Significance
Chromosomes carry our genetic material and must be precisely copied and partitioned into daughter cells during cell division. To ensure correct partitioning, a signaling cascade called the spindle assembly checkpoint delays cell division until all chromosomes are correctly attached to microtubules, the dynamic filaments that drive chromosome movements. If even a single chromosome is unattached or incorrectly attached, an enzyme, Mps1, initiates checkpoint signaling by binding to and modifying kinetochores, the large protein complexes that connect chromosomes to microtubules. Here we identify conditions that promote Mps1 release from native kinetochore particles isolated from yeast. Our findings suggest how the checkpoint “wait” signals might be turned off when kinetochores bind microtubules.
Keywords: mitosis, spindle assembly checkpoint, single molecule fluorescence
Abstract
Accurate mitosis depends on a surveillance system called the spindle assembly checkpoint. This checkpoint acts at kinetochores, which attach chromosomes to the dynamic tips of spindle microtubules. When a kinetochore is unattached or improperly attached, the protein kinase Mps1 phosphorylates kinetochore components, catalyzing the generation of a diffusible “wait” signal that delays anaphase and gives the cell time to correct the error. When a kinetochore becomes properly attached, its checkpoint signal is silenced to allow progression into anaphase. Recently, microtubules were found to compete directly against recombinant human Mps1 fragments for binding to the major microtubule-binding kinetochore element Ndc80c, suggesting a direct competition model for silencing the checkpoint signal at properly attached kinetochores. Here, by developing single-particle fluorescence-based assays, we tested whether such direct competition occurs in the context of native kinetochores isolated from yeast. Mps1 levels were not reduced on kinetochore particles bound laterally to the sides of microtubules or on particles tracking processively with disassembling tips. Instead, we found that Mps1 kinase activity was sufficient to promote its release from the isolated kinetochores. Mps1 autophosphorylation, rather than phosphorylation of other kinetochore components, was responsible for this dissociation. Our findings suggest that checkpoint silencing in yeast does not arise from a direct competition between Mps1 and microtubules, and that phosphoregulation of Mps1 may be a critical aspect of the silencing mechanism.
Accurate segregation of chromosomes into daughter cells is essential for the development and survival of all organisms. However, the process is prone to errors that must be detected and corrected to avoid chromosome missegregation events that would generate aneuploidy, a hallmark of cancer and other diseases (reviewed in ref. 1). A surveillance system called the spindle assembly checkpoint prevents errors by delaying segregation until every chromosome has made proper bioriented attachments to microtubules from opposite poles (reviewed in refs. 2 and 3). Checkpoint signaling is initiated by recruitment of a hierarchy of proteins to the kinetochore, which subsequently generate a diffusible inhibitory signal that delays anaphase. While the activation of this “wait” signal has been extensively characterized, the events that trigger its deactivation remain unclear. Both premature and abnormally late deactivation of the spindle assembly checkpoint can lead to genetic abnormalities associated with cancer (4, 5), so understanding its mechanism is relevant to human health.
The conserved protein kinase Mps1 is the most upstream component of the spindle assembly checkpoint signaling cascade, and it also regulates sister kinetochore biorientation through an independent mechanism (reviewed in ref. 6). Mps1 expression varies in a cell cycle-dependent manner, with protein levels peaking during prometaphase and then declining during anaphase (7, 8). Mps1 localizes to kinetochores by associating with the microtubule-binding protein Ndc80 (9–11), an ideal position for sensing and responding to the attachment status of the kinetochore. Mps1 kinetochore localization is highly dynamic, cycling on and off the kinetochore with a half-life of several seconds in mammalian cells (12, 13). Stable tethering of Mps1 to the central kinetochore causes constitutive activation of the spindle assembly checkpoint, suggesting that regulation of the Mps1 kinetochore localization is critical for proper checkpoint silencing (13–15). In addition, the levels of Mps1 on kinetochores are highest in prometaphase when attachments are being established, and they decline as bioriented attachments are achieved at metaphase (12, 14, 16). Thus, the release of Mps1 protein from kinetochores appears to be important for silencing the checkpoint.
A simple and elegant model for spindle assembly checkpoint silencing posits that Mps1 and microtubules compete directly for binding to Ndc80, thereby linking microtubule attachment to the release of Mps1 and deactivation of checkpoint signaling (17, 18). Although this direct competition model is supported by biochemical data collected using recombinant human protein fragments, it does not account for a residual pool of Mps1 that remains on bioriented metaphase kinetochores in cells (12, 14, 17). In addition, there is evidence for other mechanisms, such as spatial or enzymatic regulation of Mps1, which might account for the loss of Mps1 checkpoint activity on biorientation (13, 14, 19–24). Despite the evidence for multiple mechanisms controlling Mps1 localization and checkpoint activity, it has been difficult to discern their relative importance or sequential order, given the intertwined nature of these events within cells. Thus, the mechanisms that cause Mps1 release from microtubule-attached kinetochores remain incompletely understood.
To elucidate how the association and dissociation of Mps1 from kinetochores are controlled, we developed a unique reconstitution system using isolated budding yeast kinetochores, which have previously been shown to recapitulate checkpoint signaling events (25, 26) and microtubule tip-coupling activity in vitro (27). Using single-particle microscopy assays, we show that microtubules and Mps1 do not compete directly for association with isolated native kinetochores. Instead, our biochemical assays reveal that Mps1 kinase activity is sufficient for its release from kinetochores. The key substrate appears to be Mps1 itself rather than core kinetochore proteins. Together, these data lead us to propose that biorientation promotes Mps1 autophosphorylation, which decreases the affinity of Mps1 for kinetochores and thus contributes to checkpoint silencing.
Results
Kinetochores Bind Simultaneously to Mps1 and Microtubules In Vitro.
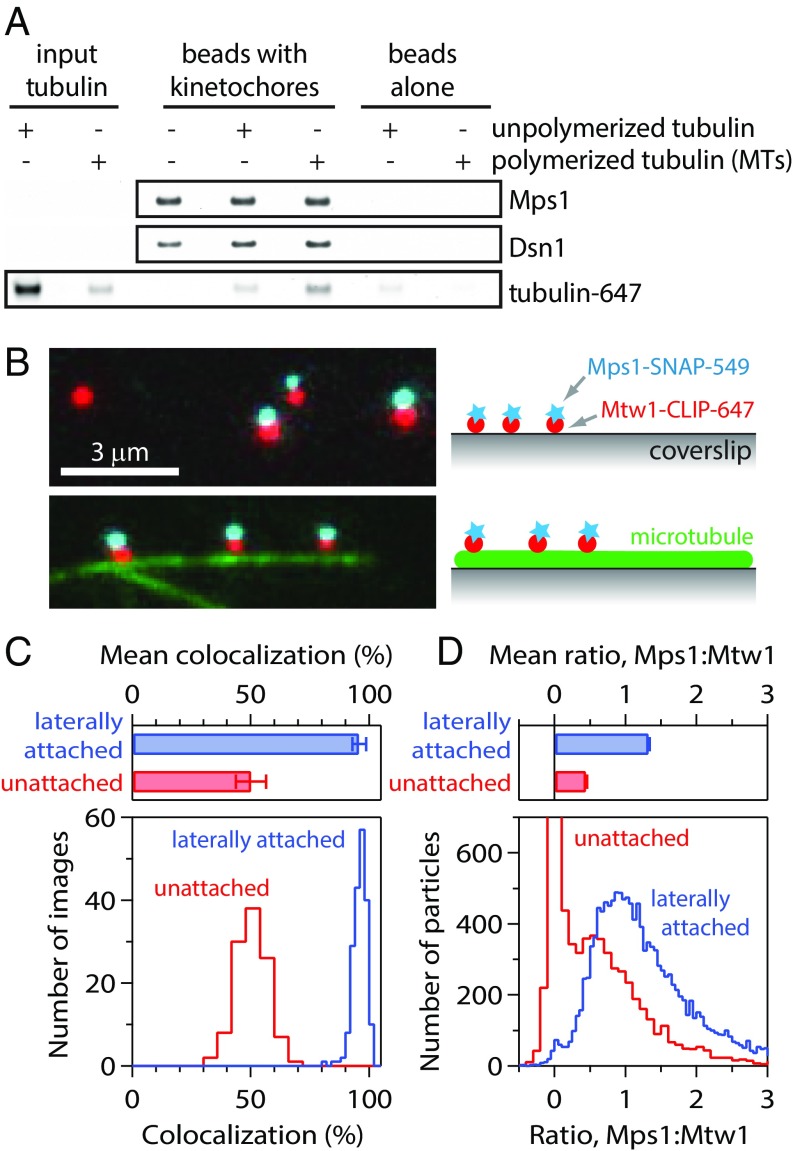
We began by testing whether microtubules and Mps1 compete against each other for binding to isolated kinetochores. We previously developed a method to purify kinetochores by immunoprecipitation of the Dsn1 kinetochore protein from budding yeast cells (26, 27). These native kinetochores copurify with a pool of Mps1 that, when activated, is sufficient to recruit the Bub3, Bub1, and Mad1 spindle assembly checkpoint proteins to the kinetochores (25, 26). In addition, association of Mps1 with the native kinetochores requires Ndc80, its kinetochore receptor (SI Appendix, Fig. S1A) (11). To directly assess the effects of microtubule binding on the kinetochore-bound levels of Mps1, kinetochores purified from cells expressing epitope-tagged Mps1 were incubated with fluorescently labeled Taxol-stabilized microtubules or with unpolymerized tubulin. As negative controls, we also incubated beads lacking kinetochores with microtubules or free tubulin. After incubation, the beads were washed, and the relative levels of fluorescent tubulin and Mps1 that remained bound were analyzed by SDS/PAGE followed by fluorescence imaging and immunoblotting, respectively (Fig. 1A). As expected, the kinetochores bound more polymerized tubulin (microtubules) than unpolymerized tubulin, and this binding required the Ndc80 protein (SI Appendix, Fig. S1B). However, there was no difference in the amount of Mps1 that remained associated with the kinetochores in the presence or absence of microtubule attachments (Fig. 1A). These data suggest that kinetochores can simultaneously bind Mps1 and microtubules, in apparent contradiction to a competitive binding model.
Fig. 1.
Isolated kinetochores retain Mps1 when attached laterally to microtubules. (A) Kinetochores isolated (from SBY9190) and bound to magnetic beads were mock-treated, incubated with unpolymerized fluorescent tubulin, or incubated with fluorescent Taxol-stabilized microtubules (MTs). The amount of tubulin retained after bead washing was assessed by fluorescence imaging. Kinetochores (represented by Dsn1) and kinetochore-associated Mps1 were detected by immunoblotting. (B) Fluorescence images of individual kinetochore particles (from SBY15285) carrying Mps1-SNAP 549 (cyan) and Mtw1-CLIP 647 (red), tethered to a coverslip (Top) or attached laterally to a microtubule (green; Bottom). Colors are offset vertically; cyan-red pairs are colocalized, dual-color particles. (C) Percentages of kinetochore particles retaining Mps1. Bars show mean ± SD values from 192 images of microtubule-attached particles and 112 images of coverslip-tethered particles. Histograms show corresponding distributions. (D) Approximate ratios of Mps1 to Mtw1 molecules, estimated from particle brightness relative to the brightness of single Mps1-SNAP 549 and Mtw1-CLIP 647 molecules. Bars show mean ± SEM ratios from 14,189 laterally attached particles and 7,830 coverslip-tethered particles. Histograms show corresponding distributions.
A Single-Particle Method to Measure Mps1 Levels on Individual Kinetochores.
Because subpopulations of microtubule-bound and -unbound kinetochores could not be distinguished in our initial experiments, we developed a single-particle method to visualize and quantify Mps1 levels on individual kinetochores. We created strains in which endogenous Mps1 and the core kinetochore protein Mtw1 were fused at their C-termini to SNAP and CLIP epitope tags (28, 29), respectively, for labeling with bright, photostable organic fluorophores. Cells expressing Mtw1-CLIP and Mps1-SNAP grew normally and activated the spindle assembly checkpoint in response to the microtubule-destabilizing drug nocodazole, confirming the functionality of the fusion proteins (SI Appendix, Fig. S2A). Kinetochores isolated from these cells were dyed with SNAP-Surface 549 and CLIP-Surface 647 and then tethered sparsely to coverslips for imaging by multicolor total internal reflection fluorescence (TIRF) microscopy. Most of the particles were single color, carrying Mtw1 but lacking Mps1. Only a small fraction of particles (typically 20 ± 5% or less) were dual color, carrying both Mps1 and Mtw1 (SI Appendix, Fig. S2 C–E). Because Mps1 and Mtw1 molecules at kinetochores are separated by only ∼50 nm or less (14), which is below the resolution limit of TIRF microscopy, their signals overlaid nearly perfectly in raw images. Therefore, to facilitate identification of colocalized particles, the 2 colors are deliberately offset in all the images presented here.
Given the relatively low colocalization, we sought to increase retention of Mps1 with the kinetochores to allow a better dynamic range for our single-particle experiments. We found that kinetochore particles purified from a mutant dsn1-2D strain, with phosphomimetic mutations S240D and S250D that have previously been shown to improve recovery of outer kinetochore proteins (30, 31), retained significantly more Mps1. The fraction of phosphomimetic Dsn1-2D particles carrying both Mps1 and Mtw1 was 47 ± 6% (SI Appendix, Fig. S2 C and D), more than double the fraction of wild-type kinetochores carrying both proteins. Particles with just one detectable Mps1 or Mtw1, identifiable by their single-step photobleaching behavior, served as internal controls, allowing normalization of particle brightness and estimation of the ratio of Mps1 molecules to Mtw1 molecules for each particle. On average, Dsn1-2D kinetochore particles carried a ratio of 0.51 ± 0.02 Mps1 molecules per Mtw1 molecule, and a substantial fraction (20%) were “high-occupancy” (i.e., with 1 or more Mps1 per Mtw1). In contrast, wild-type kinetochore particles carried only 0.11 ± 0.02 Mps1 molecules per Mtw1 molecule on average, and far fewer (3%) were high-occupancy particles (SI Appendix, Fig. S2E). We confirmed that dsn1-2D cells have normal checkpoint function (SI Appendix, Fig. S2 A and B) and used them for subsequent single-particle experiments.
Individual Kinetochores Associate Simultaneously with Mps1 and Microtubules.
If Mps1 and microtubules compete directly for binding to kinetochores, then high-occupancy particles, carrying 1 or more Mps1 molecules per Mtw1 molecule, should be inhibited from binding to microtubules. Conversely, the population of particles that bind microtubules should be enriched for low-occupancy particles (i.e., particles with fewer than 1 molecule of Mps1 per Mtw1 molecule). To test this prediction, we anchored Taxol-stabilized microtubules to coverslips, introduced the labeled kinetochores, and then allowed them to bind the sides of the filaments (Fig. 1B ) (27, 29). Contrary to our expectation, microtubule-bound kinetochores had an even higher average Mps1 occupancy than unbound particles. The fraction of microtubule-bound kinetochores that carried Mps1 was 96 ± 3% (Fig. 1C), roughly double that of the control unbound population (50 ± 3%). The microtubule lattice-bound kinetochores also carried a higher ratio of Mps1 molecules per Mtw1 molecule, 1.33 ± 0.01 on average, which was 3-fold higher than the ratio of 0.45 ± 0.01 measured for the control unbound population (Fig. 1D). Thus, microtubule attachment appears to select for, rather than against, the high-occupancy particles. We speculate that this higher retention of Mps1 might occur because binding to microtubules selects for more complete kinetochore particles.
To confirm that the simultaneous association of Mps1 and microtubules with kinetochores was not related to the Dsn1-2D mutant, we also analyzed wild-type kinetochores. Although the particles from a wild-type Dsn1 strain retained less Mps1 than seen in Dsn1-2D particles, they nevertheless bound microtubules independently of whether or not they carried Mps1 (SI Appendix, Fig. S3). These observations indicate that isolated yeast kinetochores can associate simultaneously with Mps1 and with the microtubule lattice in a noncompetitive manner.
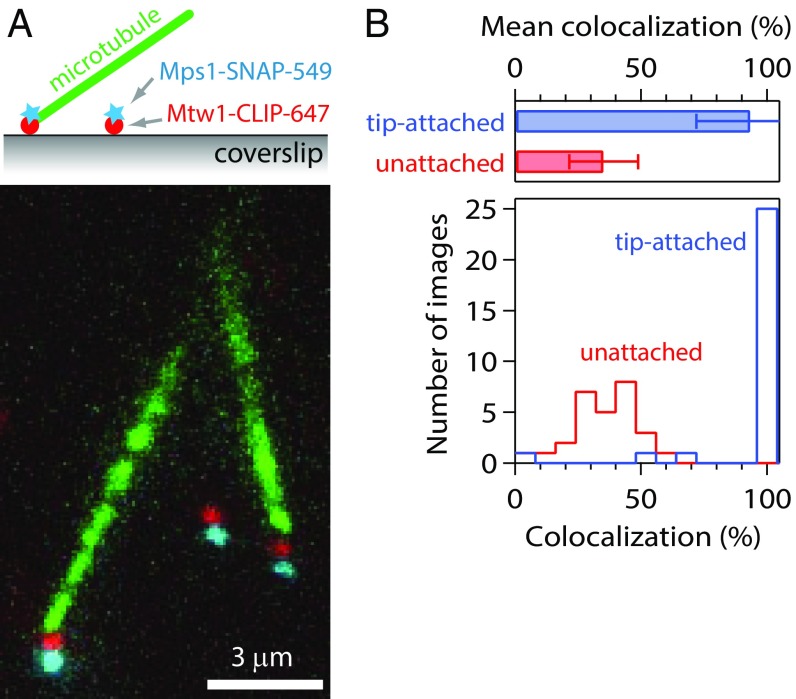
While kinetochores initially associate with the lateral sides of microtubules, they convert to end-on attachments upon biorientation. Because the checkpoint is apparently silenced only after bioriented, end-on attachments are made (32), we developed a single-particle technique to analyze Mps1 association specifically with tip-attached kinetochores. Purified, labeled kinetochores were tethered to coverslips, and fluorescent Taxol-stabilized microtubules were introduced into the chamber. Through thermal diffusion, the microtubules tended to attach via their ends to the kinetochores (Fig. 2A). Surface-tethered kinetochores in the same fields of view that failed to capture a microtubule served as internal controls. Nearly all of the kinetochores that captured microtubule ends (93 ± 21%) retained Mps1 (Fig. 2B). A smaller fraction of the control, unattached particles (35 ± 14%) retained Mps1, similar to our previous results without microtubules (Fig. 1). These data suggest that Mps1 does not compete specifically with microtubule tips.
Fig. 2.
Kinetochores retain Mps1 when attached to microtubule tips. (A) Fluorescence image of kinetochore particles (from SBY15285) carrying Mps1-SNAP 549 (cyan) and Mtw1-CLIP 647 (red) tethered to a coverslip and exposed to free, Taxol-stabilized Alexa Fluor 488 microtubules (green). By thermal diffusion, the filaments attached via their tips to the coverslip-tethered kinetochores. Colors are offset vertically; cyan-red pairs are colocalized, dual-color kinetochore particles. (B) Percentages of kinetochore particles retaining Mps1. Bars show mean ± SD values from 28 images of 40 tip-attached particles and 366 coverslip-tethered particles. Histograms show the corresponding distributions of colocalization measured for the tip-attached and coverslip-tethered particles in each individual image.
Individual Kinetochores Tracking Disassembling Microtubule Tips Retain Mps1.
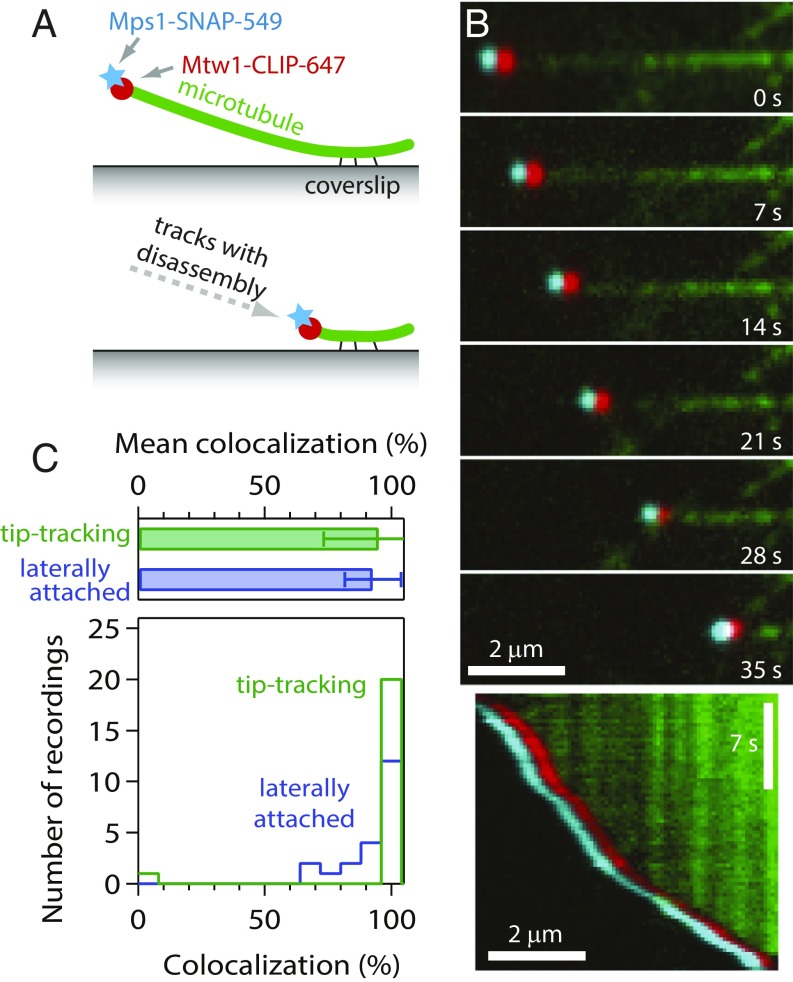
Bioriented kinetochores in vivo attach persistently to dynamic microtubule tips, an arrangement that allows them to harness tip disassembly to drive chromosome movement (33, 34) and that also might be important for silencing their checkpoint signals. To examine whether dynamic tip attachments are required to exclude Mps1 from kinetochores, we grew microtubules from coverslip-anchored seeds and allowed purified, labeled kinetochores to attach laterally to the growing filaments. We then triggered microtubule disassembly by rapid buffer exchange to remove the free tubulin (Fig. 3A). When disassembling tips encountered individual kinetochore particles, the particles began tracking with the tips and were usually carried all the way to the coverslip-anchored seed (Fig. 3B and Movie S1), as reported previously (27). Nearly all the tip-tracking particles (95 ± 22%) retained Mps1 throughout their movement (Fig. 3C). A similarly high level of Mps1 retention (93 ± 11%) was seen for laterally attached particles in the same fields of view (Fig. 3C), consistent with the experiments using Taxol-stabilized filaments. These data show that Mps1 does not compete with dynamic microtubule tips for binding to isolated kinetochores.
Fig. 3.
Kinetochores retain Mps1 when tracking with disassembling microtubule tips. (A) Schematic of the experiment. Dynamic microtubule extensions were grown from coverslip-anchored seeds and decorated with kinetochore particles. Disassembly was then induced by buffer exchange to remove free tubulin. (B) Selected frames (Top) and kymograph (Bottom) from Movie S1 showing a kinetochore particle (from SBY15285) tracking with the disassembling tip of a microtubule (green) and carrying Mps1-SNAP 549 (cyan) and Mtw1-CLIP 647 (red). Colors are offset horizontally; the cyan-red pair is a colocalized, dual-color kinetochore particle. (C) Percentages of kinetochore particles retaining Mps1. Bars show mean ± SD values from 21 recordings of 32 tip-tracking particles and 213 laterally attached particles. Histograms show corresponding distributions.
Activating Mps1 Triggers Its Release from Kinetochores.
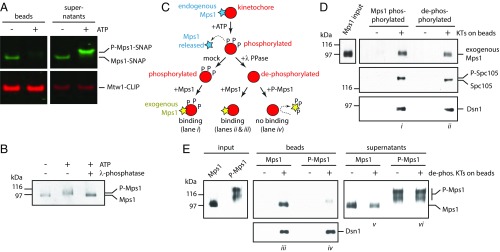
Microtubule binding did not release Mps1 from kinetochores in our reconstitution experiments, yet Mps1 levels are clearly reduced on bioriented vs. unattached kinetochores in cells (7, 12, 14). Previous research in human cells found that attenuating Mps1 activity with inhibitors increased the level of kinetochore-associated Mps1 and also reduced Mps1 turnover at kinetochores, suggesting that Mps1 release from kinetochores is phosphoregulated (13, 20–23, 35, 36). In addition, mutational analyses found that Mps1 autophosphorylation regulates its localization to kinetochores, but there were conflicting observations on whether this autophosphorylation aids or inhibits its kinetochore association (17, 23, 37). We hypothesized that Mps1 activity might be sufficient to promote its release from isolated kinetochores in vitro, and we were able to test this because Mps1 is the primary kinase activity that copurifies with the isolated yeast kinetochores (26). To do this, we purified kinetochores carrying Mps1-SNAP and Mtw1-CLIP, dyed them, and then treated them with or without ATP. We analyzed Mps1 levels that remained associated with the ATP-treated kinetochores, as well as the levels that were released into the supernatants via fluorescent gel imaging. ATP treatment was sufficient to release Mps1 from the purified kinetochores, and the electrophoretic mobility of the released Mps1 was clearly reduced (Fig. 4A), suggesting that it had become phosphorylated. To confirm that the mobility shift was due to phosphorylation, we purified Mps1 itself, treated it with ATP, and then added λ-phosphatase. Indeed, an ATP-dependent mobility shift occurred and was eliminated by phosphatase treatment (Fig. 4B). These results show that activating kinetochore-associated Mps1 triggers its autophosphorylation, as well as its release from kinetochores.
Fig. 4.
Mps1 autophosphorylation inhibits its binding to kinetochores. (A) An in vitro kinase reaction releases Mps1 from isolated kinetochores. Kinetochores carrying Mps1-SNAP 549 (green) and Mtw1-CLIP 647 (red) (from SBY15285) were immunoprecipitated on magnetic beads and incubated with or without ATP. Proteins retained on beads and released into the supernatants were analyzed by SDS/PAGE and fluorescence imaging. (B) The electrophoretic mobility shift caused by incubation of Mps1 with ATP is due to phosphorylation. Mps1 was purified (from SBY12412) via immunoprecipitation under stringent conditions to remove copurifying proteins and then incubated with or without ATP. An aliquot of the ATP-treated sample was subsequently treated with λ-phosphatase. The resulting samples were analyzed by immunoblotting. (C) Schematic illustrating the preparation of Mps1-phosphorylated and dephosphorylated kinetochores for binding to exogenous Mps1. (D) Mps1 binds equivalently to Mps1-phosphorylated or dephosphorylated kinetochores. Kinetochores (from SBY9190) and control beads lacking kinetochores were prepared, incubated with purified exogenous Mps1 (from SBY12412, as diagrammed in C), washed, and then analyzed by immunoblotting. (E) Autophosphorylated Mps1 fails to bind kinetochores. Immobilized kinetochores (from SBY9190) and beads lacking kinetochores were treated sequentially with ATP and then λ-phosphatase to release endogenous Mps1 and remove phosphates. They were then washed and incubated with native Mps1 or with autophosphorylated P-Mps1 (from SBY12412, as diagrammed in C). Native Mps1 that had not been autophosphorylated in vitro bound well to the kinetochores (lane iii), with some excess remaining in the supernatant (lane v). Autophosphorylated P-Mps1 bound poorly (lane iv). Electrophoretic mobility of the minor, kinetochore-bound P-Mps1 subfraction (lane iv) was faster than that of the major unbound fraction (lane vi), indicating that kinetochores selectively bind less-phosphorylated forms of Mps1.
Autophosphorylation Reduces the Affinity of Mps1 for Kinetochores.
Mps1 phosphorylates itself and also several major kinetochore proteins in vitro (11, 26), including Ndc80, making it unclear whether the dissociation of Mps1 was due to phosphorylation of sites on Mps1 itself, on other kinetochore proteins, or on both. To distinguish among these possibilities, we independently manipulated the phosphorylation states of isolated kinetochores and Mps1 and then tested whether they would associate in vitro.
We first tested whether Mps1-mediated phosphorylation of kinetochores alters their affinity for Mps1. We created phosphorylated kinetochores lacking endogenous Mps1 by incubating purified kinetochores with ATP (Fig. 4C). Immunoblotting confirmed the release of endogenous Mps1 (SI Appendix, Fig. S4A), and an electrophoretic mobility shift of Spc105, a known Mps1 substrate (26), served as a readout for kinetochore phosphorylation by Mps1 (Fig. 4D). To create dephosphorylated kinetochores for use as controls, we divided the ATP-treated kinetochores and treated one-half with λ-phosphatase (Fig. 4C). The phosphorylated and dephosphorylated kinetochores were then incubated with exogenous Mps1 (∼97 kD) that had been purified separately under high stringency conditions from asynchronously growing yeast cells (SI Appendix, Fig. S4B). We found that similar amounts of exogenous Mps1 bound to the kinetochores regardless of their phosphorylation state (Fig. 4D), indicating that kinetochore phosphorylation by Mps1 does not inhibit Mps1 from associating with kinetochores.
To test whether the affinity of Mps1 for kinetochores is reduced specifically by autophosphorylation, we generated autophosphorylated Mps1 and tested its binding to dephosphorylated kinetochores lacking endogenous Mps1 (Fig. 4C). To obtain autophosphorylated Mps1 (P-Mps1), we purified the native kinase and incubated it with ATP (SI Appendix, Fig. S4B). We prepared dephosphorylated kinetochores lacking endogenous Mps1 as described above by sequentially treating them with ATP to release Mps1 and then λ-phosphatase to remove phosphorylation (SI Appendix, Fig. S5). We incubated the kinetochores with the Mps1 that was either untreated or autophosphorylated. Strikingly, while native untreated Mps1 bound well to the kinetochores, the autophosphorylated P-Mps1 bound poorly (Fig. 4E and SI Appendix, Fig. S5). Only a minor subfraction bound to the kinetochores, and its electrophoretic mobility was faster than that of the major unbound fraction, indicating that the kinetochores selectively bound less-phosphorylated forms of Mps1. These results demonstrate that autophosphorylated forms of Mps1 have lower affinity for kinetochores and suggest that autophosphorylation underlies the release of Mps1 from yeast kinetochores.
Discussion
Mps1 initiates and sustains the spindle assembly checkpoint, so understanding the regulation of its activity is paramount to understanding checkpoint function. Together, the release of Mps1 from bioriented kinetochores in vivo (12, 14, 16) and the evidence that the duration of checkpoint signaling is correlated with Mps1 kinetochore localization (13–15) strongly suggest that controlling the binding and release of Mps1 to and from kinetochores is key to regulating the spindle assembly checkpoint. Here, by developing in vitro assays to monitor spindle assembly checkpoint protein colocalization with isolated yeast kinetochore particles, we show that native, kinetochore-bound Mps1 does not interfere with attachment of the kinetochores to microtubules in several different configurations, arguing against the direct competition mechanism for checkpoint silencing.
Given the dynamic association of Mps1 with kinetochores in cells, the stability of its association with isolated kinetochores in vitro is striking. Our kinetochore isolation procedure requires >60 min for the resuspension of cell lysate, anti-FLAG immunoprecipitation, washing away of free proteins, and then FLAG-peptide elution. Therefore, retention of Mps1 on 20% to 50% of the eluted particles implies a very low spontaneous release rate, roughly 1 to 3 h−1. In contrast, fluorescence recovery after photobleaching (FRAP) measurements indicate that turnover in vivo occurs much faster, at rates of 5 to 20 min−1 (12, 13). The slower turnover after isolation of the kinetochores is presumably caused, at least in part, by a lack of ATP, since treating the kinetochores with ATP triggers Mps1 release. This implies that fast turnover in vivo is likely an active, ATP-dependent process.
Indeed, prior work in human cells has shown that chemical inhibition of Mps1 causes its accumulation at kinetochores (13, 20–23, 35), and that phosphomimetic mutations in Mps1 reduce its levels at kinetochores (23). Taken together, these observations suggest that Mps1 autophosphorylation promotes its release from kinetochores. Our findings provide direct support for this view and reveal that Mps1 autorelease only requires components stably associated with kinetochores (and ATP). Autophosphorylation of Mps1 kinase could be a conserved mechanism for promoting its release from kinetochores. However, some evidence suggests that autophosphorylation of human Mps1 might promote its interaction with, rather than its release from, the core kinetochore component Ndc80 (17, 37). Given that Mps1 is heavily phosphorylated in mitotic cells, it seems possible that autophosphorylation of some sites might promote kinetochore localization while phosphorylation of others might promote release (7, 8, 13, 17, 18, 37–39). In the future, it will be crucial to identify the key autoregulatory phosphorylation sites to determine specifically how they affect Mps1 association with and release from kinetochores.
Two previous elegant studies have demonstrated that recombinant fragments of human Mps1 compete directly against Taxol-stabilized microtubules for binding to human Ndc80/Hec1 complex (17, 18). There are at least 2 possible explanations for the absence of such direct competition at the level of whole yeast kinetochore particles. First, checkpoint silencing might rely on direct competition in humans but not in yeast. While such an interspecies difference is formally possible, we do not favor this explanation, because the Ndc80 complex is a widely conserved microtubule-binding element that recruits Mps1 in both organisms. Another possibility is that Mps1 might interact with kinetochores in more than one way, and the native purified kinetochores might selectively retain only a noncompetitive fraction. Consistent with this possibility, multiple types of kinetochore interactions have been observed for human Mps1 (18, 35, 40, 41). While further work is needed to determine whether this is the case for yeast Mps1, our previous reconstitutions of key Mps1-dependent checkpoint-triggering phosphorylation events strongly suggest that the pool of Mps1 carried by the isolated yeast kinetochore particles is physiologically relevant (25, 26).
Our results lead us to speculate that molecular or physical events occurring when sister kinetochores make end-on bioriented attachments might stimulate Mps1 autophosphorylation and thus release. In support of this possibility, a previous study found that microtubules stimulate Mps1 kinase activity in vitro (42). Perhaps when a kinetochore binds a microtubule, interactions between the kinetochore-associated Mps1 and the microtubule might enable Mps1 autophosphorylation, lowering its affinity for Ndc80 and thereby causing its release from the kinetochore. Alternatively, conformational changes in the kinetochore (14) that occur when it attaches to a microtubule might directly promote Mps1 autophosphorylation. Recent work has suggested that Mps1 is prevented from reaching its checkpoint substrate KNL1/Spc105 when kinetochores bind to microtubules (14). Thus, conformational changes in the kinetochore caused by microtubule binding or tension could silence Mps1 checkpoint activity in 2 distinct ways; they might simultaneously block Mps1 activity toward checkpoint substrates and redirect its activity toward itself, triggering its own release from the kinetochore. Consistent with the idea of heightened Mps1 autoactivity during checkpoint silencing, FRAP data suggest that Mps1 is more dynamic on metaphase kinetochores compared with prometaphase kinetochores in mammalian cells (12). A model in which overall checkpoint activity is tuned by the phosphorylation state of Mps1 fits well with the kinetic evidence demonstrating that spindle assembly checkpoint signaling is graded rather than switch-like (reviewed in ref. 2). A graded regulation of Mps1 kinetochore localization, which favors a decrease in Mps1 affinity rather than its complete loss from kinetochores, might also facilitate rapid checkpoint reactivation should an attachment be lost. In the future, the in vitro assays that we have established here should be useful for further understanding how microtubule attachment affects the activity and kinetochore localization of Mps1 and other checkpoint proteins.
Methods
Native kinetochores were purified by immunoprecipitation from asynchronously growing yeast as described previously (27) with modifications to the lysis as described previously (43), and then labeled with fluorescent dyes (29). Native Mps1 was purified in essentially the same manner. For examining the levels of Mps1 on individual, unattached kinetochore particles, the fluorescent kinetochores were tethered sparsely to coverslips. For examining Mps1 levels on laterally attached kinetochore particles, the kinetochores were incubated with Taxol-stabilized microtubules that had been anchored to coverslips. To examine tip-attached kinetochores, kinetochore particles tethered sparsely to coverslips were incubated with short, Taxol-stabilized microtubules, which tended to attach to the coverslip-tethered kinetochores by their ends. To examine tip-tracking particles, dynamic microtubule extensions were grown from coverslip-anchored seeds in the presence of free kinetochores and then tubulin was removed by buffer exchange, to trigger microtubule disassembly. Kinetochores that had attached laterally to the microtubule extensions were captured and carried by the disassembling tips. For all single-particle experiments, images were recorded using an automated multicolor TIRF microscope (44) and analyzed using custom routines written in LabView (National Instruments) and Igor Pro (WaveMetrics). Additional details of the purification, fluorescent labeling, and imaging of the native kinetochores, along with descriptions of the kinase and bulk binding assays, are provided in SI Appendix, Methods.
Supplementary Material
Acknowledgments
We thank Geert Kops and Hongtao Yu for communicating results and useful discussions; Arshad Desai, Aaron Hoskins, Leon Chan, and Mark Winey for providing reagents; and Justin Kollman, John Tuthill, members of the Seattle Mitosis Group, and the C.L.A. and S.B. labs for helpful discussions and comments on the manuscript. L.B.K. was supported by an NSF graduate research fellowship (DGE-1256082). K.N.O. was supported by an NIH training fellowship (T32GM007270). N.L. was supported by an NIH interdisciplinary training grant (T32CA080416). This work was supported by Packard Fellowship 2006-30521 (to C.L.A.); by NIH Grants R01 GM079373 (to C.L.A.), P01 GM105537 (to C.L.A.), and R01 GM064386 (to S.B.); and by the Genomics and Scientific Imaging Shared Resources of the Fred Hutchinson/University of Washington Cancer Consortium (P30 CA015704). S.B. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901653116/-/DCSupplemental.
References
- 1.Gordon D. J., Resio B., Pellman D., Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet. 13, 189–203 (2012). [DOI] [PubMed] [Google Scholar]
- 2.London N., Biggins S., Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell Biol. 15, 736–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musacchio A., The molecular biology of spindle assembly checkpoint signaling dynamics. Curr. Biol. 25, R1002–R1018 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Rieder C. L., Maiato H., Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7, 637–651 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Kops G. J., Weaver B. A., Cleveland D. W., On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 5, 773–785 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Liu X., Winey M., The MPS1 family of protein kinases. Annu. Rev. Biochem. 81, 561–585 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S. T., et al. , Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores. Mol. Biol. Cell 14, 1638–1651 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palframan W. J., Meehl J. B., Jaspersen S. L., Winey M., Murray A. W., Anaphase inactivation of the spindle checkpoint. Science 313, 680–684 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Hardwick K. G., Weiss E., Luca F. C., Winey M., Murray A. W., Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273, 953–956 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Martin-Lluesma S., Stucke V. M., Nigg E. A., Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267–2270 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Kemmler S., et al. , Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 28, 1099–1110 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howell B. J., et al. , Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 14, 953–964 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Jelluma N., Dansen T. B., Sliedrecht T., Kwiatkowski N. P., Kops G. J., Release of Mps1 from kinetochores is crucial for timely anaphase onset. J. Cell Biol. 191, 281–290 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aravamudhan P., Goldfarb A. A., Joglekar A. P., The kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling. Nat. Cell Biol. 17, 868–879 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maciejowski J., et al. , Mps1 regulates kinetochore-microtubule attachment stability via the Ska complex to ensure error-free chromosome segregation. Dev. Cell 41, 143–156.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dou Z., et al. , Dynamic distribution of TTK in HeLa cells: Insights from an ultrastructural study. Cell Res. 13, 443–449 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Hiruma Y., et al. , Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 348, 1264–1267 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Ji Z., Gao H., Yu H., Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 348, 1260–1264 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Moura M., et al. , Protein phosphatase 1 inactivates Mps1 to ensure efficient spindle assembly checkpoint silencing. eLife 6, e25366 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewitt L., et al. , Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J. Cell Biol. 190, 25–34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiatkowski N., et al. , Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat. Chem. Biol. 6, 359–368 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sliedrecht T., Zhang C., Shokat K. M., Kops G. J., Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS One 5, e10251 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., et al. , Dynamic autophosphorylation of Mps1 kinase is required for faithful mitotic progression. PLoS One 9, e104723 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo R., et al. , Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res. 70, 10255–10264 (2010). [DOI] [PubMed] [Google Scholar]
- 25.London N., Biggins S., Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev. 28, 140–152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.London N., Ceto S., Ranish J. A., Biggins S., Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr. Biol. 22, 900–906 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyoshi B., et al. , Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468, 576–579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoskins A. A., et al. , Ordered and dynamic assembly of single spliceosomes. Science 331, 1289–1295 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarangapani K. K., et al. , Sister kinetochores are mechanically fused during meiosis I in yeast. Science 346, 248–251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyoshi B., Nelson C. R., Biggins S., The Aurora B kinase promotes inner and outer kinetochore interactions in budding yeast. Genetics 194, 785–789 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrova Y. N., Jenni S., Valverde R., Khin Y., Harrison S. C., Structure of the MIND complex defines a regulatory focus for yeast kinetochore assembly. Cell 167, 1014–1027.e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn J., Dumont S., Spindle assembly checkpoint satisfaction occurs via end-on but not lateral attachments under tension. J. Cell Biol. 216, 1533–1542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoué S., Salmon E. D., Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell 6, 1619–1640 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koshland D. E., Mitchison T. J., Kirschner M. W., Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature 331, 499–504 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Dou Z., et al. , Dynamic localization of Mps1 kinase to kinetochores is essential for accurate spindle microtubule attachment. Proc. Natl. Acad. Sci. U.S.A. 112, E4546–E4555 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santaguida S., Tighe A., D’Alise A. M., Taylor S. S., Musacchio A., Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 190, 73–87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Q., et al. , Regulation of kinetochore recruitment of two essential mitotic spindle checkpoint proteins by Mps1 phosphorylation. Mol. Biol. Cell 20, 10–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nijenhuis W., et al. , A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J. Cell Biol. 201, 217–231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morin V., et al. , CDK-dependent potentiation of MPS1 kinase activity is essential to the mitotic checkpoint. Curr. Biol. 22, 289–295 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Pachis S. T., Kops G. J. P. L., Leader of the SAC: Molecular mechanisms of Mps1/TTK regulation in mitosis. Open Biol. 8, 180109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pachis S. T., Hiruma Y., Tromer E. C., Perrakis A., Kops G., Interactions between N-terminal modules in MPS1 enable spindle checkpoint silencing. Cell Rep. 26, 2101–2112.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Stucke V. M., Baumann C., Nigg E. A., Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma 113, 1–15 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Miller M. P., Asbury C. L., Biggins S., A TOG protein confers tension sensitivity to kinetochore-microtubule attachments. Cell 165, 1428–1439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng Y., Asbury C. L., Simultaneous manipulation and super-resolution fluorescence imaging of individual kinetochores coupled to microtubule tips. Methods Mol. Biol. 1486, 437–467 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.