Abstract
Leptospira is a widespread zoonosis that has been linked to transmission between dogs and humans. The main purposes were to determine the seroprevalence of anti-Leptospira serum antibody and to identify the most common serovars in dogs in Spain. This is a cross-sectional study with 1,310 records of canine Leptospira testing data from Spain since 2015 to 2017. Inclusion criteria were individual cases with MAT test results for 8 serovars (Bratislava, Icterohaemorrhagiae, Australis, Pomona, Grippotyphosa, Autumnalis, Canicola and Saxkoebing) and to have the zip code data. Three hundred and thirty-eight samples (25.8%; 95%CI 23.6–28.4) were seropositive (≥1:100). According to geographic areas, North had the highest seroprevalence (38.0%; 95%CI 28.9–47.1) followed by South (29.4%; 95%CI 20.1–38.8), Center (28.6%; 95%CI 24.3–33.0), Mediterranean (22.3%; 95%CI 19.1–25.6) and Northwest (22.2%; 95%CI 7.9–36.4). Seropositivity (MAT ≥1:100) was most common to serovars Icterohaemorrhagiae (19.4%; 95%CI 17.2–21.5) and Bratislava (8.5%; 95%CI 7.0–10.0), followed by Grippotyphosa (7.2%; 95%CI 5.8–8.6), Australis (6.4%; 95%CI 5.0–7.7), Autumnalis (5.0%; 95%CI 3.8–6.2), Pomona (4.5%; 95%CI 3.3–5.6), Canicola (3.4%; 95%CI 2.4–4.4) and Saxkoebing (0.8%; 95%CI 0.3–1.3). An association was found between positivity (MAT ≥1:100) and males (P = 0.003) and dogs that were 6 years old or older were at higher risk of exposure (P = 0.001; OR 4.61; 95%CI 1.86–11.43). This study has shown that dogs in Spain are commonly exposed to Leptospira infection and points out the necessity to control the prevalence of this severe widespread zoonosis in dogs and humans.
Keywords: Microbiology, Systems biology, Zoology, Infectious disease, Veterinary medicine, Public health, Epidemiology, Infectious disease, Internal medicine, Leptospirosis, MAT, Canine leptospirosis, Europe
1. Introduction
Leptospirosis is a zoonotic disease caused by pathogenic spirochetes with worldwide distribution affecting most mammalian species (Bharti et al., 2003).
Several studies have reported variable prevalence in dogs ranging from 1.8% in Australia to 71.1% in India (Davis et al., 2008; Zwijnenberg et al., 2008; Shi et al., 2012; Ambily et al., 2013; Bier et al., 2013; Ojha et al., 2018). In Europe, the prevalence ranges from 6% in Ireland to 49% in Italy (Scanziani et al., 2002; Burriel et al., 2003; Schuller et al., 2015b; Llewellyn et al., 2016; Habus et al., 2017).
Leptospirosis in dogs has been associated with serovars Canicola and Icterohaemorraghiae but it is now clear that dogs are susceptible to infection with multiple serovars (Schuller et al., 2015a). In Europe, Grippotyphosa and Bratislava have emerged as major causes of canine leptospirosis (Ellis, 2010; Mayer-Scholl et al., 2013; Renaud et al. 2013). Based on the available antibody prevalence data in Europe, most prevalent serovars are Icterohaemorrhagiae, Grippotyphosa, Australis, Hardjo, and Canicola (Ellis, 2010). Seropositivity to the serogroup Grippotyphosa is common in mainland Europe but appears to be rare in the UK and Ireland, perhaps associated with the distribution of relevant reservoir hosts (Ellis, 2010). In Germany, the most common serovars in dogs are Grippotyphosa and Sejroe (Geisen et al., 2007), while the serovar Australis appears to be common in Italy (Mastrorilli et al. 2007; Tagliabue et al., 2016). Infection with serovar Javanica has been described in dogs and humans in Europe, however, no reports have been found correlating infection with this serovar and clinical signs in dogs (VandenBroek et al., 1991; Cacciapuoti et al., 1994). As currently available anti-leptospiral vaccines are primarily serovar specific, the recognition of leptospires commonly involved in canine exposure in specific areas is crucial in order to keep vaccination strategies up to date. Canine vaccines used in Spain contain L. interrogans serovars Canicola and Icterohaemorragiae, although since 2012 vaccines also include L. interrogans serovar Bratislava and L. kirshneri serovar Grippotyphosa (Klaasen et al., 2013). The rate of use of these vaccines has not been described in Spain, however multiple positive titers in MAT to vaccine serovars could be an indication of recent vaccination.
In contrast to other European countries, there have been limited canine seroprevalence studies in Spain. In dogs, prevalence data is available for two regions of Spain; Andalusia with 35.7% (Millán et al., 2009) and Comunidad Valenciana with 19.8% (Benito et al., 2005). Furthermore, serovars were only determined in Andalusia by indirect microscopic agglutination test (MAT), considered 1:100 the cut-point to positive sera, detecting Canicola, Icterohaemorrhagiae, and Australis (Millán et al., 2009).
A recent meta-analysis study identified being male sex and an urban dogs as major risks factors for leptospirosis (Azócar-Aedo and Monti, 2016). Dog ownership has also been identified as a risk factor for human leptospirosis in Nicaragua (Trevejo et al., 1998), Barbados (Douglin et al., 1997) and Germany (Jansen et al., 2005) suggesting transmission of Leptospira spp. from dogs to humans. Dogs could serve as an important sentinel specie for human infection, as well as indicator of the presence of leptospires in specific environments (Ghneim et al., 2007; Major et al., 2014; Schuller et al., 2015a).
The aims of this study were: 1) to determine the seroprevalence of anti-Leptospira serum antibody in owned dogs from Spain; 2) to know the most common canine serovars and if there is any relation between the region and serovars; and 3) to determine if geographic area, season, age and sex could be risk factors for exposure to pathogenic leptospires in dogs.
2. Material and methods
A cross-sectional study design was used to determine the prevalence of antibodies against various Leptospira serovars in owned dogs from Spain. Sampling was done by convenience with serological canine leptospirosis MAT results in Spain obtained through an agreement with IDEXX Laboratories (Ludwisburg). The results of 1,310 individual tests were obtained from the proprietary database. The clinical history, vaccination status and whether samples were submitted for paired MATs were unknown. All MAT results for tests conducted from January 2015 to July 2017 were included. The laboratory is accredited according to ISO 17025 and regularly participates in the International Leptospirosis Society MAT Proficiency Testing Scheme. Source of cultures was the national reference laboratory (NRL) for leptospirosis (Bundesinstitut für Risikobewertung, BfR). MAT was performed according to OIE standards (Office International des Epizooties OIE, 2008). Samples were tested for the presence of antibodies to a panel of 8 serovars (Table 1). Dogs tested against the eight serovars with zip code of origin and dates of the test done, were eligible for inclusion. MAT positivity was defined as positive reaction to at least one serovar included in the 8 serovars panel at reciprocal titers of ≥1:100 (ALL 100) or ≥1:400 (ALL 400). Two instead of one cut-off titers were chosen in the absence of a consensus to what represents an ideal cut-off titer to document exposure in a population of dogs. A dog could be classified as having more than one positive serovar titer.
Table 1.
Panel of 8 Leptospira spp. used as live antigens for microscopic agglutination testing.
| Genomospecies | Serogroup | Serovar |
|---|---|---|
| L. interrogans | Australis | Bratislava |
| Australis | Australis | |
| Autumnalis | Autumnalis | |
| Icterohaemorrhagiae | Icterohaemorrhagiae | |
| Pomona | Pomona | |
| L. borgpetersenii | Sejroe | Saxkoebing |
| L. kischeneri | Grippothyphosa | Grippothyposa |
Samples analyzed were grouped by month of diagnosis (spring - March 1st, summer - June 1st, autumn - September 1st, and winter - December 1st), by sex (female, male), and by age (categorized as 0 to <2 years, 2 to <6 years, 6 to <10 years, and 10–15 years). Cut-offs for age categories were chosen to create 4 age groups approximately corresponding to those in previous studies (Grayzel and DeBess, 2016) although no consistent agreement on age groupings was found among studies. For association and risk factor analysis, age groups were regrouped in <6 years and ≥6 years.
Geographic mapping was used to characterize the distribution of the disease in dogs throughout Spain by zip code. Spain is geographically divided into five domains (Estrada-Peña et al. 2017). These are: 1–2) North and Northwest, which are humid, with mild winters and summers, and the rough features of natural vegetation are similar in both North and Northwest, but weather is warmer and humid in the latter; 3) Center, which is a large area at high altitude (average above 800 m above the sea level and therefore has a continental type climate); 4) Mediterranean, which is also wet because of the influence of the Mediterranean Sea, with mild winters and hot summers; and 5) South, which is the warmest and driest region.
Descriptive statistics, relative prevalence calculations, univariate and multivariate analyses were performed using IBM SPSS Statistic version 25 for Mac. All statistical analyses were performed on data from 1,310 dogs and to the serovars they were exposed without exclusion of vaccinal serogrups (Icterohaemorrhagiae, Canicola, Grippotyphosa and Australis). Prevalence of different serovars in seropositive dogs was computed by geographic region. Season prevalence was determined for complete year data samples. In order to identify risk factors, the independent variables as sex (male, female), season (winter, spring, summer and autumn), age (<6 years and ≥6 years) and region (Mediterranean, Northwest, North, Center and South) with MAT seropositivity at reciprocal titers of ≥1:100 (ALL 100) or ≥1:400 (ALL 400) were assessed. Moreover, independent variables were also assessed with positive titers (≥100) to each serovar tested in MAT.
Univariate analysis was performed using χ2 or Fisher's exact test for categorical variables. A value of P < 0.05 was considered as the critical level of significance. For multivariate analysis a logistical regression was performed. Risk factor was statistically significant at a p-value <0.05.
Geographic mapping was used to characterize the distribution of the disease in dogs throughout Spain by zip code using QGIS 3.4 (QGIS Development Team, 2018. QGIS Geographic Information System. Open Source Geospatial Foundation Project).
3. Results
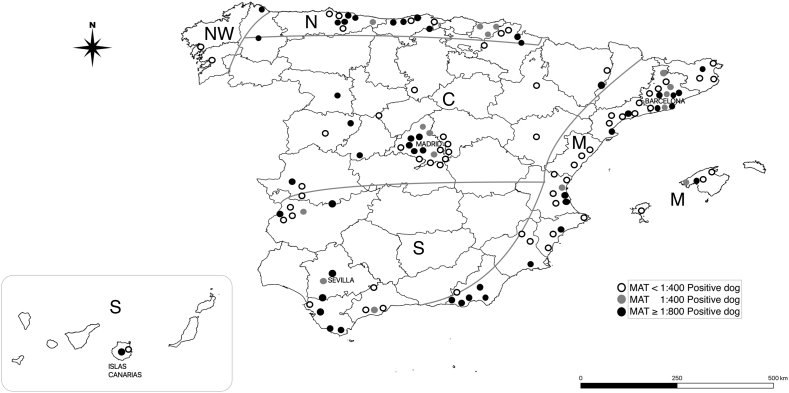
A total of 1,310 samples met the inclusion criteria. All dogs included in this study were privately owned pet dogs. Three hundred thirty-eight out of 1,310 dogs (25.8%; 95%CI 23.6–28.4) were positive titer (≥100) to at least one serovar tested. One hundred and twenty-two (9.3%; 95%CI 7.8–10.9) were positive for ≥400. One hundred and fifty-one dogs out of 338 (44.6%; 95%CI 39.3–50.0) had more than one positive titer (≥100) with a total of 723 different positive titers. The geographical distribution of positive sera across Spain is shown in Fig. 1.
Fig. 1.
The geographical distribution of positive dogs tested for serum antibodies Leptospira spp. with MAT (n = 338). Geocoding was performed based on the zip code. Geographic areas: Mediterranean (M), Northwest (NW), North (N), Center (C), and South (S).
As described in Table 2, seropositivity for MAT titer ≥100 (n = 338) was most common to serovar Icterohaemorrhagiae followed by Bratislava, Grippotyphosa, Australis, Autumnalis, Pomona and Canicola with similar prevalence, and Saxkoebing with the lowest percentage. A MAT titer ≥400 (n = 122) was quite similar among Icterohaemorrhagiae, Bratislava and Grippotyphosa, and between Pomona, Australis, Autumnalis and Canicola, with lowest prevalence of Saxkoebing. For a higher MAT titer ≥1600 (n = 44) distribution kept same patter where Icterohaemorrhagie was the most common serovar, followed by Grippotyphosa and Bratislava (Table 2).
Table 2.
Number of individuals (n), relative prevalence and 95% confidence interval (CI) per Leptospira serovar for all dogs (n = 1310). Tested serovars: Bratislava (BRA), Icterohaemorrhagie (ICT), Australis (AUS), Pomona (POM), Grippotyphosa (GRI), Autumnalis (AUT), Canicola (CAN) and Saxkoebing (SAX).
| MAT Titer | All Serovars | BRA | ICT | AUS | POM | GRI | AUT | CAN | SAX | |
|---|---|---|---|---|---|---|---|---|---|---|
| ≥1:100 | n | 338 | 111 | 254 | 84 | 59 | 94 | 66 | 44 | 11 |
| % | 25.8 | 8.5 | 19.4 | 6.4 | 4.5 | 7.2 | 5.0 | 3.4 | 0.8 | |
| CI | 23.6–28.4 | 7.0–10.0 | 17.2–21.5 | 5.0–7.7 | 3.3–5.6 | 5.8–8.6 | 3.8–6.2 | 2.4–4.4 | 0.3–1.3 | |
| ≥1:200 | n | 223 | 84 | 155 | 56 | 43 | 67 | 41 | 21 | 9 |
| % | 17.0 | 6.4 | 11.8 | 4.3 | 3.3 | 5.1 | 3.1 | 1.6 | 0.7 | |
| CI | 15.0–19.1 | 5.1–7.7 | 10.1–13.6 | 3.2–5.4 | 2.3–4.2 | 3.9–6.3 | 2.2–4.1 | 0.9–2.3 | 0.2–1.1 | |
| ≥1:400 | n | 122 | 54 | 72 | 29 | 30 | 35 | 25 | 13 | 8 |
| % | 9.3 | 4.1 | 5.5 | 2.2 | 2.3 | 2.7 | 1.9 | 1.0 | 0.6 | |
| CI | 7.8–10.9 | 3.0–5.2 | 4.3–6.7 | 1.4–3.0 | 1.5–3.1 | 1.8–3.5 | 1.2–2.6 | 0.4–1.5 | 0.2–1.0 | |
| ≥1:800 | n | 77 | 35 | 39 | 14 | 16 | 23 | 15 | 3 | 4 |
| % | 5.9 | 2.7 | 3.0 | 1.1 | 1.2 | 1.8 | 1.1 | 0.2 | 0.3 | |
| CI | 4.6–7.2 | 1.8–3.5 | 2.1–3.9 | 0.5–1.6 | 0.6–1.8 | 1.0–2.5 | 0.5–1.7 | 0.0–0.5 | 0.0–0.6 | |
| ≥1:1600 | n | 44 | 11 | 21 | 7 | 6 | 16 | 5 | 1 | 2 |
| % | 3.4 | 0.8 | 1.6 | 0.5 | 0.5 | 1.2 | 0.4 | 0.1 | 0.2 | |
| CI | 2.4–4.4 | 0.3–1.3 | 0.9–2.3 | 0.1–0.9 | 0.1–0.8 | 1.6–1.8 | 0.1–0.7 | 0.0–0.2 | 0.0–0.4 | |
| ≥1:3200 | n | 19 | 2 | 8 | 2 | 1 | 11 | - | 1 | - |
| % | 1.5 | 0.2 | 0.6 | 0.2 | 0.1 | 0.8 | - | 0.1 | - | |
| CI | 0.8–2.1 | 0.0–0.4 | 0.2–1.0 | 0.0–0.4 | 0.0–0.2 | 0.3–1.3 | - | 0.0–0.2 | - | |
| ≥1:6400 | n | 11 | 2 | 4 | - | 1 | 5 | - | 1 | - |
| % | 0.8 | 0.2 | 0.3 | - | 0.1 | 0.4 | - | 0.1 | - | |
| CI | 0.3–1.3 | 0.0–0.4 | 0.0–0.6 | - | 0.0–0.2 | 0.1–0.7 | - | 0.0–0.2 | - | |
Serogroup analyses was also performed for all samples, at lower titers Icterohaemorrhagiae was the most common serogroup found followed by Australis and Grippothyposa but at higher titers, Grippothyposa was more common than Australis (Table 3).
Table 3.
Number of individuals (n), relative prevalence and 95% confidence interval (CI) per Leptospira serogroup for all dogs (n = 1310). Tested serogroups: Australis (AUS), Icterohaemorrhagie (ICT), Pomona (POM), Grippotyphosa (GRI), Autumnalis (AUT), Canicola (CAN) and Sejroe (SAX).
| MAT Titer | All Serogroups | AUS | ICT | POM | GRI | AUT | CAN | SEJ | |
|---|---|---|---|---|---|---|---|---|---|
| ≥1:100 | n | 338 | 117 | 254 | 59 | 94 | 66 | 44 | 11 |
| % | 25.8 | 8.9 | 19.4 | 4.5 | 7.2 | 5.0 | 3.4 | 0.8 | |
| CI | 23.6–28.4 | 7.4–10.5 | 17.2–21.5 | 3.3–5.6 | 5.8–8.6 | 3.8–6.2 | 2.4–4.4 | 0.3–1.3 | |
| ≥1:200 | n | 223 | 89 | 155 | 43 | 67 | 41 | 21 | 9 |
| % | 17.0 | 6.8 | 11.8 | 3.3 | 5.1 | 3.1 | 1.6 | 0.7 | |
| CI | 15.0–19.1 | 5.4–8.2 | 10.1–13.6 | 2.3–4.2 | 3.9–6.3 | 2.2–4.1 | 0.9–2.3 | 0.2–1.1 | |
| ≥1:400 | n | 122 | 57 | 72 | 30 | 35 | 25 | 13 | 8 |
| % | 9.3 | 4.4 | 5.5 | 2.3 | 2.7 | 1.9 | 1.0 | 0.6 | |
| CI | 7.8–10.9 | 3.3–5.5 | 4.3–6.7 | 1.5–3.1 | 1.8–3.5 | 1.2–2.6 | 0.4–1.5 | 0.2–1.0 | |
| ≥1:800 | n | 77 | 37 | 39 | 16 | 23 | 15 | 3 | 4 |
| % | 5.9 | 2.8 | 3.0 | 1.2 | 1.8 | 1.1 | 0.2 | 0.3 | |
| CI | 4.6–7.2 | 1.9–3.7 | 2.1–3.9 | 0.6–1.8 | 1.0–2.5 | 0.5–1.7 | 0.0–0.5 | 0.0–0.6 | |
| ≥1:1600 | n | 44 | 15 | 21 | 6 | 16 | 5 | 1 | 2 |
| % | 3.4 | 1.1 | 1.6 | 0.5 | 1.2 | 0.4 | 0.1 | 0.2 | |
| CI | 2.4–4.4 | 0.6–1.7 | 0.9–2.3 | 0.1–0.8 | 1.6–1.8 | 0.1–0.7 | 0.0–0.2 | 0.0–0.4 | |
| ≥1:3200 | n | 19 | 3 | 8 | 1 | 11 | - | 1 | - |
| % | 1.5 | 0.2 | 0.6 | 0.1 | 0.8 | - | 0.1 | - | |
| CI | 0.8–2.1 | 0.0–0.5 | 0.2–1.0 | 0.0–0.2 | 0.3–1.3 | - | 0.0–0.2 | - | |
| ≥1:6400 | n | 11 | 2 | 4 | 1 | 5 | - | 1 | - |
| % | 0.8 | 0.2 | 0.3 | 0.1 | 0.4 | - | 0.1 | - | |
| CI | 0.3–1.3 | 0.0–0.4 | 0.0–0.6 | 0.0–0.2 | 0.1–0.7 | - | 0.0–0.2 | - | |
Total co-reactivity prevalence of positive dogs with ≥100 and ≥400 cut-off was analyzed (Table 4). The most common serovars with double exposure (≥400) were Bratislava and Icterohaemorrhagiae (7/25, 28.0%), Icterohaemorrhagie and Canicola (4/25, 16.0%), and Bratislava and Australis (3/25, 12.0%). Co-reactivity for serogroups in multivalent vaccine (Australis, Icterohaemorrhagiae, Canicola, Grippothyphosa) for titer ≥100 and ≥400 was 4/183 and 3/67, respectively.
Table 4.
Number of individuals positive against one, two or more serovars at a MAT Titer of ≥100 and ≥400.
| Nº seropositive serovars | MAT ≥1:100 n (%) | MAT ≥1:400 n (%) |
|---|---|---|
| 1 | 183 (54.2) | 55 (45.0) |
| 2 | 54 (16.0) | 25 (20.5) |
| 3 | 32 (9.5) | 20 (16.4) |
| 4 | 27 (8.0) | 11 (9.0) |
| 5 | 24 (7.1) | 9 (7.4) |
| 6 | 14 (4.1) | 2 (1.6) |
| 7 | 4 (1.2) | 0 (0) |
| 8 | 0 (0) | 0 (0) |
Age was known in 209/1310 (15.9%) of dogs and ranged from 0 to 15 years old (median 5.1 IQR 2–8). Distribution of age groups was 49/209 (23.4%) from 0 to <2 years, 64/209 (30.6%) from 2 to <6, 71/209 (34.0%) from 6 to <10, and 25/209 (12%) for ≥10. Prevalence for titers ≥100 was 8.2% (4/49) in <2 years, 21.8% (14/64) from 2 to <6, 32.3% (23/71) from 6 to <10, and 32.0% (8/25) in ≥10. Dogs with ≥6 years in ≥100 cut-off were in major risk of being seropositive (OR 4.6; 95% CI 1.9–11.4, P = 0.001) as well as for Icterohaemorrhagiae (OR 3.7; 95% CI 1.4–9.7, P = 0.007) (Table 6). Sex was available for 309/1310 (23.6%) dogs and was equally distributed with 180/309 (58.3%) male and 129/309 (41.7%) female. A total of 51/180 (28.3%) males and 18/129 (13.9%) females were positive at least to one serovar. Evidence of positive serovars were more frequently found in males (87.5%) than females (12.5%, P = 0.015) for MAT titers ≥400. This association was also found for Bratislava, Icterohaemorrhagiae and Autumnalis serovars (Table 5).
Table 6.
Multivariable analysis of the association between sex, age, season and region with positive against any serovar at MAT titers ≥100, ≥400 and Icterohaemorrhagiae (ICT) serovar for titer ≥100.
| Covariate | Levels | Cut-Off | Odds ratio | 95%CI | p-value |
|---|---|---|---|---|---|
| Sex | Female | Ref. | |||
| Male | ≥1:100 | 2.16 | 0.90–5.19 | 0.086 | |
| ≥1:400 | 4.03 | 0.44–37.14 | 0.218 | ||
| ICT | 1.53 | 0.60–3.87 | 0.367 | ||
| Age | <6y | Ref | |||
| ≥6y | ≥1:100 | 4.61 | 1.86–11.43 | 0.001* | |
| ≥1:400 | 2.73 | 0.41–18.17 | 0.300 | ||
| ICT | 3.73 | 1.42–9.77 | 0.007* | ||
| Season | W | Ref. | |||
| SP | ≥1:100 | 0.72 | 0.21–2.44 | 0.602 | |
| ≥1:400 | 1.37 | 0.09–20.15 | 0.818 | ||
| ICT | 0.62 | 0.18–2.22 | 0.467 | ||
| SU | ≥1:100 | 0.50 | 0.14–1.83 | 0.295 | |
| ≥1:400 | 1.21 | 0.09–16.31 | 0.890 | ||
| ICT | 0.73 | 0.20–2.67 | 0.634 | ||
| A | ≥1:100 | 0.52 | 0.15–1.87 | 0.319 | |
| ≥1:400 | 0.87 | 0.04–37.14 | 0.926 | ||
| ICT | 0.36 | 0.09–1.48 | 0.160 | ||
| Region | S | Ref. | |||
| M | ≥1:100 | 0.33 | 0.08–1.37 | 0.126 | |
| ≥1:400 | 0.15 | 0.01–2.70 | 0.195 | ||
| ICT | 0.40 | 0.08–1.86 | 0.242 | ||
| NW | ≥1:100 | - | - | - | |
| ≥1:400 | - | - | - | ||
| ICT | - | - | - | ||
| N | ≥1:100 | 1.27 | 0.18–8.93 | 0.813 | |
| ≥1:400 | 2.04 | 0.09–46.21 | 0.654 | ||
| ICT | 0.90 | 0.10–7.83 | 0.242 | ||
| C | ≥1:100 | 0.55 | 1.86–11.43 | 0.423 | |
| ≥1:400 | 0.68 | 0.06–8.11 | 0.760 | ||
| ICT | 0.67 | 0.14–3.21 | 0.618 |
*Statistically significant (p < 0.05).
Table 5.
Analysis of association (χ2) between season, sex, region or age with positive microscopic agglutination test against any of the tested serovars at MAT cut-offs ≥1:100 (ALL 100) and ≥1:400 (ALL 400); and against each serovar at MAT cut-off ≥1:100 for serovars Bratislava (BRA), Icterohamorrhagiae (ICT), Australis (AUS), Gripothypposa (GRI), Autumnalis (AUT), Canicola (CAN) and Saxkoebing (SAX).
| Variable | ALL100 | ALL400 | BRA100 | ICT100 | AUS100 | POM100 | GRI100 | AUT100 | CAN100 | SAX100 |
|---|---|---|---|---|---|---|---|---|---|---|
| SEASON | 0.187 | 0.060 | 0.232 | 0.254 | 0.512 | 0.149 | 0.039∗ | 0.09 | 0.373 | 0.426 |
| SEX | 0.003∗ | 0.015∗ | 0.015∗ | 0.023∗ | 0.072 | 0.407# | 1.000# | 0.023#∗ | 0.085# | 1.000# |
| REGION | 0.001∗ | 0.042∗ | 0.373 | 0.301 | 0.402 | 0.208 | 0.003∗ | 0.257 | 0.241 | - |
| AGE | 0.001∗ | 0.735# | 0.729# | 0.000∗ | 1.000# | 0.689# | 0.456# | 1.000# | 0.663# | - |
Statistically significant (p < 0.05).
Fisher's test used.
Seasonality was analyzed only for 2015 and 2016 samples. Autumn had the major prevalence with 82 positive dogs (37 and 45, respectively); followed by summer 60 (29 and 31), winter 57 (29 and 28) and spring 54 (30 and 24). Statistically significant relation was found between Grippothyposa positive titers and season (P = 0.039), higher prevalence was found in autumn and winter (9,0% and 9.6%, respectively) compared to spring (5.4%) and summer (4.0%).
According to geographic areas with a ≥100 cut-off, association is described in Table 5, statistically significant association was found with higher prevalence in North area (P = 0.001). For a ≥400 cut-off this relation was also found statistically significant (P = 0.042) with prevalence of 15.9% in North, 13.7% in South, 9.0% in Center, 8.3% in Northwest and 7.8% in Mediterranean region. In multivariate analyses no statistically significant risk factor was found between region and positivity (Table 6).
Dogs with MAT titer ≥100 were divided by serovar and region as described in Table 7, only one statistically significant association was found between Grippothyposa serovar and region (P = 0.003), with higher prevalence in Center (47/422, 11.1%) followed by South (7/95, 7.4%), North (10/113, 8.8%), Northwest (2/36, 5.4%) and Mediterranean (28/644, 4.3%).
Table 7.
Number of titers (n) and relative prevalence for MAT cut-off ≥100 divided by geographic areas and serovar. Serovars tested: Bratislava (BRA), Icterohaemorrhagie (ICT), Australis (AUS), Pomona (POM), Grippotyphosa (GRI), Autumnalis (AUT), Canicola (CAN), and Saxkoebing (SAX).
| Region | All Serovars | BRA | ICT | AUS | POM | GRI | AUT | CAN | SAX | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mediterranean | n | 147 | 48 | 115 | 40 | 20 | 28 | 27 | 21 | 3 |
| % | 22.3 | 7.4 | 17.9 | 6.2 | 3.1 | 4.3 | 4.2 | 3.2 | 0.5 | |
| CI | 19.1–25.6 | 5.4–9.5 | 14.9–20.8 | 4.3–8.1 | 1.7–4.4 | 2.7–5.9 | 2.6–5.7 | 1.9–4.6 | 0.0–1.0 | |
| Northwest | n | 8 | 3 | 4 | 2 | 2 | 2 | 2 | 1 | 0 |
| % | 22.2 | 8.3 | 11.1 | 5.6 | 5.6 | 5.6 | 5.6 | 2.8 | - | |
| CI | 7.9–36.4 | 0.0–17.8 | 0.3–21.9 | 0.0–13.4 | 0.0–13.4 | 0.0–13.4 | 0.0–13.4 | 0.0–8.4 | - | |
| North | n | 44 | 13 | 25 | 12 | 7 | 10 | 8 | 1 | 6 |
| % | 38.0 | 11.5 | 22.1 | 10.6 | 6.2 | 8.8 | 7.1 | 0.8 | 5.3 | |
| CI | 28.9–47.1 | 5.5–17.5 | 14.3–29.9 | 4.8–16.4 | 1.7–10.7 | 3.5–14.2 | 2.3–11.9 | 0.0–2.6 | 1.1–9.5 | |
| Center | n | 125 | 36 | 88 | 23 | 25 | 47 | 23 | 18 | 1 |
| % | 28.6 | 8.5 | 20.8 | 5.4 | 5.9 | 11.1 | 5.4 | 4.2 | 0.2 | |
| CI | 24.3–33.0 | 5.8–11.2 | 17.0–24.7 | 3.3–7.6 | 3.3–7.6 | 8.1–14.1 | 3.3–7.6 | 2.3–6.2 | 0.0–0.7 | |
| South | n | 27 | 11 | 22 | 7 | 5 | 7 | 6 | 3 | 1 |
| % | 29.4 | 11.5 | 23.2 | 7.4 | 7.4 | 7.4 | 6.3 | 3.2 | 1.0 | |
| CI | 20.1–38.8 | 5.0–18.1 | 14.5–31.8 | 2.0–12.7 | 2.0–12.7 | 2.0–12.7 | 1.3–11.3 | 0.0–6.7 | 0.0–3.1 | |
4. Discussion
The analyses conducted in this study evidenced that Spain's dogs are exposed to eight pathogenic Leptospira serovars. Our prevalence results (25.8% at ≥100 and 9.3% at ≥400 cut-off) were similar to previous data of seroprevalence of anti-Leptospira antibodies reported in Europe (Scanziani et al., 2002; Burriel et al., 2003; Krawczyk, 2005; Schuller et al., 2015b; Llewellyn et al., 2016; Delaude et al., 2017; Habus et al., 2017).
Leptospira prevalence in dogs from Spain has only been previously described in free-roaming dogs (10/28, 35.7%) in Andalusia (Millán et al., 2009) and in a mix (owned and un-owned) population of dogs (167/864, 16.7%) in Comunidad Valenciana (Benito et al., 2005). The prevalence in Andalusia was higher than the mean of the present study, even if it is compared with our South region's prevalence (29.4%). A possible explanation for this difference is that Millán's study (2009) evaluated rural and un-owned dog's population. However, in Comunidad Valenciana the described prevalence was lower than in the present study. A plausible explanation to this would be that dogs were randomly taken for analyses in the previous study (Benito et al., 2005). It should be noted that in our study a possible bias could raise the prevalence because samples were collected from a laboratory and we hypothesized that probably received most of them from veterinary clinicians in order to confirm or reject leptospirosis as a potential diagnosis of sick dogs.
Although there was some variability between the different geographic areas, the most frequent serovars found in this study were Icterohaemorrhagiae, Bratislava, Grippotyphosa and Australis. Serovar Icterohaemorrhagiae was the most prevalent especially when ≥100 cut-off was used. This could be explained as a result of vaccination bias; however, it is also remarkable that this high prevalence was kept in higher cut-offs (≥400) when vaccination bias could be more unlikely. This serovar has been previously described as the most frequent in wild and domestic animals in southern Spain (Millán et al., 2009).
Conversely, our study showed a low rate of positivity for Canicola serovar consistent with the wide use of vaccination could be responsible of the decrease of positivity in Italy (Scanziani et al. 1994, 2002; Tagliabue et al., 2016) and Europe (André-Fontaine, 2006). Although both vaccine serogroups (Icterohaemorrhagiae and Canicola) are expected to induce agglutinating antibodies, in our study the seroprevalence of Icterohaemorrhagiae antibodies was higher than those of Canicola similar to a previous study with canine serum samples in France (André-Fontaine, 2006). Perhaps, this could be explained by the higher infection pressure in the environment of the former strain, compared to the latter.
The second most prevalent serovar reported in this study was Bratislava. This was consistent with previous studies in dogs in Italy (Scanziani et al., 2002) and Scotland (VandenBroek et al., 1991). In Spain, Bratislava was considered the most prevalent serovar in cattle (Alonso-Andicoberry et al., 2001; Guitián et al., 2001; Atxaerandio et al., 2005).
Our study showed that the third most frequent co-reactivity was to Australis and Bratislava serovars (12.0%), both belong to the serogroup Australis. That co-reactivity could be due to a cross-reactivity in MAT because this test is good detecting serogroups, being a bias in the analysis. Moreover, it was described that Bratislava antibody titers often increase with titers to Grippotyphosa and Pomona in dogs (Brown et al., 1996; Greenlee et al., 2004) making the analysis of the infecting serovar even more challenging. Co-reactivity results for titers ≥400 also showed a high prevalence of Bratislava and Icterohaemorrhagiae (28.0%) as well as Icterohaemorrhagiae and Canicola (16.0%), all these serovars are found in multivalent vaccines and that could be an indicator of recent vaccination. Positive titers for only the four vaccinal serogroups was found in four dogs, while multiple co-reactivities with no vaccinal serogroups were found, this fact could be explained by cross-reaction in MAT test which is a challenge for MAT interpretation for vaccination without previous history of vaccination status.
In order to assess areas of increased risk, the prevalence of Leptospira serovars amongst 5 different geographic regions was compared. This geographic classification was based on weather variations found in different areas of Spain but does not consider the potentially significant regional variations as a flora and fauna or the presence of stagnant water and the wildlife populations. The prevalence was markedly higher in North region (38.9%) compared with quite similar prevalence found in the rest of areas of Spain. Furthermore, a significant relation between region and seropositive samples was found but no significant risk factor with any region was found. All this data could be explained due to the humid weather and more water sources available in North area. However, this high prevalence was not found in Northwest with similar climatological features, but the sample size from this region was too small to draw any conclusions and also the different sample size from different regions could be a bias for our study.
Higher prevalence could be biologically plausible in regions with lower altitudes due to less extreme climatic conditions throughout the year (Adler, 2015). Moreover, a recent study about Leptospira infection in Mediterranean periurban micro-mammals reported a prevalence of 12% that could be a high risk factor for infection in dogs and humans in this area (Millán et al., 2018). In our study, no risk association with Mediterranean area was found.
Leptospirosis in the United States has been characterized as having a seasonal distribution that favors late summer and early fall (Ward, 2002). A similar pattern can be found across Europe (Jansen et al., 2005; Baranton and Postic, 2006; Habus et al., 2017). In this study, only a significant association between Grippotyphosa and season was found (Table 5) with higher prevalence in autumn.
The high prevalence in male dogs observed in our study agreed with previous results described for canine leptospirosis (Azócar-Aedo and Monti, 2016). Similar to previous studies in dogs, our study also revealed correlation between age and MAT seropositivity, where being younger than 1-year-old was a protective factor and, in contrast, being 6 years old or older increased the risk of seropositivity (Harland et al., 2013; Grayzel and DeBess, 2016; Delaude et al., 2017). This relationship could be plausible as antibodies related to infection can persist for years and the probability of having contacted with Leptospira increases over time (Delaude et al., 2017).
The most important limitation of this study was the fact that vaccination status of the dogs was unknown, limiting the interpretation of the results of MAT. While the MAT is far from being a perfect method, it is currently the only tool available to document exposure in dogs (Adler, 2015). Non-infected dogs vaccinated with bivalent or quadrivalent whole cell anti leptospiral vaccines can have post-vaccinal titers of 1:6400 or higher to both vaccinal and non-vaccinal serovars (Barr et al., 2005; Midence et al., 2012; Martin et al., 2014). Although the majority of vaccinated dogs have been shown to become antibody negative by week 15 post-vaccination, vaccinal titers can persist for 12 months in a small percentage of dogs (Martin et al., 2014). Despite its limitations the MAT is well accepted and extensively used to derive information about the presence of exposition to Leptospira in sick (Boutilier et al., 2003; Ward et al., 2004; Ghneim et al., 2007; Hennebelle et al., 2013; Mayer-Scholl et al., 2013; Major et al., 2014) and healthy dogs (Davis et al., 2008; Arent et al., 2013; Schuller et al., 2015a; Llewellyn et al., 2016). In the absence of a consensus of what represents an ideal cut off titer for MAT positivity, our study used two different cut offs (≥100 and ≥400) to calculate seroprevalence in order to compare the results with previous publications worldwide. Furthermore, MAT does not discriminate between vaccination titers and titers due to exposure, thus adding further difficulty to the interpretation of canine tests, as a large proportion of dogs are vaccinated with either bivalent or multivalent Leptospira vaccines (Sykes et al., 2011). Moreover, multivalent vaccines were available in Spain since the study started which made the interpretation of the results even more challenging. In dogs with confirmed leptospirosis the sensitivity of MAT has been estimated to be 50–67% at 1:400 dilution and 22–67% at 1:800 dilution, while the specificity ranges from 69–93% at 1:400 dilution and 69–100% for 1:800 dilution (Miller et al., 2011). However, the sensitivity and specificity of the MAT in detecting chronic subclinical infection or post exposure titers has not been reported (Delaude et al., 2017). In the present study all statistical analyses were performed using both cut-off ≥100 and ≥400 in order to minimize the important limitation of vaccine interference. Finally, multiple seroconversions to two or more serovars contained in the current vaccines were detected in many dogs in this study, probably also related to vaccination. In this case the MAT was also not able to identify the real etiological agent as has been previously described (Cerri et al., 2003; Tagliabue et al., 2016).
5. Conclusions
This study shows that owned dogs in Spain are commonly exposed to Leptospira spp., with Icterohaemorrhagiae, Bratislava, Grippotyphosa and Australis being the most common serovars. Sex is associated with seropositivity and being older than 6 years old was a risk factor for seropositivity. Multiple positive serovars occurrence is very frequent and this produces difficulties to identify the real circulation of Leptospira in dogs. Since dogs are considered as sentinel of human zoonosis, it makes sense to consider a regular monitoring of them.
Declarations
Author contribution statement
M.C. López: Analyzed and interpreted the data; Wrote the paper.
A. Vila, X. Roura: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
J. Rodón: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adler B. Springer-Verlag Berlin Heidelberg; 2015. Leptospira and Leptospirosis. [Google Scholar]
- Alonso-Andicoberry C., García-Peña F.J., Ortega-Mora L.M. Epidemiología, diagnóstico y control de la leptospirosis bovina (Revisión) Investig. Agrar. Prod. Sanid. Anim. 2001;16:205–226. [Google Scholar]
- Ambily R., Mini M., Joseph S., Krishna S.V., Abhinay G. Canine leptospirosis - a seroprevalence study from Kerala, India. Vet. World. 2013;6:42–44. [Google Scholar]
- André-Fontaine G. Canine leptospirosis-Do we have a problem? Vet. Microbiol. 2006;117:19–24. doi: 10.1016/j.vetmic.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Arent Z.J., Andrews S., Adamama-Moraitou K., Gilmore C., Pardali D., Ellis W.A. Emergence of novel Leptospira serovars: a need for adjusting vaccination policies for dogs? Epidemiol. Infect. 2013;141:1148–1153. doi: 10.1017/S0950268812002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atxaerandio R., Aduriz G., Ziluaga I., Esteban J.I., Maranda L., Mainar-Jaime R.C. Serological evidence of Leptospira interrogans serovar Bratislava infection and its association with abortions in cattle in northern Spain. Vet. Rec. 2005;156:376–380. doi: 10.1136/vr.156.12.376. [DOI] [PubMed] [Google Scholar]
- Azócar-Aedo L., Monti G. Meta-analyses of factors associated with leptospirosis in domestic dogs. Zoonoses Public Health. 2016;63:328–336. doi: 10.1111/zph.12236. [DOI] [PubMed] [Google Scholar]
- Baranton G., Postic D. Trends in leptospirosis epidemiology in France. Sixty-six years of passive serological surveillance from 1920 to 2003. Int. J. Infect. Dis. 2006;10:162–170. doi: 10.1016/j.ijid.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Barr S., McDonough P., Scipioni-Ball R., Starr J. Serologic responses of dogs given a commercial vaccine against Leptospira interrogans serovar pomona and Leptospira kirschneri serovar grippotyphosa. Am. J. Vet. Res. 2005;66:1780–1784. doi: 10.2460/ajvr.2005.66.1780. [DOI] [PubMed] [Google Scholar]
- Benito M., Pérez J., Vega S., Bernabeu G., García F. Comparison of the prevalence of the infection by leptospira spp, Leishmania infantum and Ehrlichia canis in dogs in the Comunidad Valenciana (Spain) Epidémio Santé Anim. 2005;45:83–86. [Google Scholar]
- Bharti A.R., Nally J.E., Ricaldi J.E., Matthias M.A., Diaz M.M., Lovett M.A., Levett P.N., Gilman R.H., Willig M.R., Gotuzzo E., Vinetz J.M. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- Bier D., Shimakura S.E., Morikawa V.M., Ullmann L.S., Kikuti M., Langoni H., Biondo A.W., Molento M.B. Análise espacial do risco de Leptospirose canina na Vila Pantanal, Curitiba, Paraná. Pesqui. Vet. Bras. 2013;33:74–79. [Google Scholar]
- Boutilier P., Carr A., Schulman R.L. Leptospirosis in dogs: a serologic survey and case series 1996 to 2001. Vet. Ther. 2003;4:387–396. [PubMed] [Google Scholar]
- Brown C., Roberts A., Miller M., Davis D., Brown S., Bolin C., Jarecki-Black J., Greene C., Miller-Liebl D. Leptospira interrogans serovar grippotyphosa infection in dogs. J. Am. Vet. Med. Assoc. 1996;209:1265–1267. [PubMed] [Google Scholar]
- Burriel A.R., Dalley C., Woodward M.J. Prevalence of Leptospira species among farmed and domestic animals in Greece. Vet. Rec. 2003;153:146–148. doi: 10.1136/vr.153.5.146. [DOI] [PubMed] [Google Scholar]
- Cacciapuoti B., Ciceroni L., Pinto A., Apollini M., Rondinella V., Bonomi U., Benedetti E., Cinco M., Dessì S., Dettori G., Grillo R., Falomo R., Mansueto S., Miceli D., Marcuccio L., Marcuccio C., Pizzocaro P., Schivo M.L., Varaldo E., Lupidi R., Ioli A., Marzolini A., Rosmini F. Survey on the prevalence of leptospira infections in the Italian population. Eur. J. Epidemiol. 1994;10:173–180. doi: 10.1007/BF01730367. [DOI] [PubMed] [Google Scholar]
- Cerri D., Ebani V.V., Fratini F., Pinzauti P., Andreani E. Epidemiology of leptospirosis: observations on serological data obtained by a ‘diagnostic laboratory for leptospirosis’ from 1995 to 2001. New Microbiol. 2003;26:383–389. [PubMed] [Google Scholar]
- Davis M.A., Evermann J.F., Petersen C.R., VanderSchalie J., Besser T.E., Huckabee J., Daniels J.B., Hancock D.D., Leslie M., Baer R. Serological survey for antibodies to Leptospira in dogs and raccoons in Washington State. Zoonoses Public Health. 2008;55:436–442. doi: 10.1111/j.1863-2378.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Delaude A., Rodriguez-Campos S., Dreyfus A., Counotte M.J., Francey T., Schweighauser A., Lettry S., Schuller S. Canine leptospirosis in Switzerland—a prospective cross-sectional study examining seroprevalence, risk factors and urinary shedding of pathogenic leptospires. Prev. Vet. Med. 2017;141:48–60. doi: 10.1016/j.prevetmed.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Douglin C., Jordan C., Rock R., Hurley A., Levett P.N. Risk factors for severe leptospirosis in the parish of st. Andrew, Barbados. Emerg. Infect. Dis. 1997;3:78–80. doi: 10.3201/eid0301.970114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis W.A. Control of canine leptospirosis in Europe: time for a change? Vet. Rec. 2010;167:602–605. doi: 10.1136/vr.c4965. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A., Roura X., Sainz A., Miró G., Solano-Gallego L. Species of ticks and carried pathogens in owned dogs in Spain: results of a one-year national survey. Ticks Tick. Borne. Dis. 2017;8:443–452. doi: 10.1016/j.ttbdis.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Geisen V., Stengel C., Brem S., Müller W., Greene C., Hartmann K. Canine leptospirosis infections - clinical signs and outcome with different suspected Leptospira serogroups (42 cases) J. Small Anim. Pract. 2007;48:324–328. doi: 10.1111/j.1748-5827.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Ghneim G.S., Viers J.H., Chomel B.B., Kass P.H., Descollonges D.A., Johnson M.L. Use of a case-control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet. Res. 2007;38:37–50. doi: 10.1051/vetres:2006043. [DOI] [PubMed] [Google Scholar]
- Grayzel S.E., DeBess E.E. Characterization of leptospirosis among dogs in Oregon, 2007–2011. J. Am. Vet. Med. Assoc. 2016;248:908–915. doi: 10.2460/javma.248.8.908. [DOI] [PubMed] [Google Scholar]
- Greenlee J.J., Bolin C.A., Alt D.P., Cheville N.F., Andreasen C.B. Clinical and pathologic comparison of acute leptospirosis in dogs caused by two strains of Leptospira kirschneri serovar grippotyphosa. Am. J. Vet. Res. 2004;65:1100–1107. doi: 10.2460/ajvr.2004.65.1100. [DOI] [PubMed] [Google Scholar]
- Guitián F.J., García-Peña F.J., Oliveira J., Sanjuána M.L., Yusa E. Serological study of the frequency of leptospiral infections among dairy cows in farms with suboptimal reproductive efficiency in Galicia, Spain. Vet. Microbiol. 2001;80:275–284. doi: 10.1016/s0378-1135(01)00306-6. [DOI] [PubMed] [Google Scholar]
- Habus J., Persic Z., Spicic S., Vince S., Stritof Z., Milas Z., Cvetnic Z., Perharic M., Turk N. New trends in human and animal leptospirosis in Croatia, 2009–2014. Acta Trop. 2017;168:1–8. doi: 10.1016/j.actatropica.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Harland A.L., Cave N.J., Jones B.R., Benschop J., Donald J.J., Midwinter A.C., Squires R.A., Collins-Emerson J.M. A serological survey of leptospiral antibodies in dogs in New Zealand. N. Z. Vet. J. 2013;61:98–106. doi: 10.1080/00480169.2012.719212. [DOI] [PubMed] [Google Scholar]
- Hennebelle J.H., Sykes J.E., Carpenter T.E., Foley J. Spatial and temporal patterns of Leptospira infection in dogs from northern California: 67 cases (2001–2010) J. Am. Vet. Med. Assoc. 2013;242:941–947. doi: 10.2460/javma.242.7.941. [DOI] [PubMed] [Google Scholar]
- Jansen A., Schöneberg I., Frank C., Alpers K., Schneider T., Stark K. Leptospirosis in Germany, 1962-2003. Emerg. Infect. Dis. 2005;11:1048–1054. doi: 10.3201/eid1107.041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaasen H.L.B.M., Van Der Veen M., Molkenboer M.J.C.H., Sutton D. A novel tetravalent Leptospira bacterin protects against infection and shedding following challenge in dogs. Vet. Rec. 2013;172:172–181. doi: 10.1136/vr.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M. Serological evidence of leptospirosis in animals in northern Poland. Vet. Rec. 2005;156:88–89. doi: 10.1136/vr.156.3.88. [DOI] [PubMed] [Google Scholar]
- Llewellyn J.R., Krupka-Dyachenko I., Rettinger A.L. en., Dyachenko V., Stamm I., Kopp P.A., Straubinger R.K., Hartmann K. Urinary shedding of leptospires and presence of Leptospira antibodies in healthy dogs from Upper Bavaria. Berl. Munch. Tierarztl. Wochenschr. 2016 [PubMed] [Google Scholar]
- Major A., Schweighauser A., Francey T. Increasing incidence of canine leptospirosis in Switzerland. Int. J. Environ. Res. Public Health. 2014;11:7242–7260. doi: 10.3390/ijerph110707242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.E.R., Wiggans K.T., Wennogle S.A., Curtis K., Chandrashekar R., Lappin M.R. Vaccine-associated leptospira antibodies in client-owned dogs. J. Vet. Intern. Med. 2014;28:789–792. doi: 10.1111/jvim.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrorilli C., Dondi F., Agnoli C., Turba M.E., Vezzali E., Gentilini F. Clinicopathologic features and outcome predictors of Leptospira interrogans Australis serogroup infection in dogs: a retrospective study of 20 cases (2001-2004) J. Vet. Intern. Med. 2007;21:3–10. doi: 10.1892/0891-6640(2007)21[3:cfaopo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mayer-Scholl A., Luge E., Draeger A., Nöckler K., Kohn B. Distribution of leptospira serogroups in dogs from Berlin, Germany. Vector Borne Zoonotic Dis. 2013;13:200–202. doi: 10.1089/vbz.2012.1121. [DOI] [PubMed] [Google Scholar]
- Midence J.N., Chandler A.M., Goldstein R.E. Assessing the effect of recent leptospira vaccination on whole blood quantitative Pcr testing in dogs. J. Vet. Intern. Med. 2012;26:149–152. doi: 10.1111/j.1939-1676.2011.00852.x. [DOI] [PubMed] [Google Scholar]
- Millán J., Candela M.G., López-Bao J.V., Pereira M., Jiménez M.Á., León-Vizcaíno L. Leptospirosis in wild and domestic carnivores in natural areas in Andalusia, Spain. Vector Borne Zoonotic Dis. 2009;9:549–554. doi: 10.1089/vbz.2008.0081. [DOI] [PubMed] [Google Scholar]
- Millán J., Cevidanes A., Chirife A.D., Candela M.G., León-Vizcaíno L. Risk factors of Leptospira infection in Mediterranean periurban micromammals. Zoonoses Public Health. 2018;65:1–7. doi: 10.1111/zph.12411. [DOI] [PubMed] [Google Scholar]
- Miller M.D., Annis K.M., Lappin M.R., Lunn K.F. Variability in results of the microscopic agglutination test in dogs with clinical leptospirosis and dogs vaccinated against leptospirosis. J. Am. Vet. Med. Assoc. 2011;25:426–432. doi: 10.1111/j.1939-1676.2011.0704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha K.C., Singh D.K., Kaphle K., Shah Y., Pant D.K. Sero-prevalence of leptospirosis and differentiation in blood parameters between positive and negative cases in dogs of Kathmandu Valley. Trans. R. Soc. Trop. Med. Hyg. 2018;00:1–5. doi: 10.1093/trstmh/try065. [DOI] [PubMed] [Google Scholar]
- Renaud C., Andrews S., Djelouadji Z., Lecheval S., Corrao-Revol N., Buff S., Demont P., Kodjo A. Prevalence of the Leptospira serovars bratislava, grippotyphosa, mozdok and pomona in French dogs. Vet. J. 2013;196:126–127. doi: 10.1016/j.tvjl.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Scanziani E., Calcaterra S., Tagliabue S., Luini M., Giusti A.M., Tomba M. Serological findings in cases of acute leptospirosis in the dog. J. Small Anim. Pract. 1994;35:257–260. [Google Scholar]
- Scanziani E., Origgi F., Giusti A.M., Iacchia G., Vasino A., Pirovano G., Scarpas P., Tagliabue S. Serological survey of leptospiral infection in kennelled dogs in Italy. J. Small Anim. Pract. 2002;43:154–157. doi: 10.1111/j.1748-5827.2002.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Schuller S., Arent Z.J., Gilmore C., Nally J. Prevalence of antileptospiral serum antibodies in dogs in Ireland. Vet. Rec. 2015;177:126–128. doi: 10.1136/vr.102916. [DOI] [PubMed] [Google Scholar]
- Schuller S., Francey T., Hartmann K., Hugonnard M., Kohn B., Nally J.E., Sykes J. European consensus statement on leptospirosis in dogs and cats. J. Small Anim. Pract. 2015;56:159–179. doi: 10.1111/jsap.12328. [DOI] [PubMed] [Google Scholar]
- Shi D., Liu M., Guo S., Liao S., Sun M., Liu J., Wang L., Wang Z., Wang S., Yang D., Chai T. Serological survey of canine leptospirosis in southern China. Pak. Vet. J. 2012;32:280–282. [Google Scholar]
- Sykes J.E., Hartmann K., Lunn K.F., Moore G.E., Stoddard R.A., Goldstein R.E. 2010 ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J. Vet. Intern. Med. 2011;25:1–13. doi: 10.1111/j.1939-1676.2010.0654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue S., Figarolli B.M., D’Incau M., Foschi G., Gennero M.S., Giordani R., Natale A., Papa P., Ponti N., Scaltrito D., Spadari L., Vesco G., Ruocco L. Serological surveillance of Leptospirosis in Italy: two-year national data (2010-2011) Vet. Ital. 2016;52:129–138. doi: 10.12834/VetIt.58.169.2. [DOI] [PubMed] [Google Scholar]
- Trevejo R.T., Rigau-Perez J.G., Ashford D.A., McClure E.M., Jarquin-Gonzalez C., Amador J.J., de los Reyes J.O., Gonzalez A., Zaki S.R., Shieh W.J., McLean R.G., Nasci R.S., Weyant R.S., Bolin C.A., Bragg S.L., Perkins B.A., Spiegel R.A. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J. Infect. Dis. 1998;178:1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- VandenBroek A.H., Thrusfield M.V., Dobbie G.R., Ellis W.A. A serological and bacteriological survey of leptospiral infection in dogs in Edinburgh and Glasgow. J. Small Anim. Pract. 1991;32:118–124. [Google Scholar]
- Ward M.P. Seasonality of canine leptospirosis in the United States and Canada and its association with rainfall. Prev. Vet. Med. 2002;56:203–213. doi: 10.1016/s0167-5877(02)00183-6. [DOI] [PubMed] [Google Scholar]
- Ward M.P., Guptill L.F., Prahl A., Wu C.C. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997-2002) J. Am. Vet. Med. Assoc. 2004;224:1958–1963. doi: 10.2460/javma.2004.224.1958. [DOI] [PubMed] [Google Scholar]
- Zwijnenberg R.J.G., Smythe L.D., Symonds M.L., Dohnt M.F., Toribio J.A. Cross-sectional study of canine leptospirosis in animal shelter populations in mainland Australia. Aust. Vet. J. 2008;86:317–323. doi: 10.1111/j.1751-0813.2008.00324.x. [DOI] [PubMed] [Google Scholar]