Significance
How did the first cells on Earth arise? In a minimal cell, a membrane separates proteins and RNA from the surrounding aqueous environment. Cell-like membranes spontaneously assemble from simple prebiotic surfactants called fatty acids. However, fatty acid membranes are unstable in solutions containing salts that were likely present in environments of the early Earth. We find that amino acids, the building blocks of proteins, bind to fatty acid membranes and stabilize them against salts. Moreover, enhanced stabilization persists after dilution as would occur when a dehydrated pool refills with water—a likely setting for the emergence of cells. In addition to explaining how the first membranes were stabilized, our findings answer how key components of the first cells colocalized.
Keywords: prebiotic, amino acid, membrane, origin of life, protocell
Abstract
The membranes of the first protocells on the early Earth were likely self-assembled from fatty acids. A major challenge in understanding how protocells could have arisen and withstood changes in their environment is that fatty acid membranes are unstable in solutions containing high concentrations of salt (such as would have been prevalent in early oceans) or divalent cations (which would have been required for RNA catalysis). To test whether the inclusion of amino acids addresses this problem, we coupled direct techniques of cryoelectron microscopy and fluorescence microscopy with techniques of NMR spectroscopy, centrifuge filtration assays, and turbidity measurements. We find that a set of unmodified, prebiotic amino acids binds to prebiotic fatty acid membranes and that a subset stabilizes membranes in the presence of salt and Mg2+. Furthermore, we find that final concentrations of the amino acids need not be high to cause these effects; membrane stabilization persists after dilution as would have occurred during the rehydration of dried or partially dried pools. In addition to providing a means to stabilize protocell membranes, our results address the challenge of explaining how proteins could have become colocalized with membranes. Amino acids are the building blocks of proteins, and our results are consistent with a positive feedback loop in which amino acids bound to self-assembled fatty acid membranes, resulting in membrane stabilization and leading to more binding in turn. High local concentrations of molecular building blocks at the surface of fatty acid membranes may have aided the eventual formation of proteins.
In a minimal cell, a membrane sequesters proteins and RNA components from the surrounding aqueous solution. Prebiotic membranes would have spontaneously self-assembled from fatty acids (1, 2), which are known to be generated by abiotic reactions and delivered to Earth by meteorites (3, 4). However, fatty acid membranes are unstable in solutions containing salt at concentrations >200 mM or divalent cations at low millimolar concentrations (5). This presents a significant challenge in establishing the plausibility that the first protocell membranes were composed of fatty acids, because salt would have been prevalent in early oceans (6) and pools and because Mg2+ (or Fe2+) is essential for RNA catalysis (7, 8). Although glycerol monoesters (9, 10), long-chain alcohols (11, 12), decylamine (13, 14), and citrate (15) increase the stability of fatty acid vesicles (cell-like structures that separate an interior volume from the bulk solution), the prebiotic availability of these agents in sufficient quantities is uncertain (16). These observations lead to the question: what molecules that were likely found in prebiotic pools and oceans might interact with and stabilize fatty acid membranes? Amino acids are the building blocks of proteins, and 10 amino acids are deemed prebiotic (17–20). These molecules are excellent candidates for stabilizing agents of fatty acid membranes.
An additional challenge in explaining the origin of protocells is accounting for the colocalization of proteins, RNA, and membranes as a single unit. A prevalent view is that these 3 structures were formed through separate and independent processes followed by a random event that led to their colocalization. We have proposed an alternate autoamplification scenario in which fatty acid vesicles bound and concentrated the building blocks of proteins and RNA, which in turn, stabilized fatty acid vesicles, leading to further binding of the building blocks (21, 22). If the resulting conformational constraints and increased local concentration of the building blocks facilitated formation of proteins and RNA, then the colocalization of these macromolecules with fatty acid membranes would be explained. We previously reported results with RNA bases and ribose that support part of this scenario (21). Here, we seek to complete the picture by investigating interactions between amino acids and fatty acid membranes to show that all of the major components of protocells could have self-assembled into a single unit.
Our focus is on molecules that are prebiotically plausible. Previous studies have shown evidence for interactions between fatty acid vesicles and nonprebiotic amino acids and peptides (23–27). Here, we use decanoic acid as our fatty acid rather than longer-tailed versions that produce more stable membranes but are less prebiotically plausible. We choose unmodified prebiotic amino acids rather than versions altered to optimize specific reactions. We interrogate interactions between the decanoic acid membranes and the amino acids with multiple techniques: cryotransmission electron microscopy (cryo-TEM), fluorescence microscopy, NMR spectroscopy, centrifuge filtration assays, and turbidity measurements.
We find that several amino acids bind to fatty acid membranes and that a subset stabilizes fatty acid membranes in the presence of salt and Mg2+. Moreover, we find that an amino acid’s stabilization of the membrane persists after mixing and dilution to lower overall concentrations, alleviating the need for consistently high concentrations. Together, these results explain how protocells could have endured in the presence of salt and Mg2+ and provide a plausible mechanism by which the building blocks of proteins could have colocalized with early membranes.
Results
Amino Acids Bind to Decanoic Acid Vesicles.
Evidence that amino acids bind to decanoic acid vesicles emerges from 2 types of experiments: diffusion NMR spectroscopy and filtration assays. Diffusion NMR exploits the fact that, when molecules in solution bind to surfaces (even transiently), the apparent rate at which they diffuse decreases. Of the 10 amino acids that are widely regarded as prebiotically plausible (17–20), we chose 3 characteristic structures to investigate with this technique: glycine has no side chain, leucine has a hydrophobic side chain, and serine has a hydrophilic side chain. The structures of the fatty acid and amino acids that we used appear in SI Appendix, Fig. S1, and the experiments are summarized in SI Appendix, Table S1. As a positive control, we chose a positively charged amino acid, lysine, which is not considered prebiotic (17–20). Lysine should bind to negatively charged decanoic acid headgroups. We attempted to include aspartic acid (to represent amino acids with acidic side chains), but it was insoluble under our experimental conditions.
On their own in solution, amino acids move freely with single fast diffusion coefficients as shown in Fig. 1A and Table 1. When the amino acids are in the presence of decanoic acid vesicles, separate fast and slow sets of coefficients appear, which arise from the biexponential curves in Fig. 1B. The reason that the fast coefficients in Fig. 1B (with decanoic acid) are slightly lower than in Fig. 1A (without decanoic acid) is likely that decanoic acid solutions have a higher bulk viscosity than water (28). The slow set of coefficients is due to binding of amino acids to decanoic acid surfaces in accordance with other NMR diffusion results (29). This binding could be due to amino acids associating with the outer surface of vesicles and micelles (the structures of which appear in SI Appendix, Fig. S1), traversing the vesicle membrane, or associating with the inner surface of vesicles. Within Fig. 1B, stronger binding is reflected in a higher y intercept (the intensity) for the second slope; hence, leucine (which is hydrophobic) and lysine (which is positively charged) bind more strongly to decanoic acid vesicles than serine and glycine do.
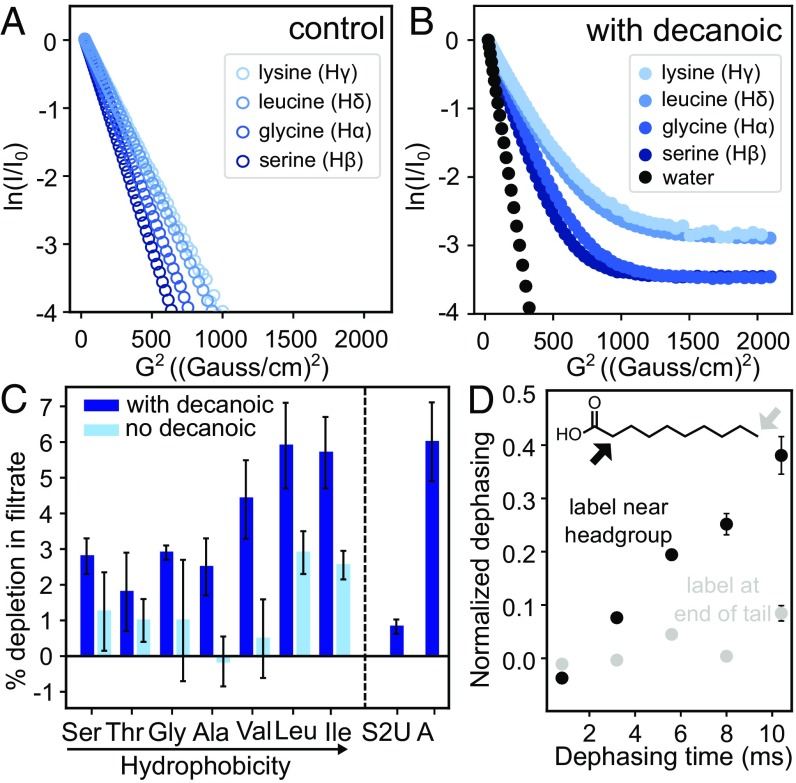
Fig. 1.
Amino acids bind to fatty acid vesicles. (A) Lysine, leucine, glycine, and serine diffuse freely in solution with 1 characteristic diffusion coefficient (m2/s) indicated by a straight line on a plot of the square of the NMR magnetic field gradient strength (G2, where G is dB/dz in units of Gauss per centimeter and B is the magnetic field) vs. normalized peak intensity (ln I/I0). The slope of the line yields the first coefficient in Table 1. (B) When the amino acids are in decanoic acid solutions containing micelles and vesicles, 2 slopes (and 2 diffusion coefficients) are distinguishable. Water, which is a negative control, because it is not expected to bind to vesicles, shows only 1 slope. (C) Amino acids are retained with decanoic acid vesicles in a centrifugal filtration assay (dark bars). Controls (light bars) were performed in the absence of decanoic acid; given that decanoic acid is a surfactant, controls may represent an overestimate of the binding to the filtration unit that occurs in the presence of decanoic acid. Error bars represent SEMs for independent experiments conducted 7 (Ser), 10 (Thr), 5 (Gly), 17 (Ala), 5 (Val), 7 (Leu), 5 (Ile), 3 (thiouracil), 3 (adenine), and 4 (controls) times. The P values for the differences between Ser, Thr, Gly, Ala, Val, Leu, and Ile and their respective controls without decanoic acid are 0.15, 0.65, 0.25, 0.15, 0.04, 0.10, and 0.04, respectively (Student’s 2-tailed t test). The P values for Ser, Thr, Gly, and Ala compared with leucine are 0.03, 0.02, 0.06, and 0.03, respecticely. Thiouracil (S2U) and adenine (A) were insufficiently soluble in the absence of decanoic acid to run controls. The hydrophobicity ranking is from ref. 40. (D) Leucine and the headgroups of decanoic acid molecules interact within a distance <5 Å in lyophilized samples. 13C{2H} REDOR dephasing occurs when decanoic acid is labeled near its carboxyl group (black symbols) and not when labeled at the terminal methyl group (gray symbols).
Table 1.
Translational diffusion coefficients for water and for amino acids in solutions with and without decanoic acid vesicles
| Test compound | Diffusion coefficient (m2/s) | ||
| No vesicles (fast) | With vesicles (fast) | With vesicles (slow) | |
| Water | 1.692 × 10−10 | 1.563 × 10−10 | Not applicable |
| Serine | 6.373 × 10−10 | 6.132 × 10−10 | 1.8 × 10−12 |
| Glycine | 7.645 × 10−10 | 7.397 × 10−10 | 1.0 × 10−12 |
| Leucine | 5.210 × 10−10 | 4.951 × 10−10 | 3.0 × 10−12 |
| Lysine | 4.888 × 10−10 | 4.580 × 10−10 | 1.0 × 10−11 |
Maximum uncertainties are ±0.63% for water, ±2.4% for fast coefficients of amino acids, and ±61% for slow coefficients. SI Appendix, Table S2 lists values for each experiment and describes how experimental uncertainties were calculated. If uncertainties are neglected, the percentage of each amino acid that binds to vesicles is calculated to be 3% for Gly, 3% for Ser, 5% for Leu, and 6% for Lys in agreement with values in Fig. 1C.
The diffusion coefficients in Table 1 tell us that the newly appearing set of (slow) coefficients is not merely due to encapsulation of amino acids within vesicles. In samples without vesicles, leucine's diffusion coefficient corresponds to a length scale of 18 µm within the NMR timescale of 0.3 s (via the 1-dimensional diffusion equation r2 = 2Dt, where r is the length, D is the diffusion coefficient, and t is the time). Limiting the length to 10 µm, the approximate size of a decanoic acid vesicle, would yield a diffusion coefficient of 1.7 × 10−10 m2/s, which is 2 orders of magnitude greater than for leucine in the presence of vesicles. Additional support that the slow diffusion coefficient is due to binding is that diffusion coefficients for highly mobile (e.g., unsaturated) phospholipids across the surface of bilayers have comparable values within uncertainty on the order of 10−12 to 10−11 m2/s (30).
Using an independent test of binding based on centrifugal filtration, we find that the 3 most hydrophobic amino acids (valine, leucine, and isoleucine) are retained to a greater extent when they are in the presence of decanoic acid vesicles and micelles (Fig. 1C). Retention of the 4 least hydrophobic amino acids (serine, threonine, glycine, and alanine) is insignificant relative to controls and is significantly less than the retention of leucine. These results support our conclusion that association of amino acids with vesicles is indeed due to binding rather than a nonspecific effect, such as encapsulation within vesicles, because encapsulation would yield the same retention in all cases. To quantify the maximum signal that we would expect from nonbinding effects, we repeated the experiment with thiouracil (which differs from the nucleobase uracil by the substitution of a sulfur for an oxygen atom). We chose thiouracil, because it is negligibly retained with decanoate micelles, which suggests that its binding to vesicles should be low as well (21). The result, depletion in the filtrate of 0.8 ± 0.2%, indicates that no more than ∼1% of the thiouracil is depleted from the filtrate due to encapsulation in vesicles. If some or all of the ∼1% is due to binding, the amount encapsulated and unbound is even lower (Fig. 1C). Our positive control, using the same assay for detection, was the nucleobase adenine, which interacts strongly with micelles and vesicles (21). As expected, adenine is strongly retained (Fig. 1C).
Our results in Fig. 1 B and C, namely that hydrophobic and positively charged amino acids bind most strongly to fatty acid vesicles, imply that the amino acids’ side chains contribute to the binding. We interrogated leucine’s mode of interaction with decanoic acid by testing if it interacts with hydrogen atoms close to the headgroup of decanoic acid, with hydrogens at the end of decanoic acid’s carbon chain, or both. To conduct this test, we used rotational echo double-resonance (REDOR) NMR spectroscopy, which measures the dipolar coupling between 13C and 2H nuclei. 13C{2H} REDOR is commonly used to probe protein–membrane interactions (31–34). We found that leucine interacts with a hydrogen near the headgroup of decanoic acid but not at the end of the tail (Fig. 1D and SI Appendix, Fig. S2). Specifically, we lyophilized solutions containing labeled leucine and decanoic acid vesicles. We measured the dephasing of 13C-labeled leucine by 2 versions of decanoic acid: one 2H labeled at the 2-carbon that adjoins the carboxyl group and one 2H labeled at the 10-carbon at the end of the carbon chain. We find significant dephasing in the former case (indicating that the moieties interact over distances <5 Å) and not in the latter.
Amino Acids Increase Vesicle Stability Against Mg2+ and/or NaCl.
What significance does binding of amino acids to fatty acid membranes hold for the origins of life on Earth? As described in the Introduction, a challenge in constructing a plausible scenario in which the first protocells incorporated fatty acid membranes is that individual ∼10-µm vesicles are unstable in the presence of divalent and monovalent ions (5) that would have been present in early oceans (6) and pools and in the case of Mg2+ or Fe+2, would have been required for RNA catalysis (7, 8). Here, we show cases in which amino acids stabilize individual large fatty acid vesicles against such cations.
Fig. 2E shows that 10 mM Mg2+ converts individual ∼10-µm vesicles into punctate structures (compare with Fig. 2A). The addition of serine or glycine, amino acids that lack hydrophobic side chains, to decanoic acid solutions with Mg2+ has an enormous positive effect: these amino acids convert the punctate structures back to intensely bright ∼10-µm vesicles (compare Fig. 2 B and C with Fig. 2 F and G). In contrast, the addition of leucine, which has a hydrophobic side chain, provides no protection against the Mg2+ (Fig. 2H).
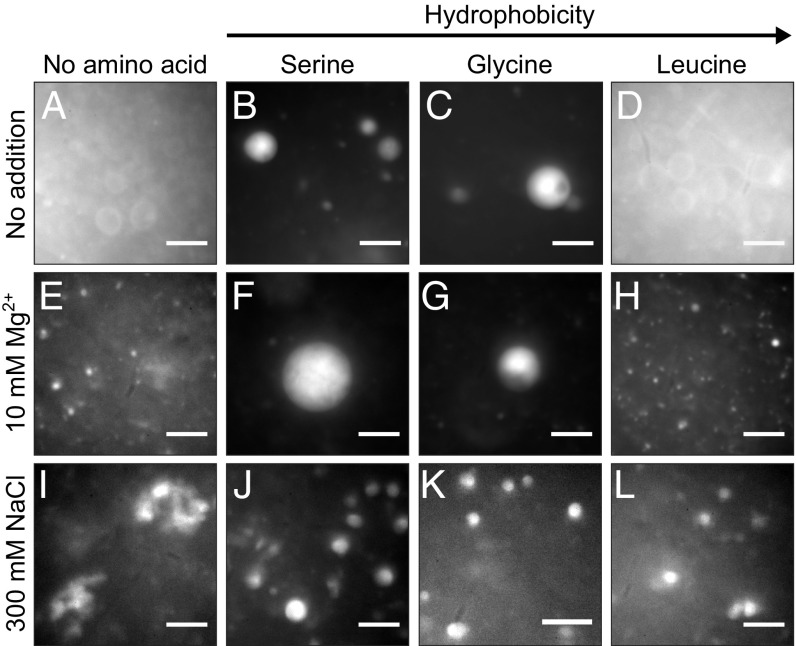
Fig. 2.
Amino acids can stabilize decanoic acid vesicles against Mg2+ and NaCl. (A) Vesicles ∼10 µm in diameter self-assemble in the decanoic acid solution described in Methods. (B and C) In the presence of 10 mM serine or glycine, these ∼10-µm vesicles appear brighter, consistent with multilamellar structures. Vesicle lumens are aqueous; they can be labeled with calcein, a soluble dye (SI Appendix, Fig. S3). (D) In contrast, in the presence of 10 mM leucine, ∼10-µm vesicles are indistinguishable from those in A. (E–H) When the decanoic acid solutions include 10 mM Mg2+, bright ∼10-µm vesicles are retained if the solutions also contain serine or glycine. In contrast, in solutions without amino acid or in the presence of leucine, micrometer-scale vesicles are replaced by punctate structures, some of which are smaller vesicles. (I–L) When 300 mM NaCl is added at room temperature to 80 mM decanoic acid at pH 7.65, vesicles flocculate. After heating the solution to 60 °C to disaggregate the flocs and then cooling to 30 °C, individual ∼5-µm vesicles reform in solutions containing 10 mM serine, glycine, or leucine. In contrast, in solutions without amino acid, flocs reform. All panels are fluorescence micrographs. (Scale bars, 10 µm.)
We conducted 3 tests to verify that the ∼10-µm vesicles observed in solutions with serine are indeed vesicles rather than oil droplets. First, we showed that, with or without Mg2+, the vesicles can encapsulate calcein and retain it through the process of size exclusion chromatography (SI Appendix, Fig. S3 C and D and S4 C and D). In contrast, oil droplets of decanoic acid exclude calcein and therefore, are visually distinct from vesicles (SI Appendix, Fig. S3 G and H). Second, we showed that the size of individual free-floating vesicles in the presence of serine and Mg2+ does not visibly increase over 24 h (SI Appendix, Fig. S5 K and L), whereas oil droplets coalesce unless they are on glass surfaces (compare with ref. 35). Third, we showed that bulk solutions containing Mg2+ (with or without serine) do not separate with a clear layer on the bottom and an oil layer on top as a solution of oil droplets does (SI Appendix, Fig. S5).
The mechanism by which Mg2+ eliminates individual ∼10-µm vesicles presumably involves binding of this cation to the carboxylate headgroups of the membrane. This mechanism forms the basis of 3 speculations: serine may block the binding of Mg2+ through interaction of its side-chain hydroxyl group and decanoic acid headgroups; glycine may block it through interaction of its amine with the headgroups (because its amine can rotate relatively freely around the α-carbon); and leucine may fail to block the binding of Mg2+, because its side chain orients in the bilayer such that leucine’s amine and/or carboxyl group is less accessible to fatty acid headgroups. Although our micrographs clearly show that Mg2+ eliminates ∼10-µm decanoic acid vesicles, it is difficult to characterize the structures that replace them. At least some of the puncta are consistent with vesicles, but few approach 1 µm in size, and poor correspondence between puncta labeled with rhodamine 6G and calcein implies that the membranes allow leakage of vesicle contents.
Individual ∼10-µm decanoic acid vesicles are also unstable in the presence of high-NaCl concentrations (5). Vesicles flocculate immediately when 300 mM NaCl is added to decanoic acid solutions at room temperature, whether or not the solutions contain amino acids. However, when these solutions contain serine, glycine, or leucine (amino acids that span a wide range of hydrophobicities) and are temperature cycled, a striking beneficial change occurs. Heating the flocs to 60 °C causes them to disaggregate, and as the solutions cool to 30 °C to 32 °C, flocs reform in solutions without amino acids (Fig. 2I), whereas serine, glycine, and leucine reduce flocculation (SI Appendix, Fig. S5 G–J), retaining many discrete, bright vesicles (Fig. 2 J–L). To confirm that these structures are vesicles rather than oil droplets, we verified that they do not behave as oil droplets: they do not float to the top of a solution in a separate layer, and they do not visibly grow over 24 h (SI Appendix, Fig. S5). Our results are important, because they imply that amino acids could have enabled large individual decanoic acid vesicles to form in early oceans or drying pools in which salt levels may have been high (6).
Vesicle Stability Against Mg2+ Correlates with an Increase in Lamellarity.
Two lines of direct evidence imply that an amino acid’s success in stabilizing individual ∼10-µm vesicles in the presence of Mg2+ correlates with an increase in the number of lamellae in each vesicle. The first line of evidence follows from the fluorescence micrographs in Figs. 2 A–C and 3 A and B, which show that individual vesicles are brighter in the presence of serine and glycine, suggesting that more decanoic acid membranes are present in each vesicle. As noted above, we know that the bright structures are not oil droplets, because they have lumens that encapsulate calcein, whereas oil droplets exclude calcein (SI Appendix, Fig. S3).
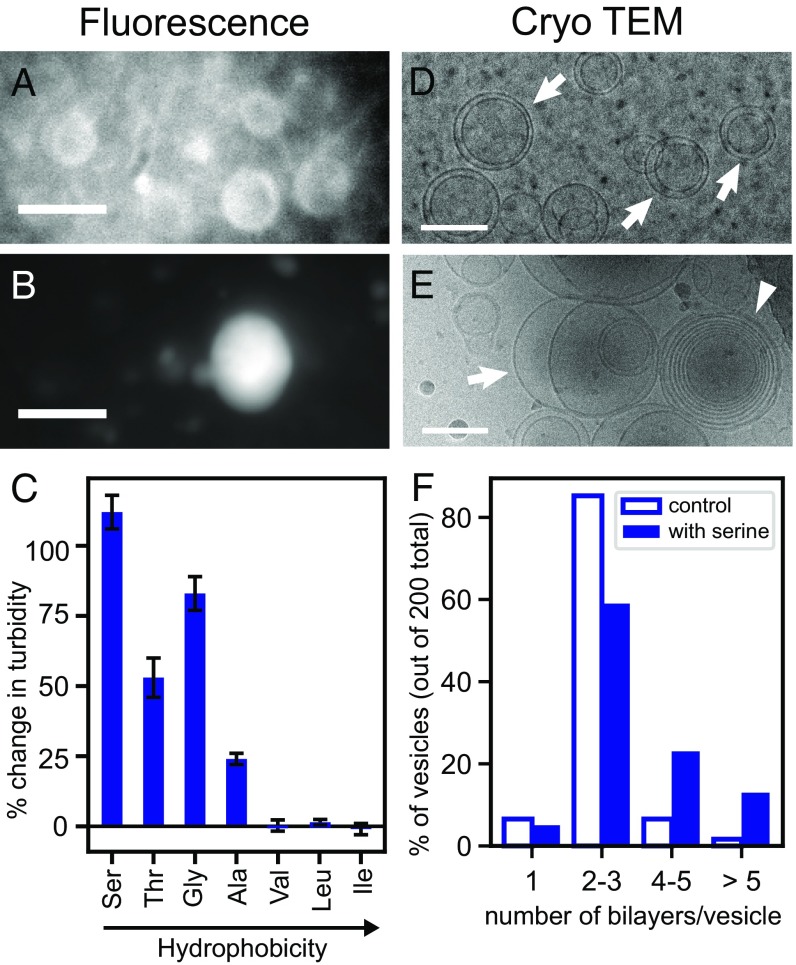
Fig. 3.
Serine increases vesicle lamellarity. (A and B) Vesicles in the decanoic acid solution were imaged by fluorescence microscopy without (A) and with (B) 10 mM serine. A and B show cropped sections of Fig. 2 A and B with linear contrast enhancement. (Scale bars, 10 µm.) (C) Amino acids were dissolved in the decanoic acid solution to yield 10 mM solutions. Turbidity was measured by absorbance 30 min later. pH was constant. The graph shows the change in turbidity relative to a control without amino acid. (D and E) Vesicle structure in decanoic acid solutions without (D) and with (E) 10 mM serine was imaged by cryo-TEM. Arrows indicate paucilamellar vesicles; the wedge indicates multilamellar vesicles. Cryo-TEM records images of vesicles that are 2 orders of magnitude smaller than fluorescence microscopy does, because vesicles >300 nm are not retained on TEM grids. (Scale bars, 100 nm.) (F) The fraction of vesicles with >3 lamellae is higher in decanoic acid solutions containing serine.
Additional evidence that serine increases lamellarity comes from cryo-TEM, which interrogates submicrometer structures. Without amino acids, >80% of submicrometer vesicles are paucilamellar, with ≤3 nested vesicles (Fig. 3 D and F). Only ∼10% of vesicles have ≥4 lamellae. When vesicle solutions include serine, which stabilizes vesicles against Mg2+, the percentage of vesicles with ≥4 lamellae jumps to >30% (Fig. 3 E and F). The increase in lamellarity induced by serine persists after the addition of Mg2+ (SI Appendix, Fig. S6).
An increase in lamellarity is also beneficial in the context of protocell growth and division. As Joyce and Szostak (16) have noted: “In contrast to the behavior of multilamellar vesicles, large unilamellar vesicles are fragile and tend to rupture with extensive loss of contents under shear stress. These features combine to make multilamellar vesicles…attractive as a means of simple, environmentally driven cycles of growth and division, requiring only episodic delivery of additional amphiphiles and a moderately turbulent environment.”
The amino acid leucine presents an interesting counterpoint. Leucine does not protect large ∼10-µm vesicles against Mg2+ (Fig. 2). However, leucine does protect against flocculation of vesicles in the presence of NaCl. Therefore, stabilization against flocculation by NaCl does not seem to rely on an increase in the number of lamellae.
Serine, Glycine, and the Other Relatively Hydrophilic Amino Acids Increase Turbidity.
Above, we showed that an increase in the number of lamellae of decanoic acid vesicles correlates with an increase in vesicle brightness when solutions contain serine (Fig. 3). To establish a correlation that can be measured with a higher-throughput technique, we also evaluated solution turbidity, specifically solution absorbance at 490 nm. As summarized in SI Appendix, Table S1, vesicle brightness (which we measured for solutions containing serine, glycine, and leucine) correlates with turbidity: the highest turbidities are observed when the 4 most hydrophilic amino acids (serine, threonine, glycine, and alanine) are mixed with the decanoic acid solution (Fig. 3C).
In these turbidity experiments, the decanoic acid solution was added to solid amino acids. Mineral surfaces (36) and ionic strength (37) are known to affect vesicle formation. To test whether the high turbidity of solutions containing the more hydrophilic amino acids results from interactions of the decanoic acid with the surfaces of the solid amino acid or from altered conditions (pH or ionic strength) in the vicinity of the dissolving amino acid, we repeated our experiments by adding amino acids as concentrated solutions rather than solids. We found that amino acids added as solutions produce the same results as when amino acids are added as solids: turbidity increases, and the most hydrophilic amino acid produces the highest increase (SI Appendix, Fig. S7).
Increases in solution turbidity due to amino acids can be long lived and can arise at low concentrations. For example, when decanoic acid solutions are prepared with 10 mM serine, elevated turbidity persists at least 4 d (SI Appendix, Fig. S8), and a significant increase occurs with serine concentrations as low as 1.25 mM (SI Appendix, Fig. S9). The cryo-TEM images in Fig. 3 D and E suggest that the serine-induced increase in turbidity is due to an increase in vesicle lamellarity. This interpretation is consistent with a recent theoretical analysis predicting a strong effect of lamellarity on turbidity (38). We rule out that the increased turbidity is due to oil drops forming (SI Appendix, Fig. S3) or a decrease in the minimum concentration at which decanoic acid forms vesicles (SI Appendix, Fig. S10). In addition, we do not observe a significant increase in the size of individual vesicles either by fluorescence microscopy (Fig. 2 and SI Appendix, Fig. S3) or by dynamic light scattering analysis of the apparent hydrodynamic diameter (which was 206 nm for decanoic acid vesicles without amino acid and 205 nm with 10 mM serine). We know that the mechanism by which serine (or any other amino acid that we tested) increases lamellarity and turbidity is not through a change in solution pH, because the pH values were the same before and after the addition of amino acid.
Amino Acids Plausibly Increase Vesicle Lamellarity in Pools Undergoing Cycles of Drying and Wetting.
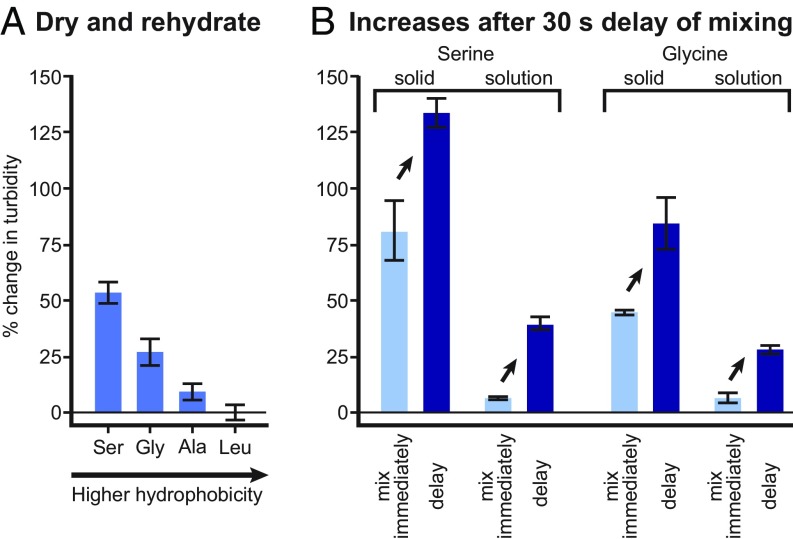
Damer and Deamer (39) propose that protocells arose in pools undergoing cycles of drying and wetting. Concentrations of amino acids and decanoic acid in these pools could have changed in at least 2 scenarios. In the first scenario, all solutes in a pool could have fully dried together and then have been rehydrated simultaneously. Fig. 4A shows that, under these conditions, the 3 relatively hydrophilic amino acids increase turbidity (and presumably, the number of lamellae in each vesicle), just as in solutions that are not subjected to drying (Fig. 3C). Therefore, the amino acids’ beneficial effects on vesicles persist through periods of drying and wetting.
Fig. 4.
Amino acids increase turbidity of vesicle solutions in 2 scenarios that recapitulate pools undergoing cycles of drying and wetting. (A) Drying and rehydration of the decanoic acid solution containing amino acids result in elevated turbidity (absorbance at 490 nm) relative to a solution without amino acids. Error bars show SEM for 4 experiments with glycine, alanine, and serine and show average error for 2 leucine experiments. (B) Delayed mixing of decanoic acid solutions and amino acid results in higher turbidity, even when final concentrations are constant. The result holds equally well when the amino acid is a solid and when it is in solution. In “solid” samples, the decanoic acid solution was added to a test tube containing solid amino acid such that the final amino acid concentration was 10 mM. In “solution” samples, 10 µL of 1 M amino acid in 50 mM sodium phosphate at pH 6.83 (or the buffer alone) was placed on top of 990 µL of the decanoic acid solution. For both types of samples, mixing by vortexing occurred either immediately or after a delay of 30 s. Error bars show average error for duplicate samples.
In the second scenario, the concentration of amino acid relative to decanoic acid could have been transiently elevated. For example, a pool containing amino acids could have partially or completely dried, and then, fluid containing decanoic acid vesicles could have flowed in. Complete mixing would not have occurred immediately, and therefore, the concentration of amino acid would have been high within a boundary layer in contact with the dried or partially dehydrated material. The turbidity experiments in Fig. 3C and SI Appendix, Fig. S7 reproduce this scenario in vitro through the addition of vesicle solutions to solid amino acids or by the deposition of a concentrated amino acid solution on a vesicle solution, with a brief delay before mixing.
To determine whether the delay is required for the increased turbidity in the second scenario, we repeated the experiments in parallel with samples that were mixed immediately. The solutions mixed after a delay were indeed more turbid (and presumably contained more multilamellar vesicles) than solutions that were mixed immediately (Fig. 4B) whether the amino acid was serine or glycine. It is important to note that all final concentrations were equivalent whether the solutions were mixed immediately or after a delay. These results imply that vesicle structures that form during a period of transiently high amino acid concentration, established either as the amino acid dissolves from a solid or as it diffuses from a concentrated solution, persist after dilution caused by mixing. In other words, systems of amino acids and fatty acid vesicles require a long time to reach equilibrium, even when the vesicles are composed of dynamic molecules, like decanoic acid, and even when solutions are mixed by vortexing.
Overall, the implication of the results in this section is that amino acids could plausibly have had large effects on protocell structure even if they were at low abundance; amino acids merely needed to be transiently held at higher concentrations as could have occurred in pools undergoing dehydration and rehydration.
Discussion and Conclusions
We used multiple techniques to find that a set of unmodified prebiotic amino acids binds to decanoic acid membranes and stabilizes large ∼10-µm vesicles in the presence of Mg2+ or NaCl. These results are important for 2 reasons: (i) they help explain how simple prebiotic vesicles could have survived in the presence of divalent cations and salt, and (ii) they help explain how the building blocks of biological polymers colocalized with early membranes. In a prebiotic scenario, several types of small molecules likely bound to fatty acid membranes, with varying affinities and different contributions toward stabilizing the large vesicles. Because we found dramatic differences in the consequences of binding (e.g., serine and glycine preserved ∼10-µm vesicles in the presence of Mg2+, whereas leucine did not), large vesicles with a distinct repertoire of bound amino acids could have emerged even if most amino acids had equal binding affinities.
Our fluorescence microscopy, cryo-TEM, and turbidity data suggest that serine and glycine (amino acids that lack hydrophobic side chains) stabilize individual large ∼10-µm fatty acid vesicles against Mg2+ by increasing the number of lamellae. In contrast, an amino acid that has a hydrophobic side chain (leucine) does not protect the membranes from Mg2+ and does not increase vesicle lamellarity (based on fluorescence microscopy and turbidity).
To our knowledge, no other researchers have previously addressed the question of whether prebiotic compounds that stabilize prebiotic fatty acid membranes also increase lamellarity. Monoacyl glycerols and long-chain alcohols probably increase membrane stability by hydrogen bonding with fatty acid headgroups (10, 11). Membrane stabilization by amino acids may also entail hydrogen bonding, particularly in the cases of serine and threonine, since the side chain of each bears a hydroxyl group. However, serine does not lower the critical vesicle concentration, whereas monoacyl glycerols and long-chain alcohols do (5, 10, 11). Several other mechanisms of membrane stabilization have been proposed, but none explain our results in full. Citrate increases membrane stability by chelating Mg2+ (15); the structures of the amino acids that we tested do not suggest chelation as a likely mechanism. Decylamine presumably stabilizes membranes via an electrostatic interaction with fatty acid headgroups (13). This mechanism may also be relevant to amino acids given that the amine group should be positively charged under conditions typically used for formation of fatty acid vesicles. Since membranes that contain a mixture of amphiphiles are more stable than pure membranes (5, 9–12), it will be interesting to determine whether amino acids stabilize large ∼10-µm vesicles of mixed composition in addition to vesicles of decanoic acid alone.
Our results (summarized in SI Appendix, Table S1) are particularly compelling in the context of the hypothesis that protocells arose in pools undergoing cycles of drying and rehydration (39). Such cycles would have produced transiently high concentrations of amino acids. We find that transiently high serine and glycine concentrations cause increases in the turbidity of a decanoic acid solution (a correlate of lamellarity) that persist after dilution. Our results open up exciting research questions about chemical reactions that would enable the next stage of protocell evolution—does self-assembly of vesicles and amino acids increase synthesis of peptides, and is the system sufficiently robust to support divalent cation-dependent ribozyme activity? Moreover, these discoveries reinforce our previous report that RNA bases bind to and stabilize fatty acid membranes (21). Taken together, those findings and the results reported here support a coherent scenario in which aggregates of fatty acids, which would have spontaneously self-assembled, bound the building blocks of RNA and proteins (nucleobases, ribose, and amino acids), generating the concentration and conformational constraints required for abiotic synthesis of RNA and protein.
Methods
Decanoic Acid Solution.
Decanoic acid was dissolved, with heating, in 190 mM NaOH to yield a 180 mM stock solution. This stock, 0.5 M monosodium phosphate, and 4 M NaCl were diluted into water to yield a solution of 50 mM decanoic acid, 30 mM sodium phosphate, and 100 mM NaCl. The pH was then adjusted to 6.83 by adding a small volume of 1 M HCl.
Diffusion NMR.
Pulsed field gradient NMR experiments were performed on a Bruker Avance III 700-MHz NMR instrument with a 5-mm Broadband Observe probe at 25 °C.
Filtration Assay.
Solutions were placed in an Amicon Ultra-4 3K filter and centrifuged in a swinging bucket centrifuge at 3,000 × g for 10 min at 21 °C.
Turbidity.
Turbidity was measured by determining absorbance at 490 nm.
Supplementary Material
Acknowledgments
C.E.C. and A.M. were funded by National Institutes of General Medical Sciences of the NIH Awards T32GM008268 (to C.E.C.) and T32-GM007750 (to A.M.). A.R. and M.G. were supported as undergraduate researchers by NSF Grant MCB-1402059 (to S.L.K.). J.A.W. and K.K.L. were supported by NIH Grant R01-GM099989. The laboratories of G.P.D. and S.L.K. were supported by NASA Grant NNX17AK86G (Exobiology). We thank our anonymous reviewers for their particularly careful reading of our manuscript and for their in-depth comments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900275116/-/DCSupplemental.
References
- 1.Deamer D., Dworkin J. P., Sandford S. A., Bernstein M. P., Allamandola L. J., The first cell membranes. Astrobiology 2, 371–381 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Morigaki K., Walde P., Fatty acid vesicles. Curr. Opin. Coll. Int. Sci. 12, 75–80 (2007). [Google Scholar]
- 3.Proskurowski G., et al. , Abiogenic hydrocarbon production at lost city hydrothermal field. Science 319, 604–607 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Lawless J., Yuen G., Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282, 396–398 (1979). [Google Scholar]
- 5.Monnard P.-A., Apel C. L., Kanavarioti A., Deamer D. W., Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2, 139–152 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Knauth L. P., Temperature and salinity history of the precambrian ocean: Implications for the course of microbial evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 219, 53–69 (2005). [Google Scholar]
- 7.Szostak J. W., The eightfold path to non-enzymatic RNA replication. J. Syst. Chem. 3, 2 (2012). [Google Scholar]
- 8.Jin L., Engelhart A. E., Zhang W., Adamala K., Szostak J. W., Catalysis of template-directed nonenzymatic RNA copying by Iron(II). J. Am. Chem. Soc. 140, 15016–15021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen I. A., Salehi-Ashtiani K., Szostak J. W., RNA catalysis in model protocell vesicles. J. Am. Chem. Soc. 127, 13213–13219 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer S. E., Deamer D. W., Boncella J. M., Monnard P.-A., Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 9, 979–987 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Apel C. L., Deamer D. W., Mautner M. N., Self-assembled vesicles of monocarboxylic acids and alcohols: Conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta 1559, 1–9 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Mansy S. S., Szostak J. W., Thermostability of model protocell membranes. Proc. Natl. Acad. Sci. U.S.A. 105, 13351–13355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namani T., Deamer D. W., Stability of model membranes in extreme environments. Orig. Life Evol. Biosph. 38, 329–341 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Maurer S. E., et al. , Vesicle self-assembly of monoalkyl amphiphiles under the effects of high ionic strength, extreme pH, and high temperature environments. Langmuir 34, 15560–15568 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Adamala K., Szostak J. W., Nonenzymatic template-directed RNA synthesis inside model protocells. Science 342, 1098–1100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce G. F., Szostak J. W., Protocells and RNA self-replication. Cold Spring Harb. Perspect. Biol. 10, a034801 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longo L. M., Blaber M., Protein design at the interface of the pre-biotic and biotic worlds. Arch. Biochem. Biophys. 526, 16–21 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Doi N., Kakukawa K., Oishi Y., Yanagawa H., High solubility of random-sequence proteins consisting of five kinds of primitive amino acids. Protein Eng. Des. Sel. 18, 279–284 (2005). [DOI] [PubMed] [Google Scholar]
- 19.McDonald G. D., Storrie-Lombardi M. C., Biochemical constraints in a protobiotic earth devoid of basic amino acids: The “BAA(-) world”. Astrobiology 10, 989–1000 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Cleaves H. J., 2nd, The origin of the biologically coded amino acids. J. Theor. Biol. 263, 490–498 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Black R. A., et al. , Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells. Proc. Natl. Acad. Sci. U.S.A. 110, 13272–13276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black R. A., Blosser M. C., A self-assembled aggregate composed of a fatty acid membrane and the building blocks of biological polymers provides a first step in the emergence of protocells. Life (Basel) 6, E33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamala K., Szostak J. W., Competition between model protocells driven by an encapsulated catalyst. Nat. Chem. 5, 495–501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murillo-Sánchez S., Beaufils D., González Mañas J. M., Pascal R., Ruiz-Mirazo K., Fatty acids’ double role in the prebiotic formation of a hydrophobic dipeptide. Chem. Sci. 7, 3406–3413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamat N. P., Tobé S., Hill I. T., Szostak J. W., Electrostatic localization of RNA to protocell membranes by cationic hydrophobic peptides. Angew. Chem. Int. Ed. Engl. 54, 11735–11739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blocher M., Liu D., Walde P., Luisi P. L., Liposome-assisted selective polycondensation of alpha-amino acids and peptides. Macromolecules 32, 7332–7334 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Mayer C., et al. , Molecular evolution in a peptide-vesicle system. Life (Basel) 8, E16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valeri D., Meirelles A. J. A., Viscosities of fatty acids, triglycerides and their binary mixtures. J. Am. Oil Chem. Soc. 74, 1221–1226 (1997). [Google Scholar]
- 29.Zhang W., et al. , Quantifying binding of ethylene oxide-propylene oxide block copolymers with lipid bilayers. Langmuir 33, 12624–12634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindblom G., Orädd G., Filippov A., Lipid lateral diffusion in bilayers with phosphatidylcholine, sphingomyelin and cholesterol. An NMR study of dynamics and lateral phase separation. Chem. Phys. Lipids 141, 179–184 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Jia L., et al. , REDOR solid-state NMR as a probe of the membrane locations of membrane-associated peptides and proteins. J. Magn. Reson. 253, 154–165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L., Ghosh U., Schmick S. D., Weliky D. P., Residue-specific membrane location of peptides and proteins using specifically and extensively deuterated lipids and 13C-2H rotational-echo double-resonance solid-state NMR. J. Biomol. NMR 55, 11–17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gullion T., Kishore R., Asakura T., Determining dihedral angles and local structure in silk peptide by 13C-2H REDOR. J. Am. Chem. Soc. 125, 7510–7511 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Luthra S. A., et al. , Carbon-deuterium rotational-echo double-resonance NMR spectroscopy of lyophilized aspartame formulations. J. Pharm. Sci. 101, 283–290 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Monnard P.-A., Deamer D. W., Preparation of vesicles from nonphospholipid amphiphiles. Methods Enzymol. 372, 133–151 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Hanczyc M. M., Mansy S. S., Szostak J. W., Mineral surface directed membrane assembly. Orig. Life Evol. Biosph. 37, 67–82 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Maurer S., The impact of salts on single chain amphiphile membranes and implications for the location of the origin of life. Life 7, 44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang A., Chan Miller C., Szostak J. W., Core-shell modeling of light scattering by vesicles: Effect of size, contents, and lamellarity. Biophys. J. 116, 659–669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damer B., Deamer D., Coupled phases and combinatorial selection in fluctuating hydrothermal pools: A scenario to guide experimental approaches to the origin of cellular life. Life (Basel) 5, 872–887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones D. D., Amino acid properties and side-chain orientation in proteins: A cross correlation approach. J. Theor. Biol. 50, 167–183 (1975). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.