Abstract
The data supplied in this work are related to the research article entitled “IL-17A neutralizing antibody regulates monosodium urate crystal-induced gouty inflammation” [1]. This data article presents the results of the gating strategy applied to identify Treg population in peripheral blood of mice injected with MSU crystals and MSU crystals + interleukin-17 antibody (IL-17Ab). Lastly, this article provides in-depth immunophenotyping data relating to all specific and isotype control antibodies used in the phenotypical characterization of circulating Treg (defined as CD4+CD25+Foxp3+), Th17 (defined as CD4+IL-17+) cells and joint-infiltrated (in situ) inflammatory monocytes (defined as B220−GR1highF480highCD115+).
Keywords: Gout, IL-17A, Monocytes, Monosodium urate crystals, Neutralizing antibody, Th17, Treg
Specifications Table
| Subject | Pharmacology |
| Specific subject area | Immunopharmacology |
| Type of data | Imaging flow cytometry data, image analysis of dot plots |
| How data were acquired | BriCyte E6 flow cytometer with MRFlow and FlowJo software operation |
| Data format | Analysed data, raw data |
| Parameters for data collection | N = 6 samples from digested ankle joints and peripheral blood in each experimental condition |
| Description of data collection | The data obtained were expressed as the mean ± SD of flow cytometry raw data |
| Data source location | ImmunoPharmaLab, Department of Pharmacy, School of Medicine and Surgery, University of Naples Federico II, Naples, Italy |
| Data accessibility | Data are presented in this article |
| Related research article | F. Raucci, A.J. Iqbal, A. Saviano, P. Minosi, M. Piccolo, C. Irace, F. Caso, R. Scarpa, S. Pieretti, N. Mascolo, F. Maione. IL-17A neutralizing antibody regulates monosodium urate crystal-induced gouty inflammation. Pharmacol Res. 2019 Jul 14:104351. https://doi.org/10.1016/j.phrs.2019.104351. |
Value of the data
|
1. Data
Our data indicate a correlation between the administration of IL-17 neutralizing antibody (IL-17Ab) and a reduction in circulating Th17 cells (defined as CD4+IL-17+) and in situ infiltrated inflammatory monocytes (defined as B220−GR1highF480highCD115+) in a mouse model of gouty inflammation. However, administration of IL-17Ab did not interfere with circulating Treg levels (defines as CD4+CD25+Foxp3+) [1].
The methodology for the determination of these cell subsets is based on a standardized flow cytometry gating strategy and data analysis of related isotype control antibodies. The data presented here contribute to the understanding of the real percentage of Th17 cells and inflammatory monocytes presented in our recent published paper [1].
In particular, to identify the systemic Treg and Th17 balance, we stained isolated peripheral lymphocytes cells with an anti-CD4, an accessory protein for MHC class-II antigen/T-cell receptor interaction, an anti-CD8, a marker that identifies cytotoxic/suppressor T-cells that interacts with MHC class I. We also stained isolated peripheral lymphocytes with an anti-CD4/IL-17 and anti-CD4/CD25/Foxp3 to identify Th17 and Treg population respectively.
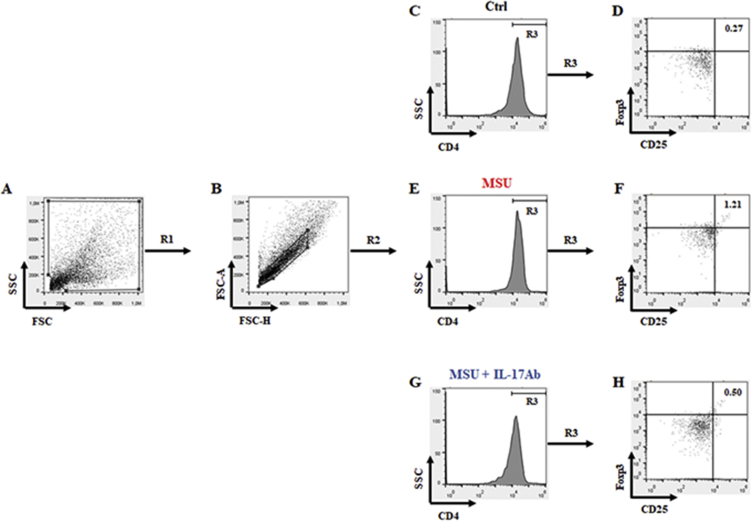
For Treg, we first established the gate for cells that were positive for CD4 and then gated for CD25 and Foxp3 (Fig. 1). Dot plots values indicate the percentage of positively stained cells in the different experimental conditions and are presented as means ± SD of n = 6 mice per group (Table 1).
Fig. 1.
Gating strategy applied to identify CD4/CD25/Foxp3 positive cells. Lymphocytes isolated from whole blood by Ficoll-Paque Plus gradient method were washed, gated in their totality (A, gate R1) and singlets (B, gate R2) for the identification of CD4 positive cells (C, E and G). Finally, CD4+ cells (gate R3) were plotted for CD25 and Foxp3 (D, F and H). Dot plots values indicated the percentage of positively stained cells in the different experimental conditions. FACS pictures are representative of six samples with similar results. Data are presented as means ± SD of n = 6 mice per group.
Table 1.
Raw data related to CD4/CD25/Foxp3 positive cells.
| CD25/Foxp3 | Ctrl | MSU | MSU + IL-17Ab |
| 0.20 | 1.35 | 0.40 | |
| 0.21 | 1.27 | 0.26 | |
| 0.28 | 1.20 | 0.33 | |
| 0.30 | 1.19 | 0.61 | |
| 0.27 | 1.41 | 0.54 | |
| 0.36 | 0.84 | 0.86 | |
| Mean ± SD | 0.27 ± 0.06 | 1.21 ± 0.20 | 0.50 ± 0.22 |
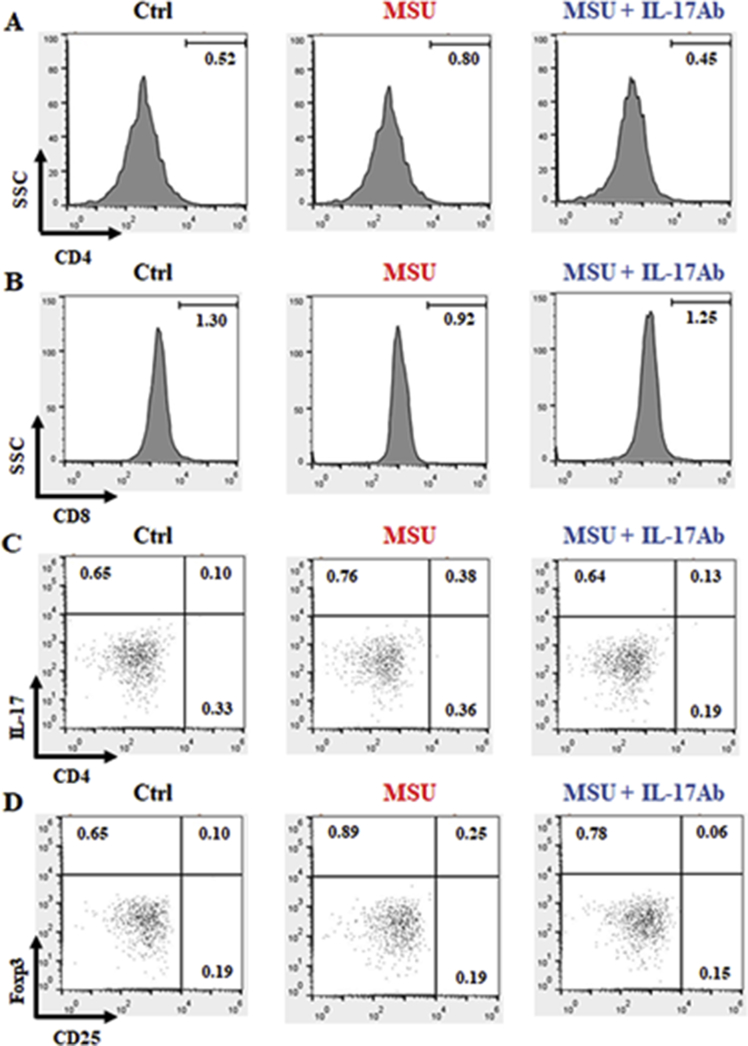
These values were strengthened by low percentage of positive cells found in all the staining for the related isotype control antibodies (Fig. 2). Data are presented as means ± SD of n = 6 mice per group (Table 2).
Fig. 2.
Strategy applied to identify potential positive cells with isotype control antibodies. Lymphocytes isolated from whole blood by Ficoll-Paque Plus gradient method were washed, stained with CD4 (A), CD8 (B), CD4/IL-17 (C) or CD25/Foxp3 (D) isotype control antibodies and analysed by FACS. The numbers in the histograms and dot plots indicated the percentage of positively stained cells in the different experimental conditions. FACS pictures are representative of six samples with similar results. Data are presented as means ± SD of n = 6 mice per group.
Table 2.
Raw data related to CD4, CD8, CD4/IL-17 and CD25/Foxp3 positive cells with isotype control antibodies.
| CD4 | Ctrl | MSU | MSU + IL-17Ab |
| 0.38 | 0.94 | 0.31 | |
| 0.42 | 0.86 | 0.49 | |
| 0.81 | 1.12 | 0.29 | |
| 0.36 | 0.74 | 0.64 | |
| 0.54 | 0.46 | 0.26 | |
| 0.61 | 0.68 | 0.71 | |
| Mean ± SD | 0.52 ± 0.17 | 0.80 ± 0.23 | 0.45 ± 0.19 |
| CD8 | Ctrl | MSU | MSU + IL-17Ab |
| 1.62 | 1.12 | 1.34 | |
| 1.13 | 0.96 | 1.29 | |
| 1.26 | 0.84 | 1.57 | |
| 0.95 | 0.75 | 1.20 | |
| 1.38 | 1.23 | 1.12 | |
| 1.46 | 0.62 | 0.98 | |
| Mean ± SD | 1.30 ± 0.24 | 0.92 ± 0.23 | 1.25 ± 0.20 |
| CD4/IL-17 | Ctrl | MSU | MSU + IL-17Ab |
| 0.10 | 0.52 | 0.26 | |
| 0.04 | 0.14 | 0.06 | |
| 0.09 | 0.29 | 0.14 | |
| 0.25 | 0.41 | 0.09 | |
| 0.11 | 0.69 | 0.21 | |
| 0.01 | 0.23 | 0.02 | |
| Mean ± SD | 0.10 ± 0.08 | 0.38 ± 0.20 | 0.13 ± 0.09 |
| CD25/Foxp3 | Ctrl | MSU | MSU + IL-17Ab |
| 0.02 | 0.33 | 0.01 | |
| 0.12 | 0.23 | 0.04 | |
| 0.19 | 0.17 | 0.09 | |
| 0.08 | 0.09 | 0.03 | |
| 0.11 | 0.46 | 0.08 | |
| 0.08 | 0.22 | 0.11 | |
| Mean ± SD | 0.10 ± 0.06 | 0.25 ± 0.13 | 0.06 ± 0.04 |
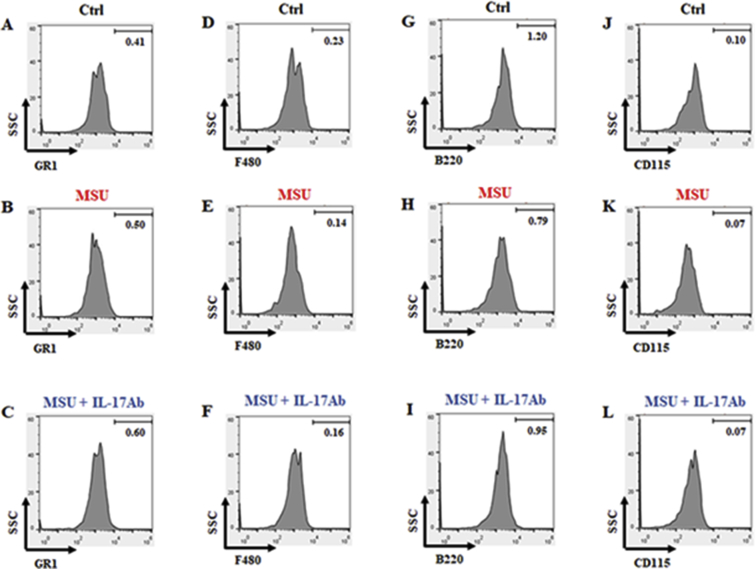
Finally, we also report the percentage of positive cells found in all the staining for the isotype control antibodies related to B220−GR1highF480highCD115+cells (Fig. 3). Data are presented as means ± SD of n = 6 mice per group (Table 3).
Fig. 3.
Strategy applied to identify potential positive cells with isotype control antibodies. Cells obtained from ankle joints digestion protocol were washed and successively stained with GR1 (A–C), F480 (D–F), B220 (G–I) and CD115 (J–L) isotype control antibodies and analysed by FACS. The numbers in the histograms indicated the percentage of positively stained cells in the different experimental conditions. FACS pictures are representative of six samples with similar results. Data are presented as means ± SD of n = 6 mice per group.
Table 3.
Raw data related to GR1, F480, B220 and CD115 positive cells with isotype control antibodies.
| GR1 | Ctrl | MSU | MSU + IL-17Ab |
| 0.24 | 0.62 | 0.62 | |
| 0.41 | 0.28 | 0.39 | |
| 0.68 | 0.54 | 0.42 | |
| 0.24 | 0.36 | 0.91 | |
| 0.37 | 0.51 | 0.54 | |
| 0.52 | 0.69 | 0.72 | |
| Mean ± SD | 0.41 ± 0.17 | 0.50 ± 0.15 | 0.60 ± 0.19 |
| F480 | Ctrl | MSU | MSU + IL-17Ab |
| 0.21 | 0.14 | 0.21 | |
| 0.08 | 0.08 | 0.16 | |
| 0.34 | 0.21 | 0.34 | |
| 0.51 | 0.11 | 0.09 | |
| 0.10 | 0.05 | 0.11 | |
| 0.14 | 0.25 | 0.05 | |
| Mean ± SD | 0.23 ± 0.17 | 0.14 ± 0.08 | 0.16 ± 0.10 |
| B220 | Ctrl | MSU | MSU + IL-17Ab |
| 1.27 | 0.64 | 1.12 | |
| 1.33 | 0.81 | 0.94 | |
| 1.06 | 0.52 | 1.26 | |
| 1.11 | 1.21 | 0.84 | |
| 0.97 | 0.47 | 0.44 | |
| 1.46 | 1.09 | 1.10 | |
| Mean ± SD | 1.20 ± 0.18 | 0.79 ± 0.30 | 0.95 ± 0.29 |
| CD115 | Ctrl | MSU | MSU + IL-17Ab |
| 0.01 | 0.01 | 0.02 | |
| 0.12 | 0.09 | 0.09 | |
| 0.09 | 0.11 | 0.11 | |
| 0.15 | 0.04 | 0.01 | |
| 0.21 | 0.06 | 0.07 | |
| 0.02 | 0.11 | 0.12 | |
| Mean ± SD | 0.10 ± 0.08 | 0.07 ± 0.04 | 0.07 ± 0.05 |
2. Experimental design, materials, and methods
CD-1 male mice (Charles River, Italy) weighing 25–30 g were used in all the experiments. Mice were housed in colony cages (six mice per cage) under standard conditions of light, temperature, and relative humidity for at least one week before the start of experimental procedures. Food and water were available ad libitum. All experiments were performed according to Legislative Decree 26/14, which implements the European Directive 2010/63/UE on laboratory animal protection in Italy, and were approved by the local Ethics Committee. According to the protocol described by Akitsu and colleagues [2] ankle joints of CD-1 mice were removed and digested with hyaluronidase (2.4 mg ml−1) and collagenase (1 mg ml−1) in RPMI 1640 plus 10% FBS for 1 h at 37 °C after the induction of experimental gouty inflammation. The model was induced by the intra-articular (i.a.) administration of MSU crystals (200 μg/20 μl) into the right knee joint of mice under isoflurane anaesthesia. Control animals received an i.a. injection of PBS (20 μl) or IL-17Ab isotype control (20 μl). Animals from IL-17Ab-treated groups received the selected dose of 10 μg (i.a.) of neutralizing antibody 30 minutes after MSU crystals administration. The cells obtained at 18h post model induction (peak of inflammation) were filtered through a cell strainer with a 70-μm nylon mesh (Becton Dickinson, Franklin Lakes, NJ, USA) and washed with RPMI 1640 plus 10% FBS. Thereafter, collected cells were washed in PBS before flow cytometry procedure and analysis as previously described [3], [4]. For the characterization of joint-infiltrating cells we performed additional stainings with GR1 (1:300; clone RB6-8C5), F480 (1:300; clone BM8), B220 (1:200; clone RA3-6B2) and CD115 (1:200; clone AFS98) antibody for 60 minutes at 4 °C. Inflammatory monocytes were defined as B220−GR1highF480highCD115+cells. Lymphocytes isolated from whole blood by Ficoll-Paque Plus gradient method were washed in FACS buffer (PBS containing 1% BSA and 0.02% NaN2) and directly stained with the following conjugated antibodies (all from BioLegend, London, UK): CD4 (1:200; clone GK1.5), CD8 (1:200; clone 5H10-1), CD25 (1:200; clone 3C7) for 60 minutes at 4 °C. After washing, cells were fixated, permeabilized, and stained intracellularly with IL-17 (1:200; clone TC11-18H10.1) and Foxp3 antibodies (1:200: clone MF-14). Th17 and Treg population were defined as CD4+IL-17+ and CD4+CD25+Foxp3+ cells respectively. At least 1 × 104 cells were analysed per sample, and determination of positive and negative populations was performed based on the staining obtained with related IgG isotypes. Flow cytometry was performed on BriCyte E6 flow cytometer (Mindray Bio-Medical Electronics, Nanshan, China) using MRFlow and FlowJo software operation. The data and statistical analysis presented here comply with the international recommendations on experimental design and analysis in pharmacology and data sharing and presentation in preclinical pharmacology [5], [6], [7]. The results obtained were expressed as the mean ± SD. Statistical analysis were performed by using one-way ANOVA followed by Bonferroni or Dunnett's post-test, when comparing more than two groups. GraphPad Prism 7.0 software (San Diego, CA, USA) was used for analysis. Data were considered statistically significant when a value of P ≤ 0.05 was achieved.
Acknowledgments
This work was supported by MIUR (PRIN 2017; 2017A95NCJ/2017A95NCJ_002, “Stolen molecules - Stealing natural products from the depot and reselling them as new drug candidates”). We would like to thank Dr. Antonio Baiano, Mr. Giovanni Esposito and Mr. Angelo Russo for animal care and technical assistance.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Raucci F., Iqbal A.J., Saviano A., Minosi P., Piccolo M., Irace C., Caso F., Scarpa R., Pieretti S., Mascolo N., Maione F. IL-17A neutralizing antibody regulates monosodium urate crystal-induced gouty inflammation. Pharmacol. Res. 2019 Jul 14:104351. doi: 10.1016/j.phrs.2019.104351. [DOI] [PubMed] [Google Scholar]
- 2.Akitsu A., Ishigame H., Kakuta S., Chung S.H., Ikeda S., Shimizu K., Kubo S., Liu Y., Umemura M., Matsuzaki G., Yoshikai Y., Saijo S., Iwakura Y. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vγ6(+)γδ T cells. Nat. Commun. 2015;25(6):7464. doi: 10.1038/ncomms8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maione F., Iqbal A.J., Raucci F., Letek M., Mauer B., D'Acquisto F. Repetitive exposure of IL-17 into the murine air pouch favors the recruitment of inflammatory monocytes and the release of IL-16 and TREM-1 in the inflammatory fluids. Front. Immunol. 2018;9:2752. doi: 10.3389/fimmu.2018.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 5.Curtis M.J., Alexander S., Cirino G., Docherty J.R., George G.C.H., Giembycz M.A., Hoyer D., Insel P.A., Izzo A.A., Ji Y., MacEwan D.J., Sobey C.G., Stanford S.C., Teixeira M.M., Wonnacott S., Ahluwalia A. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018;175(7):987–993. doi: 10.1111/bph.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maione F., Cantone V., Pace S., Chini M.G., Bisio A., Romussi G., Pieretti S., Werz O., Koeberle A., Mascolo N., Bifulco G. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br. J. Pharmacol. 2017;174(11):1497–1508. doi: 10.1111/bph.13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristiano C., Volpicelli F., Lippiello P., Buono B., Raucci F., Piccolo M., Iqbal A.J., Irace C., Miniaci M.C., Perrone Capano C., Calignano A., Mascolo N., Maione F. Neutralization of IL-17 rescues amyloid-β-induced neuroinflammation and memory impairment. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]