Significance
Environmental conditions strongly influence pathogen–host plant interactions. Rice blast erupts in overcast and rainy conditions, due not only to favorable environmental conditions but also to insufficient light, which reduces host resistance. Due to the importance of breeding of blast-resistant rice varieties, elucidation of light-regulated rice immunity is an important research goal. We revealed that light induces protein phosphorylation of a harvesting complex II protein, LHCB5, upon infection by the rice blast fungus Magnaporthe oryzae. Resistance governed by LHCB5 phosphorylation cosegregates with the progenies harboring the desirable haplotype promoter. This establishes the genetic basis of LHCB5-regulated resistance mediated by phosphorylation. Our study highlights a mechanism for light-dependent rice blast resistance that promises future breeding of blast-resistant rice varieties.
Keywords: light-harvesting complex II, phosphorylation, Magnaporthe oryzae, broad-spectrum resistance
Abstract
Environmental conditions are key factors in the progression of plant disease epidemics. Light affects the outbreak of plant diseases, but the underlying molecular mechanisms are not well understood. Here, we report that the light-harvesting complex II protein, LHCB5, from rice is subject to light-induced phosphorylation during infection by the rice blast fungus Magnaporthe oryzae. We demonstrate that single-nucleotide polymorphisms (SNPs) in the LHCB5 promoter control the expression of LHCB5, which in turn correlates with the phosphorylation of LHCB5. LHCB5 phosphorylation enhances broad-spectrum resistance of rice to M. oryzae through the accumulation of reactive oxidative species (ROS) in the chloroplast. We also show that LHCB5 phosphorylation-induced resistance is inheritable. Our results uncover an immunity mechanism mediated by phosphorylation of light-harvesting complex II.
Environmental conditions strongly influence pathogen–host plant interactions, and changes in a range of environmental factors can directly determine the outcomes of such interactions. In the 1960s, George McNew proposed a “disease triangle” concept, comprising the host, pathogen, and environment as the 3 fundamental components that affect crop diseases. Many studies have subsequently demonstrated that environmental conditions, such as light, temperature, and humidity, can affect disease development and plant innate immunity. For example, the wheat (Triticum aestivum) Yr36 gene confers broad-spectrum resistance to stripe rust at high temperatures, but not at low temperatures (1), while moderate temperatures have been shown to enhance pathogen-associated molecular patterns (PAMPs)-triggered immunity (PTI) in Arabidopsis thaliana (2). Other studies have shown that high humidity promotes bacterial infection by also modulating plant immunity (3). Rice (Oryza sativa) blast caused by Magnaporthe oryzae is often triggered by the overcast weather and lack of sunlight conditions. A study documenting rice blast of over 55 y in Jiangsu, China, showed that fewer hours of sunlight directly correlated with the seriousness of the blast disease (4, 5). However, the underlying molecular mechanisms linking light to infection are not well understood.
Notably, chloroplasts are not only the organelles where light sensing and photosynthesis take place, but also the site of biosynthesis of defense-related molecules, such as salicylic acid (SA) and jasmonic acid (JA), as well as secondary messengers, including calcium and reactive oxygen species (ROS) (6–9). Photosynthetic electron transduction in the chloroplast can result in the reduction of O2 and the formation of ROS, such as singlet oxygen (1O2), which is generated from the chlorophyll and photosystems II (PSII) antenna complex (10). PSII, which is central to the conversion of light into chemical energy, is surrounded by the light-harvesting complex II (LHCII) and the monomeric light-harvesting proteins LHCB4 (CP29), LHCB5 (CP26), and LHCB6 (CP24) (11, 12). It has been established that LHCB4, LHCB5, and LHCB6 are all involved in energy dissipation in A. thaliana (13, 14) and that LHCB5 functions as a trimeric protein complex rescuing a defect in LHCII function due to the absence of LHCB1 and LHCB2 (15). However, in contrast to its known function in energy transduction, the role of LHCII in plant immunity has not been well elucidated.
Results
LHCB5 Is Involved in Light Regulation of Resistance to M. oryzae in Different Rice Varieties.
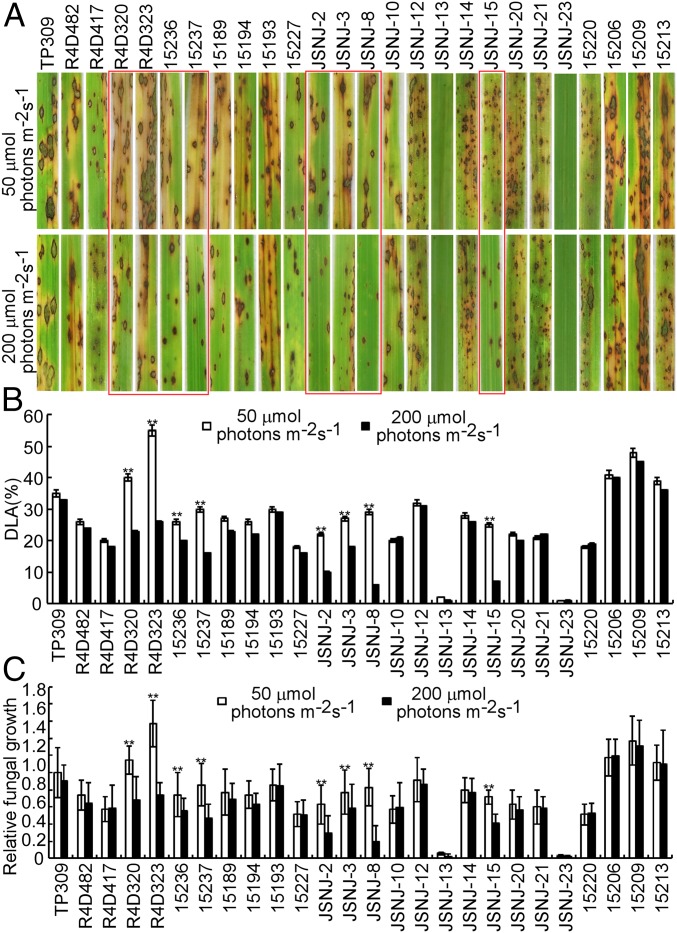
To investigate whether rice resistance to M. oryzae is light-dependent, 25 rice varieties, collected worldwide, were screened for blast resistance under 2 different light intensities. These varieties showed various degrees of susceptibilities to the wild-type M. oryzae Guy11 under the low light intensity of 50 μmol photons m−2s−1 (Fig. 1), based on both the diseased leaf area (DLA) and the pathogen biomass assay. However, 8 varieties showed significantly increased resistance when the assessment was conducted under the higher light intensity of 200 μmol photons m−2s−1 (Fig. 1). In contrast, the remaining 17 varieties did not show any significant differences in light-associated blast resistance (Fig. 1).
Fig. 1.
Identification of blast-resistant rice varieties in a light-dependent manner. (A) The wild-type TP309 and 25 rice varieties collected worldwide were used for blast resistance screen. The rice varieties inoculated with Guy11 were cultivated in a light incubator with different light intensity (50 and 200 μmol photons m−2s−1). Red boxes indicate rice varieties with resistant variations under different light intensity. (B) Disease lesion area (DLA) was assessed by Image J. Lesions were photographed and measured or scored at 7 d postinoculation (dpi). The experiments were repeated twice with similar results. (C) Fungal growth and severity of blast were evaluated by quantifying M. oryzae genomic 28S rDNA relative to rice genomic Rubq1 DNA. The mean values of 3 determinations with SDs are shown. The asterisks indicate a significant difference according to Student’s t test (P < 0.01).
This light intensity-associated resistance prompted us to investigate the possible roles of the LHCII proteins, which are required for absorbing and transferring the light energy (16, 17). We first tested the transcript levels of 6 LHCB genes in the nonresponsive TP309 variety rice and found that all of them were significantly up-regulated under the lighted conditions, compared to the dark, suggesting that all LHCB genes are light-inducible (SI Appendix, Fig. S1). We also observed that the LHCB5 transcript level was uniformly up-regulated in all 8 light-dependent resistant varieties, but not in TP309 (SI Appendix, Fig. S2), as was the accumulation of LHCB5 protein (SI Appendix, Fig. S3). LHCB5 transcript levels gradually increased at different time points until 96 h postinoculation (hpi) (SI Appendix, Fig. S4A), and this accumulation also corresponded with the levels of the LHCB5 protein. These results indicated that LHCB5 is light- and blast pathogen-responsive and may play a role in light-responsive blast resistance.
LHCB5-Mediated Resistance Is Highly Associated with Promoter Sequence Divergence Leading to Different Expression Levels among Diverse Rice Germplasm.
The various levels of the LHCB5 transcript and protein in different rice varieties prompted us to investigate the variance of its promoter and the coding sequences from 3,000 sequenced rice genomes (18). No nonsynonymous sequence mutations were found in LHCB5 coding sequences, suggesting high conservation (Dataset S1). In contrast, 11 single-nucleotide polymorphisms (SNPs) were identified that were separated into 18 groups (SNP ≥ 1) using O. sativa ssp japonica cv. Nipponbare (NPB) as the control (SNP = 0) (Dataset S2). The SNPs were mainly discovered in the indica rice varieties, and most of these varieties showed variations in 7 of the SNPs identified (SI Appendix, Fig. S5 A and B). The difference in SNP variation suggested a causal relationship with a lower expression in indica than in japonica (SI Appendix, Fig. S6A). To test this hypothesis, 238 rice varieties were selected for analyzing the relationship between SNPs and transcription levels (Dataset S3). The expression of LHCB5 in SNP variation rice lines (SNP ≥ 1) was significantly lower than that in nonvariation varieties (SNP = 0) (SI Appendix, Fig. S5C), and this was, in particular, true among 7 of the 11 SNP positions (1–4, 9–11) (SI Appendix, Fig. S5D). To validate the correlation between SNPs and the LHCB5 expression levels, we expressed GFP (green fluorescent protein) reporter gene driven by different promoters (p35S:GFP, pLHCB5SNP = 0:GFP and pLHCB5SNP = 7:GFP) in rice protoplast. The result showed that constructs p35S:GFP and pLHCB5SNP = 0:GFP had a much higher level of green fluorescent; the Western blot also showed a higher GFP protein level under the control of 35S and SNP = 0 promoter than SNP = 7 promoter, indicating that the promoter SNPs are indeed associated with higher LHCB5 expression (SI Appendix, Fig. S7). When 238 rice varieties were inoculated with 2 rice blast isolates, Mo15-125 and Mo15-9, a correlation between higher LHCB5 expression and smaller disease lesion area was found (SI Appendix, Fig. S8). These data indicated that SNP variations in indica rice varieties result in reduced LHCB5 expression and lower resistance to blast.
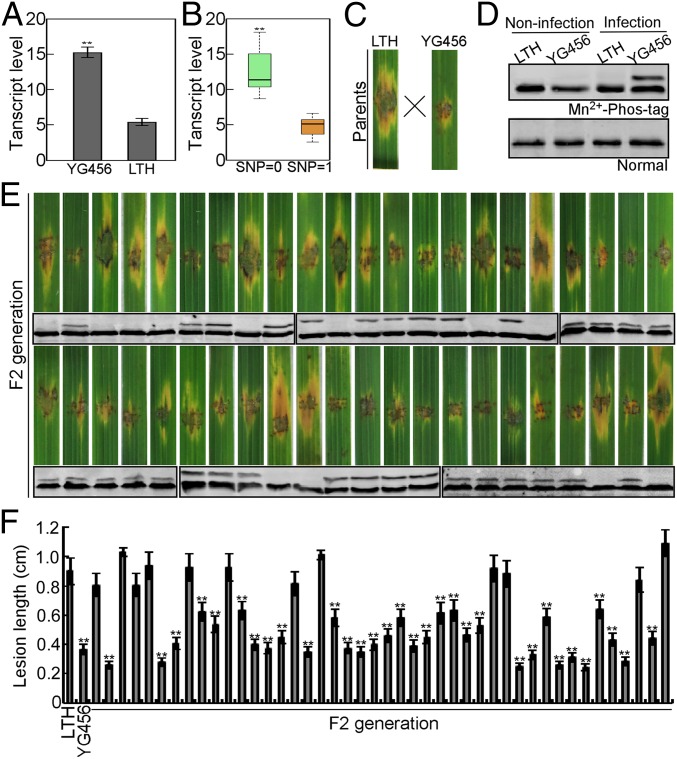
To further investigate the genetic association between the SNP pattern and expression of LHCB5, we used an F2 population of 44 progenies derived from a cross between YG456 (Yangeng456, SNP = 0) and LTH (Lijiangxintuanheigu, SNP ≥ 1, containing all 7 candidate SNPs mentioned above). As indicated in Fig. 2 A and B, the parental line YG456 and 32 progenies containing the YG456-type promoter showed significantly higher expression levels than LTH and the 12 progenies containing the LTH-type promoter. This revealed a cosegregation between the expression level and the promoter type in the F2 population.
Fig. 2.
Genetic association between the SNP pattern and expression of LHCB5 and the inheritance of LHCB5 phosphorylation. (A) The transcript analysis of LHCB5 in YG456 and LTH. qRT-PCR on LHCB5 in YG456 in comparison with LTH. (B) The transcript analysis of LHCB5 in an F2 population derived from a cross between YG456 and LTH. The expression level of 44 progeny was analyzed by qRT-PCR. (C) Blast resistance of LTH and YG456 plants. The leaves of 4-wk-old plants were inoculated using method of punch. Photos were taken at 6 dpi. (D) Detection of LHCB5 phosphorylation in LTH and YG456. LTH and YG456 plants were inoculated with (infection) or without (noninfection) Guy11 after 48 hpi. The protein extracts were subjected to Phos-tag SDS/PAGE and normal SDS/PAGE followed by immunoblotting with the anti-LHCB5 polyclonal antibody. (E) Rice blast resistance is correlated with phosphorylation assess in the F2 generation of LTH and YG456. Blast resistance of 44 F2 generations using punch inoculation. Phosphorylation assay was performed as above. (F) Lesion length was measured 6 dpi. Values are the means of 3 replications, and error bars represent the SD (n = 3). The asterisks indicate a significant difference according to Student’s t test (P < 0.01).
YG456 was also found to be resistant to the virulent rice blast isolate Guy11, which had a lesion length 40% smaller than the lesion of the susceptible rice variety LTH (Fig. 2 C and F). Moreover, all of the progenies with a higher LHCB5 expression level showed resistance to Guy11, whereas those with a lower LHCB5 expression level were susceptible (Fig. 2 E and F). Taken together, these results revealed that the elevated level of LHCB5 governed by the promoter (SNP = 0) contributes to enhanced resistance to blast, and the trait is genetically transferable.
Expression of LHCB5 in Transgenic Rice Lines Correlates with Resistance to Infection.
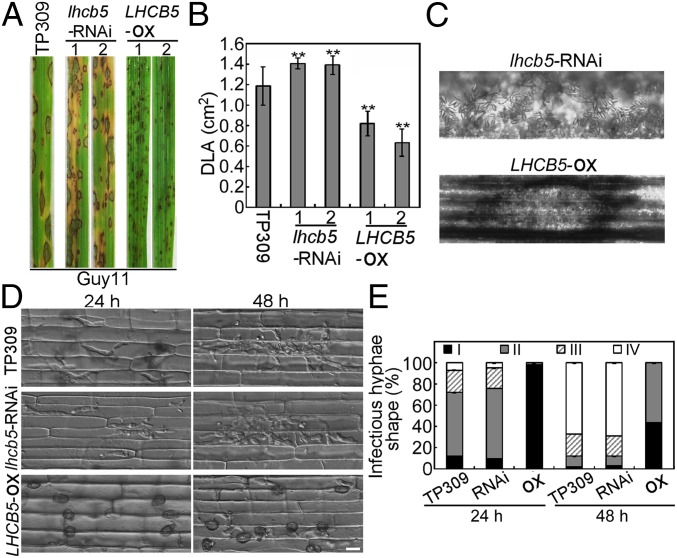
To further investigate the relationship between LHCB5 expression and resistance, we characterized transgenic rice lines with expression differences. We first generated transgenic LHCB5 RNAi-silenced (lhcb5-RNAi) and overexpression (LHCB5-OX) rice lines in the TP309 background. The lhcb5-RNAi lines #1 and #2 showed significantly reduced LHCB5 transcript levels in the leaves, whereas LHCB5-OX lines #1 and #2 showed significantly increased levels, compared to TP309 (SI Appendix, Fig. S9A). Western blot analysis showed that the amount of the LHCB5 protein was 3- to 4-fold higher in the LHCB5-OX lines than in TP309, while the lhcb5-RNAi lines had 20 to 25% of the levels of TP309 (SI Appendix, Fig. S9B).
In infection, the LHCB5-OX rice lines showed strong resistance to blast, with punctate and significantly reduced lesion areas, whereas the lhcb5-RNAi lines were more susceptible (Fig. 3 A and B). In addition, lesions on LHCB5-OX lines failed to produce any conidia (Fig. 3C). A rice sheath infection assay for invasive hyphal growth (19) revealed that >90% of penetration was at the level I in the LHCB5-OX rice lines, in contrast to 11% and 10% in TP309 and lhcb5-RNAi lines at 24 hpi, respectively. The infectious hyphae on the LHCB5-OX rice lines failed to expand, even at 48 hpi, and showed level II penetration (Fig. 3 D and E). Furthermore, we used the CRISPR/Cas9 technology to knock out the endogenous LHCB5 gene (LHCB5-KO) in TP309, and the infection phenotype of LHCB5-KO lines was as susceptible as lhcb5-RNAi lines (SI Appendix, Fig. S10). Together, these data indicated that LHCB5 overexpression enhances basal resistance.
Fig. 3.
The function of LHCB5 in blast resistance. (A) Blast resistance of lhcb5-RNAi and LHCB5-OX plants using spraying inoculation. Numbers “1” and “2” mean 2 independent transformants. (B) The DLA of the leaves infected by Guy11 was assessed by Image J. Lesions were photographed and measured or scored at 7 dpi, and experiments were repeated twice with similar results. Values are the means of 3 replications, and error bars represent the SD (n = 3). The asterisks indicate a significant difference according to Student’s t test (P < 0.01). (C) Assays for fungal growth on surface-sterilized rice leaves inoculated by spray with Guy11. (D) Typical infection sites of TP309, lhcb5-RNAi, and LHCB5-OX leaf sheath inoculated with Guy11 strain, showing greater fungal proliferation and tissue invasion in TP309 and lhcb5-RNAi by the wild-type strain and restricted in LHCB5-OX leaf sheath. Infectious growth was observed at 30 hpi. (Scale bar, 10 μm). (E) Statistics of invasive hyphal growth at 100 appressorium penetration sites by rating the hyphal growth from level I to IV (I, no penetration; II, with primary invasive hypha; III, secondary invasive hypha does not extend to the neighboring plant cells; IV, invasive hypha extended into neighboring plant cells).
Induced Immune Response in LHCB5-OX Lines Is Broad Spectrum to M. oryzae.
To explore the mechanism by which LHCB5 mediates resistance, we inoculated TP309, LHCB5-OX, and lhcb5-RNAi leaves with Guy11 and the incompatible strain 51# and stained the leaf cells with 3, 3′-diamino-benzidine (DAB) and Trypan Blue (TB). We observed that LHCB5-OX accumulated higher levels of H2O2, accompanied by cell death (SI Appendix, Fig. S11A). We also used a luminol-based chemiluminescence assay to monitor the generation of ROS induced by purified mycelia (PM) as the elicitor (20) and obtained a similar result (SI Appendix, Fig. S11B). Finally, we performed a rice sheath infection assay to examine ROS accumulation and cell death and found that the DAB and TB stain signals were 4 to 5 times stronger in LHCB5-OX sheaths than in those of TP309 and lhcb5-RNAi. Pretreating LHCB5-OX rice sheaths with the catalase of Aspergillus niger (CAG) (21) and reduced glutathione (GSH) (22) partially suppressed the production of ROS and rescued the invasive growth (SI Appendix, Fig. S11 C–E). Moreover, qRT-PCR revealed that the expression levels of PR genes, including PR1, PBZ1, AOS2, and LOX1, as well as 2 NADPH oxidases (RBOHA and RBOHB), were significantly up-regulated in the LHCB5-OX lines (SI Appendix, Fig. S11 F and G).
We next examined TP309, lhcb5-RNAi, and LHCB5-OX resistant to 21 M. oryzae isolates containing various AVR genes (SI Appendix, Fig. S12) (23) and found that the LHCB5-OX lines showed greater resistance to all 21 isolates (SI Appendix, Fig. S13). To investigate whether these lines have also been more resistant to other pathogens, we inoculated them with the pathogenic fungus Bipolaris oryzae and the pathogenic bacterium Xanthomonas oryzae pv oryzae PXO99. We observed that the LHCB5-OX lines were not resistant to either of these pathogens (SI Appendix, Fig. S14A), suggesting that the basal resistance in the LHCB5-OX lines, associated with ROS production, was M. oryzae-specific.
LHCB5 Phosphorylation Regulates Basal Immunity Independent of R Genes Pia and Pizt.
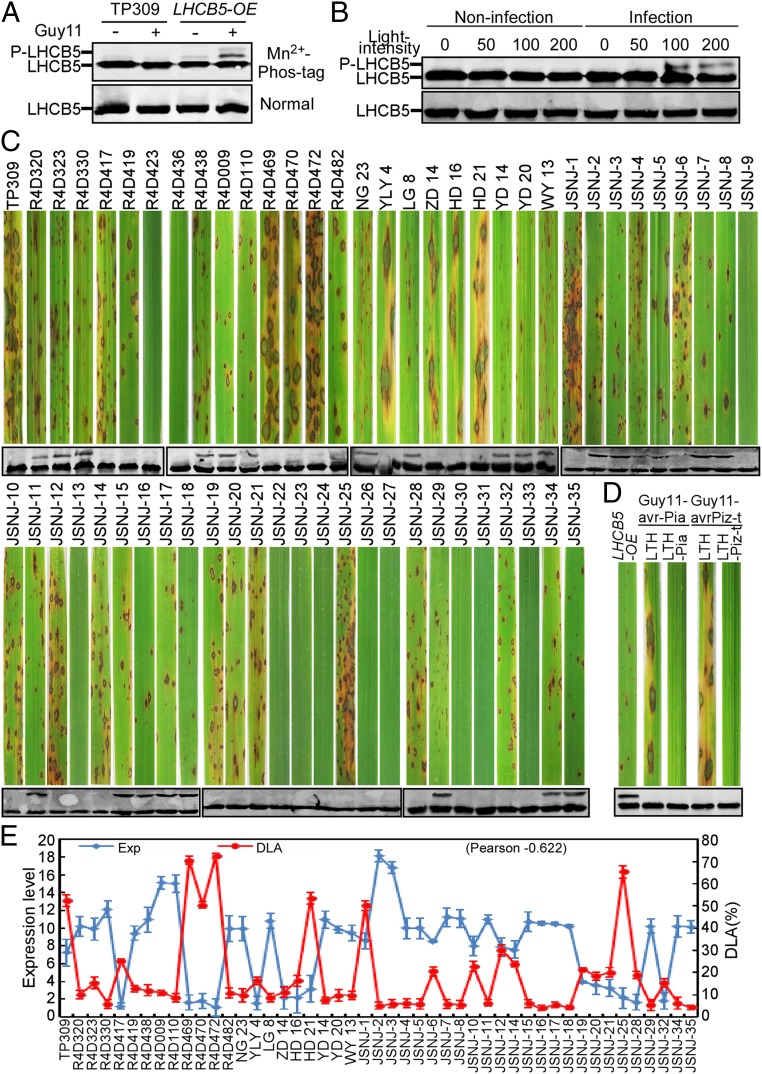
Previous studies have suggested that LHCII is phosphorylated to balance light excitation energy between photosystem I and photosystem II (24). In the alga Chlamydomonas reinhardtii, a marked increase in phosphorylation of the chlorophyll protein CP26 (LHCB5 homolog) was observed in state II (25). We analyzed the phosphorylation of LHCB5 in TP309 and LHCB5-OX lines that were uninfected, or that had been infected by Guy11, using Mn2+-Phos-tag gel electrophoresis (26). We found that LHCB5 phosphorylation was induced by Guy11 in the LHCB5-OX lines, but not in TP309 (Fig. 4A), and that this phosphorylation was M. oryzae-specific (SI Appendix, Fig. S14B). To further test whether the LHCB5 phosphorylation was light-dependent, we examined LHCB5 phosphorylation at various light intensities and found that it was induced at 100 and 200 μmol photons m−2s−1, but not below 50 μmol photons m−2s−1 (Fig. 4B).
Fig. 4.
Phosphorylation of LHCB5 regulates blast resistance. (A) LHCB5 was phosphorylated in LHCB5-OX plants inoculated with Guy11. Proteins from TP309 and the LHCB5-OX cell extracts were subjected to Phos-tag SDS/PAGE and normal SDS/PAGE followed by immunoblotting with LHCB5 polyclonal antibody. (B) LHCB5 phosphorylation was light-dependent. The LHCB5-OX plants inoculated with or without Guy11 were cultivated in a light incubator with different light intensity (0, 50, 100, and 200 μmol photons m−2s−1). (C) The phosphorylation is correlated with blast resistance. Blast resistance of 59 rice varieties using spraying inoculation. The phosphorylation of LHCB5 was detected at 48 hpi. Lesions were photographed and measured or scored at 7 dpi. (D) Phosphorylation assay of LHCB5 during interaction between Avr-Pia/Pia and AvrPiz-t/Piz-t. LTH-Pia and LTH-Pizt plants were inoculated with Guy11/Avr-Pia and Guy11/AvrPiz-t, respectively. (E) The expression level is associated with blast resistance. The DLA was measured by Image J, and the expression (Exp) level was detected by qRT-PCR. The Pearson correlation coefficient was −0.622. Values are the means of 3 replications, and error bars represent the SD (n = 3).
We next carried out a blast-resistance assay with 14 randomly selected lines out of 3,000 rice accessions (18) and 45 lines of the Chinese origin to determine whether phosphorylation was associated with resistance. We observed that only LHCB5-phosphorylated lines exhibited resistance (Fig. 4C), and a total of 12 lines showed no observable lesions, indicative of the typical R resistance gene-mediated immune response (corresponding to the AVR protein in Guy11) (Fig. 4C). Further, we observed that this correlates with LHCB5 not being phosphorylated during interactions between Avr-Pia/Pia and AvrPiz-t/Piz-t (Fig. 4D). These findings suggested that LHCB5 phosphorylation regulates basal immunity and that the resistance is independent of the R genes Pia and Pizt.
Finally, we examined the expression of LHCB5 in the above-mentioned 47 rice lines (excluding the 12 lines showing no lesions) and found higher LHCB5 expression levels correlated with smaller lesion sizes (0.622 Pearson Correlation Coefficient) (Fig. 4E), which is consistent with the results shown in SI Appendix, Fig. S7. Since LHCB5 expression was significantly reduced in indica compared to japonica (SI Appendix, Fig. S6A), we next determined whether LHCB5 phosphorylation similarly differed, which we tested using 9 high and low LHCB5 expression varieties from each subgroup. After M. oryzae infection, LHCB5 phosphorylation was found to mainly occur in the highly expressing japonica varieties (SI Appendix, Fig. S6B).
Phosphorylation of LHCB5 Correlates with Enhanced Resistance of Progeny Containing the YG456-Type Promoter.
To confirm the relationship between the phosphorylation of LHCB5 and resistance to M. oryzae, we investigated LHCB5 phosphorylation in the same F2 population as used for the genetic association experiments. First, we analyzed the phosphorylation of LHCB5 in the LTH and YG456 lines, with or without infection, and found that LHCB5 was phosphorylated in infected YG456 (Fig. 2D). Of all 42 progenies, LHCB5 phosphorylation was only detected in resistant progenies (Fig. 2E), indicating that these traits cosegregated. Taken together with the observed close association between the YG456-type promoter and resistance to rice blast, we speculate that the resistance governed by the phosphorylation of LHCB5 is genetically controlled by the LHCB5 promoter in YG456.
Phosphorylation of LHCB5 24th Threonine (T24) Activates an ROS Burst in Chloroplasts.
To study the phosphorylation mechanism, we first compared the LHCB5 sequence to those of other plant species and predicted 3 possible phosphorylation sites at the less conserved N-terminal domain, using DISPHOS 1.3 and NetPhos 3.1 server (SI Appendix, Fig. S15A). We showed that the constitutively activated LHCB5 T24 (LHCB5T24D) allele induced ROS production and cell death in Nicotiana benthamiana leaves, in contrast to the nonphosphorylated LHCB5 (LHCB5T24A) (SI Appendix, Fig. S15 B and C), or LHCB5 with constitutive phosphorylation at 2 other sites (SI Appendix, Fig. S16). The phosphorylation of LHCB5 was observed in the cytoplasm, but not in the chloroplast (SI Appendix, Fig. S17). We further expressed the rice LHCB5 and LHCB5T24A protein with a FLAG peptide tag at the C terminus (OsLHCB5:Flag and OsLHCB5T24A:Flag) in lhcb5-RNAi protoplasts induced by PM and detected mobility shifts representing phosphorylated LHCB5 in OsLHCB5, but not OsLHCB5T24A (SI Appendix, Fig. S15D). Using a luminol-based chemiluminescence assay, we observed higher ROS levels in the presence of constitutively activated LHCB5 (LHCB5T24D) than with inactivated LHCB5 (LHCB5T24A) in protoplasts (SI Appendix, Fig. S15E). These results further suggested that LHCB5 24T phosphorylation is important for LHCB5 function.
To confirm the elevated production of ROS in chloroplasts, we expressed the empty plasmid pBIN:GFP (as a marker for subcellular localization), as well as OsLHCB5T24D:GFP, and OsLHCB5T24A:GFP in rice protoplasts, and found that both OsLHCB5T24D:GFP and OsLHCB5T24A:GFP were localized to the chloroplast. Nitroblue tetrazolium (NBT) staining revealed an accumulation of superoxide (O2•−) in OsLHCB5T24D:GFP expressing protoplasts, but not in pBIN:GFP or OsLHCB5T24A:GFP expressing protoplasts (SI Appendix, Fig. S15F), consistent with LHCB5 phosphorylation that results in O2•− accumulation in the chloroplast and activation of the immune response.
Phosphorylation Facilitates LHCB5 Accumulation and Trimerization in Chloroplasts.
We noticed that the phosphorylation site was located within the region of the LHCB5 polypeptide responsible for chloroplast transit, which is important for the import of precursor proteins (27, 28). To investigate whether phosphorylation influences the accumulation of LHCB5 in the chloroplast, we separated chloroplast and cytoplasmic protein fractions extracted from TP309, lhcb5-RNAi, and LHCB5-OX lines, with or without Guy11 inoculation. Western blot analysis indicated the increased accumulation of LHCB5 in the chloroplasts of LHCB5-OX lines inoculated with Guy11 (SI Appendix, Fig. S18). Furthermore, we expressed pBIN-LHCB5-Flag, pBIN- LHCB5T24A-Flag, and pBIN- LHCB5T24D-Flag in the LHCB5-KO rice protoplast treated with or without PM. As shown in SI Appendix, Fig. S19 A and B, inactivated LHCB5 (LHCB5T24A) cannot stimulate host immunity. Moreover, we found that pBIN-LHCB5-Flag and pBIN- LHCB5T24D-Flag showed higher protein accumulation in chloroplast than pBIN- LHCB5T24A-Flag when treated with PM (SI Appendix, Fig. S19C). Together, the results indicated that phosphorylation of 24th threonine facilitates LHCB5 accumulation in chloroplast to stimulate the immune response.
Since a previous study found that A. thaliana LHCB5 is organized into trimeric complexes in the absence of LHCB1 and LHCB2 (15), we next tested whether increased LHCB5 accumulation also led to the accumulation of trimers. Using native-PAGE analysis, we found that LHCB5 is mostly present in the form of monomers and dimers in TP309 and that trimeric forms were only founded in infected LHCB5-OX lines (SI Appendix, Fig. S20A). When a His-tagged form of LHCB5, LHCB5-His, was expressed in Escherichia coli BL21 and then purified (SI Appendix, Fig. S20B), fractionated, and analyzed by native-PAGE analysis, we also observed LHCB5-His in a trimeric complex (SI Appendix, Fig. S20C). Together, these data indicated that phosphorylation facilitates the accumulation of LHCB5 into chloroplasts and that LHCB5 exists in multimeric conformations, including a trimer.
Trimeric LHCB5 Conformation Affects PsbS Binding and the Electron Transport Rate in Chloroplasts.
It was reported that rice plants deficient in the PSII protein PsbS had higher levels of chloroplastic superoxide and hydrogen peroxide and were more resistant to M. oryzae infection (29, 30). In A. thaliana, PsbS binds to LHCB5 to control nonphotochemical quenching (NPQ) (31). We therefore tested the interaction between LHCB5 and PsbS in the LHCB5-OX lines and found that this is indeed also the case in rice. However, the Co-IP analysis showed that the LHCB5-PsbS interaction does not occur upon infection by Guy11 (SI Appendix, Fig. S21). We hypothesized that multimeric forms of LHCB5 interact less with PsbS than does the LHCB5 monomer. To test this, we performed an in vitro pull-down assay with varying amounts of LHCB5. Consistent with our hypothesis, PsbS binding decreased as LHCB5 concentration increased (SI Appendix, Fig. S22).
To validate O2•− accumulation in the chloroplast, we measured photosynthetic parameters in the TP309, G-TP309 (Guy11-infected), lhcb5-RNAi, G-lhcb5-RNAi (Guy11-infected), LHCB5-OX, and G-LHCB5-OX (Guy11-infected) lines using a Chlorophyll Fluorescence Imager (Ecotek, Beijing). The electron transport rate (ETR) was significantly reduced in G-LHCB5-OX lines (SI Appendix, Fig. S23A). Moreover, a transmission microscopic analysis of chloroplast morphology revealed that the structure was less compact in G-TP309 and G-lhcb5-RNAi lines when compared to noninfected lines, while no difference was detected in the G-LHCB5-OX lines (SI Appendix, Fig. S23B). These data suggested that LHCB5 may form trimeric complexes during infection to maintain normal morphology and may affect PsbS binding, thereby influencing O2•− accumulation.
Discussion
Rice blast threatens rice production worldwide, and serious outbreaks can destroy whole crop harvests. Rice blast erupts in overcast and rainy conditions, due not only to favorable environmental conditions but also to insufficient light, which reduces host resistance (32). The mechanistic basis of the relationship between resistance and light levels has not been elucidated until now. Our study establishes the relationships among light conditions, light-harvesting complex protein LHCB5, and disease resistance.
It is known that transcription is often associated with SNP variations in the promoter and that nucleotide polymorphism in either the promoter or gene can affect resistance (33, 34). For example, it was reported that an A to G transition in the promoter region of the BSR-D1 gene results in lower expression, resulting in elevated resistance (35). A survey of SNPs among LHCB5 promoter sequences of 3,000 sequenced rice genomes suggested that SNP variation is associated with the differential expression of LHCB5 and, accordingly, resistance (Fig. 4E and SI Appendix, Fig. S8). It has been demonstrated that japonica rice varieties from the Chinese Yuanyang terraces have higher basal immunity, but a lower content of major resistance (Pi) genes than do indica varieties (36). Our results suggested that LHCB5 expression was higher in japonica (SI Appendix, Fig. S6A). Moreover, a higher LHCB5 expression leads to its phosphorylation upon challenge with the blast fungus, particularly in japonica (SI Appendix, Fig. S6B). We propose that SNP variation among japonica and indica varieties causes differences in LHCB5 expression patterns, resulting in the differential phosphorylation of LHCB5. This is consistent with the elevated basal immunity observed in japonica rice. Whereas the evidence for a direct connection remains to be established, our studies suggest a strong correlation between promoter SNP and LHCB5 phosphorylation.
Biotic stress typically stimulates the production of ROS, such as O2•−, in the chloroplast, (37) and ROS generation is associated with resistance to rice blast (35). Here, we showed that the ROS burst in the chloroplast is correlated with the phosphorylation of LHCB5, and that mediated broad-spectrum blast resistance (SI Appendix, Fig. S15). A widely used strategy to protect rice against pathogens is through the breeding of resistant varieties (38, 39), despite that the rapid variation of rice blast can lessen the effectiveness of this approach (40). To evaluate LHCB5 phosphorylation-induced resistance, we assessed the inheritance of this trait and found that it cosegregated with resistance (Fig. 2). Our studies indicated no differences between LHCB5 transgenic and wild-type rice lines in the plant height, grain weight, and seed-setting rate (SI Appendix, Fig. S24), which promises for future breeding of high yield and blast-resistant rice varieties.
We propose a model where during the interaction between rice and M. oryzae, the host monitors the progression of infection through the phosphorylation of LHCB5 in a light-dependent manner. M. oryzae challenge results in phosphorylated LHCB5 accumulation in chloroplasts, which helps maintain chloroplast function by reducing the binding of LHCB5 to PsbS, which in turn leads to reduced ETR for ROS production (SI Appendix, Fig. S25).
Materials and Methods
The plant strains and blast isolates are listed in SI Appendix, Materials and Methods. Resistance test and infection assessment were performed as described (19). The ROS and cell death observation was performed as described (19). The phosphorylation assay was performed as described (41). Purified recombinant proteins from Escherichia coli were used for in vitro pull-down assays (41). All other materials and details of experimental methods can be found in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This research was supported by the Key program of Natural Science Foundation of China (NSFC) (Grant No: 31530063, to Z.Z.), Innovation Team Program for NSFC (Grant No: 31721004), Innovation Team Program for Jiangsu Universities (2017), and Jiangsu Agricultural Science and Technology Innovation Fund (CX[18]1003). B.Z. acknowledges the support of the Swiss Programme for Research on Global Issues for Development (IZ07Z0-160877). We thank Dr. Jianmin Wan of Nanjing Agricultural University and Dr. Zhongqiang Qi of Jiangsu Academy of Agricultural Sciences for providing rice seeds.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905123116/-/DCSupplemental.
References
- 1.Fu D., et al. , A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323, 1357–1360 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng C., et al. , Plant immune response to pathogens differs with changing temperatures. Nat. Commun. 4, 2530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xin X. F., et al. , Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539, 524–529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren Y., Gao P., Zhu F., Meteorological grade prediction of occurrence degree of rice blast in Jiangsu. Jiangsu Agr. Sci. 44, 151 (2016). [Google Scholar]
- 5.Zhang H., Zheng X., Zhang Z., The Magnaporthe grisea species complex and plant pathogenesis. Mol. Plant Pathol. 17, 796–804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dempsey D. A., Vlot A. C., Wildermuth M. C., Klessig D. F., Salicylic Acid biosynthesis and metabolism. Arabidopsis Book 9, e0156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca S., et al. , (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 5, 344–350 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Strawn M. A., et al. , Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J. Biol. Chem. 282, 5919–5933 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Shapiguzov A., Vainonen J. P., Wrzaczek M., Kangasjärvi J., ROS-talk–How the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 3, 292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pospíšil P., Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 1817, 218–231 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Hankamer B., et al. , Isolation and biochemical characterisation of monomeric and dimeric photosystem II complexes from spinach and their relevance to the organisation of photosystem II in vivo. Eur. J. Biochem. 243, 422–429 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Boekema E. J., van Breemen J. F., van Roon H., Dekker J. P., Arrangement of photosystem II supercomplexes in crystalline macrodomains within the thylakoid membrane of green plant chloroplasts. J. Mol. Biol. 301, 1123–1133 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Andersson J., Walters R. G., Horton P., Jansson S., Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: Implications for the mechanism of protective energy dissipation. Plant Cell 13, 1193–1204 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bianchi S., Dall’Osto L., Tognon G., Morosinotto T., Bassi R., Minor antenna proteins CP24 and CP26 affect the interactions between photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 20, 1012–1028 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruban A. V., et al. , Plants lacking the main light-harvesting complex retain photosystem II macro-organization. Nature 421, 648–652 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Jansson S., The light-harvesting chlorophyll a/b-binding proteins. Biochim. Biophys. Acta 1184, 1–19 (1994). [DOI] [PubMed] [Google Scholar]
- 17.Horton P., Ruban A. V., Walters R. G., Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Li J. Y., Wang J., Zeigler R. S., The 3,000 rice genomes project: New opportunities and challenges for future rice research. Gigascience 3, 8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi Z., et al. , The syntaxin protein (MoSyn8) mediates intracellular trafficking to regulate conidiogenesis and pathogenicity of rice blast fungus. New Phytol. 209, 1655–1667 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Ayers A. R., Ebel J., Valent B., Albersheim P., Host-pathogen interactions: X. Fractionation and biological activity of an elicitor isolated from the mycelial walls of phytophthora megasperma var. sojae. Plant Physiol. 57, 760–765 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe S., Nishizawa Y., Minami E., Effects of catalase on the accumulation of H(2)O(2) in rice cells inoculated with rice blast fungus, Magnaporthe oryzae. Physiol. Plant. 137, 148–154 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Li J., et al. , Hydrogen peroxide regulates elicitor PB90-induced cell death and defense in non-heading Chinese cabbage. Physiol. Mol. Plant Pathol. 67, 220–230 (2005). [Google Scholar]
- 23.Chen Q. H., Wang Y. C., Zheng X. B., Genetic diversity of Magnaporthe grisea in China as revealed by DNA fingerprint haplotypes and pathotypes. J. Phytopathol. 154, 361–369 (2006). [Google Scholar]
- 24.Lemeille S., et al. , Analysis of the chloroplast protein kinase Stt7 during state transitions. PLoS Biol. 7, e45 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Depège N., Bellafiore S., Rochaix J. D., Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299, 1572–1575 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T., Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5, 749–757 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Lamberti G., Drurey C., Soll J., Schwenkert S., The phosphorylation state of chloroplast transit peptides regulates preprotein import. Plant Signal. Behav. 6, 1918–1920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May T., Soll J., 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell 12, 53–64 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zulfugarov I. S., et al. , Production of superoxide from Photosystem II in a rice (Oryza sativa L.) mutant lacking PsbS. BMC Plant Biol. 14, 242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zulfugarov I. S., et al. , Enhanced resistance of PsbS-deficient rice (Oryza sativa L.) to fungal and bacterial pathogens. J. Plant Biol. 59, 616–626 (2016). [Google Scholar]
- 31.Sacharz J., Giovagnetti V., Ungerer P., Mastroianni G., Ruban A. V., The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching. Nat. Plants 3, 16225 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y., et al. , Genetic diversity and disease control in rice. Nature 406, 718–722 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Bimolata W., et al. , Nucleotide diversity analysis of three major bacterial blight resistance genes in rice. PLoS One 10, e0120186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rashid M. A., Zhao Y., Zhang H., Li J., Li Z., Nucleotide diversity, natural variation, and evolution of Flexible culm-1 and Strong culm-2 lodging resistance genes in rice. Genome 59, 473–483 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Li W., et al. , A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 170, 114–126.e15 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Liao J., et al. , Pathogen effectors and plant immunity determine specialization of the blast fungus to rice subspecies. eLife 5, e19377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su J., et al. , Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity. PLoS Biol. 16, e2004122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuoka S., et al. , Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325, 998–1001 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Zhou B., et al. , The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol. Plant Microbe Interact. 19, 1216–1228 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Huang J., Si W., Deng Q., Li P., Yang S., Rapid evolution of avirulence genes in rice blast fungus Magnaporthe oryzae. BMC Genet. 15, 45 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L., et al. , MoCAP proteins regulated by MoArk1-mediated phosphorylation coordinate endocytosis and actin dynamics to govern development and virulence of Magnaporthe oryzae. PLoS Genet. 13, e1006814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.