Abstract
Chitosan-based nanocomposites films with different clay loadings (0, 5, 10, 15 wt %), with (10, 20, 30 wt%) and without glycerol as plasticizer, were prepared by solution casting. The effects of the addition of clay and glycerol on the thermal, mechanical, water absorption, and antimicrobial activity properties of chitosan/clay nanocomposites films were investigated in this study. XRD results indicated that the intercalated structure was obtained in the chitosan/clay nanocomposites with and without glycerol. The thermal stability of the chitosan was significantly enhanced by the presence of clay and glycerol. It was found that the addition of clay into the chitosan improved significantly the tensile strength and tensile modulus. The highest values in strength and stiffness were achieved for the chitosal/clay nanocomposites with 5 wt% of clay and 20 wt% of glycerol. The addition of both clay and glycerol reduced drastically the ductility of chitosan. The best water resistance was obtained for the chitosan film containing 5 wt% of clay and 20 wt% of glycerol. The chitosan/clay nanocomposite film had potential for application of alternative food packing materials.
Keywords: Materials science, Antimicrobial activity, Chitosan, Water sorption, Glycerol, Mechanical properties, Clay
1. Introduction
Chitosan is one of the most interesting biopolymer for application of alternative food packaging material because of its biodegradability, biocompatibility, antimicrobial properties and non-toxicity [1, 2, 3, 4]. However, chitosan has low water resistance, poor mechanical and thermal properties limiting its usage in functional films. Theses disadvantages of chitosan are attributed to the hydrophilic nature of chitosan [5, 6]. A commonly used method to improve the mechanical and barrier properties of chitosan is by the addition of nanoscale reinforcements into chitosan chains [7, 8, 9, 10, 11]. Montmorillonite clay has been widely used as an effective reinforcement in polymer/clay nanocomposites due to consisted of the several silicate layers, high aspect ratio and strong interaction with polymer matrix [12, 13, 14, 15].
Many studies on chitosan/montmorillonite clay nanocomposites have been reported previously [6, 15, 16, 17, 18, 19, 20]. It has been demonstrated that the tensile strength of chitosan/clay was increased significantly with increasing the clay loading [6, 20, 21, 22]. In addition, the thermal stability of chitosan/clay was increased with increase in the clay loading [6, 16, 24, 25]. Eventhough the presence of clay improves strength and thermal stability of chitosan but the ductility and toughness are drastically decreased. Therefore, the improvement of toughness of chitosan/clay nanocomposites should be done. A usual method for increasing the toughness of chitosan/clay nanocomposites films is by the addition of plasticizers such as poly (vinylalcohol) (PVA) [4], glycerol [7, 23], oleic acid [26], and ionic liquid [27]. Besides mechanical properties, another important property for food packaging application of chitosan nanocomposite films is the antimicrobial activity behavior. Study on the antimicrobial activity of chitosan nanocomposite films with different reinforcement materials have been demonstrated by some previous researchers. Wu et al. [28] reported that novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging was successfully synthesized, showed good antimicrobial activity and effectively extended the storage life of litchi as a packaging. Deng et al. [29] investigated quaternized chitosan-layered silicate intercalated composites based nanofibrous mats and the results showed that the antibacterial activity of the electrospun mats was enhanced when the amount of the layered silicates increased. Another application of chitosan/layered silicate composites is for as efficient absorbents for double-Stranded DNA as reported by Li et al. [30]. Chai et al. [31] prepared the copper-chelate quaternized carboxymethylchitosan/organic rectorite nanocomposites for algae inhibition. They reported that the thermal stability and algae inhibition activity of copper-chelate quaternized carboxymethylchitosan/organic rectorite nanocomposites were improved significiantly. Another application of chitosan/layered silicates films is for heavy metal removal. Xin et al. [32] prepared the novel use of bio-electrosprayingtechnique to immobilize S. cerevisiae onto poly (Ɛ-caprolactone)/chitosan/layered silicates ternary composites based nanofibrous mats mats and successfully developed reusable and cost-effective mats for heavy metal removal. Tu et al. [33] developed chitosan/cellulose composites as dye adsorbents with highly cost-effective, high-strength hydrogels, and biodegradable. Although several papers on chitosan/clay nanocomposites films have well been documented, there are still limited studies of the combined effect of both clay and glycerol on the mechanical properties of chitosan/clay nanocomposites. Most previous studies were done using sodium montmorillonite (NaMMT) clay for the chitosan/clay nanocomposites films and organically modified montmorillonite clay was still limited to be used.
The aim of this study is to elaborate the effects of the organically modified montmorillonite clay and glycerol addition on the thermal stability, mechanical, water sorption and antimicrobial activity properties of chitosan/clay nanocomposites. Chitosan/clay nanocomposites were prepared by mixing the different clay (0, 5, 10, 15 wt%) and with and without glycerol (10, 20, and 30 wt%). The structure of nanocomposites was characterized using X-ray diffraction (XRD). The mechanical properties were studied through the tensile test to find the tensile strength, elastic modulus, and elongation at break. The thermal stability was evaluated using thermogravimetric analysis. The water sorption behavior and antimicrobial activity were also investigated.
2. Experimental
2.1. Materials
High molecular weight chitosan (CS) in powder form (310–375 kDa, with viscosity of 800–2000 cP, 1 wt. % in 1% acetic acid 25 °C with a deacetylation degree greater than 75%) was purchased from Sigma-Aldrich, Singapore. Glacial acetic acid was brought from Sigma Aldrich, Singapore to produce acetic acid solutions. Glycerol used as plasticizer was purchased from Sigma-Aldrich, Singapore. Montmorillonite (MMT) clay (Nanomer I.28E) used in this work was montmorillonite clay modified by quaternary trimethylstearylammonium ions having an approximate aspect ratio of 75–120 μm, purchased from Nanocor Co., USA.
2.2. Preparation of chitosan/clay nanocomposites
Chitosan solution was prepared by dissolving 2 g of CS powder in 100 ml of aqueous acetic acid solution (1%, v/v), using a magnetic stirring plate at 90 °C and 150rpm for 1 hours and then cooled to room temperature. Chitosan nanocomposite films were obtained by dispersing selected amounts of clay (0, 5, 10, and 15% (wt/wt) on solid CS) in 100 ml of 1% (v/v) aqueous acetic acid solution for 2 h at room temperature. This dispersion was added to the CS solution and stirred for 1 h at room temperature. The dispersion was then poured onto glass discs (diameter = 14 cm) and dried at ambient conditions for 3 days, until the water was completely evaporated. Chitosan solution and its nanocomposite then were plasticized with the addition of glycerol (10, 20, and 30 wt%) on solid CS and the stirred for 2 hours at 60 °C. The sample designation and amounts of chitosan, clay, and glycerol used for each sample were summarized in Table 1.
Table 1.
Sample designation and composition.
| Sample | Composition | Parts (wt%) |
|---|---|---|
| C/0Cl | Chitosan/clay | 100/0 |
| C/5Cl | Chitosan/clay | 95/5 |
| C/10Cl | Chitosan/clay | 90/10 |
| C/15Cl | Chitosan/clay | 85/15 |
| C/5Cl/10Gl | Chitosan/clay/glycerol | 85/5/10 |
| C/5Cl/20Gl | Chitosan/clay/glycerol | 75/5/20 |
| C/5Cl/30Gl | Chitosan/clay/glycerol | 65/5/30 |
2.3. X-ray diffraction (XRD) analysis
The X-ray diffraction analysis of the clay and chitosan nanocomposites films was performed in reflection mode using X-ray diffractometer (micromeritics ASAP 2020) at a scan rate of 3°/min in a 2θ range of 0–40° and operated at 40 kV and 50 mA.
2.4. Fourier transform ifra-red (FT-IR) spectra analysis
FI-IR spectra of pure chitosan and its nanocomposites with and without glycerol films were done in transmission mode by using a FT-IR spectrophotometer in the range of 4000-400 cm−1 at a resolution of 4 cm−1.
2.5. Thermal stability
Thermal degradation processes of pure chitosan and its nanocomposite films were investigated using thermogravimetry analysis (TGA) (Perkin Elmer) in the range temperature of 30–800 °C at heating rate of 10 °C/min in nitrogen.
2.6. Tensile measurements
Tensile tests were performed according to ASTM D638 type IV using universal testing machine at a crosshead speed of 5 mm/min. Tensile strength, elastic modulus, and elongation at break were determined.
2.7. Water sorption behavior
Chitosan films were cut in small pieces (1.2 cm × 1.2 cm), desiccated overnight under vacuum and weighed to determine their dry mass. The weighed films were placed in closed beakers containing 30 ml of water and stored at the room temperature. The swelling kinetic was evaluated by periodically measuring the weight increment of the samples with respect to dry films with a balance accurate to 0.001 g, after gently bottling the surface with a tissue, until equilibrium was reached. The water gain (W.G.) was calculated as follows:
| (1) |
where mwet film and mdry flm are the weight of the wet and dry film, respectively.
2.8. Antimicrobial activity
The antimicrobial activity of the pure chitosan and its nanocomposite films was evaluated using the solid diffusion method. The representative food pathogenic bacterium (Escherichia coli) was used for testing the antimicrobial activity. The solutions of the pure chitosan and the nanocomposite films were prepared in acetat buffer at concentration of 1% (w/v). The cells of Escherichia coli were cultivated on nutrient agar and incubated at 37 °C for 24 hours. The test samples were systematically diluted from 0.1% (w/v), 0.05% (w/v), 0.025% (w/v), and 0.0125% (w/v) to determine the minimum inhibition concentration (MIC) values.
3. Results and discussion
3.1. XRD analysis
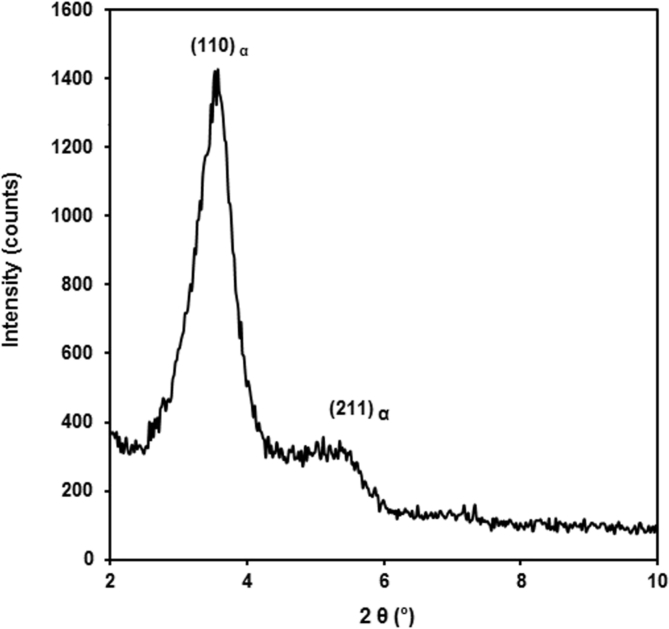
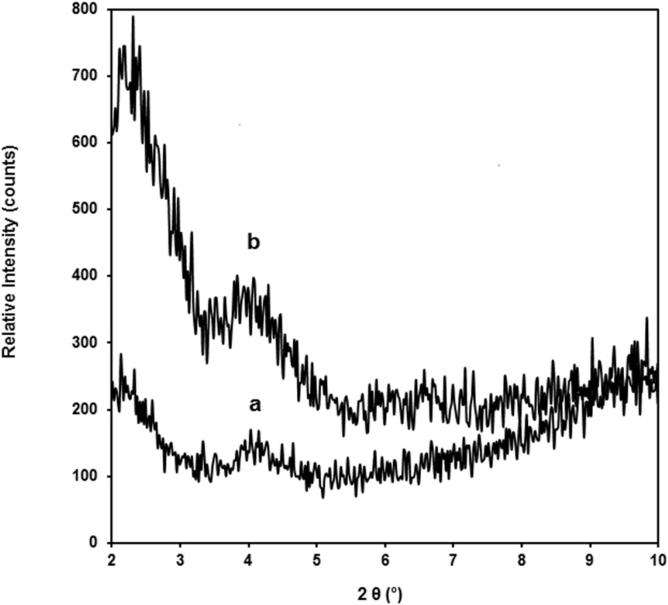
Fig. 1 presents the XRD pattern of clay. The XRD pattern of clay shows a characteristic (001) diffraction peak at 2θ = 3.48°, which corresponds to a basal spacing of 2.48 nm. The broad peak observed at 5.56° is attributed to the unmodified montmorillonite that the inorganic cations of clay were not fully replaced by organic ions [34]. The XRD patterns of the chitosan nanocomposites films containing 5 wt% of clay with and without glycerol are shown in Fig. 2. The characteristic peak of clay can be observed in the chitosan/clay nanocomposite film with and without glycerol (Figs. 2a and 2b). These indicate that the intercalated structure and or clay agglomeration are found in these chitosan nanocomposites films.
Fig. 1.
XRD pattern of clay.
Fig. 2.
XRD patterns of chitosan/clay nanocomposites film with: a. 5% wt of clay. b. 5% wt of clay and 20% wt of glycerol.
3.2. FT-IR analysis
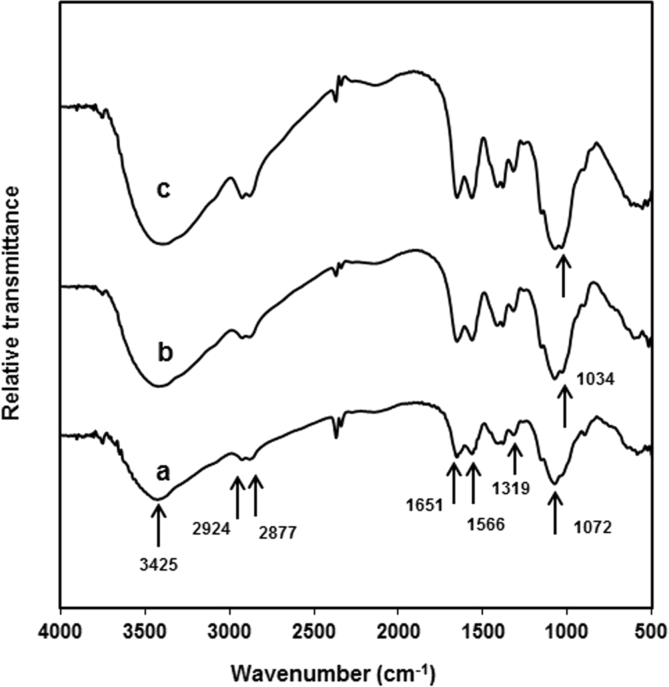
Fig. 3 shows the FT-IR spectra of pure chitosan film, chitosan nanocomposite film containing 5 wt% of clay and chitosan nanocomposite film containing 5 wt% of clay and 20 wt% of glycerol. From Fig. 3, it can be seen that all samples show the same scpectra. The absorption peaks at 3425 cm−1 corresponds to the stretching vibration of N–H while peaks at 2924 and 2877 cm−1 are assigned to the typical of C–H stretching vibration in –CH2 and –CH3 of chitosan, respectively. In addition, the peak at 1651 cm−1 corresponds to C=O strechting (amide I), the peak at 1566 cm−1 is assigned to N–H bending (amide II), the peak at 1319 cm−1 is assigned to C–N stretching (amide III) and the peak at 1072 cm−1 is assigned to Si–O bond of chitosan [3]. In the spectra of chitosan nanocomposites films with and without glycerol (Figure 3b and c), the peak at 1034 cm−1 is attributed to the Si–O–Si vibration of clay indicating the presence of clay in the nanocomposites films. The peak at 1034 cm−1 is not observed in the spectra of pure chitosan.
Fig. 3.
FT-IR spectra of: a. Pure chitosan. b. Chitosan nanocomposite film with 5 wt% of clay. c. Chitosan nanocomposite film with 5 wt% of clay and 20 wt% of glycerol.
3.3. Thermal stability
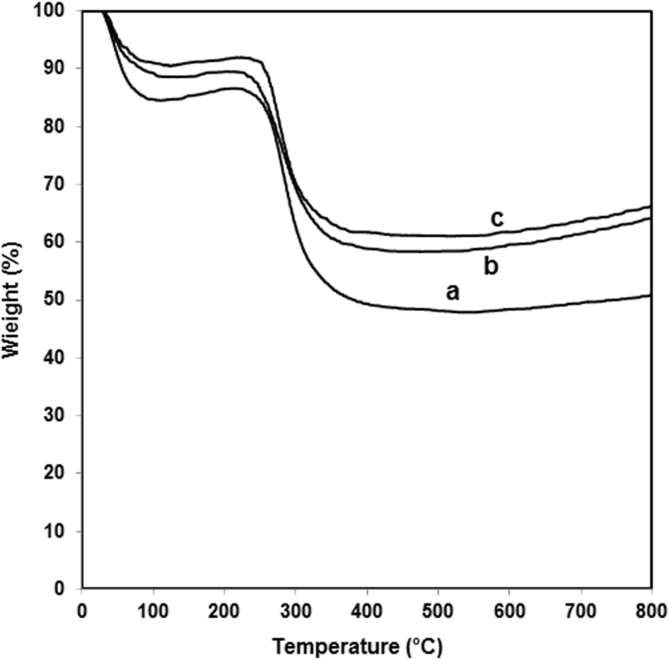
The thermal stability of pure chitosan and its nanocomposites film containing 5 wt% of clay with and without 20 wt% of glycerol were evaluated by TGA analysis as shown in Fig. 4. It can be seen that all samples present a weight loss in two stages. The first stage (30–150 °C) is related to the evaporation of absorbed water in the chitosan. The second stage (200–365 °C) is attributed to the chemical degradation and deacetylation of chitosan [35, 36]. From the thermogravimetric analysis, the temperature at which thermal degradation causes a loss of 20% is determined. The increased thermal stability can be observed for the chitosan/clay nanocomposites with and without glycerol (Figs. 4b and 4c). The degradation temperature of chitosan is improved about 4 °C and 10 °C by the presence of 5 wt% of clay. This means that the chitosan/clay nanocomposites with and without glycerol exhibited better thermal stability than the pure chitosan. The improved thermal stability may be attributed to the strong interaction between the chitosan and clay [37]. The nano layer silicates of clay with high aspect ratio acted as barriers may strongly inhibit the volatility of the decomposed products obtained from pyrolysis and limit the continuous decomposition of chitosan [38]. Furthermore, the higher increased degradation temperature about 10 °C is also obtained by the addition of glycerol plasticizer (Fig. 4c). This is attributed to the formation of the cross-link network induced by the hydrogen bonds between the chitosan and glycerol and also to an improved intercalation of chitosan molecules into the silicate galleries of clay [8].
Fig. 4.
TGA curves of: a. Pure chitosan. b. Chitosan nanocomposite film with 5 wt% of clay. c. Chitosan nanocomposite film with 5 wt% of clay and 20 wt% of glycerol.
3.4. Tensile properties
Fig. 5 shows the effect of clay loading on the tensile strength of chitosan/clay nanocomposites. It can be seen that the addition of 5 wt% of clay increased significantly the tensile strength of chitosan but drastically decreased with further clay addition. The highest value in tensile strength was obtained at the clay content of 5 wt%. This improvement may be attributed to the formation of intercalated structure as confirmed by XRD patterns (Fig. 2b). In addition, highest tensile strength at the clay content of 5 wt% may be due to more efficient stress transfer between the adjacent chitosan chains due to strong electrostatic bonding the –NH2 and –NH3 groups. The enhancement in mechanical properties of polymer/clay nanocomposites may be mainly attributed to the homogenous dispersion of high rigid clay particles with high aspect ratio in the polymer matrix, and subsequently resulting in ultra-high interfacial adhesiom and ionic bonds between clay and polymer matrix [21]. On the other hand, the tensile strength was drastically reduced by the presence of clay more than 5 wt%. The some agglomerates of clay within the chitosan matrix induced local stress concentration were believed to be responsible for the decrease in tensile strength.
Fig. 5.
Tensile strength of chitosan/clay nanocomposites films as a function of clay content.
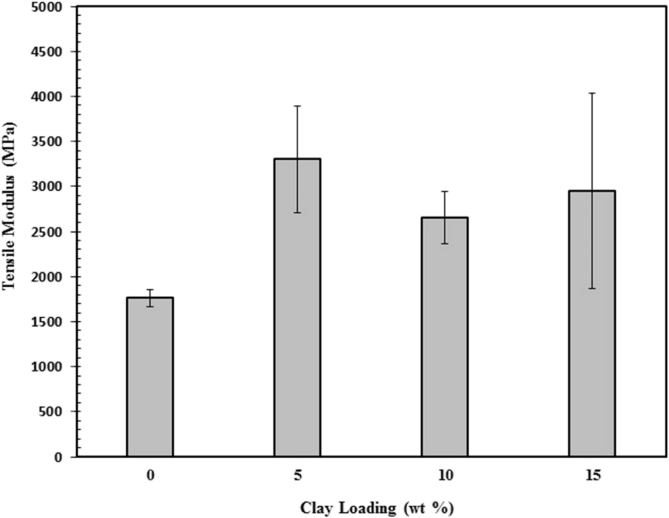
The tensile modulus as a function of clay loading of the chitosan/clay nanocomposites films is shown in Fig. 6. It can be seen that the elastic modulus of chitosan was increased with the presence of clay 5 wt% and however decreased with increasing clay content. A similar behavior to that of the tensile strength was observed. The highest elastic modulus was found for 5 wt% of clay content where the elastic modulus was improved by 87%. The improvement in elastic modulus for the clay loading of 5 wt % may be related to reinforcing effect of silicate layers of clay, its high aspect ratio and surface area, good dispersion of clay layers throughout the chitosan matrix, as well as strong interaction between chitosan matrix and silicate layers via the formation of hydrogen bonds [39]. At 15 wt% of clay content, the reduction in elastic modulus may be due to some agglomeration of clay particles within the chitosan matrix.21 The mechanical properties as a function of clay loading have been reported in various biopolymer-based nanocomposites films such as stracth [40, 41], chitosan [6], and agar [42].
Fig. 6.
Tensile modulus of chitosan/clay nanocomposites films as a function of clay content.
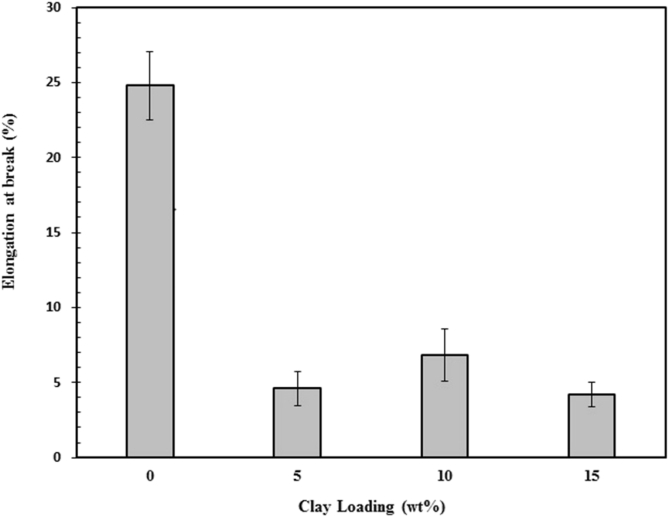
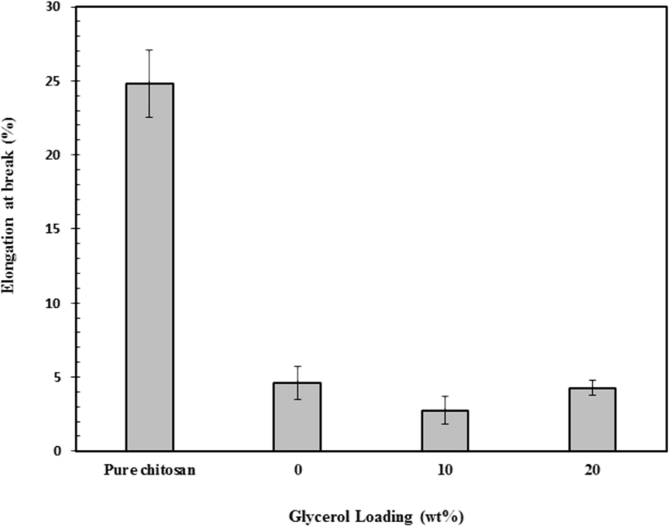
Fig. 7 displays the effect of clay loading on the elongation at break of chitosan/clay nanocomposites films. As it can be observed that the elongation at break of chitosan was decreased drastically by the addition of clay. It indicates that ductility of chitosan was decreased by the presence of clay. This may be attributed to the restricted mobility of chitosan chains by the presence of high rigid clay particle and finally results in the reduced ductility. From Fig. 7, it can also be seen that the elongation at break of chitosan/clay nanocomposites films unchanged with increasing clay content. These findings are good agreement with the previously reported results where the presence of clay in the chitosan matrix leads to a reduction in ductility [42].
Fig. 7.
Elongation at break of chitosan/clay nanocomposites films as a function of clay content.
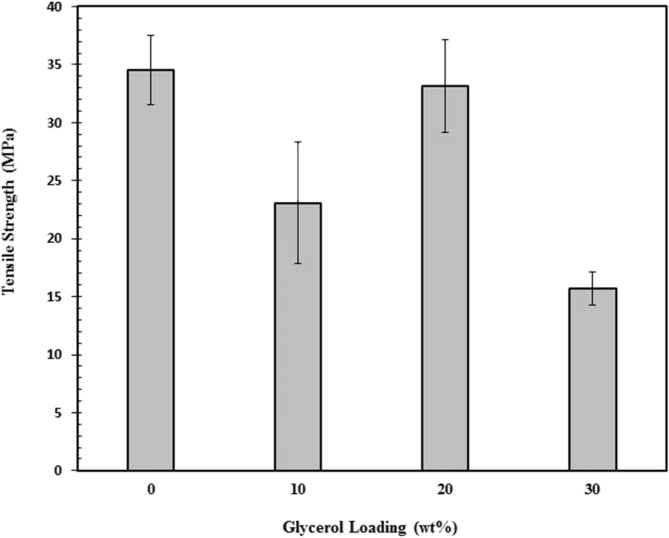
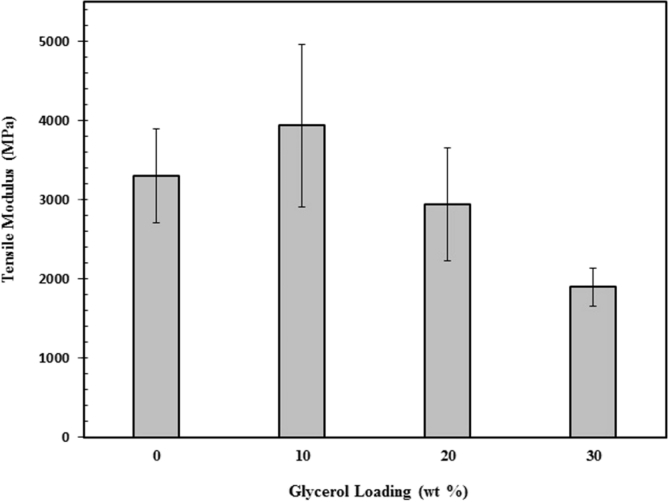
Fig. 8 depicts the effect of glycerol addition on tensile strength of chitosan/clay nanocomposites films. It can be seen that the addition of glycerol reduced the tensile strength of chitosan/clay nanocomposites. This is attributed to less hydrogen bonding formed between –OH and –NH2 of chitosan and –OH groups of glycerol that results in phase separated systems [42]. Therefore, the glycerol plays as plasticizer resulting in a pronounced reduction in tensile strength. Furthermore, the tensile modulus as a function of glycerol loading is displays in Fig. 9. The addition of 10 wt% of glycerol leads to an increase in tensile modulus of chitosan/clay nanocomposite films but decreases with increasing the glycerol content. The improved dispersion of clay for 10 wt% of glycerol is believed to be responsible for highet tensile modulus. However, the presence of glycerol above 10 wt% results in reduction in tensile modulus. The plasticizing effect of gycerol may play more dominant at higher content of glycerol. Fig. 10 demonstrates the effect of glycerol addition on the elongation at break of the chitosan/clay nanocomposite films. The elongation at break of chitosan/clay/glycerol film nanocomposites is much lower than that of pure chitosan. This means that the chitosan/clay/glycerol film nanocomposites are very brittle. The plasticizing effect was not found as expected where the addition of 10 wt% of glycerol decreased the elongation at break. Moreover, the ductility was unchanged by the presence of 20 wt% of glycerol. It can be concluded that the introduction of glycerol into the chitosan/clay nanocomposite film has no effect significantly. This may be attributed to more dominant role of rigid particle of clay compared to the presence of glycerol.
Fig. 8.
Tensile strength of chitosan/clay/glycrol nanocomposites films as a function of glycerol content.
Fig. 9.
Tensile modulus of chitosan/clay/glycrol nanocomposites films as a function of glycerol content.
Fig. 10.
Elongation at break of chitosan/clay/glycrol nanocomposites films as a function of glycerol content.
3.5. Water sorption
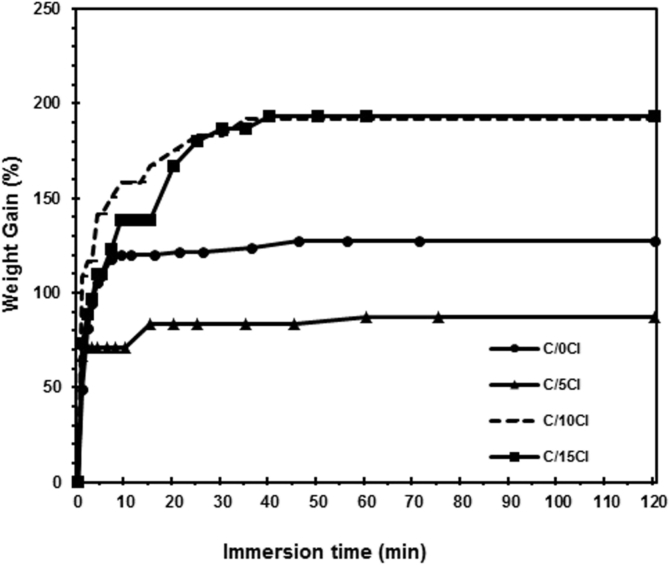
Fig. 11 shows the percentage water gains as a function of immersion time for the pure chitosan and its nanocomposite films containing different clay loading. An initial linear relationship between water gain and immersion time was observed in each case, followed by saturation. This indicates that the water absorption behavior of nanocomposites obeyed Fick's law. From Fig. 11, it can also be observed that the lowest water sorption was found in the chitosan/clay nanocomposite film containing 5 wt% of clay. This may be attributed to the best dispersion of clay in the chitosan matrix as shown in XRD results. This result is in line with the tensile results where the highest tensile strength was found at 5 wt% of clay (Fig. 5). The silicate layers of clay dispersed in the nanometer in a polymer matrix can create a tortuous pathway for water molecules to diffuse into the composites [43]. According to previous reserachers [44], the speed of moisture absorption in the polyamide 6/clay nanocomposites was reduced with increasing amounts of exfoliated silicate, due to barrier properties. Because of the high aspect ratio and large surface area of the exfoliated silicate layers, the silicate layers acted as efficient barriers against transport through the material. However, the addition of 10 and 15 wt% of clay into the pure chitosan leads to increase in the water gain. This may be related to the lowered dispersion of clay and or agglomeration of clay resulting in the decrease in the tortuosity effect.
Fig. 11.
Water sorption curves of pure chitosan and its nanocomposite films with different clay content.
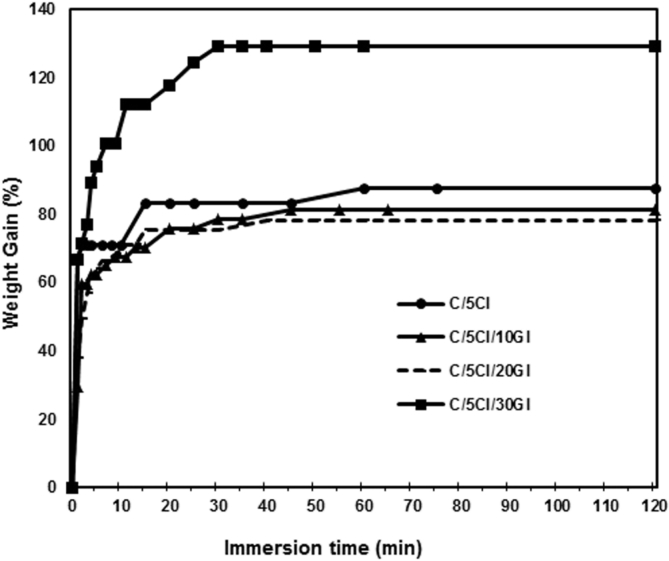
The water sorption curves of the chitosan/clay nanocomposite films containing different glycerol content are shown in Fig. 12. It is very interesting to note that the addition of glycerol up to 20 wt% reduces the water sorption. This may be related to the interaction between hydroxyl groups of glycerol and the functional groups of chitosan to form a network which does not allow water to further interact with the polar groups of chitosan and prevents the water molecules penetrating into the films [26]. However, the presence of 30 wt% of glycerol increases the water sorption. This may be attributed to higher hydrophilic molecules with the presence of 30 wt% of glycerol.
Fig. 12.
Water sorption curves of pure chitosan and its nanocomposite films with different glycerol content.
3.6. Antimicrobial activity
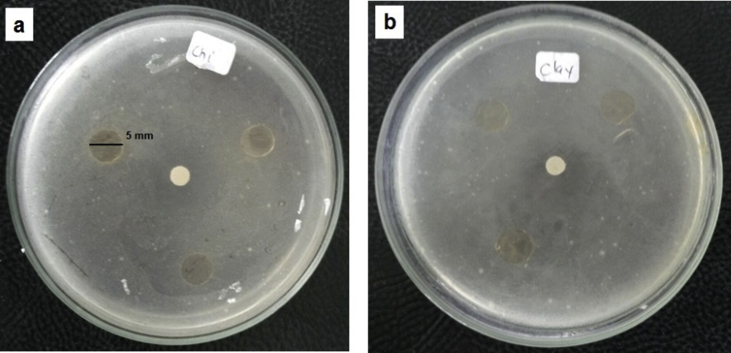
The antimicrobial activity of pure chitosan and chitosan/clay nanocomposite film has been tested against pathogenic Escherichia coli. The disc agar diffusion method was selected to perform the antimicrobial activity test. The agar diffusion method is a commonly method used to examine antimicrobial activity regarding the diffusion of the compound tested through water-containing agar plate. The inhibitory activity was measured based on the presence or not of the clear inhibition zone. In order to evaluate the effect of the addition of clay on the antimicrobial properties of chitosan, film with 5 wt% of clay was selected. The antimicrobial activity of pure chitosan and chitosan/clay film containing 5wt% of clay are presented in Figure 13a and b, respectively. It can be seen that the the absence of clear inhibition zone was observed in both pure chitosan and chitosan films containing clay. This indicates that there is no inhibitory effect shown by pure chitosan and chitosan nanocomposite film containing clay. This can be concluded that the chitosan/clay nanocomposites film can be a potential application of biodegradable food packaging films. The absence of inhibition zone on chitosan based films containing sodium montmorillonite (Na-MMT) clay was also reported by previous researchers [26].
Fig. 13.
Antimicrobial activity of pure chitosan (a) and chitosan/clay nanocomposite film (b) against Escherichia coli.
4. Conclusion
Chitosan-based nanocomposites at different clay contents, with and without glycerol as plasticizer, were successfully produced by solution casting. The interacalaed structures were observed in the chitosan/clay/glycerol nanocomposites film. It was found that the addition of clay significantly increased the strength and stiffness of neat chitosan films. The presence of glycerol above 10 wt% into the chitosan decreased drastically the stiffness and had no effect on the ductility. The combined use of clay and glycerol produced the highest values in strength and stiffness of the chitosan/clay/glycerol nanocomposites with 5 wt% of clay and 20 wt% of glycerol. However, the ductility of chitosan was drastically decreased by the introduction of clay and glycerol. The presence of glycerol led to the improved intercalation of chitosan chains into the galleries of silicate layers of clay. The enhanced thermal stability of the chitosan was obtained by the addition of both clay and glycerol. Chitosan nanocomposite film containing clay may be an alternative promising material as a packaging film.
Declarations
Author contribution statement
Kusmono: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ian Abdurrahim: Performed the experiments.
Funding statement
This work was supported by the Department of Mechanical and Industrial Engineering, Universitas Gadjah Mada through research grant (contract no. 832/H1.17/TMI/LK/2017).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors also would like to thank Dr. Alva Edy Tontowi as head of Bioceramic Laboratory, Department of Mechanical and Industrial Engineering, Faculty of Engineering, Universitas Gadjah Mada for his help with the mechanical properties measurement and Mr. E.S.S. Asianto for his assistant in collecting data.
References
- 1.Mohammed A. Chitosan application for active bio-based films production and potential in the food industry: review. LWT - Food Sci. Technol. 2010;43:837–842. [Google Scholar]
- 2.Lago M.A., Sendón R., Rodríguez-Bernaldo de Quirós A., Sanches-Silva A., Costa H.S., Sánchez-Machado D.I., Soto Valdez H., Angulo I., Aurrekoetxea G.P., Torrieri E., López-Cervantes J., Paseiro P. Preparation and characterization of antimicrobial films based on chitosan for active food packaging applications. Food Bioprocess Technol. 2014;7:2932–2941. [Google Scholar]
- 3.Leceta I., Guerrero P., Ibarburu I. Characterization and antimicrobial analysis of chitosan-based films original research article. J. Food Eng. 2013;116:889–899. [Google Scholar]
- 4.Tripathi S., Mehrotra G.K., Dutta P.K. Physicochemical and bioactivity of cross-linked chitosan–PVA film for food packaging applications. Int. J. Biol. Macromol. 2009;45:372–376. doi: 10.1016/j.ijbiomac.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y., Ren X., Hanna M.A. Chitosan/clay nanocomposite film preparation and characterization. J. Appl. Polym. Sci. 2006;99:1684–1691. [Google Scholar]
- 6.Lewandowska K., Sionkowska A., Kaczmarek B., Furtos G. Characterization of chitosan composites with various clays. Int. J. Biol. Macromol. 2014;65:534–541. doi: 10.1016/j.ijbiomac.2014.01.069. [DOI] [PubMed] [Google Scholar]
- 7.Marroquin B. Jason, Rhee K.Y., Park S.J. Chitosan nanocomposite films: enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013;92:1783–1791. doi: 10.1016/j.carbpol.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Lavorgna M., Attianese I., Buonocore G.G. MMT-supported Ag nanoparticles for chitosan nanocomposites: structural properties and antibacterial activity. Carbohydr. Polym. 2014;102:385–392. doi: 10.1016/j.carbpol.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Muzzarelli C., Muzzarelli R.A.A. Natural and artificial chitosan–inorganic composites. J. Inorg. Biochem. 2002;92:89–94. doi: 10.1016/s0162-0134(02)00486-5. [DOI] [PubMed] [Google Scholar]
- 10.Muzzarelli R.A.A. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr. Polym. 2011;83:1433–1445. [Google Scholar]
- 11.Mallakpour S., Madani M. Synthesis, structural characterization, and tensile properties of fructose functionalized multi-walled carbon nanotubes/chitosan nanocomposite films. J. Plastic Film Sheeting. 2016;32:56–73. [Google Scholar]
- 12.Fu X., Qutubuddin S. Polymer–clay nanocomposites: exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer. 2001;42:807–813. [Google Scholar]
- 13.Kalaitzidou K., Fukushima H., Miyagawa H., Drzal L.T. Flexural and tensile moduli of polypropylene nanocomposites and comparison of experimental data to Halpin-Tsai and Tandon-Weng models. Polym. Eng. Sci. 2007;47:1796–1803. [Google Scholar]
- 14.Shakesheff K.M., Cannizzaro S.M., Langer R. Creating biomimetic micro-environments with synthetic polymer-peptide hybrid molecules. J. Biomater. Sci. Polym. 1998;9:507–518. doi: 10.1163/156856298x00596. [DOI] [PubMed] [Google Scholar]
- 15.Kusmono M.W., Wildan Z.A., Ishak Mohd. Preparation and properties of clay-reinforced epoxy nanocomposites. Int. J. Polym. Sci. 2013;2013:1–7. ID 690675. [Google Scholar]
- 16.Darder M., Colilla M., Ruiz-Hitzky E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003;15:3774–3780. [Google Scholar]
- 17.Wang S.F., Shen L., Tong Y.J., Chen L., Phang I.Y., Lim P.Q., Liu T.X. Biopolymer chitosan/montmorillonite nanocomposites: preparation and characterization. Polym. Degrad. Stab. 2005;90:123–131. [Google Scholar]
- 18.Pandey S., Mishra S.B. Organic–inorganic hybrid of chitosan/organoclay bionanocomposites for hexavalent chromium uptake. J. Colloid Interface Sci. 2011;361:509–520. doi: 10.1016/j.jcis.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Wang S., Chen L., Tong Y. Structure–property relationship in chitosan-based biopolymer/montmorillonite nanocomposites. J. Polym. Sci. A: Polym. Chem. 2006;44:686–696. [Google Scholar]
- 20.Lin K.-F., Hsu Ch.-Y., Huang T.-S., Chiu W.-Y., Lee Y.-H., Young T.-H. A novel method to prepare chitosan/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2005;98:2042–2047. [Google Scholar]
- 21.Xu Y., Ren X., Hanna M.A. Chitosan/clay nanocomposite film preparation and characterization. J. Appl. Polym. Sci. 2006;99:1684–1691. [Google Scholar]
- 22.Abdolmohammadi S., Yunus W.M.Z.W., Rahman M.Z.A.B., Ibrahim N.A. Effect of organoclay on mechanical and thermal properties of polycaprolactone/chitosan/montmorillonite nanocomposites. J. Reinf. Plast. Compos. 2011;30:1045–1054. [Google Scholar]
- 23.Lavorgna M., Piscitelli F., Mangiacapra P., Buonocore G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010;82:291–298. [Google Scholar]
- 24.Günister E., Pestreli D., Ünlü C.H., Atici O., Güngör N. Synthesis and characterization of chitosan-MMT biocomposite systems. Carbohydr. Polym. 2007;67:358–365. [Google Scholar]
- 25.Giannakas A., Grigoriadi K., Leontiou A., Barkoula N.-M., Ladavos A. Preparation, characterization, mechanical and barrier properties investigation of chitosan-clay nanocomposites. Carbohydr. Polym. 2014;108:103–111. doi: 10.1016/j.carbpol.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Vlacha M., Giannakas A., Katapodis P., Stamatis H., Ladavos A., Barkoula N.-M. On the efficiency of oleic acid as plasticizer of chitosan/clay nanocomposites and its role on thermo-mechanical, barrier and antimicrobial properties-comparison with glycerol. Food Hydrocolloids. 2016;57:10–19. [Google Scholar]
- 27.Boesel L.F. Effect of plasticizers on the barrier and mechanical properties of biomimetic composites of chitosan and clay. Carbohydr. Polym. 2015;115:356–363. doi: 10.1016/j.carbpol.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z., Huang X., Li Yi-Chen, Xiao H., Wang X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018;199:210–218. doi: 10.1016/j.carbpol.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Deng H., Lin P., Xin S., Huang R., Li W., Du Y., Zhou X., Yang J. Quaternized chitosan-layered silicate intercalated composites based nanofibrous mats and their antibacterial activity. Carbohydr. Polym. 2012;89:307–313. doi: 10.1016/j.carbpol.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Li X., Han Y., Ling Y., Wang X., Sun R. Assembly of layered silicate loaded quaternized chitosan/reduced graphene oxide composites as efficient absorbents for double-stranded DNA. ACS Sustain. Chem. Eng. 2015;3:1846–1852. [Google Scholar]
- 31.Cai J., Ye W., Wang X., Lin W., Lin Q., Zhang Q., Wu F. Preparation of copper-chelate quaternized carboxymethylchitosan/organic rectorite nanocomposites for algae inhibition. Carbohydr. Polym. 2016;151:130–134. doi: 10.1016/j.carbpol.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 32.Xin S., Zeng Z., Zhou X., Luo W., Shi X., Wang Q., Deng H., Du Y. Recyclable Saccharomyces cerevisiae loaded nanofibrous mats withsandwich structure constructing via bio-electrospraying for heavymetal removal. J. Hazard Mater. 2017;324:365–372. doi: 10.1016/j.jhazmat.2016.10.070. [DOI] [PubMed] [Google Scholar]
- 33.Tu H., Yu Y., Chen J., Shi X., Zhou J., Deng H., Dua Y. Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: chitosan and cellulose. Polym. Chem. 2017;8:2913–2921. [Google Scholar]
- 34.González I., Eguiazábal J.I., Nazábal J. Rubber-toughened polyamide 6/clay nanocomposites. Compos. Sci. Technol. 2006;66:1833–1843. [Google Scholar]
- 35.Wang X., Du Y., Yang J., Wang X., Shi X., Hu Y. Preparation, characterization and antimicrobial activity of chitosan/layered silicate nanocomposites. Polymer. 2006;47:6738–6744. [Google Scholar]
- 36.Han Y.S., Lee S.H., Choi K.H., Park I. Preparation and characterization of chitosan-clay nanocomposites with antimicrobial activity. J. Phys. Chem. Solids. 2010;71:464–467. [Google Scholar]
- 37.Yu L., Li L., Wei’an Z., Yue’e F. A new hybrid nanocomposite prepared by graft copolymerization of butyl acrylate onto chitosan in the presence of organophilic montmorillonite. Radiat. Phys. Chem. 2004;69:467–471. [Google Scholar]
- 38.Almasi H., Ghanbarzadeh B., Entezami A.A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010;46:1–5. doi: 10.1016/j.ijbiomac.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Ayana B., Suin S., Khatua B. Highly exfoliated eco-friendly thermoplastic starch (TPS)/poly (lactic acid)(PLA)/clay nanocomposites using unmodified nanoclay Carbohydr. Polymer. 2014;110:430–439. doi: 10.1016/j.carbpol.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Rhim J.W. Mechanical and water barrier properties of agar/κ-carrageenan/konjac glucomannan ternary blend biohydrogel films. Carbohydr. Polym. 2011;86:691–699. doi: 10.1016/j.carbpol.2013.03.083. [DOI] [PubMed] [Google Scholar]
- 41.Ghelejlu S.B., Esmaiili M., Almasi H. Characterization of chitosan–nanoclay bionanocomposite active films containing milk thistle extract. Int. J. Biol. Macromol. 2016;86:613–621. doi: 10.1016/j.ijbiomac.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Giannakas A., Vlacha M., Salmas C. Preparation, characterization, mechanical, barrier and antimicrobial properties of chitosan/PVOH/clay nanocomposites. Carbohydr. Polym. 2016;140:408–415. doi: 10.1016/j.carbpol.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H., Li R.K.Y. Effect of water absorption on the mechanical and dielectric properties of nano-alumina filled epoxy nanocomposites. Composites A. 2008;39:602–611. [Google Scholar]
- 44.Vlasveld D.P.N., Groenewold J., Bersee H.E.N., Picken S.J. Moisture absorption in polyamide-6 silicate nanocomposites and its influence on themechanical properties. Polymer. 2005;46:12567–12576. [Google Scholar]