235:185–193. doi: 10.1016/j.tox.2007.03.014. [DOI] [PubMed] [Google Scholar]
- El-Sharaky A.S., Newairy A.A., Kamel M.A., Eweda S.M. Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats. Food Chem. Toxicol. 2009;47(7):1584–1590. doi: 10.1016/j.fct.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Farag A.G., Elhalwagy M.E., Farid H.E. Effect of ginger supplementation on developmental toxicity induced by fenitrothioninsecticide and/or lead in albino rats. Pestic. Biochem. Physiol. 2010;97(3):267–274. [Google Scholar]
- Fawcett J.K., Scott J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gena P., Calamita G., Guggino W.B. cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ. Health Perspect. 2010;118:1551–1556. doi: 10.1289/ehp.0901874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Trujano E., Navarrete A. Effect of zinc on the cadmium acute intoxication in the gastric injury induced in rats. Rev. Latinoamer. Quím. 2011;39(1–2):45–54. [Google Scholar]
- Goyall R.K., Kadnur S.V. Beneficial effects of Zingiber officinale on old thioglucose induced obesity. Fitoterapia. 2006;77(3):160–163. doi: 10.1016/j.fitote.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hamden K., Carreau S., Ellouz F., Masmoudi H., El Feki A. Improvement effect of green tea on hepatic dysfunction, lipid peroxidation and antioxidant defence depletion induced by cadmium. Afr. J. Biotech. 2009;8:4233–4238. [PubMed] [Google Scholar]
- Hamed M.A., Ali S.A., El-Rigal N.S. Therapeutic potential of Ginger against renal injury induced by carbon tetrachloride in rats. Sci. World J. 2012;2012:840421. doi: 10.1100/2012/840421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniadka R., Saldanha E., Sunita V., Palatty P.L., Fayad R., Baliga M.S. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe) Food Funct. 2013;4:845–855. doi: 10.1039/c3fo30337c. [DOI] [PubMed] [Google Scholar]
- Haniadka R., Saxena A., Shivashankara A.R., Fayad R., Palatty P.L. Ginger protects the liver against the toxic effects of xenobiotic compounds: preclinical observations. J. Nutr. Food Sci. 2013;3:226. [Google Scholar]
- Höferl M., Stoilova I., Wanner J., Schmidt E., Jirovetz L., Trifonova D., Stanchev V., Krastanov A. Composition and comprehensive antioxidant activity of ginger (Zingiber officinale) essential oil from Ecuador. Nat. Prod. Commun. 2015;10(6):1085–1090. [PubMed] [Google Scholar]
- Ippoushi K., Azuma K., Ito H., Horie H., Higashio H. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci. 2003;73:3427–3437. doi: 10.1016/j.lfs.2003.06.022. [DOI] [PubMed] [Google Scholar]
- Järup L., Hellström L., Alfvén T., Carlsson M.D., Grubb A., Persson B., Pettersson C., Spång G., Schütz A., Elinder C.G. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup. Environ. Med. 2000;57(10):668–672. doi: 10.1136/oem.57.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T., Kong Q., Ye T., Wu X., Nordberg G.F. Renal dysfunction of cadmium-exposed workers residing in a cadmium-polluted environment. Biometals. 2004;17(5):513–518. doi: 10.1023/b:biom.0000045730.01633.45. [DOI] [PubMed] [Google Scholar]
- Johari H., Delirnasab F., Sharifi E., Hemayat-Khah V., Pourdanesh M., Kargar H., Maryam Nikpour M., Yazdani M. The effects of hydro-alcoholic extract of zingiber officinale on prevention from plumbism in kidney tissue of neonatal rats. Zahedan J. Res. Med. Sci. (ZJRMS) 2013;15(8):13–17. [Google Scholar]
- Karmakar R., Chatterjee M. Cadmium-induced timedependent oxidative stress in liver of mice: a correlation with kidney. Environ. Toxicol. Pharmacol. 1998;6:201–207. doi: 10.1016/s1382-6689(98)00035-0. [DOI] [PubMed] [Google Scholar]
- Khaki A.A., Khaki A. Antioxidant effect of ginger to prevents lead-induced liver tissue apoptosis in rat. J. Med. Plants Res. 2010;4(14):1492–1495. [Google Scholar]
- Lamien-Meda A., Lamie C.E., Compaoré M.M.Y., Meda R.N., Kiendrebeogo M., Zeba B., Millogo J.F., Nacoulma O.G. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules. 2008;13:581–594. doi: 10.3390/molecules13030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Azou B., Dubus I., Ohayon-Courtès C., Labouyrie J., Perez L., Pouvreau C., Juvet L., Cambar J. Cadmium induces direct morphological changes in mesangial cell culture. Toxicology. 2002;15:233–245. doi: 10.1016/s0300-483x(02)00374-8. [DOI] [PubMed] [Google Scholar]
- Lee C., Park G.H., Kim C.Y., Jang J.H. [6]-Gingerol attenuates β-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2011;6:1261–1269. doi: 10.1016/j.fct.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Lin J., Li Xican, Chen L., Lu W., Chen X., Han L., Chen D. Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of [6]-gingerol: a chemical study. Bull. Kor. Chem. Soc. 2014;35(6):1633–1638. [Google Scholar]
- Liu J., Huang H.L., Zhang W.C., Li H. Cadmium-induced increase in uterine wet weight and its mechanism. Birth Defects Res. B. Dev. Reprod. Toxicol. 2010;89:43–49. doi: 10.1002/bdrb.20233. [DOI] [PubMed] [Google Scholar]
- Liu J., Qian S.Y., Guo Q., Jiang J., Waalkes M.P., Mason R.P., Kadiiska M.B. Cadmium generates reactive oxygen- and carbon-centered radical species in rats: insights from in vivo spin-trapping studies. Free Radic. Biol. Med. 2008;45:475–481. doi: 10.1016/j.freeradbiomed.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez V., Akerreta S., Casanova E., García-Mina J.M., Cavero R.Y., Calvo M.I. In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum. Nutr. 2007;62:151–155. doi: 10.1007/s11130-007-0056-6. [DOI] [PubMed] [Google Scholar]
- Lu P., Lai B.S., Liang P., Chen Z.T., Shun S.Q. Antioxidation activity and protective effection of ginger oil on DNA damage in vitro. Zhongguo Zhong Yao Za Zhi. 2003;28(9):873–875. [PubMed] [Google Scholar]
- Maghsoudi S., Gol A., Dabiri S., Javadi A. Preventive effect of ginger (Zingiber officinale) pretreatment on renal ischemia-reperfusion in rats. Eur. Surg. Res. 2011;46:45–51. doi: 10.1159/000321704. [DOI] [PubMed] [Google Scholar]
- Mao W.P., Zhang N.N., Zhou F.Y., Li W.X., Liu H.Y., Feng J., Zhou L., Wei C.J., Pan Y.B., He Z.J. Cadmium directly induced mitochondrial dysfunction of human embryonic kidney cells. Hum. Exp. Toxicol. 2011;30:920–929. doi: 10.1177/0960327110384286. [DOI] [PubMed] [Google Scholar]
- Masuda Y., Kikuzaki H., Hisamoto M., Nakatani N. Antioxidant properties of gingerol related compounds from ginger. Biofactors. 2004;21:293–296. doi: 10.1002/biof.552210157. [DOI] [PubMed] [Google Scholar]
- Messaoudi I., El Heni J., Hammouda F., Said K., Kerkeni A. Protective effects of selenium, zinc, or their combination on cadmium-induced oxidative stress in rat kidney. Biol. Trace Elem. Res. 2009;130:152–161. doi: 10.1007/s12011-009-8324-y. [DOI] [PubMed] [Google Scholar]
- Minta M., Wlodarczyk B., Biernacki B., Szkoda J., Juszkiewicz T. Selenium embryotoxicity in hamsters following exposure to sodium selenite. Acta Poloniae Toxicol. 1995;3(2):134. [Google Scholar]
- Mothana R.A. Anti-inflammatory, antinociceptive and antioxidant activities of the endemic Soqotraen Boswelliaelongata Balf. f. and JatrophaunicostataBalf. f. in different experimental models. Food Chem. Toxicol. 2011;49:2594. doi: 10.1016/j.fct.2011.06.079. [DOI] [PubMed] [Google Scholar]
- Obianime A.W., Roberts I.I. Antioxidants, cadmium-induced toxicity, serum biochemical and the histological abnormalities of the kidney and testes of the male wistar rats. Nigerian J. Physiol. Sci. 2009;24(2):177–185. doi: 10.4314/njps.v24i2.52910. [DOI] [PubMed] [Google Scholar]
- Ognjanović B.I., Marković S.D., Ethordević N.Z., Trbojević I.S., Stajn A.S., Saicić Z.S. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: protective role of coenzyme Q (10) and vitamin E. Reprod. Toxicol. 2010;29:191–197. doi: 10.1016/j.reprotox.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Ognjanović B.I., Marković S.D., Ethordević N.Z., Trbojević I.S., Stajn A.S., Saicić Z.S. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: protective role of coenzyme Q(10) and vitamin E. Reprod. Toxicol. 2010;29:191–197. doi: 10.1016/j.reprotox.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Ohno I. Relationship between hyperuricemia and chronic kidney disease. Nucleosides, Nucleotides Nucl. Acids. 2011;30:1039–1044. doi: 10.1080/15257770.2011.611484. [DOI] [PubMed] [Google Scholar]
- Onwuka F.C., Erhabor O., Eteng M.U., Umoh I.B. Protective effects of ginger toward cadmium-induced testes and kidney lipid peroxidation and hematological impairment in albino rats. J. Med. Food. 2011;14(7–8):817–821. doi: 10.1089/jmf.2010.0106. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Cooperation and Development (OECD), 1999. OECD Guidelines for Testing of Chemicals. Acute Oral Toxicity, OECD: 401.
- Palatty P.L., Haniadka R., Valder B., Arora R., Baliga M.S. Ginger in the prevention of nausea and vomiting: a review. Crit. Rev. Food Sci. Nutr. 2013;53:659–669. doi: 10.1080/10408398.2011.553751. [DOI] [PubMed] [Google Scholar]
- Patra R.C., Rautray A.K., Swarup D. Oxidative stress in leadand cadmium toxicity and its amelioration. Vet. MedInt. 2011;20:457327. doi: 10.4061/2011/457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policegoudra R.S., Aradhya S.M., Singh L. Mango ginger (Curcuma amada Roxb.), a promising spice for phytochemicals and biological activities. J. Biosci. 2011;36:739–748. doi: 10.1007/s12038-011-9106-1. [DOI] [PubMed] [Google Scholar]
- Renugadevi J., Prabu S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010;62:171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Rodrigo R., Bosco C. Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. A review. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2006;142:317–327. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarwat M., Das S., Srivastava P.S. Estimation of genetic diversity and evaluation of relatedness through molecular markers among medicinally important trees: Terminalia arjuna, T. chebula and T. bellerica. Mol. Biol. Rep. 2011;38:5025–5036. doi: 10.1007/s11033-010-0649-2. [DOI] [PubMed] [Google Scholar]
- Satarug S., Ujin P., Vanavanitkun Y., Baker J.R., Moore J.R. Influence of body iron-ore status and cigarette smoking on cadmium body burden of healthy thai women and men. Toxicol. Lett. 2004;148:177–185. doi: 10.1016/j.toxlet.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Schwerdtle T., Ebert F., Thuy C., Richter C., Mullenders L.H., Hartwig A. Genotoxicity of soluble and particulate cadmium compounds: impact on oxidative DNA damage and nucleotide excision repair. Chem. Res. Toxicol. 2010;23:432–442. doi: 10.1021/tx900444w. [DOI] [PubMed] [Google Scholar]
- Shan B., Cai Y.Z., Sun M., Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Shin S.G., Kim J.Y., Chung H.Y., Jeong J.C. Zingerone as an antioxidant against peroxynitrite. J. Agric. Food Chem. 2005;53:7617–7622. doi: 10.1021/jf051014x. [DOI] [PubMed] [Google Scholar]
- Stoilova I., Krastanov A., Stoyanova A., Denev P., Gargova S. Antioxidant activity of a ginger extract (Zingiber officinale) Food Chem. 2007;102:764–770. [Google Scholar]
- Sullivan J.B., Krieger G.R. 2nd ed. Williams & Wilkins; USA: 2001. Clinical Environmental Health and Toxic Exposures. [Google Scholar]
- Swarup D., Naresh R., Varshney V.P., Balagangatharathilagar M., Kumar P., Nandi D., Patra R.C. Changes in plasma hormones profile and liver function in cows naturally exposed to lead and cadmium around different industrial areas. Res. Vet. Sci. 2007;82:16–21. doi: 10.1016/j.rvsc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Teitz N.W. 3rd ed. WB Saunders Company; Philadelphia (PA): 1987. Fundamentals of Clinical Chemistry. [Google Scholar]
- Telisman S., Colak B., Pizent A., Jurasović J., Cvitković P. Reproductive toxicity of low-level lead exposure in men. Environ. Res. 2007;105(2):256–266. doi: 10.1016/j.envres.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Tormanen C.D. Inhibition of rat liver and kidney arginase by cadmium ion. J. Enzyme Inhib. Med. Chem. 2006;21:119–123. doi: 10.1080/14756360500483420. [DOI] [PubMed] [Google Scholar]
- Utley H.C., Bernheim F., Hochslein P. Effect of sulfhydryl reagent on peroxidation in microsome. Arch Biochem. Biophys. 1967;260:521. [Google Scholar]
- Valverde M., Trejo C., Rojas E. Is the capacity of lead acetate and cadmium chloride to induce genotoxic damage due to direct DNA-metal interaction? Mutagenesis. 2001;16:265–270. doi: 10.1093/mutage/16.3.265. [DOI] [PubMed] [Google Scholar]
- Wang L., Chen D., Cao J., Liu Z. Protective effect of N-acetylcysteine on experimental chronic cadmium nephrotoxicity in immature female rats. Hum. Exp. Toxicol. 2009;28:221–229. doi: 10.1177/0960327109102365. [DOI] [PubMed] [Google Scholar]
- Waskmundzka M., Wianowska D., Szewczyk K., Oniszczuk A. Effect of sample-preparation methods on the HPLC quantitation of some phenolic acids in plant materials. Acta Chromatogr. 2007;19:227. [Google Scholar]
- Yang G., Zhong L., Jiang L., Geng C., Cao J., Sun X., Liu X., Chen M., Ma Y. 6-gingerol prevents patulin-induced genotoxicity in HepG2 cells. Phytother. Res. 2011;10:1480–1485. doi: 10.1002/ptr.3446. [DOI] [PubMed] [Google Scholar]