Significance
Gastrointestinal (GI) failure is a serious dose-limiting side effect observed in patients undergoing lower-abdominal radiotherapy with no approved protective measure/cure. Here, we show that increased p53-induced p21 activity protects against radiation-induced GI toxicity in a genetically engineered mouse model. This radioprotection can be recapitulated in wild-type mice by pretreating them with RG7112, a pharmacological compound that enhances p53 activity by disrupting p53–Mdm2 interaction. These results signify that transient enhancement of wild-type p53 activity by pharmacological means could be exploited as a potential prophylactic strategy to alleviate GI toxicity issues in patients undergoing radiotherapy in the clinic.
Keywords: RG7112, gastrointestinal syndrome, shielded body radiation, stem cells, p21
Abstract
Gastrointestinal (GI) syndrome is a serious side effect and dose-limiting toxicity observed in patients undergoing lower-abdominal radiotherapy. Previous mouse studies show that p53 gene dosage determines susceptibility to GI syndrome development. However, the translational relevance of p53 activity has not been addressed. Here, we used a knock-in mouse in which the p53–Mdm2 negative feedback loop is genetically disrupted. These mice retain biallelic p53 and thus, normal basal p53 levels and activity. However, due to the lack of p53-mediated Mdm2 transcription, irradiated Mdm2P2/P2 mice exhibit enhanced acute p53 activity, which protects them from GI failure. Intestinal crypt cells residing in the +4 and higher positions exhibit decreased apoptosis, increased p21 expression, and hyperproliferation to reinstate intestinal integrity. Correspondingly, pharmacological augmentation of p53 activity in wild-type mice with an Mdm2 inhibitor protects against GI toxicity without affecting therapeutic outcome. Our results suggest that transient disruption of the p53–Mdm2 interaction to enhance p53 activity could be a viable prophylactic strategy for alleviating GI syndrome in patients undergoing radiotherapy.
Despite being a mainstay in cancer treatment, the full therapeutic benefit of ionizing radiation (IR) is limited by normal tissue toxicity. In particular, rapidly proliferating cells of hematopoietic (HP) and gastrointestinal (GI) systems are highly sensitive to radiation effects (1). In contrast to lethal HP injury, which can be rescued by bone marrow transplantation, there is no approved treatment or preventive measure for GI damage. This is a significant dose-limiting factor in abdominal and pelvic radiotherapy.
The intestinal epithelium performs the important function of nutrient absorption and is continuously replenished with new cells from the underlying crypt-based stem cells. High-dose radiation impairs the ability of crypt stem cells to repair the absorptive function of the epithelial layer, which leads to electrolyte imbalance, infections, and fatality (2). Previous studies have suggested that radiation-induced intestinal cell death occurs in 2 distinct phases. Early after IR, affected crypt cells undergo p53-dependent apoptosis before undergoing cell cycle arrest (2, 3). Later, at the time of reentry into the cell cycle, unresolved damage promotes cells to undergo aberrant mitosis and death by mitotic catastrophe (4, 5).
The p53 tumor suppressor is one of the major effectors of the cellular response to radiation (6). Radiation exposure triggers p53-dependent transcriptional activation of downstream targets that determine radiation sensitivity of affected cells/tissues. Interestingly, in vivo studies have also shown that p53 plays a paradoxical role in determining radiation susceptibility of tissues (5, 7). In comparison with p53-null mice, mice with 2 or more copies of p53 are highly sensitive and succumb to HP failure after radiation exposure (3, 7). However, the same mice when exposed to high-dose (>15 Gy) GI toxic radiation survive, while p53-null mice do not (3, 7). Nonetheless, these gene dosage studies have limited translational relevance, and additional studies are needed to address the role of acute p53 activity in GI radioprotection.
p53 is tightly regulated by Mdm2, which inhibits its transcriptional activity and also acts as an E3 ligase that targets it for degradation (8–12). Incidentally, Mdm2 is also a downstream target of p53 (13). Under stress conditions, p53 binds and transactivates the Mdm2 promoter, thereby creating a feedback loop wherein both proteins regulate each other (14). We recently generated a knock-in mouse in which we disrupted the p53–Mdm2 negative feedback loop by mutating the p53 response elements in the Mdm2 P2 promoter (15). These mice (Mdm2P2/P2) have normal biallelic expression of p53 and thus, normal basal p53 levels and activity. However, after DNA damage, these mice exhibit enhanced p53 activity in most tissues and radiation sensitivity due to insufficient Mdm2 levels.
Here, we have used Mdm2P2/P2 mice to examine the role of p53 activity in GI protection after exposure to a toxic dose of radiation. We show that, in comparison with wild-type mice, Mdm2P2/P2 mice with increased p53 activity are protected from radiation-induced GI toxicity. Furthermore, we provide evidence that pretreatment with an inhibitor of the p53–Mdm2 interaction enhances p53 activity and imparts endurance against GI toxic radiation in wild-type mice. We propose that transient enhancement of wild-type p53 activity by pharmacological means could, therefore, be exploited to alleviate GI toxicity issues in patients undergoing radiotherapy in the clinic.
Results
Enhanced p53 Activity Protects Mdm2P2/P2 Mice from Radiation-Induced GI Toxicity.
Previous animal studies suggest that p53 gene dosage correlates with sensitivity against radiation-induced GI toxicity. To further clarify the role of acute p53 transcriptional activity in protection against radiation-induced GI damage, we devised a shielded body radiation (SBR) strategy using a modified jig and lead shield. This allowed us to target radiation to the lower-abdominal area of the mice, while the anterior end, including the head, sternum, and the bone marrow in front limbs, was protected with a lead shield. This strategy alleviated the undesired effects of radiation-induced bone marrow toxicity in experimental animals (SI Appendix, Fig. S1A). Radiation dosage and delivery were confirmed by a radiation physicist, and GI toxic SBR dose of 17 Gy for wild-type mice in C57BL/6 background was determined experimentally.
We irradiated 6- to 8-wk-old Mdm2+/+ (wild-type) and Mdm2P2/P2 mice in C57BL/6 background with 17 Gy SBR using the above methodology and monitored their survival. In agreement with previously published studies, the majority of wild-type mice (26 of 30) exposed to lethal GI radiation succumbed to this regimen. Surprisingly, Mdm2P2/P2 mice that exhibit elevated p53 activity after IR fared much better, with >95% (20 of 21) mice still alive (P < 0.0001) at the end of observation period (30 d) (Fig. 1A).
Fig. 1.
Radiation differentially impacts wild-type and Mdm2P2/P2 mice. (A) Kaplan–Meier survival curve of wild-type and Mdm2P2/P2 mice after 17 Gy SBR. (B) Kaplan–Meier survival curves of Mdm2P2/P2 and Mdm2P2/P2p53 −/− mice after 17 Gy SBR. (C) Representative H&E-stained intestinal sections of wild-type and Mdm2P2/P2 mice at various post-IR time points. (Magnification, 20×.)
Next, we examined whether the above-mentioned post-IR survival of Mdm2P2/P2 mice was p53 dependent. To test this, we generated Mdm2P2/P2p53 −/− mice and exposed them to the same 17-Gy SBR regimen. Indeed, 100% of Mdm2P2/P2p53 −/− mice died within the first week after 17 Gy SBR, the same setting in which Mdm2P2/P2 mice thrived (P < 0.0001) (Fig. 1B).
GI Toxic SBR Differentially Impacts Wild-Type and Mdm2P2/P2 Mouse Intestines.
Based on the differences in survival of wild-type and Mdm2P2/P2 mice after 17 Gy SBR, we examined the pathological effects of radiation on small intestines of wild-type and Mdm2P2/P2 mice. We irradiated wild-type and Mdm2P2/P2 mice with 17 Gy SBR and harvested intestines at various time points. Pathological assessment of hematoxylin and eosin (H&E)-stained sections of intestine clearly showed a progressive disintegration of the GI in both mouse genotypes starting at 24 h post-IR (Fig. 1C). Both sets of mouse intestines showed characteristic lymphocyte infiltration, loss of crypt cellularity, thinning of lamina propria, and unhealthy looking villi after IR exposure. The severity of intestinal damage continued to increase up to the 72-h post-IR time point. Surprisingly, however, by 96 h, Mdm2P2/P2 intestines completely recovered with new multicellular crypts and healthy looking villi, whereas wild-type intestines were completely disintegrated with no crypts visible in multiple microscope fields (Fig. 1C).
To further refine the time point when differences between recovered and obliterated GI sections become evident, we repeated the experiment and harvested intestines from irradiated wild-type and Mdm2P2/P2 mice after 84 and 96 h of 17 Gy SBR. At 84 h, we noted some signs of cellular reorganization around the crypt location in both mouse genotypes, but the stark difference in crypt cellularity at 96 h clearly set them apart (Fig. 1C). At 96 h, only Mdm2P2/P2 mouse crypts appeared healthy and regained full cellularity. This suggests that major intestinal recovery after IR occurs during the last 84- to 96-h time period.
Mdm2P2/P2 Intestines Exhibit Decreased Apoptotic Cell Death and Increased Proliferation to Counter IR-Induced Toxicity.
Cell death and proliferation rate are the 2 major phenomenon that define post-IR cellularity of an organ. To identify the role of apoptosis in leading to cellularity differences between the irradiated wild-type and Mdm2P2/P2 mouse intestines, we performed cleaved Caspase 3 (CC3) staining of intestine sections at early post-IR time points. Cleavage of Caspase 3 marks an early event in apoptosis. Unirradiated intestinal sections were used as control. In the absence of IR, no CC3 staining was visible in both wild-type and Mdm2P2/P2 mouse intestines, indicating a normal course of damage-free cellular proliferation for this organ (Fig. 2A). In contrast, at both 4- and 24-h time points, irradiated wild-type intestines showed higher levels of CC3 staining than Mdm2P2/P2 intestine (Fig. 2A). To further confirm increased apoptosis in wild-type intestines, we also carried out terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling (TUNEL) assay on these sections. TUNEL marks double-stranded DNA breaks, a relatively late event in apoptosis. At 4 h after IR, TUNEL-positive cells increased in both genotypes, but the numerical differences were not remarkable (SI Appendix, Fig. S1B). However, at 24 h post-IR, a statistically significant difference in TUNEL-positive cells was observed between the 2 genotypes. Again, more positive staining was evident in wild-type intestinal sections compared with Mdm2P2/P2 genotype (SI Appendix, Fig. S1B). These data indicated that intestinal cells from Mdm2P2/P2 mice were somehow less susceptible to apoptotic cell death after IR.
Fig. 2.
Mdm2P2/P2 mice exhibit decreased apoptotic cell death and increased proliferation. (A, Left) Representative CC3 staining of intestinal sections from irradiated wild-type and Mdm2P2/P2 mice at different time points. (Magnification, 20×.) (A, Right) Quantification of CC3-positive cells from irradiated wild-type and Mdm2P2/P2 mice (n = 3 each, ±SEM) at different time points. At least 100 crypts were counted. Statistical significance was determined by Student’s t test. (B) Representative Ki-67–stained intestinal sections from wild-type and Mdm2P2/P2 mice at various post-IR time points. (Magnification, 20×.) (C) Representative BrdU-stained intestinal sections from wild-type and Mdm2P2/P2 mice at various post-IR time points. (Magnification, 40×.)
Next, we investigated the differences in cell proliferation between wild-type and Mdm2P2/P2 intestinal cells after IR. To this end, we performed Ki-67 immunohistochemistry (IHC) on intestines harvested at different time points after 17 Gy SBR. Before radiation exposure, both wild-type and Mdm2P2/P2 mouse intestines showed positive staining in the crypts, indicating proliferation (Fig. 2B). After IR, Ki-67–positive cell numbers gradually decreased from 24- to 84-h time points, correlating with crypt cell death in both mouse genotypes. However, at 96 h, intense Ki-67 staining was exclusively limited to reestablished crypts cells in the Mdm2P2/P2 intestines. Notably, the revived crypts of Mdm2P2/P2 mouse intestines were enlarged and intensely stained, indicating cellular hyperproliferation (Fig. 2B). In contrast, no staining was apparent in the wild-type intestines that were completely obliterated by this time point.
We further performed 5-bromo-2′-deoxyuridine (BrdU) staining on intestine sections from irradiated wild-type and Mdm2P2/P2 mice that were injected with BrdU 2 h before euthanasia. Results indicated that only proliferating Mdm2P2/P2 intestinal crypt cells incorporated BrdU at the 96-h time point (Fig. 2C). Together, these results confirmed that actively replicating cells were responsible for the reemergence of intestinal crypts in Mdm2P2/P2 mice.
p53-Dependent p21 Activity Protects Mdm2P2/P2 Mice.
The p53 transcription factor activates multiple downstream targets that influence tissue sensitivity to radiation. Previous studies have implicated canonical p53 target genes p21 and Puma in sensitizing bone marrow after radiation (15–18). Similarly, reports have also implicated these p53 downstream targets in intestinal recovery after whole-body radiation (19, 20).
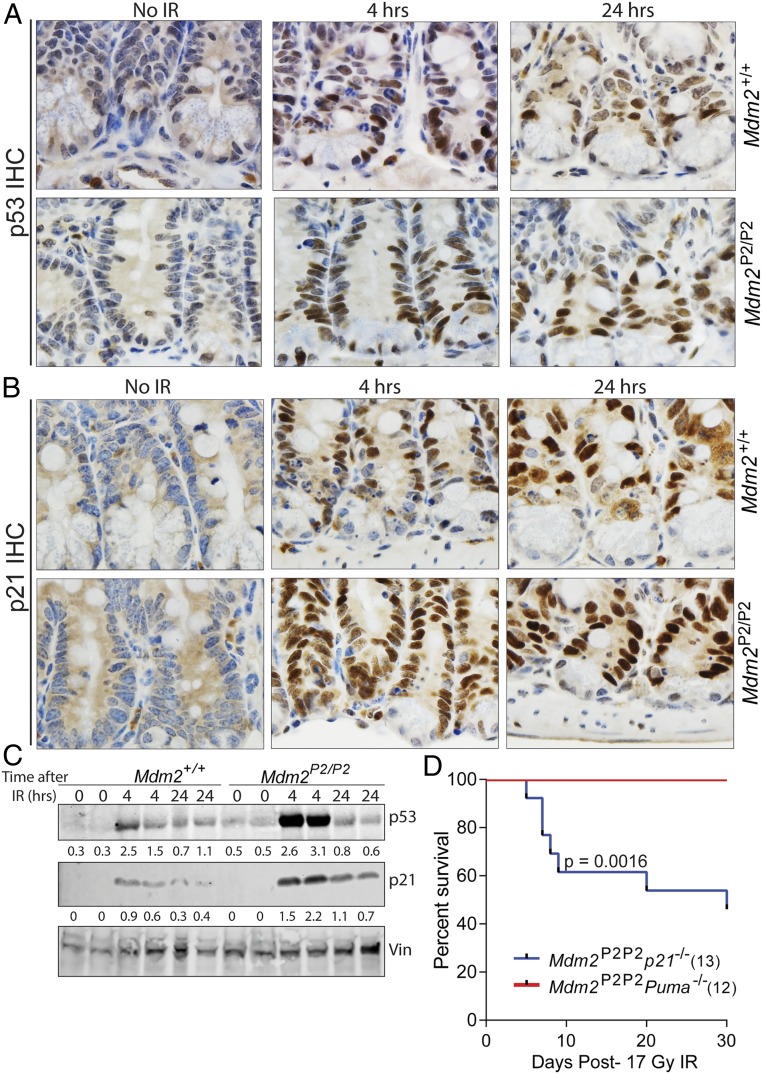
To further understand how increased activation of p53 in Mdm2P2/P2 mouse intestine hampered apoptosis and promoted cell cycle arrest, we carried out IHC analysis for p53 and p21 in wild-type and Mdm2P2/P2 mouse intestinal sections. As expected, unirradiated sections did not stain for either protein. However, 4 h after IR, p53 up-regulation was clearly evident in the intestinal crypts of both mouse genotypes (Fig. 3A). Notably, more crypt cells in Mdm2P2/P2 intestine stained positive for p53 than wild-type controls. The relative difference in staining was maintained at 24 h. p21 IHC on corresponding intestinal sections also showed relatively more intense and widespread staining in Mdm2P2/P2 intestinal crypts compared with the wild type (Fig. 3B). Specifically, more cells primarily located at +4 and higher crypt positions stained positive for both p53 and p21 at these time points. Intriguingly, cells localized in this region also previously showed less apoptotic death by CC3 staining and TUNEL assay (Fig. 2A and SI Appendix, Fig. S1B). Comparative western blot analyses for p53 and p21 on intestinal cells from the jejunum region of wild-type and Mdm2P2/P2 mice also reflected these results (Fig. 3C). This suggested that these cells do not undergo immediate cell death after radiation exposure and may be involved in intestinal recovery after IR.
Fig. 3.
p53-dependent p21 activity protects Mdm2P2/P2 mice from GI toxicity. (A) Representative sections showing p53 IHC in intestinal sections from wild-type and Mdm2P2/P2 mice at various post-IR time points. (Magnification, 100×.) (B) Representative intestinal sections from wild-type and Mdm2P2/P2 mice showing p21 IHC at different post-IR time points. (Magnification, 100×.) (C) Western blot analysis of p53 and p21 in crypt cells from the intestinal jejunum region. Numbers underneath each blot depict relative protein quantification of the corresponding band. (D) Kaplan–Meier survival curve of Mdm2P2/P2 p21 −/− and Mdm2P2/P2 Puma −/− mice after 17 Gy SBR.
Next, to genetically ascertain the importance of these p53 targets in protecting Mdm2P2/P2 mice from IR-induced GI toxicity, we crossed Mdm2P2/P2 mice with either p21 or Puma-null mice. Post-IR survival of Mdm2P2/P2 Puma−/− mice treated with 17 Gy SBR showed that loss of Puma, one of the major p53-induced proapoptotic genes, did not alter the survival of irradiated Mdm2P2/P2 mice. However, loss of p21, a p53-induced cell cycle arrest gene, sensitized Mdm2P2/P2 mice to 17 Gy SBR, with more than 50% (7 of 13) of Mdm2P2/P2p21 −/− mice dying prematurely (P = 0.0016) after IR exposure (Fig. 3D). In light of death of all Mdm2P2/P2p53 −/− mice after 17 Gy SBR (Fig. 1B), partial sensitivity of Mdm2P2/P2p21 −/− mice under similar conditions emphasizes a major role of p21 in GI radioprotection but also, suggests involvement of other p53 targets.
Enhanced p53 Activity Provides Long-Term Protection against IR-Induced GI Syndrome.
Since a few wild-type and most Mdm2P2/P2 mice escaped the immediate death fate after exposure to 17 Gy SBR, we monitored these mice to examine the long-term impact of high-dose IR on lifespan. Eight wild-type and 20 Mdm2P2/P2 mice that survived the first 30 d after 17 Gy SBR were followed for over 390 d. Again, we observed that, overall, Mdm2P2/P2 mice survived longer than their wild-type counterparts (SI Appendix, Fig. S2A). While the difference in survival was statistically significant (P = 0.02), the exact cause of death in these mice remains undetermined. These irradiated mice did not develop any tumors, and pathological assessment of major murine tissues did not identify anything amiss in these mice. Complete blood counts, bone marrow, and GI tissues analyzed by pathology were also not different between the 2 genotypes (SI Appendix, Fig. S3). Nonetheless, this suggests that enhanced acute p53 activity differentially impacts overall lifespan of these mouse genotypes.
We also monitored the survival of Mdm2P2/P2p21 −/− and Mdm2P2/P2 Puma−/− mice that survived the initial 17 Gy SBR for another 390 d (SI Appendix, Fig. S2B). Here too, irradiated Mdm2P2/P2p21 −/− mice had an overall shorter lifespan (P = 0.0001) compared with the Mdm2P2/P2 Puma −/− mice, further implying a role for p21, a p53-dependent cell cycle arrest gene, in long-term survival of irradiated mice.
A Reserve Stem Cell Population in the Middle of the Crypt Restores Mouse Intestinal Integrity.
The rapidly proliferating GI system is highly sensitive to radiation damage. The intestinal epithelium continually self-renews and can rapidly regenerate after damage owing to the unparalleled division potential of rapidly proliferating crypt cells. Cells in the crypts are organized into a stem cell zone near the base; a differentiation zone in the middle; and a transit-amplifying zone at the top, which feeds into the newly formed villus structure (Fig. 4A). Two distinct stem cell populations inhabit the intestinal crypts. At the base, interspersed by Paneth cells are crypt base columnar cells (CBCs) that express leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) (21). These represent the mitotically active intestinal stem cell population that is responsible for intestinal homeostasis (22). Lineage tracing experiments have shown that CBCs divide every 24 h and are capable of generating all different types of intestinal cells. Studies have also shown that these cells are radiosensitive and succumb to high-dose (>15 Gy) radiation (23). A second set of stem cells resides above the Paneth cells at +4 to +6 position relative to the crypt base (24). These Lgr5low cells are relatively quiescent, radioresistant, and marked by Bmi1 or Krt19 expression to compensate for loss of Lgr5+ CBCs under homesostatic and stress conditions (22, 25–27).
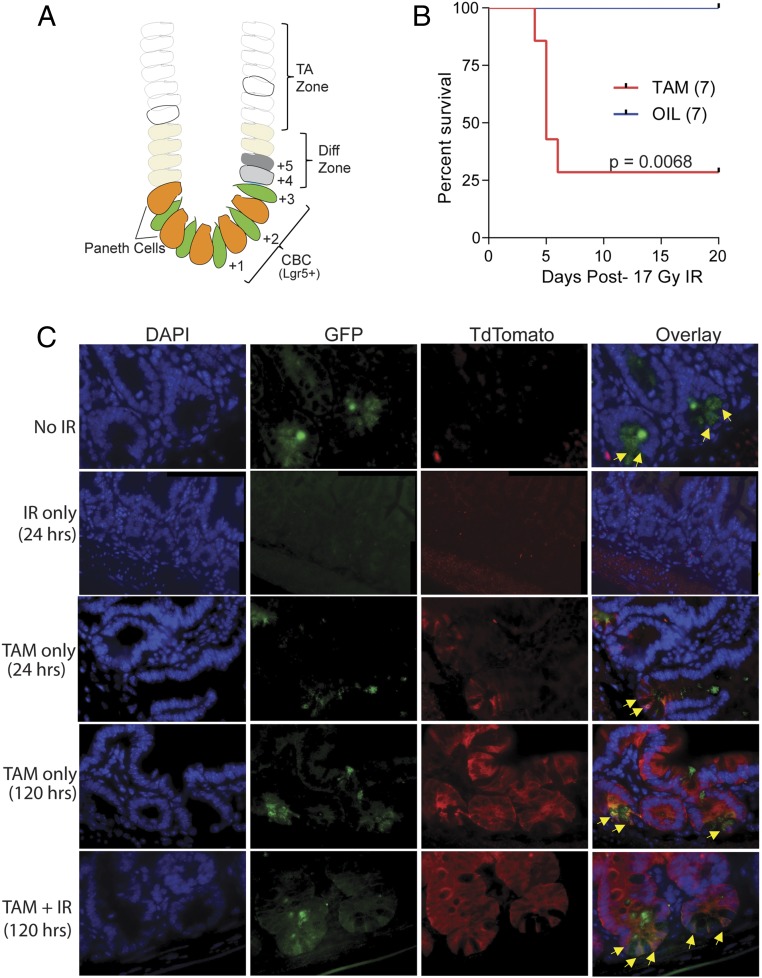
Fig. 4.
Intestinal stem cells dedifferentiate to promote intestinal recovery. (A) Schematic showing relative cell positions in intestinal crypt. Diff zone, differentiation zone; TA zone, transit amplifying zone. (B) Kaplan–Meier survival curve of Mdm2P2/P2p53fx/fx Lgr5GFPcreERT2 mice treated with either tamoxifen (TAM) or vehicle before 17 SBR. (C) Representative images of intestinal sections from Mdm2P2/P2Lgr5GFPcreERT2:Tdtomato mice treated under different conditions and harvested at either 24 or 120 h posttreatment. Yellow arrows mark the dual-labeled CBCs. (Magnification, 60×.)
To confirm that a subset of intestinal crypt cells is protected by high p53 activity and is responsible for restoring intestinal integrity, we generated Mdm2P2/P2p53flox/floxLgr5GFPcreERT2 mice. These mice express green fluorescent protein (GFP) and tamoxifen-inducible cre recombinase under the control of Lgr5 promoter in the basal CBCs. In addition, these mice also carry a floxed p53 allele. We injected Mdm2P2/P2p53flox/floxLgr5GFPcreERT2 mice with tamoxifen to delete p53 in Lgr5+ cells and their immediate progeny 24 h before 17 Gy SBR. Control mice were injected with corn oil. Interestingly, 5 of 7 tamoxifen-injected mice died within 5 d after 17 Gy SBR, while all control mice (7 of 7) survived (P = 0.0068) (Fig. 4B). This further implicated p53 in radioprotection of Lgr5+ CBCs and their progeny cells in the crypt. These results are in agreement with previous studies, which show that Lgr5+ cells quickly disappear after IR and that other cells from the crypt are involved in intestinal recovery (22, 27).
To further recognize the role of crypt cells that promote recovery of Mdm2P2/P2 mouse intestine, we generated compound Mdm2P2/P2Lgr5GFPcreERT2:Tdtomato mice by crossing Mdm2P2/P2Lgr5GFPcreERT2 and conditional LSL-Rosa26TdTomato (mice carry a conditional Tdtomato allele at the Rosa26 locus that is activated by Cre recombinase) reporter mice. We injected these mice with tamoxifen to induce recombination in the Lgr5-expressing cells 24 h before 17 Gy SBR. Mice were euthanized at various post-IR time points, and their intestines were collected. Tamoxifen-naïve mice were used as controls. We examined the intestinal sections from these experimental mice for GFP and TdTomato expression. In the absence of tamoxifen or IR treatment, GFP-labeled Lgr5+ CBCs light up at the crypt base (Fig. 4C). Tamoxifen treatment led to coexpression of red fluorescence in the CBC cells, while 17-Gy SBR-only treatment completely ablated these cells by 24 h, with no fluorescent signal visible at the crypt base. Surprisingly, 120 h after tamoxifen and 17 Gy SBR combination treatment, dual-labeled CBCs in the crypt base reappeared, while cells higher up in the crypt continued expressing red color (Fig. 4C). Concurring with the results of previously published studies (22, 25–27), this further eludes to the plastic nature of intestinal cells that reside above CBCs at +4 to +6 position in the crypt.
RG7112 Treatment of Wild-Type Mice Phenocopies Mdm2P2/P2 Results.
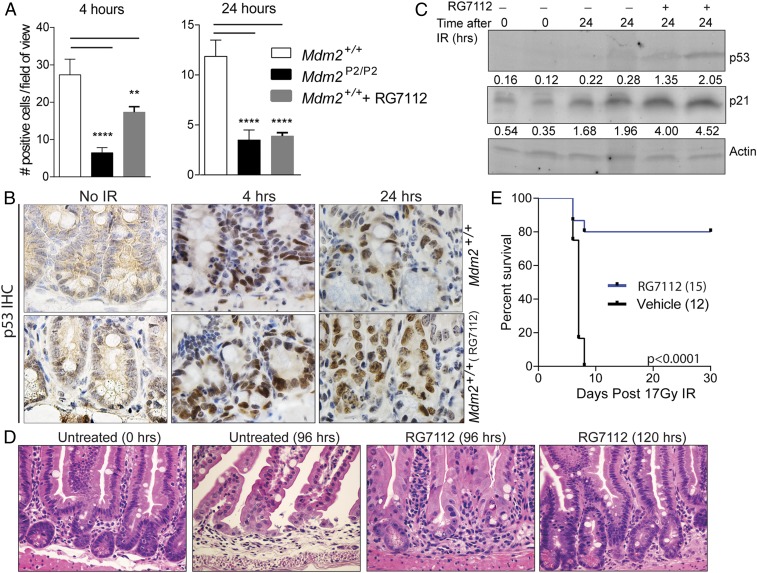
Next, we explored whether transient activation of p53 by pharmacological means could protect wild-type mice against radiation damage and promote recovery. To this end, we utilized RG7112, an analog of Nutlin-3 that binds to the p53 binding pocket of Mdm2 and thereby, inhibits p53–Mdm2 interaction. RG7112 has recently been safety tested in human phase 1 clinical trials (28). We treated wild-type mice with RG7112 6 h before 17 Gy SBR followed by another dose 6 h later and performed CC3 staining on intestines at different time points. Compared with untreated mice, RG7112 treatment lead to reduced apoptosis in wild-type mouse intestines at 4 and 24 h after 17-Gy SBR time points (Fig. 5A). At 24 h, reduction in apoptosis was similar to Mdm2P2/P2 mice. TUNEL staining of these sections also yielded similar results (SI Appendix, Fig. S4A). Next, we compared cell proliferation differences in 17 Gy SBR mice that were either pretreated or untreated with RG7112. Ki-67 staining was more prominent in RG7112-treated mouse intestines compared with untreated controls at 120 h (SI Appendix, Fig. S4B). Lastly, immunohistochemical staining of p53 and p21 in the RG7112-treated wild-type mouse intestines was also notably different from that in untreated wild-type mice (Fig. 5B and SI Appendix, Fig. S4C). More cells located in the middle and higher positions in the crypt stained positive for p53 and p21 in RG7112-treated wild-type mouse intestines, comparable with Mdm2P2/P2 intestine levels (Fig. 3B). Western blot analysis of crypt cells from the jejunum region also confirmed a 2.3-fold increase in p53 activity in RG7112-treated wild-type mice post-SBR (Fig. 5C), similar to the 2.4-fold increase in p53 activity observed in the gut of Mdm2P2/P2 mice after DNA damage (Fig. 3C). Altogether, these results clearly suggest that RG7112 pretreatment augments p53 activity/expression levels and changed the apoptosis and proliferation profiles of wild-type mouse intestine to levels comparable with those of Mdm2P2/P2 mice.
Fig. 5.
RG7112 treatment promotes intestinal recovery in wild-type mice. (A) Quantification of CC3-positive cells from irradiated wild-type, Mdm2P2/P2, and RG7112-treated wild-type mice (n = 3 each, ±SEM) at different time points. At least 100 crypts were counted. Statistical significance between wild-type mice and other samples was determined by 1-way ANOVA test. **P < 0.01; ****P < 0.0001. (B) Representative p53-stained slides of intestinal sections from wild-type mice treated with RG7112 before 17 Gy SBR at different time points. (Magnification, 100×.) (C) Western blot analysis of p53 and p21 in crypt cells from the intestinal jejunum region. Numbers underneath each blot depict relative protein quantification of the corresponding band. (D) Representative H&E-stained intestine sections of wild-type mice either untreated or treated with RG7112 before 17 Gy SBR and harvested at 96 or 120 h. (Magnification, 40×.) (E) Kaplan–Meier survival curve of wild-type mice treated with either RG7112 or vehicle before 17 Gy SBR.
RG7112 Pretreatment Protects Wild-Type Mice from GI Toxicity.
Next, we carried out histological assessment of RG7112 treatment on irradiated wild-type mouse intestines. Examination of H&E-stained slides showed a drastic difference in intestinal architecture between radiated mice that were RG7112 treated compared with untreated mice; 120 h after 17 Gy SBR, RG7112-treated mice displayed a dense crypt–villi arrangement compared with the untreated mouse intestines, which showed loss of cellularity instead (Fig. 5D). Of note, RG7112-treated mouse intestines fully recovered 120 h after IR, slightly later than Mdm2P2/P2 intestines, which typically recuperated by 96 h (Fig. 1C). A similar recovery delay was also noted in Mdm2P2/P2 Lgr5GFPcreERT2 mice as well (Fig. 4C). This could be either due to the combinatorial effect of RG7112 and radiation or due to the combination of multiple genotypes on radiation recovery altering the kinetics.
Lastly, we tested whether transient activation of p53 could be utilized for protection against radiation-induced GI toxicity in vulnerable wild-type mice. To this end, we treated wild-type mice with RG7112 6 h before 17 Gy SBR and retreated them 6 h later. Control wild-type mice were treated with vehicle alone following the same schedule. Remarkably, 12 of 15 mice treated with RG7112 did not develop GI toxicity and survived the 30-d monitoring period, while 12 of 12 vehicle-treated mice perished (Fig. 5E). RG7112-pretreated mice that survived 17 Gy radiation remained alive for over a year, and bone marrow and GI tissues analyzed at that time showed no significant pathologies (SI Appendix, Fig. S4D). Overall, these results implicate RG7112-mediated enhancement of acute p53 activity in mitigating the effects of radiation-induced GI toxicity.
RG7112 Pretreatment Does Not Adversely Affect Treatment Outcomes in Mice.
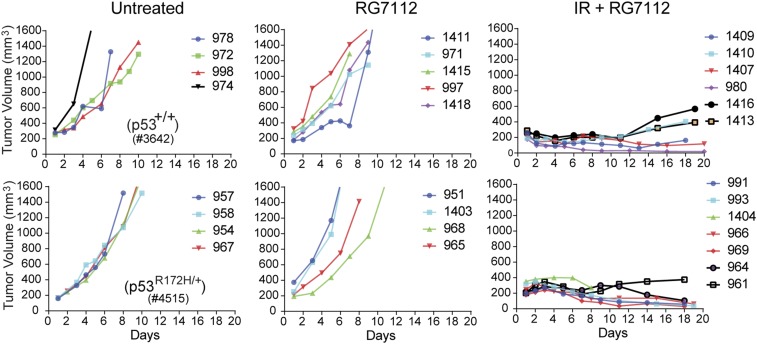
Finally, we tested whether RG7112 pretreatment might have adverse effect on tumor therapy. To test this, we transplanted MMTV-Wnt1–driven mammary tumors carrying either wild-type or mutant p53 (29) in immunocompetent wild-type mice and monitored tumor growth. When tumors reached ∼200 mm3 in size, we randomly distributed tumor-bearing mice into different treatment regimen groups and monitored tumor growth. As expected, both wild-type and mutant p53 tumors rapidly grew in the group that was not treated, reaching the maximum permissible limit in just a few days (Fig. 6). Similarly, tumor growth in mice that were pretreated with only RG7112 was also very rapid and indistinguishable from that in untreated mice. However, mice carrying p53 wild-type and mutant tumors that were pretreated with RG7112 before 17 Gy IR showed similar extent of reduced tumor growth and survived the critical 3-wk monitoring window with no additional issues. This experiment thus confirmed that RG7112 pretreatment is not likely to interfere with tumor treatment and can be safely incorporated into a radiotherapy regimen.
Fig. 6.
RG7112 pretreatment does not adversely affect treatment outcomes. (Upper) Transplant tumor growth in wild-type mice carrying MMTV-Wnt1 tumors with wild-type p53 with and without RG7112 treatment. (Lower) Transplant tumor growth in wild-type mice carrying MMTV-Wnt1 tumors with mutant p53R172H with and without RG7112 treatment.
Discussion
Radiation-induced GI syndrome is a serious side effect that constrains the use of optimal radiation dose for lower-abdominal therapy in patients. Higher gene dosage of the p53 tumor suppressor in mice protects against radiosensitivity. A better understanding of the p53 role in the intestine is crucial for developing therapeutic strategies and mitigating the side effects of radiation in patients. Here, we used Mdm2P2/P2 mice, which exhibit enhanced p53 activity in most tissues after DNA damage, to ascertain the role of p53 activity in GI protection after IR.
Mdm2P2/P2 mice exposed to high-dose (17 Gy) SBR of the lower abdomen (to overcome HP toxicity) survive, while wild-type mice perish, which clearly implicates enhanced p53 activity in protection against GI toxicity. A notable difference in cell apoptosis and proliferation between wild-type and Mdm2P2/P2 mouse intestines showed that p53 activity differentially impacts crypt cells in the 2 genotypes. p53 induces multiple downstream targets. While genetic crosses attest to the significant role played by p53-induced p21 in protection against radiation toxicity in the intestinal cells of Mdm2P2/P2 mice here, they also suggest that other presently unknown targets contribute to the response. This result is similar to a previous report, wherein p21 was shown to protect super p53 mice (3 copies of p53) from GI syndrome (4). Nonetheless, our study addresses the role of p21 in a normal biallelic p53 setting. More pronounced up-regulation of p21 in Mdm2P2/P2 mice likely extends cell cycle arrest in cells located at +4 and higher positions in the middle of the intestinal crypt. A reserve stem cell population has previously been identified to reside at this location. Increased p21 protects these reserve stem cells from apoptosis, allowing them to dedifferentiate and replenish the CBC pool, which is indispensable for intestinal recovery after IR. These data are consistent with the published role of reserve stem cells (22, 25–27) and agree with the results of a recent publication, wherein pretreatment with a cdk4/6 inhibitor protected mice from GI toxicity after single high-dose radiation exposure (30). Furthermore, we did not detect any residual fluorescent signal at the crypt base (where Lgr5+ CBCs reside) 24 h after IR and yet, saw expansion of red-colored cells in the villi with reappearance of dual fluorescent cells in the crypt base of Mdm2P2/P2Lgr5GFPcreERT2:Tdtomato mice at later time points, suggesting that dedifferentiation of reserve stem cells (Lgr5low) located at +4 to +6 position likely reinstates Lgr5+ CBCs. Multipotent CBCs hyperproliferate and revive the crypt architecture and intestinal integrity in Mdm2P2/P2 mice. Conditional deletion of p53 in Lgr5+ and progeny cells impairs their ability to regenerate CBCs, resulting in death. Thus, our study confirms the plasticity of intestinal stem cells and identifies a role of p53 in protecting the reserve stem cell population from radiation, which in turn, indispensably regulates intestinal recovery.
In addition, we also show that the initial p53 activity in Mdm2P2/P2 mice also impacts their long-term survival compared with wild-type escapees. Surprisingly, even with slightly longer survival, Mdm2P2/P2 mice did not present any tumor phenotype as a side effect of high-dose radiation exposure. This suggests that the spike in p53 activity likely protects cells in other tissues too from accumulating genotoxic mutations that can alter the tissue health and long-term survival. It is also possible that high p53 activity immediately eradicates the affected cells that could lead to future malignancies. Nonetheless, irradiated mice had a shorter lifespan than unirradiated mice, and the cause of death in these mice remains a mystery. Previous publications have highlighted late-occurring kidney defects and heart-related issues as bystander effects of radiation. Radiation-linked tissue fibrosis or atrophy, possibly p53 dependent, could be the reason for shorter lifespan of these mice (31). Interestingly, absence of cell cycle arrest gene p21 impacts the post-IR long-term survival of Mdm2P2/P2 mice and uncovers a major role of the p21 acute cell cycle arrest function in protection against long-term radiation damage.
Notably, our data provide proof that pharmacologically enhancing p53 activity in the intestine of mice can protect against negative effects of radiation treatment and protect GI toxicity. Moreover, the data also affirm that limited activation of p53 does not adversely affect therapeutic outcomes and should be further explored.
Developing novel compounds that can mitigate radiation effects is a growing research field. Many different cytokines, growth factors, and inhibitors have been proposed for this function (32–38). These agents either act on the Wnt pathway necessary for CBC function or otherwise, affect the overall health of intestine after radiation exposure. Our data clearly suggest that transient p53 activity could be harnessed for protecting against GI toxicity in patients needing lower-abdominal radiotherapy.
A limitation of this preclinical study is that it utilizes a single dose of high-energy IR that covers a relatively large intestinal area to promote GI toxicity in mouse. This is in contrast to the highly focused small fractionated dose regimen that leaves a smaller footprint utilized in the clinic for therapeutic purposes. Before initiating human studies, the radioprotective role of RG7112 with fractionated radiation dose regimen will have to be examined. Indeed, pretreatment with the cdk4/6 inhibitor, palbociclib, protects against radiation-induced damage to the GI tract in mice treated with a single high dose of radiation but exacerbates damage to the GI tract in mice treated with fractionated radiation (30). Nonetheless, our study clearly demonstrates that transient enhancement of wild-type p53 activity is GI protective. Interestingly, a number of biosimilar compounds/inhibitors of the p53–Mdm2/Mdm4 interaction are also currently being assessed in the clinic for wild-type p53 reactivation. Many of these drugs have already cleared phase 1 trials with minimal side effects (39). We propose testing them as prophylactic GI-radioprotective agents in the lower-abdominal radiotherapy regimen of patients. Clinically proven safety and efficacy of these compounds at low dosage make them ideal candidates to prevent radiation-induced bystander effects in other normal tissues with wild-type p53.
Materials and Methods
Mouse Studies.
Generation and characterization of Mdm2P2/P2 mice has been described previously (15). p21 and Puma-null mice were obtained from T. Jacks, Massachusetts Institute of Technology, Cambridge, MA, and G. Zambetti, St. Jude Children’s Research Hospital, Memphis, TN, respectively. p53flox/flox, Lgr5GFPcreERT2, and LSL-Rosa26TdTomato mice were purchased from Jackson Laboratories. Mice were maintained in >90% C57BL/6 background. All studies were approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee.
SBR Strategy.
Radiation strategy was devised under the supervision of a radiation physicist (R.C.T.). Briefly, unanesthetized mice were restrained in a jig, and their anterior portion was shielded with a 1-inch-thick lead shield. Radiation dosage and rate were determined using a mouse phantom by thermoluminescent dosimetry. Mice were irradiated in a Cesium-137 irradiator and euthanized at different time points. For survival studies, radiated mice were given Baytril in water and moist pellet diet for the initial 2 wk to prevent infections.
Histopathology and IHC/Immunofluorescence.
Intestines harvested from Mdm2+/+ and Mdm2P2/P2 mice were flushed with ice cold phosphate-buffered saline and fixed in 10% buffered formalin saline before paraffin embedding; 7-μm sections were stained by H&E and examined by light microscopy. For BrdU staining, irradiated mice were injected with 100 mg/kg of BrdU (Invitrogen) 2 h before euthanasia and tissue harvesting. Signal was detected using anti-BrdU antibody (BD Immunocytometry). IHC for p53 (Leica Biosystems), p21 (Agilent), Ki-67 (Leica Biosystems), and CC3 (Cell Signaling) was performed using standard protocols (40). Antigen was detected using a VECTASTAIN kit and ABC (Vector Laboratories). Images were taken on a Nikon Eclipse-Ci microscope using 20×, 40×, or 100× (with oil) objectives.
For immunofluorescence, GFP (chicken anti-GFP; Abcam) and TdTomato (rabbit anti-RFP; Rockland Antibody & Assays) antibodies were used. Signal was detected using goat anti-chicken 488 and goat anti-rabbit 555 antibodies (Molecular Probes), and images were taken on a Nikon 80i microscope.
TUNEL Assay.
TUNEL assay was carried out using the FragEL DNA fragmentation detection kit (EMD Millipore) following the manufacturer’s recommended protocol.
Tamoxifen Injection.
Mice were intraperitoneally injected with a single dose of 3 mg/kg tamoxifen in corn oil 24 h before irradiation.
RG7112 Gavage.
Mice were given either 50 mg/kg of RG7112 or an equal volume of vehicle by oral gavage 6 h before irradiation followed by a second dose 6 h later.
Western Blot.
CBCs were isolated from intestinal crypts as described by Sato and Clevers (41). Protein lysates prepared in radioimmunoprecipitation assay buffer were resolved by 15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Antibodies to detect p53 (CM5, 1:1,000; Vector Laboratories), p21 (556431, 1:500; BD Biosciences), Vinculin (V9131, 1:1,000; Sigma), and Actin (A2066, 1:4,000; Sigma) were used. Blots were visualized using fluorescent-labeled secondary antibodies in a Bio-Rad Chemidoc instrument (Life Sciences Research), and bands were quantified using ImageJ software.
Tumor Implantation and Growth.
MMTV-Wnt1–driven mammary tumors carrying either wild-type or mutant p53 were provided by J. G. Jackson, Tulane University, New Orleans, LA. Fresh murine tumors were chopped and dispersed into a cell suspension, filtered through 70-µm filters, mixed with Matrigel, and injected into mammary fat pads of immunocompetent wild-type C57BL/6 mice. Tumor size was noted using an electronic Vernier caliper and volume calculated as described earlier (40). Treatment was initiated when tumors reached ∼200 mm3 in size.
Statistical Analysis.
Statistical analysis was carried out using Graphpad Prism 6 (Graphpad Software). Survival difference was calculated using log rank (Mantel–Cox test). Significance was calculated using either Student’s t test or 1-way ANOVA test with Boneferroni’s correction. A P value of <0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. L. Vassilev (Roche Diagnostics) and Dr. M. Andreeff (MD Anderson Cancer Center, Houston, TX) for providing RG7112 and vehicle control for the study. Studies were supported by NIH Grant CA47296 (to G.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909550116/-/DCSupplemental.
References
- 1.Mettler F. A. Jr, Voelz G. L., Major radiation exposure–What to expect and how to respond. N. Engl. J. Med. 346, 1554–1561 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Potten C. S., Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 269, 518–521 (1977). [DOI] [PubMed] [Google Scholar]
- 3.Kirsch D. G., et al. , p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327, 593–596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan J. M., et al. , p21 protects “Super p53” mice from the radiation-induced gastrointestinal syndrome. Radiat. Res. 177, 307–310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merritt A. J., Allen T. D., Potten C. S., Hickman J. A., Apoptosis in small intestinal epithelial from p53-null mice: Evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene 14, 2759–2766 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Gudkov A. V., Komarova E. A., The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer 3, 117–129 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Komarova E. A., et al. , Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 23, 3265–3271 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Oliner J. D., et al. , Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 362, 857–860 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Haupt Y., Maya R., Kazaz A., Oren M., Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J., The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69, 1237–1245 (1992). [DOI] [PubMed] [Google Scholar]
- 11.Honda R., Tanaka H., Yasuda H., Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Kubbutat M. H., Jones S. N., Vousden K. H., Regulation of p53 stability by Mdm2. Nature 387, 299–303 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Barak Y., Juven T., Haffner R., Oren M., mdm2 expression is induced by wild type p53 activity. EMBO J. 12, 461–468 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendrysa S. M., Perry M. E., The p53 tumor suppressor protein does not regulate expression of its own inhibitor, MDM2, except under conditions of stress. Mol. Cell. Biol. 20, 2023–2030 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pant V., et al. , The p53-Mdm2 feedback loop protects against DNA damage by inhibiting p53 activity but is dispensable for p53 stability, development, and longevity. Genes Dev. 27, 1857–1867 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y. V., et al. , Fine-tuning p53 activity through C-terminal modification significantly contributes to HSC homeostasis and mouse radiosensitivity. Genes Dev. 25, 1426–1438 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H., et al. , Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 115, 3472–3480 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komarova E. A., Christov K., Faerman A. I., Gudkov A. V., Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene 19, 3791–3798 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Leibowitz B. J., et al. , Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol. Cancer Res. 9, 616–625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu W., et al. , PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2, 576–583 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker N., et al. , Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Tian H., et al. , A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hua G., et al. , Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 143, 1266–1276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L., Clevers H., Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan K. S., et al. , The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. U.S.A. 109, 466–471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asfaha S., et al. , Krt19(+)/Lgr5(-) cells are radioresistant cancer-initiating stem cells in the colon and intestine. Cell Stem Cell 16, 627–638 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalfe C., Kljavin N. M., Ybarra R., de Sauvage F. J., Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14, 149–159 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Andreeff M., et al. , Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin. Cancer Res. 22, 868–876 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonnessen-Murray C., et al. , p53 mediates vast gene expression changes that contribute to poor chemotherapeutic response in a mouse model of breast cancer. Transl. Oncol. 11, 930–940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C. L., et al. , Blocking cyclin-dependent kinase 4/6 during single dose versus fractionated radiation therapy leads to opposite effects on acute gastrointestinal toxicity in mice. Int. J. Radiat. Oncol. Biol. Phys. 102, 1569–1576 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barjaktarovic Z., et al. , Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiother. Oncol. 106, 404–410 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Kim K. A., et al. , Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309, 1256–1259 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Zsebo K. M., et al. , Radioprotection of mice by recombinant rat stem cell factor. Proc. Natl. Acad. Sci. U.S.A. 89, 9464–9468 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrell C. L., et al. , Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 58, 933–939 (1998). [PubMed] [Google Scholar]
- 35.Burdelya L. G., et al. , An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320, 226–230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi C. M., et al. , PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci. Transl. Med. 6, 236ra64 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei L., et al. , Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J. Clin. Invest. 126, 4076–4087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhanja P., Norris A., Gupta-Saraf P., Hoover A., Saha S., BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res. Ther. 9, 26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y., Aguilar A., Bernard D., Wang S., Small-molecule inhibitors of the MDM2-p53 protein-protein interaction (MDM2 Inhibitors) in clinical trials for cancer treatment. J. Med. Chem. 58, 1038–1052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson J. G., et al. , p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell 21, 793–806 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato T., Clevers H., Primary mouse small intestinal epithelial cell cultures. Methods Mol. Biol. 945, 319–328 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.