Significance
Viral infections have different outcomes in different individuals. They could be cleared spontaneously, turn chronic, or cause host death due to tissue damage. Identifying what determines these outcomes has been a longstanding challenge in immunology. Many factors, including viral inoculum size and host genetics, that influence the outcomes have been identified, suggesting that a complex interplay of numerous factors determines the outcomes. In striking contrast, we argue that the outcomes are determined by a dynamical motif comprising a few, essential interactions between viral antigens and CD8 T cells. The other factors influence the outcomes indirectly by modulating these essential interactions. The motif presents a conceptual understanding of the outcomes, explains several confounding experimental observations, and proposes interventions.
Keywords: acute infection, chronic infection, immunopathology, mathematical model, bistability
Abstract
Some viral infections culminate in very different outcomes in different individuals. They can be rapidly cleared in some, cause persistent infection in others, and cause mortality from immunopathology in yet others. The conventional view is that the different outcomes arise as a consequence of the complex interactions between a large number of different factors (virus, different immune cells, and cytokines). Here, we identify a simple dynamical motif comprising the essential interactions between antigens and CD8 T cells and posit it as predominantly determining the outcomes. Viral antigen can activate CD8 T cells, which in turn, can kill infected cells. Sustained antigen stimulation, however, can cause CD8 T-cell exhaustion, compromising effector function. Using mathematical modeling, we show that the motif comprising these interactions recapitulates all of the outcomes observed. The motif presents a conceptual framework to understand the variable outcomes of infection. It also explains a number of confounding experimental observations, including the variation in outcomes with the viral inoculum size, the evolutionary advantage of exhaustion in preventing lethal pathology, the ability of natural killer (NK) cells to act as rheostats tuning outcomes, and the role of the innate immune response in the spontaneous clearance of hepatitis C. Interventions that modulate the interactions in the motif may present routes to clear persistent infections or limit immunopathology.
Viral infection can have different outcomes in different individuals. Three frequently observed outcomes are clearance after acute infection, long-term persistence, and host mortality due to immunopathology. With hepatitis C virus (HCV), for instance, 25% of the individuals infected clear the infection spontaneously, whereas the rest become chronically infected (1, 2). Hepatitis B virus (HBV) causes persistent infection in neonates but is typically self-limiting in adults (3). The extent of pathology after infection can also vary; for example, respiratory syncytial virus infection causes severe immunopathology in some children but not in others (4). Why can infection with a given virus have different outcomes in different individuals, and what determines the outcome?
Murine models of lymphocytic choriomeningitis virus (LCMV) infection recapitulate many of these outcomes and consequently, have been used to better understand the factors that determine the outcomes. A seminal study over 25 y ago showed the effect of viral inoculum size on the outcome of LCMV infection (5). Infections initiated with a small virus inoculum were cleared; infections with intermediate inocula caused severe immunopathology, leading to death; and infections with large inocula led to survival, albeit with the persistence of high titers of the virus (5, 6). Soon after, CD8 T cells were identified to be critical to the control of LCMV infections: CD8 T-cell deficiency resulted in persistent infection of mice that otherwise would have rapidly cleared the virus (7). More recently, natural killer (NK) cells have been argued to act as rheostats tuning outcomes: NK cell depletion can change the outcome of infection with a high dose of LCMV from persistence of the virus with low levels of pathology to rapid host death (6). In other studies, regulatory T cells (8), factors contributing to CD8 T-cell dysfunction (9, 10), type I interferon (IFN) signaling (11, 12), and most recently, host genetics (13) have been argued to influence the outcomes of LCMV infection. A plethora of factors thus seems to be involved in determining the outcomes of this and other infections (14–17), and it is unclear how the interplay between these factors determines which outcome is reached.
Here, we identify a dynamical motif comprising the essential interactions of antigens and CD8 T cells and posit it as central to defining the outcomes of viral infections. Our reasoning follows from observations that suggest that the latter interactions may be both necessary and adequate to recapitulate the outcomes. Antigens exert competing influences on CD8 T cells. Antigen stimulation via viral peptides presented by class I major histocompatibility complex molecules on infected cells activates CD8 T cells and triggers a response that can kill the infected cells (18, 19). Sustained stimulation, however, when a high level of antigen is present for an extended duration can cause CD8 T-cell dysfunction via exhaustion (5, 20–22). The relative strengths of these competing influences together with the strength of the CD8 T-cell response seem to define the outcomes realized. While a strong CD8 T-cell response is critical to viral clearance (7), persistent infections are typically associated with a dysfunctional CD8 T-cell response (7, 18, 23). Furthermore, T-cell exhaustion has been proposed to be an altered differentiation program to combat chronic infection while preventing immunopathology (24–26). The exhausted state is maintained by inhibitory molecules, such as PD-1 (10), and can be partly reversed by blocking the inhibitory molecules or lowering antigen burden (9, 10, 27), which in turn, can alter the disease state from persistence to clearance (28). We hypothesized, therefore, that the dynamical motif due to these underlying interactions governs the outcomes. Furthermore, we suggest that the other factors that influence the outcomes do so by modulating the interactions in the motif.
Results
The Dynamical Motif and Its Mathematical Model.
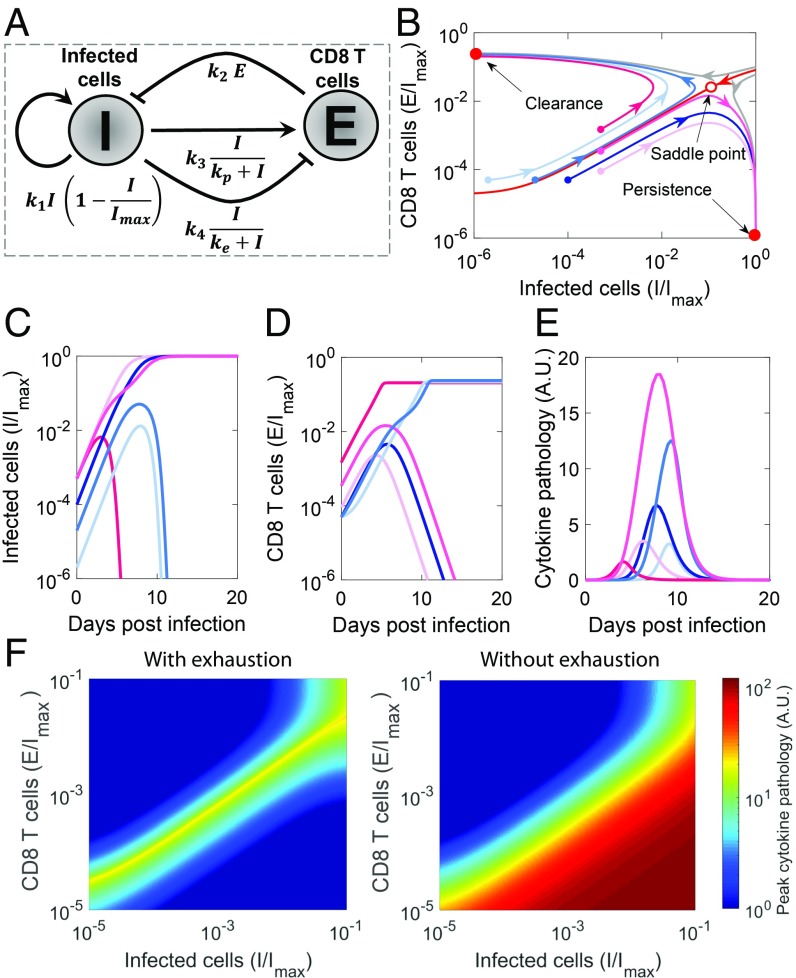
To test the hypothesis, we constructed the dynamical motif comprising the competing influences of antigen on CD8 T cells and the suppressive influence of CD8 T cells on antigen (Fig. 1A). We developed the following minimal mathematical model to analyze the motif:
| [1] |
We modeled the growth of infected cells (the source of antigenic stimulation) using a logistic term with a per capita proliferation rate and a carrying capacity . Infected cells are killed by activated CD8 T cells, , with the second-order rate constant . Following previous models (29, 30), we described T-cell activation and exhaustion by infected cells using Hill functions with maximal per capita rates and and scaling constants and , respectively. To ensure that activation happens at lower antigen levels than exhaustion and that the latter dominates at high antigen levels, we let and (30). Thus, as antigen levels rise, they first activate CD8 T cells, whereas continued increase of antigen levels triggers CD8 T-cell exhaustion. Our key findings are robust to alternative formulations, where exhaustion is triggered by cumulative rather than instantaneous antigen stimulation (24) (SI Appendix, Text S1 and Fig. S1). We show below that the motif recapitulates the observed outcomes of viral infections.
Fig. 1.
Dynamical motif underlying the outcomes of viral infections. (A) Schematic of the motif depicting the essential interactions between infected cells, , and CD8 T cells, . (Note that arrows and blunt arrows imply positive and negative regulation, respectively.) (B) Phase portrait of the system, where regions leading to the 2 stable steady states (filled red dots), clearance and persistence, are separated by a basin boundary (red line). The unstable saddle (open red dot) underlies immunopathology. Also shown are trajectories with increasing initial antigen load (light to dark blue) and increasing initial effector pool size (light to dark pink) and the corresponding time course of (C) infected cells, (D) effector pool size, and (E) pathology. (F) Heat maps of peak pathology for different initial conditions on the phase portrait with and without exhaustion. Parameters values are in SI Appendix, Table S1.
Viral Clearance and Persistence as Steady States of the Motif.
To describe the long-term outcomes of infection, we solved the model equations (Eq. 1) for its steady states. The model admits 3 steady states. The steady states can be derived analytically when the exhaustion rate is far from saturation () and can be approximated using the mass action term . The resulting steady states are (i) and , (ii) and , and (iii) and . Using linear stability analysis, we found the first 2 steady states to be stable, and the third to be an unstable saddle (SI Appendix, Text S2). The bistability existed across wide parameter ranges (SI Appendix, Fig. S2). A phase portrait shows the 2 stable states separated by a basin boundary on which the saddle lies (Fig. 1B). The first steady state has no antigen and thus, marks the clearance of infection. The second, in contrast, has maximal antigen and no effector cells, which indicate suppression of the immune response and persistent infection. The basin boundary (red line in Fig. 1B) separates conditions that lead to one outcome from those that lead to the other. Infections initiated in the region above the basin boundary, which marks higher than a threshold CD8 T cells for a given antigen load, lead to viral clearance (Fig. 1 C and D). In contrast, infections initiated in the region below the basin boundary lead to persistence.
Immunopathology as a Dynamical Outcome of the Motif.
Immunopathology can result from a variety of processes associated with the destruction of virus-infected cells or the release of cytokines resulting in a “cytokine storm.” Immunopathology is high when the infection spreads to a sizeable proportion of target cells, and there is a large population of CD8 T cells that kill these cells, directly producing tissue damage or indirectly causing pathology due to cytokine release. We thus modeled immunopathology with the equation , letting increase in proportion to the rate of contact between and and recover with a natural first-order decay. We found immunopathology to be maximum with intermediate initial antigen load and CD8 T-cell counts (Fig. 1E). When the initial antigen load is small, CD8 T cells eliminate antigen before it can spread substantially. When the load is large, CD8 T cells become exhausted before they can kill a sizeable proportion of infected cells. At intermediate antigen loads, the CD8 T-cell population is not capable of clearing the infection before it spreads significantly. At the same time, the antigen does not accumulate to extents that can trigger significant CD8 T-cell exhaustion. Thus, the antigen spreads and is continuously eliminated by CD8 T cells, which results in sizeable tissue damage.
From a dynamical systems viewpoint, immunopathology results when infection commences close to the basin boundary (Fig. 1 B and E). When the infection commences away from the boundary, it quickly settles into one of the stable steady states. When it starts close to the boundary, it traverses long trajectories along the boundary, causing a buildup of both antigen and CD8 T-cell levels and triggering immunopathology before deviating toward one of the stable steady states. Spanning a wide range of initial conditions, we found that conditions near the basin boundary yielded the most pathology (Fig. 1F).
CD8 T-cell exhaustion has been argued to be an evolutionary adaptation to prevent immunopathology (24–26). To test this argument, we repeated our calculations in the absence of exhaustion (). In contrast to the maximum seen above, we found that pathology now monotonically increased with viral inoculum and effector pool sizes (Fig. 1F), demonstrating that exhaustion curtails pathology associated with high viremia and enables host survival. The price, however, is viral persistence.
The motif thus recapitulates the outcomes of viral infections observed. We examined next whether it can describe the roles of key factors known to influence the outcomes.
Influence of the Viral Inoculum and Initial Effector Pool Sizes.
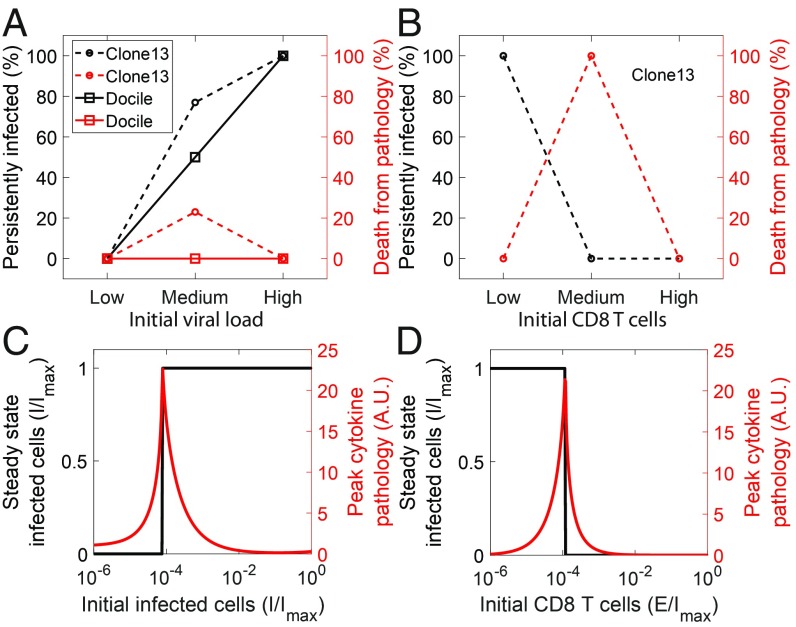
In experiments, infection with low-dose LCMV Docile or clone 13 led to clearance, whereas infection with high dose led to persistence in 100% of the mice infected (5) (Fig. 2A). With intermediate-dose Docile infection, 50% mice cleared the infection, and 50% became persistently infected (5), whereas with clone 13 infection, 20% died due to immunopathology, and the remaining became persistently infected (6). A small CD8 T-cell population at the start of infection, introduced by adoptive transfer, did not prevent persistent LCMV clone 13 infection, whereas a large population led to clearance in the absence of immune escape mutations (31) (Fig. 2B). With intermediate effector pool sizes, all of the mice infected died due to immunopathology (31).
Fig. 2.
Dependence of outcomes of infection on viral inoculum and initial effector pool sizes. Experimental observations of outcomes with different (A) initial LCMV inoculum sizes (5, 6) and (B) effector pool sizes (31). (C and D) Corresponding model predictions of steady state-infected cells and peak cytokine pathology. Parameter values are the same as in Fig. 1.
Our model recapitulated these observations. It predicted clearance with small and persistence with large viral inocula for fixed initial effector pool sizes (Fig. 2C). With fixed inocula, clearance resulted with large and persistence resulted with small initial effector pool sizes (Fig. 2D). Immunopathology was maximum with intermediate inocula or initial effector pool sizes. This is understood from our predictions above (Fig. 1). Host mortality depends on the severity and the criticality of the associated tissue damage. As the initial conditions moved to higher effector cell pool sizes along the basin boundary, we found more severe immunopathology (Fig. 1E). Consistent with these predictions, with clone 13 infection, host mortality increased from 20% without to 100% with adoptive transfer of CD8 T cells. With the LCMV Docile infection, the tissue damage was perhaps not critical, resulting in the even split between clearance and persistence, possibly due to stochastic effects and interhost variations.
To describe the influence of other factors, we constructed suitable embellishments of the motif.
Influence of NK Cells.
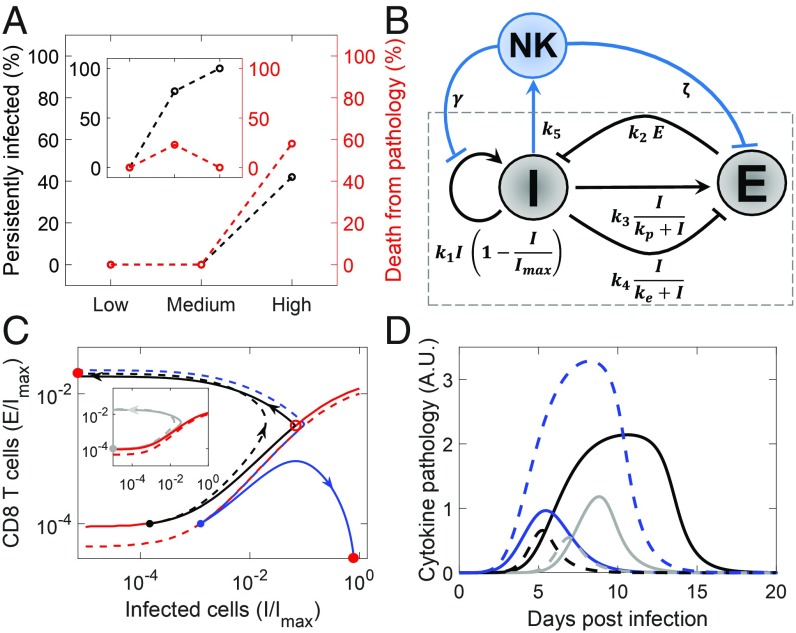
NK cells have been found to influence the outcomes as follows (6). With a normal NK cell response, 25% of mice subjected to intermediate-dose LCMV clone 13 infection died due to immunopathology, whereas 100% mice administered high-dose inocula survived but with persistent infection (Fig. 3A, Inset). In stark contrast, depleting NK cells resulted in 100% survival with intermediate dose and 60% mortality with high-dose infection (Fig. 3A). NK cells seem inconsequential with low-dose infection.
Fig. 3.
The influence of NK cells. (A) Outcomes observed experimentally in mice infected with different doses of LCMV clone 13 with (Inset) and without NK cells (6). (B) Schematic of the motif embellished to incorporate the influence of NK cells. (C) Projections of trajectories corresponding to low- (gray; Inset), medium- (black), and high-dose (blue) infection with (solid) and without (dashed) NK cells. Projections of the basin boundaries are in red. Three-dimensional versions are in SI Appendix, Fig. S3. (D) The corresponding time evolution of cytokine pathology. Parameter values are in SI Appendix, Table S1.
NK cells can kill virus-infected cells. However, they can also cytolytically eliminate CD4 T cells, which can compromise the CD8 T-cell response (6), because activation of CD8 T cells typically relies on CD4 T-cell help. We incorporated these effects in the motif by letting NK cells both kill infected cells and suppress the CD8 T-cell response (Fig. 3B).
Solving the resulting equations (SI Appendix, Text S3), we found that, whereas with normal NK cells, intermediate-dose infection led to immunopathology and high dose led to persistence and host survival, NK cell depletion reversed the trend with heightened immunopathology with high-dose infection (Fig. 3 C and D). On the phase portrait, depleting NK cells shifted the basin boundary, expanding the basin of attraction of the steady state representing clearance (Fig. 3C and SI Appendix, Fig. S3). This was because the suppression of the effector response by NK cells was relieved with NK cell depletion. (The direct killing of infected cells by NK cells seemed to have less significant an effect on the outcomes.) For the parameters chosen, the intermediate-dose infection commenced close to the basin boundary with NK cells but became removed from the boundary when NK cells were depleted. Conversely, the high-dose infection moved closer to the basin boundary when NK cells were depleted (Fig. 3C). Thus, immunopathology decreased with intermediate doses and increased with high doses after NK cell depletion (Fig. 3D). With low-dose infection, NK cells had a minimal influence (Fig. 3 C and D). Our motif thus recapitulates experimental observations and explains how NK cells function as rheostats (6): they limit the effector response in the dynamical motif to tune the outcome.
Influence of the Innate Immune Response.
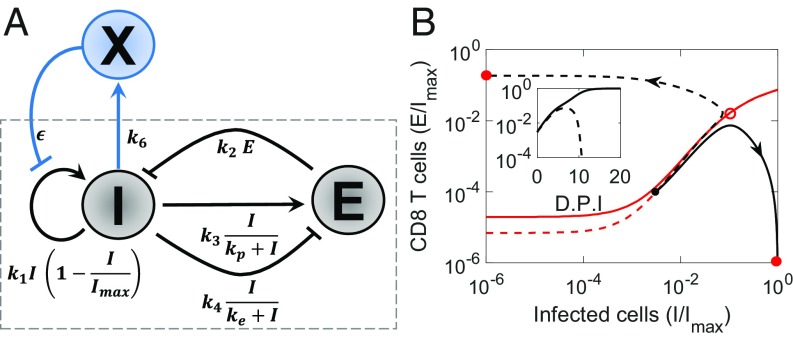
The innate immune response is typically mounted early after infection and begins to exert viremic control before the CD8 T-cell response is triggered. For instance, IFN, a key innate immune signaling molecule, is secreted after viral infection and triggers the expression of several hundred genes that collectively create an antiviral state in cells, which controls viral replication in infected cells and prevents the infection of uninfected cells (32). The early innate immune response may be viewed thus as a way to suppress viremia before the CD8 T-cell response is activated. A strong innate immune response would result in a low antigen load at the onset of the CD8 T-cell response, which based on our understanding of the motif above, would facilitate the clearance of infection. To test this hypothesis, we constructed a phenomenological description of the innate immune response, where the innate immune response is triggered by antigen and in turn, suppresses the growth of antigen (Fig. 4A). A key difference from the CD8 T-cell response is the absence of proliferative amplification, the latter a hallmark of adaptive immune responses.
Fig. 4.
Influence of the innate immune response. (A) Schematic of the motif with the embellishment representing the innate immune response, . (B) Projections of trajectories and time evolution of pathogen load (Inset) with strong (dashed) and weak (solid) innate immune responses. D.P.I., days post-infection. Red lines represent the projections of the corresponding basin boundaries. Three-dimensional versions are in SI Appendix, Fig. S4. Parameters values are in SI Appendix, Table S1.
Solving the resulting model equations (SI Appendix, Text S4), we found that a strong innate immune response resulted in clearance and a weak response in persistence (Fig. 4B, Inset). Akin to the NK cell response, a strong innate immune response expanded the basin of attraction of the steady state representing clearance (Fig. 4B and SI Appendix, Fig. S4). Unlike the NK cell response, however, which altered the CD8 T-cell response, the innate immune response modulated the outcomes by suppressing the antigen load and tilting the balance in favor of effector cells. We considered a realization of the latter phenomenon next.
Spontaneous Clearance of HCV Infection.
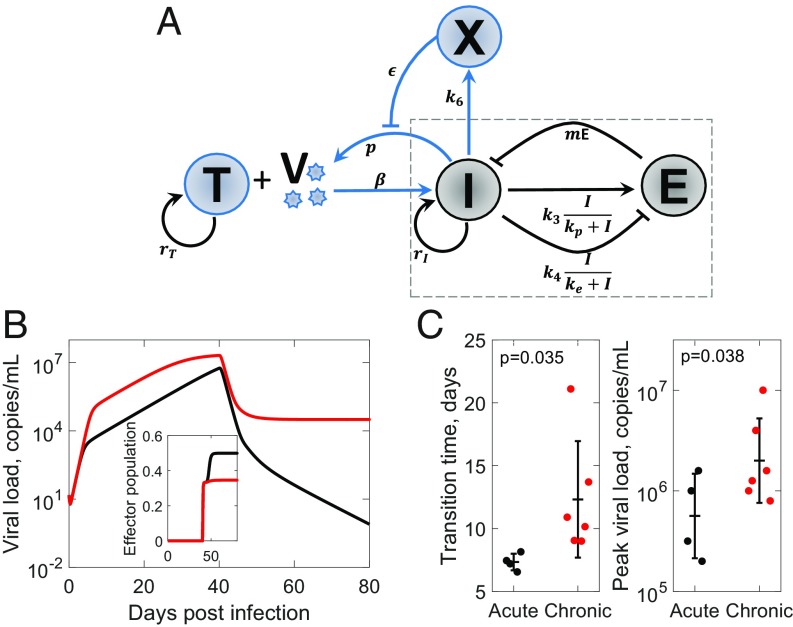
Spontaneous clearance of HCV infection has been attributed to strong IFN responses (33, 34). It is also known to require strong CD8 T-cell responses (35). To test whether spontaneous clearance is a manifestation of the influence of the innate immune response described above, we constructed a model of HCV dynamics that included the innate immune response. The model combined standard models of HCV kinetics (36, 37) with our motif above (Fig. 5A). We solved the resulting equations (SI Appendix, Text S5) using parameter values representative of HCV infection. We found that, with a weak innate immune response, the infection became persistent, whereas with a strong innate immune response, the infection was cleared after an acute phase (Fig. 5B). We observed further that the rise of viremia during the acute phase was biphasic with a sharp initial rise followed by a slower, more gradual rise to the peak viremia. The arrest of the sharp initial rise was due to the innate immune response. The decline after peak viremia was attributable to the CD8 T-cell response (Fig. 5B, Inset). The CD8 T-cell response was potentiated by the strong innate immune response, which suppressed viremia and limited exhaustion.
Fig. 5.
Innate immune response and the outcomes of HCV infection. (A) Schematic of the motif including viral dynamics. (B) Time course of viral load and (Inset) effector pool size for strong (black) and weak (red) innate immune responses. Parameters used are given in SI Appendix, Table S2. (C) Experimental observations (37, 38) of (Left) transition time from the first- to second-phase rise of viremia and (Right) peak viral load in chimpanzees infected with HCV resulting in clearance (black) or persistence (red) of the infection. One-tailed t tests with equal variance yielded P values.
Interestingly, the biphasic rise of viremia after infection has been seen in chimpanzees infected with HCV (38). Of the 10 animals challenged, 6 had persistent infection, whereas 4 cleared the infection after the acute phase. We reasoned that the earlier the transition from the first- to the second-phase rise of viremia, the stronger the innate immune response must be. The resulting peak viremia, which sets the antigen load for the CD8 T cells, would be correspondingly lower, facilitating cure. From previous fits to the viral load data (37), we estimated the transition times and peak viral loads in each animal. We found, remarkably, that the 6 animals that suffered persistent infection had significantly delayed transition times (mean 12 vs. 7 d; P = 0.035) and higher peak viral loads (mean 6.3 vs. 5.8 copies per milliliter; P = 0.038) than the 4 that cleared the infection (Fig. 5C). Our hypothesis of the innate immune response modulating the outcomes by lowering the antigen load with which the CD8 T cells must contend is thus consistent with the data.
Discussion
The quest for an understanding of the outcomes of viral infections has led, over the years, to the identification of an array of factors, including the size of the viral inoculum (5), the innate and NK cell responses (6, 34), and host genetic variations (13), that can potentially influence the outcomes. Mathematical models have been developed to explain the outcomes under specific circumstances, such as protection after vaccination (24), posttreatment control of HIV infection (30), and posttreatment cure of HCV infection (39). Here, we argue that underlying the effects of these factors is an essential dynamical motif (comprising the key interactions between antigens and CD8 T cells) that governs the outcomes. Using minimal mathematical models of the motif, we recapitulated the different outcomes observed. Broadly, when the CD8 T-cell response is strong, the infection is cleared, whereas when the antigen induces significant CD8 T-cell exhaustion, long-term persistence results. When the 2 effects are comparable, substantial spread of infection and associated CD8 T-cell killing ensue, causing tissue damage and pathology. Furthermore, using suitable embellishments of the motif, we showed that other factors could influence the outcomes by modulating the interactions in the motif. A conceptual understanding of the outcomes of viral infections thus emerges. An implication is that interventions that can manipulate the interactions in the motif may present routes to clear persistent infections or limit immunopathology.
One intervention strategy of great interest today is to use immune checkpoint inhibitors to block inhibitory receptors and reverse CD8 T-cell exhaustion. The strategy is being actively explored for treating persistent infections and cancers (40). Our study predicts an advantage to using immune checkpoint inhibitors early in infection. Infections typically commence with small antigen loads and small pools of antigen-specific CD8 T cells. At this stage, immune checkpoint inhibitors may be more readily able to reverse, if not prevent, exhaustion. From a dynamical systems viewpoint, a small increase in effector strength under these circumstances could push the infection across the basin boundary and alter the outcome from persistence to clearance. After the infection has progressed, however, the level of exhaustion is expected to be higher. Furthermore, the infection is likely to have veered away from the basin boundary toward persistence, requiring a much larger push to drive it to clearance. Our study also sounds a cautionary note. Reinvigorating the immune response in the presence of significant viral antigen may increase pathology. The pathology is likely to be specific to infected tissues and thus, distinct from the broad immune-related pathologies seen with checkpoint inhibitors during cancer treatments (41).
As an alternative strategy, antiviral drugs could be used to lower antigen levels, which could alter the outcome from persistence to clearance. Indeed, treatment with the direct-acting antiviral agents has been found recently to lead to the clearance of HCV infection in many individuals with detectable virus at the end of treatment (42). The mechanisms underlying this posttreatment cure are being investigated (39, 43, 44). The phenomenon is consistent, however, with our expectation of clearance after a sufficient reduction in antigen load as also suggested earlier (39). Posttreatment control has also been seen in a small percentage of HIV-infected individuals treated early with antiretroviral therapy (ART) (45). The interpretation, however, is more involved than HCV because of latently infected cells formed by HIV, which escape ART and host immune responses and can reignite infection posttreatment. One hypothesis is that ART lowers viremia, which reinvigorates exhausted CD8 T cells and enables control when reignition happens from a small latent cell pool (30). A large latent cell pool can result in large surges of viremia after reactivation, causing exhaustion again and compromising control.
In a series of elegant studies, paradoxical elements, where a stimulus can both activate and suppress its target, have been identified as designs prevalent in biological systems, including immune cell circuits (46–48). For instance, the cytokine interleukin-2 can induce the proliferation as well as the death of CD8 T cells. Such paradoxical pleiotropy has been argued to help maintain homeostatic cell concentrations and robustness in differentiation processes (46, 47). We recognize paradoxical pleiotropy in our motif, where antigen can both activate and suppress CD8 T cells. An additional feature of our motif is that the target can suppress the stimulus; CD8 T cells can kill infected cells. Our motif thus represents paradoxical pleiotropy coupled with a double-negative feedback loop, the latter of which is also present in many biological networks (49). Furthermore, paradoxical pleiotropy is evident also in the role of NK cells, which can kill infected cells and also compromise effector responses by destroying CD4 T cells (6, 50). Outcomes of viral infections thus represent a manifestation of paradoxical pleiotropy embedded in a more involved motif including double-negative feedback.
Our study has limitations. While our study recapitulates the different outcomes observed, it does not describe the frequencies with which they occur. A distribution of the strength of IFN responses across individuals has been argued to explain the frequency of the spontaneous clearance of HCV infection (33). Variations in other factors as well as in the strengths of the interactions in our motif, which remain to be quantified, may explain the frequencies observed with other infections. Interhost variations have been implicated in the differences in the outcomes realized with the same virus (13). In the extreme, a single outcome may be realized, such as with measles virus infection, which is nearly always cleared (51), or HIV infection, which invariably leads to persistence (52). Other infections, like influenza, may admit 2 outcomes, clearance and host mortality, the factors responsible for which seem to be similar to those considered in our study. Poor IFN responses have been implicated in the lethality of the 1918 influenza virus (17). High viral titers (16) and CD8 T-cell responses (53), the latter together with innate immune responses, dictated the outcomes with other influenza viruses. Virus-induced cytopathicity may also contribute to pathology in some instances (54). While we have addressed the roles of key factors, such as NK cells and innate immune responses, further embellishments of our motif are necessary to describe the influence of other factors that may underlie the above variations. For instance, IFN is known to have both antiviral and immune-suppressive effects. The latter effect, not considered here, is dominant late in infection and can be relieved by IFN blockade to achieve clearance of persistent infections (11–13). Similarly, CD4 T cells may influence the interactions in our motif and modulate outcomes. They have been found to influence the outcomes of LCMV infection (6, 7) and may also underlie the inverse dependence of the outcome on inoculum size seen with HBV infection of chimpanzees (14). We speculate, in the latter context, that small inoculum sizes weakly stimulate CD4 T cells, whereas large sizes may trigger their exhaustion (55) and/or NK cell killing (6). In both cases, CD4 T-cell help could be compromised or delayed, resulting in suboptimal CD8 T-cell responses and poor control of infection. Intermediate inocula may optimally stimulate CD4 T cells, leading to potent CD8 T-cell responses and rapid viral clearance, consistent with observations (14). Finally, whereas our model lets CD8 T-cell exhaustion be fully reversible, recent studies have identified subsets of exhausted CD8 T cells that cannot be reversed, especially with checkpoint inhibitors (40). These subsets must be considered in devising accurate strategies to clear persistent infections by manipulating the interactions in our motif.
Supplementary Material
Acknowledgments
The study was supported by NIH Grants U19 AI117891 (to R.A.) and R01AI110720 (to R.A.) and Wellcome Trust/DBT (Department of Biotechnology) India Alliance Senior Fellowship IA/S/14/1/501307 (to N.M.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902178116/-/DCSupplemental.
References
- 1.Micallef J., Kaldor J., Dore G., Spontaneous viral clearance following acute hepatitis C infection: A systematic review of longitudinal studies. J. Viral Hepatitis 13, 34–41 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Hajarizadeh B., Grebely J., Dore G. J., Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 10, 553–562 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Ganem D., Prince A. M., Hepatitis B virus infection—natural history and clinical consequences. N. Engl. J. Med. 350, 1118–1129 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Nair H., et al. , Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 375, 1545–1555 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskophidis D., Lechner F., Pircher H., Zinkernagel R. M., Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362, 758–761 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Waggoner S. N., Cornberg M., Selin L. K., Welsh R. M., Natural killer cells act as rheostats modulating antiviral T cells. Nature 481, 394–398 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matloubian M., Concepcion R. J., Ahmed R., CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund J. M., Hsing L., Pham T. T., Rudensky A. Y., Coordination of early protective immunity to viral infection by regulatory T cells. Science 320, 1220–1224 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay S. S., et al. , Antigen expression level threshold tunes the fate of CD8 T cells during primary hepatic immune responses. Proc. Natl. Acad. Sci. U.S.A. 111, E2540–E2549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wherry E. J., Kurachi M., Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teijaro J. R., et al. , Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson E. B., et al. , Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldstone M. B. A., et al. , Lymphocytic choriomeningitis virus clone 13 infection causes either persistence or acute death dependent on IFN-1, cytotoxic T lymphocytes (CTLs), and host genetics. Proc. Natl. Acad. Sci. U.S.A. 115, E7814–E7823 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asabe S., et al. , The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J. Virol. 83, 9652–9662 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan S., Thomas P. G., Balancing immune protection and immune pathology by CD8+ T-cell responses to influenza infection. Front. Immunol. 7, 25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong M. D., et al. , Fatal outcome of human influenza a (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobasa D., et al. , Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445, 319–323 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Zinkernagel R. M., Doherty P. C., MHC-restricted cytotoxic T cells: Studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 27, 51–177 (1979). [DOI] [PubMed] [Google Scholar]
- 19.Crawford A., Wherry E. J., The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr. Opin. Immunol. 21, 179–186 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zajac A. J., et al. , Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wherry E. J., Blattman J. N., Murali-Krishna K., van der Most R., Ahmed R., Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wherry E. J., T cell exhaustion. Nat. Immunol. 131, 492–499 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B., Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson P. L. F., et al. , Vaccination alters the balance between protective immunity, exhaustion, escape, and death in chronic infections. J. Virol. 85, 5565–5570 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornberg M., et al. , Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front. Immunol. 4, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speiser D. E., et al. , T cell differentiation in chronic infection and cancer: Functional adaptation or exhaustion? Nat. Rev. Immunol. 14, 768–774 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Utzschneider D. T., et al. , T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14, 603–610 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Barber D. L., et al. , Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Bonhoeffer S., Rembiszewski M., Ortiz G. M., Nixon D. F., Risks and benefits of structured antiretroviral drug therapy interruptions in HIV-1 infection. AIDS 14, 2313–2322 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Conway J. M., Perelson A. S., Post-treatment control of HIV infection. Proc. Natl. Acad. Sci. U.S.A. 6, 5467–5472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blattman J. N., Wherry E. J., Ha S. J., van der Most R. G., Ahmed R., Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 83, 4386–4394 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haller O., Kochs G., Weber F., The interferon response circuit: Induction and suppression by pathogenic viruses. Virology 344, 119–130 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venugopal V., Padmanabhan P., Raja R., Dixit N. M., Modelling how responsiveness to interferon improves interferon-free treatment of hepatitis C virus infection. PLoS Comput. Biol. 14, e1006335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raja R., Baral S., Dixit N. M., Interferon at the cellular, individual, and population level in hepatitis C virus infection: Its role in the interferon-free treatment era. Immunol. Rev. 285, 55–71 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdelwahab S. F., Cellular immune response to hepatitis-C-virus in subjects without viremia or seroconversion: Is it important? Infect. Agent Cancer 11, 23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann A. U., et al. , Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon- therapy. Science 282, 103–107 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Dahari H., et al. , Mathematical modeling of primary hepatitis C infection: Noncytolytic clearance and early blockage of virion production. Gastroenterology 128, 1056–1066 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Major M. E., et al. , Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology 39, 1709–1720 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Baral S., Roy R., Dixit N. M., Modeling how reversal of immune exhaustion elicits cure of chronic hepatitis C after the end of treatment with direct-acting antiviral agents. Immunol. Cell Biol. 96, 969–980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto M., et al. , CD8 T cell exhaustion in chronic infection and cancer: Opportunities for interventions. Ann. Rev. Med. 69, 301–318 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Postow M. A., Sidlow R., Hellmann M. D., Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Kohli A., et al. , Virological response after 6 week triple-drug regimens for hepatitis C: A proof-of-concept phase 2a cohort study. Lancet 385, 1107–1113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goyal A., et al. , Modeling HCV cure after an ultra-short duration of therapy with direct acting agents. Antivir. Res. 144, 281–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen T. H. T., et al. , The paradox of highly effective sofosbuvir-based combination therapy despite slow viral decline: Can we still rely on viral kinetics? Sci. Rep. 7, 10233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sáez-Cirión A., et al. , Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy anrs visconti study. PLoS Pathog. 9, e1003211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hart Y., Antebi Y. E., Mayo A. E., Friedman N., Alon U., Design principles of cell circuits with paradoxical components. Proc. Natl. Acad. Sci. U.S.A. 109, 8346–8351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart Y., et al. , Paradoxical signaling by a secreted molecule leads to homeostasis of cell levels. Cell 158, 1022–1032 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Sontag E. D., A dynamic model of immune responses to antigen presentation predicts different regions of tumor or pathogen elimination. Cell Syst. 4, 231–241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padmanabhan P., Garaigorta U., Dixit N. M., Emergent properties of the interferon-signalling network may underlie the success of hepatitis C treatment. Nat. Commun. 5, 3872 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Keşmir C., De Boer R. J., Clonal exhaustion as a result of immune deviation. Bull. Math. Biol. 65, 359–374 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Laksono B., de Vries R., McQuaid S., Duprex W., de Swart R., Measles virus host invasion and pathogenesis. Viruses 8, 210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabin C. A., Lundgren J. D., The natural history of HIV infection. Curr. Opin. HIV AIDS 8, 311–317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawelek K. A., et al. , Modeling within-host dynamics of influenza virus infection including immune responses. PLoS Comput. Biol. 8, e1002588 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daidoji T., et al. , H5N1 avian influenza virus induces apoptotic cell death in mammalian airway epithelial cells. J. Virol. 82, 11294–11307 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raziorrouh B., et al. , Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One 9, e105703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.