Abstract
The susceptibility of Escherichia coli from community onset urinary tract infection (UTI) was evaluated by dividing community onset UTI into the simple community acquired-UTI (CA-UTI) and healthcare associated UTI (HCA-UTI) groups for a period of 10 years. The susceptibility of E. coli to most antibiotics, except amikacin and imipenem, continued to decrease. In the CA-UTI group, the susceptibility to cefotaxime was 88% in 2015, but rapidly decreased to 79.3% in 2017. The susceptibility to cefepime and piperacillin-tazobactam were 88.8% and 90.5% in 2017, respectively. In the HCA-UTI group, the susceptibility to most antibiotics markedly decreased to less than 60% by 2017. The incidence of ESBL-producing E. coli increased to 23.3% in the CA-UTI group in 2017.
Keywords: Urinary Tract Infection, Escherichia coli, Antibacterial Agents
Graphical Abstract
Urinary tract infection (UTI) is a common infection in communities. The use of an initial appropriate antibiotic usually improves a patient's prognosis. Earlier studies have reported that the indiscriminate use of antibiotics for treating UTIs resulted in a lower cure rate, longer hospital stay, higher relapse rate, and accelerated development of antibiotic resistance.1,2,3 In previous studies in Korea, in 2010, the Escherichia coli susceptibility to ciprofloxacin had decreased to less than 80% while that to cefotaxime remained high.4,5 Hence, cefotaxime was used to treat community-onset UTIs and the susceptibility to cefotaxime may be decreased. Moreover, there has been an increase in the incidence of extended-spectrum β-lactamase (ESBL)-producing E. coli in community-onset UTIs.6,7 Residence in a healthcare facility is an independent risk factor of ESBL-producing E. coli infections.8 The clinical features and the antibiotic susceptibility pattern of the causative pathogens differ from patients residing in a healthcare facility and those living at home.9 Therefore, it is necessary to classify the community-onset UTI into simple community-acquired UTI (CA-UTI) and healthcare-associated community acquired UTI (HCA-UTI) when investigating the antibiotic susceptibility pattern of E. coli.
In this study, we evaluated the antimicrobial susceptibility pattern of E. coli in community-onset UTI, which were divided into CA-UTI and HCA-UTI between 2008 and 2017. This study was approved by the Institutional Review Board (IRB) of Wonkwang University Hospital (Board approval No. WKUHIRB 2018-11-013).
The patients who visited the emergency room of Wonkwang University Hospital with community-onset UTI caused by E. coli between January 2008 and December 2017 were selected for the study. We retrospectively reviewed the patients' medical records to exclude subjects with asymptomatic bacteriuria, patients who had been transferred from other acute care hospitals, and those who relapsed within 14 days of completing therapy. We used 12 antimicrobial agents (ampicillin, cefazolin, cefotaxime, cefepime, aztreonam, gentamicin, piperacillin-tazobactam [PIP-TAZ], ciprofloxacin, TMP-SMX, amikacin, ertapenem, and imipenem) for the susceptibility test. The antibiotic susceptibility test was performed using a VITEK 2 system (bioMérieux, Hazelwood, MO, USA) according to the guidelines prescribed by the Clinical and Laboratory Standards Institute (CLSI).10,11,12 The CLSI breakpoints of 2010 and 2014 were applied in May 2012 and May 2014, respectively.
Asymptomatic bacteriuria was defined as the presence of bacteria in the properly collected urine sample of patients who had no local or systemic signs and symptoms, such as fever, chills, malaise, acute altered mental status, flank pain, back pain, or costo-vertebral angle pain or tenderness. Community-onset UTI was defined as the infection that was diagnosed within 48 hours of admission into the hospital. The patients with community-onset UTI were further grouped into the CA-UTI and HCA-UTI groups. Patients in the HCA-UTI group were defined using the modified criteria of Friedman et al.9: 1) hospitalized in an acute-care hospital for 2 or more days within 90 days, 2) attending a hospital for hemodialysis or receiving intravenous chemotherapy within 30 days, 3) residing in a nursing home or long-term care facility, 4) having a long-term indwelling urethral catheter or suprapubic cystostomy.
We compared the annual antibiotic susceptibility of E. coli using χ2 test. The data were considered statistically significant when the P value was less than 0.05 (two-sided). All statistical analyses were performed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
In this study, we included 1,496 patients (aged > 18 years) with community onset UTI caused by E. coli. There was no previous history of UTI in 96% of the patients. The proportion of patients who had been hospitalized for at least 48 hours was 73.2%. The mean age of the patients was 65.5 ± 17.8 years and 85.6% of the patients were women. 95.7% of the patients were diagnosed with pyelonephritis, and 4.3% of the patients were diagnosed with cystitis. We classified 31% of the patients into the HCA-UTI group. The proportion of patients with complicated UTI was 24.4%, and 24.5% of the patients had a long-term indwelling urethral catheter or suprapubic cystostomy.
The susceptibility of E. coli to cefazolin, cefotaxime, cefepime, aztreonam, PIP-TAZ, gentamicin, and ciprofloxacin decreased significantly with each year during the study period. The susceptibility of E. coli to cefotaxime declined continuously during the study period and was less than 70% in 2017. The susceptibility E. coli to cefepime was more than 90% until 2013 but rapidly decreased to less than 80% in 2015. Similarly, the susceptibility of E. coli to aztreonam was less than 80% in 2014. E. coli was highly susceptible to PIP-TAZ until 2015, but it decreased to about 87% in 2016. The susceptibility to TMP-SMX was above 60% during the study period with minimal change. The susceptibility to ciprofloxacin decreased to less than 60% in 2014. The susceptibility to TMP-SMX was higher than that to ciprofloxacin in 2010. The susceptibility to amikacin and imipenem was very high during the study period. The incidence of ESBL-producing E. coli steadily increased from 4.5% to 33.1% (Table 1).
Table 1. Antibiotic susceptibility and incidence of ESBL-producing Escherichia coli from 2008 to 2017.
| Susceptibility, % (Total = 1,496) | 2008 (n = 88) | 2009 (n = 90) | 2010 (n = 94) | 2011 (n = 124) | 2012 (n = 164) | 2013 (n = 195) | 2014 (n = 190) | 2015 (n = 146) | 2016 (n = 224) | 2017 (n = 181) | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of patients with susceptibility to antibiotics | ||||||||||||
| Ampicillin | 37.5 | 35.6 | 41.5 | 34.7 | 32.9 | 36.1 | 32.8 | 34.2 | 27.2 | 35.4 | 0.508 | |
| PIP-TAZ | 98.9 | 96.7 | 96.8 | NA | 96.2 | 91.8 | 94.7 | 93.8 | 87.9 | 87.3 | < 0.001 | |
| Cefazolin | 89.8 | 76.7 | NA | 64.5 | 70.7 | 79.5 | 74.2 | 65.8 | 62.5 | 62.7 | < 0.001 | |
| Cefotaxime | 95.5 | 91.1 | 87.1 | 85.5 | 81.7 | 81.5 | 78.4 | 69.7 | 67.9 | 68.0 | < 0.001 | |
| Cefepime | 95.5 | 93.3 | 95.7 | 95.2 | 94.5 | 92.8 | 87.8 | 78.8 | 77.7 | 78.5 | < 0.001 | |
| Aztreonam | 97.7 | 92.2 | 92.5 | 94.4 | 89.0 | 87.2 | 79.5 | 79.5 | 78.6 | 76.2 | < 0.001 | |
| Imipenem | 98.9 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | ||
| Ertapenem | NA | NA | NA | NA | NA | 99.5 | 100.0 | 99.3 | 99.5 | 100.0 | ||
| Gentamicin | 79.5 | 75.6 | 74.5 | 77.4 | 75.0 | 75.4 | 69.2 | 69.2 | 63.4 | 65.7 | 0.018 | |
| Amikacin | 100.0 | 98.9 | 97.9 | 97.6 | 98.8 | 100.0 | 99.3 | 99.3 | 99.1 | 98.9 | ||
| TMP-SMX | 68.2 | 63.3 | 72.3 | 69.4 | 68.9 | 70.8 | 62.3 | 62.3 | 62.5 | 65.7 | 0.568 | |
| Ciprofloxacin | 79.5 | 71.1 | 66.0 | 67.7 | 71.3 | 64.6 | 58.9 | 58.9 | 51.8 | 58.6 | < 0.001 | |
| ESBL | 4.5 | 10.0 | 13.8 | 12.9 | 18.9 | 15.9 | 18.9 | 28.1 | 29.9 | 33.1 | < 0.001 | |
ESBL = extended spectrum β-lactamase, E. coli = Escherichia coli, NA = not applicable, PIP-TAZ = piperacillin-tazobactam, TMP-SMX = trimethoprim-sulfamethoxazole.
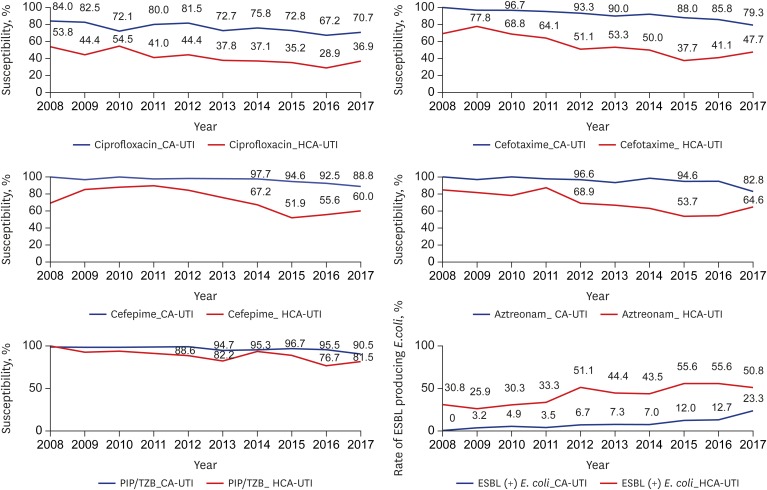
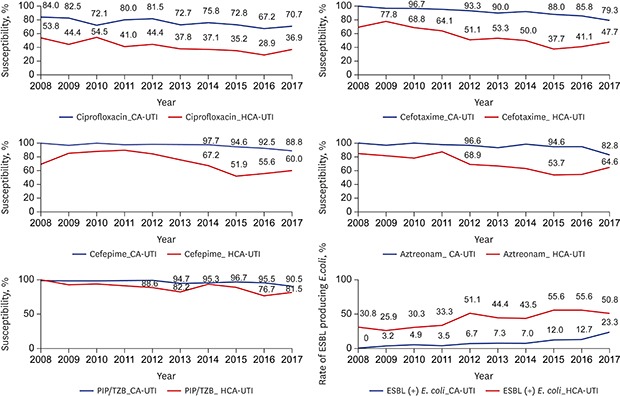
The susceptibility to most antibiotics, except amikacin and imipenem, was different between the CA-UTI and HCA-UTI groups. The susceptibility to cefotaxime in the CA-UTI group was around 90% until 2015 (88% in 2015). However, the susceptibility to cefotaxime of this group decreased to less than 80% (79.3% in 2017). The susceptibility to cefotaxime in HCA-UTI group was 50% in 2012. The susceptibility to cefepime in the CA-UTI group was 88.8% in 2017. However, the susceptibility to cefepime in the HCA-UTI group remarkably decreased to below 80% in 2013 and dropped to less than 60% in 2015. The susceptibility PIP-TAZ in the CA-UTI group was 90.5% in 2017, but that in the HCA-UTI group dropped to below 90% in 2015, and the lowest susceptibility was observed in 2016 (76.7%). The susceptibility to aztreonam in the CA-UTI group was 94.8% in 2016 but decreased to 82.8% in 2017. The susceptibility to aztreonam in the HCA-UTI group significantly decreased to below 70% in 2012 and the lowest susceptibility was observed in 2015 (53.7%). The susceptibility to ciprofloxacin in the CA-UTI group decreased to 70.7% in 2017. However, the susceptibility to ciprofloxacin in the HCA-UTI group decreased to less than 40% in 2013, and the lowest susceptibility was observed in 2016 (28.9%). The susceptibility to TMP-SMX in the CA-UTI and in HCA-UTI groups was 67.2% and 63.1%, respectively in 2017. The incidence of ESBL-producing E. coli markedly significantly increased from 0.0% to 23.3% in the CA-UTI group (P < 0.001). In the HCA-UTI group, the incidence of ESBL-producing E. coli began to exceed 50% in 2012 (Fig. 1).
Fig. 1. The annual antibiotics susceptibility rate and ESBL producing E. coli rate.
ESBL = extended spectrum β-lactamase, E. coli = Escherichia coli, CA-UTI = community acquired urinary tract infection, HCA-UTI = healthcare associated urinary tract infection.
During the study period, the susceptibility of E. coli to most antibiotics continued to decrease while the incidence of ESBL-producing E. coli increased markedly. The susceptibility to most antibiotics was different between the CA-UTI and HCA-UTI groups. In this study, we observed that the susceptibility to cefotaxime markedly decreased in patients with community-onset UTI. The production of ESBL is an important mechanism for developing cefotaxime resistance in E. coli.13 In this study, the incidence of ESBL-producing E. coli increased sharply after 2015. Concurrently, a decreased susceptibility to cefotaxime was observed after 2015. As reported in earlier studies,6,7 we observed that the incidence of ESBL-producing E. coli in the CA-UTI group was around 10% until 2016. However, the incidence rapidly increased, nearly doubling by the year 2017 (from 12.7% to 23.3%). Hence, it is necessary to constantly monitor the rapid incidence of ESBL-producing E. coli. Carbapenems are generally considered the drug of choice for treating of ESBL-producing E. coli infection.14,15 Ciprofloxacin or TMP-SMX can be used for treating UTI caused by ESBL-producing E. coli.16 However, in this study, most ESBL-producing E. coli had co-resistance to ciprofloxacin (79.5%, 245/308) and TMP-SMX (53.9%, 166/308). PIP-TAZ can be as effective as carbapenem in treating UTI caused by ESBL-producing E. coli.17,18 The susceptibility to PIP-TAZ remained high in the CA-UTI group during this study period, but in the HCA-UTI group decreased to 80% in 2016. The susceptibility to ciprofloxacin continuously decreased to 70% in the CA-UTI group and was less than 40% in the HCA-UTI group. However, there was very little change in the susceptibility to TMP-SMX during the study period and was similar that to ciprofloxacin in 2010.
This study has several limitations. During this study, the twice changed CLSI guidelines were applied to the susceptibility test, and the breakpoints for E. coli were lowered in May 2012. This was a retrospective observational study performed at a single university hospital. This study included a high proportion of patients who had severe UTI and needed hospitalization, which may limit the generalization of our results to cystitis or UTI in outpatient clinics. This study demonstrated the change in antibiotic susceptibility pattern in a 10-year study period. Our study also classified the patients based on CA-UTI and HCA-UTI. The results of this study may aid in selecting the appropriate early antibiotics for treating community-onset UTIs.
Footnotes
Presentation: Some data were presented in part at ASM 2019 in San Francisco, CA, USA on 20-24 June 2019 (Saturday-AAR-555).
Funding: This paper was supported in part by the Foundation of Wonkwang University in 2018.
Disclosure: The authors have no potential conflicts of interest to disclose
- Conceptualization: Lee JH.
- Data curation: Cho JH.
- Formal analysis: Kim YJ.
- Investigation: Lee JH, Kim YG.
- Methodology: Lee JM, Lee JH.
- Software: Lee JH.
- Validation: Lee JH.
- Writing - original draft: Kim YG, Lee JH.
- Writing - review & editing: Lee JH, Kim YG.
References
- 1.Shin J, Kim J, Wie SH, Cho YK, Lim SK, Shin SY, et al. Fluoroquinolone resistance in uncomplicated acute pyelonephritis: epidemiology and clinical impact. Microb Drug Resist. 2012;18(2):169–175. doi: 10.1089/mdr.2011.0139. [DOI] [PubMed] [Google Scholar]
- 2.Bosch-Nicolau P, Falcó V, Viñado B, Andreu A, Len O, Almirante B, et al. A cohort study of risk factors that influence empirical treatment of patients with acute pyelonephritis. Antimicrob Agents Chemother. 2017;61(12):e01317-17. doi: 10.1128/AAC.01317-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi JH. The meaning and impact of appropriate use of antibiotics. Infect Chemother. 2012;44(5):331–337. [Google Scholar]
- 4.Wie SH, Ki M, Kim J, Cho YK, Lim SK, Lee JS, et al. Clinical characteristics predicting early clinical failure after 72 h of antibiotic treatment in women with community-onset acute pyelonephritis: a prospective multicentre study. Clin Microbiol Infect. 2014;20(10):O721–O729. doi: 10.1111/1469-0691.12500. [DOI] [PubMed] [Google Scholar]
- 5.Kim B, Kim J, Seo MR, Wie SH, Cho YK, Lim SK, et al. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection. 2013;41(3):603–612. doi: 10.1007/s15010-013-0441-z. [DOI] [PubMed] [Google Scholar]
- 6.Park JJ, Seo YB, Lee J. Antimicrobial susceptibilities of Enterobacteriaceae in community-acquired urinary tract infections during a 5-year period: a single hospital study in Korea. Infect Chemother. 2017;49(3):184–193. doi: 10.3947/ic.2017.49.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang CI, Cha MK, Kim SH, Ko KS, Wi YM, Chung DR, et al. Clinical and molecular epidemiology of community-onset bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli over a 6-year period. J Korean Med Sci. 2013;28(7):998–1004. doi: 10.3346/jkms.2013.28.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YS, Ku CH, Lin JC, Shang ST, Chiu CH, Yeh KM, et al. Impact of Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae on the outcome of community-onset bacteremic urinary tract infections. J Microbiol Immunol Infect. 2010;43(3):194–199. doi: 10.1016/S1684-1182(10)60031-X. [DOI] [PubMed] [Google Scholar]
- 9.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 17th Informational Supplement (M100-S17) Wayne, PA: CLSI; 2007. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 24th Informational Supplement (M100-S24) Wayne, PA: CLSI; 2014. [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 21st Informational Supplement (M100-S21) Wayne, PA: CLSI; 2011. [Google Scholar]
- 13.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027–1052. doi: 10.2165/00003495-200767070-00006. [DOI] [PubMed] [Google Scholar]
- 15.Rupp ME, Fey PD. Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: considerations for diagnosis, prevention and drug treatment. Drugs. 2003;63(4):353–365. doi: 10.2165/00003495-200363040-00002. [DOI] [PubMed] [Google Scholar]
- 16.Kang CI. Antimicrobial therapy for infections caused by multidrug-resistant gram-negative bacteria. Korean J Med. 2015;88(5):502–508. [Google Scholar]
- 17.Yoon YK, Kim JH, Sohn JW, Yang KS, Kim MJ. Role of piperacillin/tazobactam as a carbapenem-sparing antibiotic for treatment of acute pyelonephritis due to extended-spectrum β-lactamase-producing Escherichia coli . Int J Antimicrob Agents. 2017;49(4):410–415. doi: 10.1016/j.ijantimicag.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Ko JH, Lee NR, Joo EJ, Moon SY, Choi JK, Park DA, et al. Appropriate non-carbapenems are not inferior to carbapenems as initial empirical therapy for bacteremia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: a propensity score weighted multicenter cohort study. Eur J Clin Microbiol Infect Dis. 2018;37(2):305–311. doi: 10.1007/s10096-017-3133-2. [DOI] [PubMed] [Google Scholar]