Abstract
Effective combination antiretroviral therapy (ART) has enabled human immunodeficiency virus (HIV) infection to evolve from a generally fatal condition to a manageable chronic disease. This transition began two decades ago in high-income countries, and has more recently begun in lower income, HIV endemic countries (HIV-ECs). With this transition, has come a concurrent shift in clinical and public health burden from AIDS-related complications and opportunistic infections, to those associated with well-controlled HIV disease, including cardiovascular disease (CVD). In the current treatment era, traditional CVD risk factors and HIV-related factors both contribute to an elevated risk of myocardial infarction, stroke, heart failure, and arrhythmias. In HIV-ECs, the high prevalence of persons living with HIV and growing prevalence of CVD risk factors will contribute to a growing epidemic of HIV-associated CVD. In this review, we discuss the epidemiology and pathophysiology of cardiovascular complications of HIV and the resultant implications for public health efforts in HIV-ECs..
Introduction
Over the past two decades, increased availability of antiretroviral therapy (ART) has allowed HIV to transition from a generally fatal disease into a chronic and manageable disease. Whereas only 2% of persons living with HIV (PLWH) worldwide were taking ART in 2001, 40% of PLWH (15 million total persons) were on ART by 2015. [1]. Much of this increase has been driven by improved ART availability in HIV-endemic areas of sub-Saharan Africa (SSA); however, other HIV endemic countries (HIV-EC) have witnessed substantial increases in access to ART among PLWH. For example, ART coverage increased in Thailand from 8% to 72% over 10 years and in Cambodia from almost 0% to 70% over a 13-year period [2–5]. As longevity has improved for PLWH due to effective and available ART, PLWH are now experiencing chronic non-communicable diseases such as cardiovascular diseases (CVDs), for which PLWH are at elevated risk compared with uninfected persons [1, 6–12]. This residual risk for CVD has been attributed in part to traditional CVD risk factors and in part to HIV-associated chronic inflammation and immune activation.
The content of this review is summarized in Table 1. In the first part, we discuss the general epidemiology and pathophysiology of CVD in PLHIV, and in the second part, we focus specifically on HIV-ECs. We define HIV-ECs as those countries where the epidemic is generalized throughout the entire population. More specifically, HIV-ECs are countries with HIV prevalence >1.0% where at least 50% of cases are due to heterosexual transmission and the ratio of male to female cases is less than two..[13] Broadly, this definition includes most countries in SSA plus several countries in the Caribbean and Central/South America. In Asia, only three countries – Cambodia, Myanmar and Thailand—fit this definition.
Table:
Manuscript overview by section with key points
| Part I: Cardiovascular Complications of HIV/AIDS | |
|---|---|
| Epidemiology | |
|
|
| Pathophysiology | |
| Atherosclerotic CVD (ASCVD): Traditional Risk Factors |
|
| ASCVD: HIV-Related Factors |
|
| Non-Atherosclerotic CVD |
|
| Part II: HIV-Associated CVD in HIV Endemic Countries (HIV-ECs) | |
| Patterns of Cardiovascular Morbidity and Mortality in HIV-ECs |
|
| Traditional CVD Risk Factors in HIV-ECs |
|
| Unique CVD Risk Considerations among PLWH in HIV-ECs |
|
|
Preparing for an Increased Burden of Cardiovascular Risk in HIV-Endemic Countries
in the Modern ART Era | |
| CVD Risk Stratification Tools |
|
| Health Systems Strengthening |
|
Cardiovascular Complications of HIV/AIDS
Epidemiology of CVD among PLWH
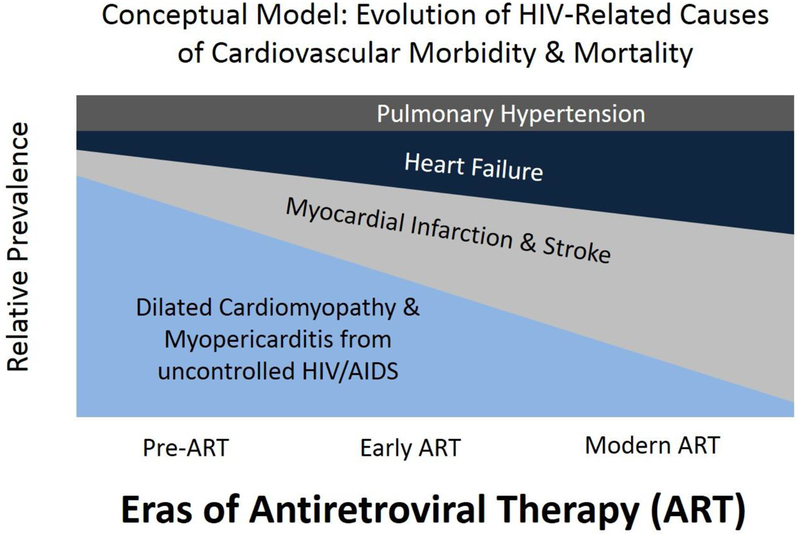
Many studies have demonstrated that PLWH are at increased risk of various manifestations of CVD compared with uninfected persons.[6] Figure 1 is a conceptual model depicting the transition in relative prevalence of HIV-associated CVD complications from the pre-ART era through the present; as access to ART has become more widespread, CVD-related complications of advanced HIV/AIDS (particularly HIV/AIDS cardiomyopathy) have become less common and been largely replaced by CVD manifestations found commonly in the general population. However, PLHIV appear to bear a substantially greater burden of common CVD manifestations – including myocardial infarction (MI), stroke, and heart failure, and arrhythmias – compared to uninfected persons.
Figure 1. Conceptual Model: Evolution of HIV-Related Causes of Cardiovascular Morbidity and Mortality.
Dilated cardiomyopathy and myopericarditis were common complications of advanced HIV/AIDS in the pre-ART era. As PLHIV are living longer due to ART, they are increasingly at risk for more common cardiovascular complications, such as myocardial infarction, stroke, and heart failure (including heart failure with preserved ejection fraction). These complications account for an increasing proportion of HIV-related cardiovascular disease.
Rates of myocardial infarction (MI) appear to be consistently higher for PLWH than uninfected persons even after adjustment for demographics and relevant clinical covariates. In an analysis of 82,459 veterans (33% HIV+) from the Veterans Aging Cohort Study (VACS), HIV-infected veterans had 1.48 times [95% confidence interval (CI) 1.27–1.72] greater risk for incident MI compared with uninfected veterans after adjustment for cardiovascular risk factors, clinical comorbidities, and substance use. [7] This elevated risk for MI persisted among HIV-infected veterans with successful viral control (HIV-1 viral load <500 copies/mL), who had adjusted hazards ratios for incident MI of 1.39 (95% CI 1.17–1.60) compared with uninfected veterans. [7] Among VACS participants with no major cardiovascular risk factors, HIV-infected veterans had a two-fold (HR 2.0, 95% CI 1.0–3.9) increased risk for MI compared with uninfected veterans. [11] Similarly, an analysis of 3,851 PLHIV and 1,044,589 uninfected patients in the Partners HealthCare System (Boston, MA) found that hazards ratios for incident MI for HIV-infected versus uninfected (adjusted for demographics, hypertension, diabetes, and dyslipidemia) were 1.40 (95% CI 1.16–1.67) for men and 2.98 (95% CI 2.33–3.75) for women, respectively. [9]
Heart failure also appears to be particularly common among PLWH. Prior to the widespread use of ART, the primary recognized HIV-associated manifestation of heart failure was nonischemic cardiomyopathy, which was generally described as severe and symptomatic systolic dysfunction in the setting of advanced HIV/AIDS. [14–16] In a study of 8486 veterans (28.6% HIV-infected) followed for a median of 7.3 years, HIV-infected veterans had significantly greater risks for incident heart failure (HR 1.81, 95% CI 1.39–2.36) after adjustment for demographics and relevant clinical covariates. [17] Heart failure risk was particularly high among HIV-infected veterans with poor viral control; HIV-infected veterans with baseline HIV viral load ≥500 copies/mL had 2.28-fold greater risk for incident HF (95% CI 1.57–3.32) compared with uninfected veterans. [17] The increased risk of heart failure associated with HIV persists when ischemic etiology and alcohol abuse are excluded[17], is similar for preserved vs. reduced ejection fraction[18], and appears to be greater in women than in men[19]. Several cohort studies from the ART era have also found relatively high rates of systolic and diastolic dysfunction (often asymptomatic) among PLWH. a meta-analyses of several of these cohort studies that included 2242 PLWH from 11 studies primarily from North America and Europe found a 8.3% prevalence of systolic dysfunction and 43.3% prevalence of diastolic dysfunction. [20–24]
Other CVD manifestations might also be more common among PLWH. Pulmonary hypertension was identified in the pre-ART era as being substantially more common among PLWH than uninfected persons. [25, 26] Subsequent analyses from the modern ART era have also demonstrated an elevated prevalence of pulmonary hypertension among PLWH, though potential mechanisms of HIV-associated pulmonary hypertension remain under debate. [27–31] Ischemic stroke also appears to be more common in HIV infection. A recent analysis from the Partners HealthCare System found significantly greater risks for stroke among PLHIV versus uninfected persons after adjustment for demographics and stroke risk factors (HR 1.21, 95% CI 1.01–1.46). [10] HIV may also be associated with arrhythmias. Atrial fibrillation was significantly more common among PLWH with uncontrolled HIV (HIV viral load >100,000 copies/mL vs. <500 copies/mL: HR 1.4, 95% CI 1.1–1.8) and severe immune dysfunction (CD4+ cell count <200 cells/mm3 vs. >350 cells/mm3: HR 1.7, 95% CI 1.2–2.4) in the VACS. [32] Additionally, an analysis of data from a public HIV clinic in San Francisco found that rates of sudden cardiac death for PLWH were 4.5-fold greater than expected based on region and demographic composition of the cohort. [12]
While the evidence for heightened CVD risk in HIV is strong, we acknowledge that trends toward early initiation of ART may result in lower CVD risk among PLHIV over time. There also is emerging evidence that aggressively managing CVD risk among HIV-infected patients may lead to declining rates of CVD events. For example, in the Northern and Southern California Kaiser Permanente system, there has been a declining relative risk for MI among HIV+ individuals with access to care which may be partly attributable to awareness raising campaigns among primary providers. [33] Furthermore, HIV-infected patients who are engaged in care may be more likely to receive preventive services during mid-life (ages 30–60) compared to a demographically similar HIV-uninfected population.
Pathophysiology of CVD among PLWH
Atherosclerotic CVD: Traditional CVD risk factors associated with HIV
The higher CVD risk observed in PLHIV is partially mediated by traditional risk factors. Dyslipidemia is quite common in the setting of untreated and treated HIV. Patients with uncontrolled HIV/AIDS generally have a characteristic lipid phenotype of high triglycerides, low high density lipoprotein cholesterol (HDL-c), and low low-density lipoprotein cholesterol (LDL-c). [34–36] Subsequently, once ART is initiated, LDL-c and total cholesterol tend to rise and peak approximately two years after ART initiation. [37–39] In particular, protease inhibitors (particularly early-generation protease inhibitors) have been linked to hypertriglyceridemia, hyperlipidemia, and metabolic syndrome, and as many as 80% of PLWH taking protease inhibitors experience elevation in plasma lipid concentrations. [37, 40–43]. For this and other reasons related to tolerability, protease inhibitor-based regimens have largely been replaced by integrase inhibitor based regimens as the first-line standard of care in resource-rich settings.[44] Unboosted integrase inhibitors have more favorable effects on lipids compared to current generation protease inhibitors.[45] There is substantial heterogeneity in the lipid-altering effects of nucleoside reverse transcriptase inhibitors (NRTIs). For instance, tenofovir is associated with lower levels of total cholesterol, LDL-c, and triglycerides compared with other NRTIs. [46] In addition to dyslipidemia, cigarette smoking is also particularly common among some populations of PLWH, and associated with a significant excess in CVD as well as all-cause mortality; [47] a recent study in a Danish cohort found that the population-attributable risk of death associated with smoking is twice that for PLHIV compared with the uninfected population. [48] Finally, blood pressure is typically lower during untreated HIV infection but rises after ART initiation, and long-term ART use is associated with higher rates of hypertension compared to uninfected controls[49]. Many of the most recent studies on blood pressure changes with ART have been performed in HIV-ECs and are discussed below.
Atherosclerotic CVD: HIV-related factors
In addition to traditional risk factors, there are HIV-specific factors that increase CVD risk. Although studies from the pre-modern ART era raised the concern that some ARTs may be associated with risk of MI, [50–52] it has become increasingly apparent that HIV-related factors such as viral replication, inflammation, and immunodeficiency drive atherosclerotic CVD risk. This may be due, in part, to fewer cardiovascular side effects of contemporary ART. However, several studies over the past decade demonstrated that early initiation of ART reduces all-cause mortality and appears to decrease inflammation, improve endothelial function, and curtail progression of carotid intima-media thickness [53–56] Nevertheless, even with successful viral suppression through effective ART or elite controller status (no detectable viral load or decrease in CD4 count despite not taking ART), PLHIV have substantial residual inflammation and atherosclerosis. [54, 57–59] A likely mechanism for this residual risk is that low-level viral replication persists in HIV reservoirs even when HIV viremia is suppressed, thereby driving chronic HIV-related inflammation. [60]
Chronic immune activation – particularly T-cell activation – and inflammation occur even in treated HIV [61–63] and are associated with atherosclerosis, endothelial dysfunction, and adverse cardiovascular events. PLHIV have significantly elevated levels of C-reactive protein (CRP, a general inflammatory biomarker) compared with uninfected persons; among PLWH, greater CRP (or high-sensitivity CRP: hsCRP) is associated with significantly greater atherosclerosis and MI risk. [64, 65] Interleukin 6 (IL-6), an inflammatory cytokine strongly associated with coronary heart disease risk, [66] is significantly elevated among PLWH, as are D-dimer (a coagulation marker associated with thrombosis [67]) and cystatin C (a marker of kidney impairment for which elevated levels have been associated with CVD). [68, 69] Notably, IL-6 and D-dimer are strongly associated with all-cause mortality in the setting of HIV. [70] PLHIV also have elevated levels of monocyte and macrophage activation markers – such as soluble CD163 and soluble CD14, which are associated with arterial inflammation and coronary plaque among PLWH [57, 71] – as well as monocyte chemoattractant protein 1 (MCP-1), which is associated with greater coronary atherosclerosis among PLWH. [72] In light of this chronic inflammation and immune activation, it is perhaps unsurprising that PLHIV are at elevated risk for coronary and carotid atherosclerosis [58, 73–75] as well as atherosclerotic events such as MI. [7, 9] Greater HIV viral load and lower CD4+ T-cell counts are associated with inflammation, endothelial dysfunction, and atherosclerosis, [54, 76–78] and initiation of ART decreases levels of circulating inflammatory biomarkers and improves endothelial function. [53, 54, 79]
Non-Atherosclerotic Cardiovascular Complications of HIV
PLHIV are at elevated risk for several non-atherosclerotic forms of CVD that occur through various mechanisms. Pulmonary arterial hypertension is a long-recognized complication of HIV, as discussed above, which appears to be driven by several potential mechanisms, including HIV-related endothelial dysfunction, chronic immune activation, co-infection, and potentially other traditional CVD risk factors. [27–29, 80, 81] There are several proposed mechanisms underlying the pathogenesis of systolic and diastolic heart failure in HIV. These mechanisms include HIV-specific factors (such as viral replication, immune activation, and chronic inflammation), environmental/behavioral factors (such as smoking, substance/alcohol use, and poor nutrition), ART-associated dyslipidemia and metabolic disturbances, and co-infection with other viruses and opportunistic infections. [17, 22, 24, 82–86] Atrial fibrillation may also be particularly common among HIV-infected persons and appears to associate with HIV disease severity, but mechanisms underlying this potential link are unclear. [32] Future investigations into potential HIV-related mechanisms of heart failure and arrhythmia – such as myocardial scar and epicardial adipose tissue accumulation [24, 87, 88] – may help clarify these questions.
HIV-Associated CVD in HIV-ECs
HIV-ECs carry a heavy burden of HIV and its sequelae. The majority of HIV-ECs are located in SSA; 69% of all PLWH in the world live on the African continent. [86] In HIV-ECs, PLWH have a disproportionately higher incidence of heart failure and other CVD than their uninfected counterparts. [89, 90] As discussed above, the burden of CVD has begun to shift from primarily complications of uncontrolled viremia and opportunistic infections (HIV/AIDS cardiomyopathy) to chronic CVDs brought about by cardiometabolic disturbances, chronic immune activation and inflammation, and traditional CVD risk factors. [86] Greater access to ART in HIV-ECs has dramatically improved longevity among PLWH, who are increasingly at risk for morbidity and mortality from non-communicable diseases such as CVD. [91, 92] Although CVD prevalence is growing for the aging population of PLWH in HIV-ECs, [93] many of these countries do not have the infrastructure in place to adequately address and manage this double burden of disease [94]. The remainder of this review article discusses studies that have evaluated cardiovascular complications of HIV in HIV-ECs.
Patterns of Cardiovascular Morbidity and Mortality in HIV-Endemic Countries
As communicable diseases such as HIV are controlled in HIV-ECs, these countries are now experiencing a double burden of both endemic and newly emerging causes of CVD. Although the majority of CVD in SSA remains secondary to non-atherosclerotic causes, ischemic heart disease is on the rise. [86, 95] In 2002, THESUS-HF studied 1006 patients with HF across 9 African countries and found that as a group, emerging causes [hypertensive (45.4%), ischemic (7.7%), and other emerging (3.4%)] were slightly more common than endemic causes [idiopathic dilated cardiomyopathy (18.8%), rheumatic heart disease (14.3%), peripartum cardiomyopathy (7.7%), pericardial disease (6.8%), other endemic (4.0%), HIV cardiomyopathy (2.6%), and endomyocardial fibrosis (1.3%)]. Another recent study [96] found that heart failure is more than twice as likely to be caused by ischemic etiologies than by uncontrolled HIV in rural SSA – a dramatic change from the early 2000s, when end-stage HIV/AIDS was responsible for a substantial proportion of heart failure in the region. [86, 95, 96] Interestingly, the Heart of Soweto study found that coronary artery disease was less prevalent for PLWH compared with uninfected participants (2.4% vs. 12.0%; p-value<0.001). [97]
Cardiovascular diseases are not only frequent, but also appear to occur earlier in HIV-ECs. Cardiovascular diseases tend to occur roughly a decade earlier in HIV-ECs such as Cambodia and Thailand, [98] Caribbean, [99] and SSA [96, 100, 101] than in developed countries, and that the majority of CVD in SSA occurs between the ages of 30 and 69. [86, 95, 96, 102] Etiologies of cardiomyopathy also vary considerably by age. For instance, in THESUS-HF, there was a mean age difference of 15 years (mean age 45 vs. 60) for patients with endemic CVD (e.g., HIV/AIDS cardiomyopathy or rheumatic heart disease) versus emerging CVD (e.g., ischemic or hypertensive heart disease). [95]
Traditional Cardiovascular Risk Factors in HIV-Endemic Countries
Lifestyle changes due to urbanization and industrialization in recent decades in low and middle income countries (LMICs), including HIV-ECs, have contributed to an increasing prevalence of obesity, diabetes, hypertension, and dyslipidemia in these regions. [103–106] While prevalence of hypertension has historically been lower in HIV-ECs than high-income countries, the prevalence of hypertension is rising and thought to be as high as 46% for adults in certain areas of Africa. [107–109] In the Caribbean, the overall prevalence of hypertension is estimated at 30–40% and as high as 50% in Jamaica; [110], this has been attributed to increasing obesity, physical inactivity, and high prevalence of tobacco and alcohol use. [111–113] In Cambodia, the prevalence of HTN is much lower but rising (recently estimated at 12.3%). [114] With increasing globalization and the adoption of “Western lifestyles” in HIV-ECs, tobacco and alcohol use, sedentary lifestyles, and atherogenic fast food consumption are becoming more common, thus contributing to the increasing burden of CVD in these countries. [105, 114]
Unique CVD Risk Considerations among PLHIV in HIV-Endemic Countries
In addition to traditional CVD risk factors, HIV-related factors may contribute to CVD in HIV-ECs. As discussed in detail above, HIV-related inflammation and immune dysfunction are implicated in endothelial dysfunction, atherosclerosis, and overt CVD even in the setting of well-controlled HIV. A meta-analysis in 2013 by Dillon et al. noted that HIV was associated with lower BMI, lower systolic and diastolic BP, lower HDL levels, and higher TG levels. [95] Many studies included in this analysis failed to adequately distinguish between PLHIV who were well controlled on ART and those with more advanced immunosuppression and AIDS. Advanced AIDS is typically associated with wasting, lower blood pressure, and lower lipid levels. For a substantial proportion of HIV-infected persons in HIV-ECs still without access to ART and/or with uncontrolled HIV, known complications associated with later stage disease such as HIV/AIDS cardiomyopathy remain relevant. [50, 52, 55] Fortunately, use of metabolically toxic first generation protease inhibitors and older NRTIs (such as stavudine or zidovudine) is absent or declining in HIV-ECs; however, access to newer integrase-inhibitors remains limited.
Initiation of ART – which is increasingly common for PLWH in HIV-ECs as a result of increased access to HIV testing and linkage to care – is associated with obesity, hypertension, metabolic syndrome, and diabetes. [49, 107, 115–119] Many epidemiologic studies of blood pressure in HIV-ECs suggest that HIV infection is generally associated with lower blood pressure [118, 120–123]. Similar to resource-rich settings, ART initiation is associated with increased BP and the development of hypertension is not uncommon. [124, 125] A study of PLHIV in Kenya revealed important gender differences, with hypertension prevalence of 7% and 11% among women and men, respectively.[126]. The pathophysiology underlying the cause of increased hypertension with ART is still unknown, although various mechanisms have been proposed, such as a “return to health” phenomenon or chronic residual inflammation and direct vascular remodeling. In order to evaluate this question, a study evaluated 500 participants in SSA who recently started ART and found that blood pressure increased six months after initiation of ART and was associated with increased weight gain, but not HIV viral load. [124] Importantly, there was also a reduction in inflammatory biomarkers (including IL-6, sCD14, and D-dimer) six months after initiation of therapy. [124, 127] A propensity matched analysis of PLWH exposed and naïve to ART estimates the average treatment effect on those treated with ART to be an increase of 8 mmHg in systolic and 7 mmHg in diastolic blood pressure. [125] Further studies are needed to better elucidate the influence of active viremia and new-generation ART on the development and progression of CVD in HIV-ECs.
Preparing for an Increased Burden of Cardiovascular Risk in HIV-Endemic Countries in the Modern ART Era
As the burden of HIV-related cardiovascular complications increases in HIV-ECs, it is necessary to develop strategies to predict, prevent, and treat CVD in these regions. Here we discuss potential approaches to CVD risk stratification, prevention, and treatment in HIV-ECs.
Detection of Clinically Significant CVD Disease
Most available risk prediction tools for CVD used in high-income countries require laboratory-related measures, which may be too costly to be realistically applied throughout HIV-ECs at this juncture. [128] Therefore, non-laboratory-based methods to stratify CVD risk and identify persons who would derive the greatest benefit from CVD prevention programs and pharmacological intervention may represent a reasonable approach in HIV-ECs. Studies comparing CVD risk stratification tools that include laboratory-based measures (such as the Framingham Risk Score or the 2013 American College of Cardiology/American Heart Association ASCVD Risk Estimator) versus non-laboratory-based tools have demonstrated fairly similar performance between the laboratory-based and non-laboratory based tools. Although studies in South African [129, 130] and Thai [131] cohorts have demonstrated strong agreement between laboratory-based and non-laboratory-based CVD risk estimation tools, whether these findings are generalizable across HIV-ECs remains unknown. HIV-specific risk calculators such as the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study equation exist, but their discrimination and calibration are not well validated, there is poor agreement with other risk models, and they require laboratory measures[132, 133]. Perhaps more importantly, given the cultural heterogeneity and diversity of medical-social milieus across HIV-ECs, further research is needed to evaluate whether the use of risk estimation tools for screening in HIV-ECs will improve clinical outcomes. Community health workers could be integral to such a strategy[134].
Health Systems Strengthening
Currently, interventions at both the individual and the population level in HIV-ECs are scarce. Clinics are not equipped with trained professionals or the diagnostic tools to evaluate patients for CVD. [93, 94] Infrastructure in resource-poor settings has not been able to keep up with the growing burden of cardiovascular risk factors – particularly hypertension and diabetes – in HIV-ECs. Patients hospitalized with serious complications of diabetes – such as diabetic ketoacidosis – are often discharged with days to weeks’ worth of medication but no available follow-up or chronic care. [116] Yet, a recent systematic review would suggest that CVD care quality can be substantially improved with interventions that target the patient/provider level and the health systems in low- and middle-income countries. [135]
One opportunity to improve CVD care in HIV-ECs is to leverage existing HIV/AIDS infrastructure. HIV-ECs have made considerable progress in creating sustainable clinics and healthcare systems to provide HIV patients with ART and continuing care. Ideally, screening of CVD and its risk factors would be integrated into chronic HIV care to provide comprehensive care to patients at highest risk and then expand to incorporate non-HIV-infected patients as well [101, 136–138] Projects addressing these concerns are already underway. In 2002, Medecins San Frontieres (MSF) and the Cambodian Ministry of Health established chronic disease clinics that integrated HIV/AIDS care with diabetes and HTN management in hopes to provide better access to NCD management.[116] Retention rate for patients with diabetes was 70%. The project demonstrated the effectiveness of utilizing integrated clinic models that use effective strategies learned from the HIV program model in HIV-EC to address the growing burden of CVD across the world. The Academic Model Providing Access to Healthcare (AMPATH) program in Western Kenya has similarly used HIV counselors to screen for diabetes and hypertension in patients’ homes and with community-based screening.[139] More study is needed to evaluate the impact of strong HIV/AIDS care systems on non-AIDS outcomes in SSA.
Conclusions
CVD is emerging as a leading cause of morbidity and mortality among PLHIV throughout the world, particularly in HIV-ECs. Yet, very little of the research on CVD in PLHIV has been conducted in HIV-ECs. One advantage of conducting research on cardiovascular complications of HIV in HIV-ECs is the relative ease with which observational studies of well-matched uninfected controls may be performed. Demographics and other CVD risk factors may be more evenly distributed among PLWH and uninfected persons in HIV-ECs than in non-endemic countries (such as the United States), thus limiting the potential for unmeasured confounding. Furthermore, relatively larger numbers of HIV-infected women in HIV-ECs (over half of the 26 million PLWH in SSA are women[140]) allows for well-powered research on sex differences in HIV and CVD.
Future work should also focus on adapting public health policy to address the growing CVD burden in HIV-ECs. Public health interventions could provide education in schools, rural areas (likely through the use of community health workers), and integrated clinic care models that can provide both treatment and preventive interventions to the community. To succeed, these efforts must also be accompanied by substantial investment in clinical infrastructure, human resources, diagnostics, and therapeutics that are locally relevant and scalable. Ultimately, if stakeholders can expand upon the successes of HIV treatment and prevention programs in HIV-ECs and direct resources and infrastructure to prevention and treatment of chronic complications of HIV, it may be possible to curb the growing burden of chronic diseases – including CVD – in these countries.
REFERENCES
- 1.World Health Organization. Global Health Observatory Data: Antiretroviral therapy (ART) coverage among all age groups. In; 2015.
- 2.Kazooba P, Kasamba I, Baisley K, Mayanja BN, Maher D. Access to, and uptake of, antiretroviral therapy in a developing country with high HIV prevalence: a population-based cohort study in rural Uganda, 2004–2008. Trop Med Int Health 2012,17:e49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral therapy coverage in sub-Saharan Africa. In; 2016.
- 4.The World Bank Group. Antiretroviral therapy coverage (% of people living with HIV). 2016.
- 5.Himakalasa W, Grisurapong S, Phuangsaichai S. Access to antiretroviral therapy among HIV/AIDS patients in Chiang Mai province, Thailand. HIV AIDS (Auckl) 2013,5:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palella FJ Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006,43:27–34. [DOI] [PubMed] [Google Scholar]
- 7.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013,173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr 2009,51:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007,92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012,60:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV Infection, Cardiovascular Disease Risk Factor Profile, and Risk for Acute Myocardial Infarction. J Acquir Immune Defic Syndr 2015,68:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012,59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canada PHAo. HIV/AIDS Epi Updates. Chapter 13: HIV/AIDS in Canada among people from countries where HIV is endemic. In; 2012.
- 14.De Castro S, d’Amati G, Gallo P, Cartoni D, Santopadre P, Vullo V, et al. Frequency of development of acute global left ventricular dysfunction in human immunodeficiency virus infection. J Am Coll Cardiol 1994,24:1018–1024. [DOI] [PubMed] [Google Scholar]
- 15.Herskowitz A, Vlahov D, Willoughby S, Chaisson RE, Schulman SP, Neumann DA, et al. Prevalence and incidence of left ventricular dysfunction in patients with human immunodeficiency virus infection. Am J Cardiol 1993,71:955–958. [DOI] [PubMed] [Google Scholar]
- 16.Levy WS, Simon GL, Rios JC, Ross AM. Prevalence of cardiac abnormalities in human immunodeficiency virus infection. Am J Cardiol 1989,63:86–89. [DOI] [PubMed] [Google Scholar]
- 17.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011,171:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freiberg M, Chang CC, Oursler KA, Gottdiener J, Gottlieb S, Warner A, et al. The Risk of and Survival with Preserved vs. Reduced Ejection Fraction Heart Failure by HIV Status In: 20th Conference on Retroviruses and Opportunistic Infections. Atlanta, GA, USA; 2013. [Google Scholar]
- 19.Al-Kindi SG, ElAmm C, Ginwalla M, Mehanna E, Zacharias M, Benatti R, et al. Heart failure in patients with human immunodeficiency virus infection: Epidemiology and management disparities. Int J Cardiol 2016,218:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaylock JM, Byers DK, Gibbs BT, Nayak G, Ferguson M, Tribble DR, et al. Longitudinal assessment of cardiac diastolic function in HIV-infected patients. Int J STD AIDS 2012,23:105–110. [DOI] [PubMed] [Google Scholar]
- 21.Cerrato E, D’Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J 2013,34:1432–1436. [DOI] [PubMed] [Google Scholar]
- 22.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail 2010,3:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, et al. High Prevalence of Echocardiographic Abnormalities among HIV-infected Persons in the Era of Highly Active Antiretroviral Therapy. Clin Infect Dis 2011,52:378–386. [DOI] [PubMed] [Google Scholar]
- 24.Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis 2015,212:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opravil M, Pechere M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 1997,155:990–995. [DOI] [PubMed] [Google Scholar]
- 26.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 1991,100:1268–1271. [DOI] [PubMed] [Google Scholar]
- 27.Almodovar S, Knight R, Allshouse AA, Roemer S, Lozupone C, McDonald D, et al. Human Immunodeficiency Virus nef signature sequences are associated with pulmonary hypertension. AIDS Res Hum Retroviruses 2012,28:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnett CF, Hsue PY. Human immunodeficiency virus-associated pulmonary arterial hypertension. Clin Chest Med 2013,34:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS 2008,22:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008,177:108–113. [DOI] [PubMed] [Google Scholar]
- 31.Zuber JP, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, et al. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis 2004,38:1178–1185. [DOI] [PubMed] [Google Scholar]
- 32.Hsu JC, Li Y, Marcus GM, Hsue PY, Scherzer R, Grunfeld C, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol 2013,61:2288–2295. [DOI] [PubMed] [Google Scholar]
- 33.Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015,60:1278–1280. [DOI] [PubMed] [Google Scholar]
- 34.Feingold KR, Krauss RM, Pang M, Doerrler W, Jensen P, Grunfeld C. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low density lipoprotein subclass pattern B. J Clin Endocrinol Metab 1993,76:1423–1427. [DOI] [PubMed] [Google Scholar]
- 35.Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med 1989,86:27–31. [DOI] [PubMed] [Google Scholar]
- 36.Grunfeld C, Kotler DP, Shigenaga JK, Doerrler W, Tierney A, Wang J, et al. Circulating interferon-alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med 1991,90:154–162. [PubMed] [Google Scholar]
- 37.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999,353:2093–2099. [DOI] [PubMed] [Google Scholar]
- 38.Riddler SA, Li X, Chu H, Kingsley LA, Dobs A, Evans R, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med 2007,8:280–287. [DOI] [PubMed] [Google Scholar]
- 39.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS 2007,21:1137–1145. [DOI] [PubMed] [Google Scholar]
- 40.Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, Korner T, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS 1999,13:F63–70. [DOI] [PubMed] [Google Scholar]
- 41.Calza L, Manfredi R, Chiodo F. Hyperlipidaemia in patients with HIV-1 infection receiving highly active antiretroviral therapy: epidemiology, pathogenesis, clinical course and management. Int J Antimicrob Agents 2003,22:89–99. [DOI] [PubMed] [Google Scholar]
- 42.Distler O, Cooper DA, Deckelbaum RJ, Sturley SL. Hyperlipidemia and inhibitors of HIV protease. Curr Opin Clin Nutr Metab Care 2001,4:99–103. [DOI] [PubMed] [Google Scholar]
- 43.Mulligan K, Grunfeld C, Tai VW, Algren H, Pang M, Chernoff DN, et al. Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection. J Acquir Immune Defic Syndr 2000,23:35–43. [DOI] [PubMed] [Google Scholar]
- 44.Services USDoHaH. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. In; 2015.
- 45.Ofotokun I, Na LH, Landovitz RJ, Ribaudo HJ, McComsey GA, Godfrey C, et al. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin Infect Dis 2015,60:1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crane HM, Grunfeld C, Willig JH, Mugavero MJ, Van Rompaey S, Moore R, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS 2011,25:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petoumenos K, Worm S, Reiss P, de Wit S, d’Arminio Monforte A, Sabin C, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*). HIV Med 2011,12:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis 2013,56:727–734. [DOI] [PubMed] [Google Scholar]
- 49.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005,19:953–960. [DOI] [PubMed] [Google Scholar]
- 50.Sabin CA. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D : A : D study: a multi-cohort collaboration (vol 371, pg 1417, 2008). Lancet 2008,372:292–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundgren JD, Neuhaus J, Babiker A, Cooper D, Duprez D, Ei-Sadr W, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. Aids 2008,22:F17–F24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003,349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 53.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr 2011,56:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho JE, Deeks SG, Hecht FM, Xie Y, Schnell A, Martin JN, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS 2010,24:1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strategies for Management of Antiretroviral Therapy Study G, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006,355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 56.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dube MP, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008,52:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011,204:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010,24:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009,23:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatano H, Strain MC, Scherzer R, Bacchetti P, Wentworth D, Hoh R, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J Infect Dis 2013,208:1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009,338:a3172. [DOI] [PubMed] [Google Scholar]
- 62.Hunt PW, Landay AL, Sinclair E, Martinson JA, Hatano H, Emu B, et al. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One 2011,6:e15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003,187:1534–1543. [DOI] [PubMed] [Google Scholar]
- 64.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, et al. Carotid Intima-Media Thickness Progression in HIV-Infected Adults Occurs Preferentially at the Carotid Bifurcation and Is Predicted by Inflammation. J Am Heart Assoc 2012,1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009,51:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med 2008,5:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabrhel C, Mark Courtney D, Camargo CA Jr., Plewa MC, Nordenholz KE, Moore CL, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med 2010,17:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neuhaus J, Jacobs DR Jr., Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010,201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010,55:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008,5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA 2012,308:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr., Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015,211:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS 2009,23:1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsue PY, Ordovas K, Lee T, Reddy G, Gotway M, Schnell A, et al. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am J Cardiol 2012,109:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Post WS, Budoff M, Kingsley L, Palella FJ Jr., Witt MD, Li X, et al. Associations Between HIV Infection and Subclinical Coronary Atherosclerosis. Ann Intern Med 2014,160:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho JE, Scherzer R, Hecht FM, Maka K, Selby V, Martin JN, et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS 2012,26:1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis 2011,53:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation 2004,109:316–319. [DOI] [PubMed] [Google Scholar]
- 79.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Melbourne K, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS 2012,26:1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC, Lung HIVS. Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc 2011,8:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension: an increasingly recognized complication of hereditary hemolytic anemias and HIV infection. JAMA 2008,299:324–331. [DOI] [PubMed] [Google Scholar]
- 82.Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 2015,116:354–367. [DOI] [PubMed] [Google Scholar]
- 83.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015,2:e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 1995,91:161–170. [DOI] [PubMed] [Google Scholar]
- 85.White JR, Chang CC, So-Armah KA, Stewart JC, Gupta SK, Butt AA, et al. Depression and Human Immunodeficiency Virus Infection Are Risk Factors for Incident Heart Failure Among Veterans: Veterans Aging Cohort Study. Circulation 2015,132:1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloomfield GS, Alenezi F, Barasa FA, Lumsden R, Mayosi BM, Velazquez EJ. Human Immunodeficiency Virus and Heart Failure in Low- and Middle-Income Countries. JACC Heart Fail 2015,3:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013,128:814–822. [DOI] [PubMed] [Google Scholar]
- 88.Iacobellis G, Pellicelli AM, Sharma AM, Grisorio B, Barbarini G, Barbaro G. Relation of subepicardial adipose tissue to carotid intima-media thickness in patients with human immunodeficiency virus. Am J Cardiol 2007,99:1470–1472. [DOI] [PubMed] [Google Scholar]
- 89.El Hattaoui M, Charei N, Boumzebra D, Aajly L, Fadouach S. [Prevalence of cardiomyopathy in HIV infection: prospective study on 158 HIV patients]. Med Mal Infect 2008,38:387–391. [DOI] [PubMed] [Google Scholar]
- 90.Olusegun-Joseph DA, Ajuluchukwu JN, Okany CC, Mbakwem AC, Oke DA, Okubadejo NU. Echocardiographic patterns in treatment-naive HIV-positive patients in Lagos, south-west Nigeria. Cardiovasc J Afr 2012,23:e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eholie SP, Lacombe K, Krain A, Diallo Z, Ouiminga M, Campa P, et al. Metabolic disorders and cardiovascular risk in treatment-naive HIV-infected patients of sub-saharan origin starting antiretrovirals: impact of westernized lifestyle. AIDS Res Hum Retroviruses 2015,31:384–392. [DOI] [PubMed] [Google Scholar]
- 92.Dillon DG, Gurdasani D, Riha J, Ekoru K, Asiki G, Mayanja BN, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013,42:1754–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mensah GA, Roth GA, Sampson UK, Moran AE, Feigin VL, Forouzanfar MH, et al. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013. Cardiovasc J Afr 2015,26:S6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Narayan KM, Miotti PG, Anand NP, Kline LM, Harmston C, Gulakowski R 3rd, et al. HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: a vital agenda for research in low- and middle-income country settings. J Acquir Immune Defic Syndr 2014,67 Suppl 1:S2–7. [DOI] [PubMed] [Google Scholar]
- 95.Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med 2012,172:1386–1394. [DOI] [PubMed] [Google Scholar]
- 96.Bloomfield GS, DeLong AK, Akwanalo CO, Hogan JW, Carter EJ, Aswa DF, et al. Markers of Atherosclerosis, Clinical Characteristics, and Treatment Patterns in Heart Failure: A Case-Control Study of Middle-Aged Adult Heart Failure Patients in Rural Kenya. Glob Heart 2016,11:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2012,33:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood) 2007,26:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rivera-Andrade A, Luna MA. Trends and heterogeneity of cardiovascular disease and risk factors across Latin American and Caribbean countries. Prog Cardiovasc Dis 2014,57:276–285. [DOI] [PubMed] [Google Scholar]
- 100.Barnighausen T, Welz T, Hosegood V, Batzing-Feigenbaum J, Tanser F, Herbst K, et al. Hiding in the shadows of the HIV epidemic: obesity and hypertension in a rural population with very high HIV prevalence in South Africa. J Hum Hypertens 2008,22:236–239. [DOI] [PubMed] [Google Scholar]
- 101.Malaza A, Mossong J, Barnighausen T, Newell ML. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One 2012,7:e47761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Temu TM, Kirui N, Wanjalla C, Ndungu AM, Kamano JH, Inui TS, et al. Cardiovascular health knowledge and preventive practices in people living with HIV in Kenya. BMC Infect Dis 2015,15:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guthold R, Louazani SA, Riley LM, Cowan MJ, Bovet P, Damasceno A, et al. Physical activity in 22 African countries: results from the World Health Organization STEPwise approach to chronic disease risk factor surveillance. Am J Prev Med 2011,41:52–60. [DOI] [PubMed] [Google Scholar]
- 104.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 2012,380:247–257. [DOI] [PubMed] [Google Scholar]
- 105.Kavishe B, Biraro S, Baisley K, Vanobberghen F, Kapiga S, Munderi P, et al. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med 2015,13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet 2009,374:934–947. [DOI] [PubMed] [Google Scholar]
- 107.Kotwani P, Kwarisiima D, Clark TD, Kabami J, Geng EH, Jain V, et al. Epidemiology and awareness of hypertension in a rural Ugandan community: a cross-sectional study. BMC Public Health 2013,13:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cappuccio FP, Miller MA. Cardiovascular disease and hypertension in sub-Saharan Africa: burden, risk and interventions. Intern Emerg Med 2016,11:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Opie LH, Seedat YK. Hypertension in sub-Saharan African populations. Circulation 2005,112:3562–3568. [DOI] [PubMed] [Google Scholar]
- 110.Tulloch-Reid MK, Younger NO, Ferguson TS, Francis DK, Abdulkadri AO, Gordon-Strachan GM, et al. Excess Cardiovascular Risk Burden in Jamaican Women Does Not Influence Predicted 10-Year CVD Risk Profiles of Jamaica Adults: An Analysis of the 2007/08 Jamaica Health and Lifestyle Survey. PLoS One 2013,8:e66625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diaz AA, Tringler MF. Prevalence of hypertension in rural populations from Ibero-America and the Caribbean. Rural Remote Health 2014,14:2591. [PubMed] [Google Scholar]
- 112.James J, Soyibo AK, Hurlock L, Gordon-Strachan G, Barton EN. Cardiovascular risk factors in an eastern Caribbean island: prevalence of non-communicable chronic diseases and associated lifestyle risk factors for cardiovascular morbidity and mortality in the British Virgin Islands. West Indian Med J 2012,61:429–436. [DOI] [PubMed] [Google Scholar]
- 113.Pierce L, Shannon A, Sonnenfeld J, Pearlmutter M, Previl H, Forrester JE. Hypertension prevalence and knowledge assessment in rural Haiti. Ethn Dis 2014,24:213–219. [PubMed] [Google Scholar]
- 114.Otgontuya D, Oum S, Palam E, Rani M, Buckley BS. Individual-based primary prevention of cardiovascular disease in Cambodia and Mongolia: early identification and management of hypertension and diabetes mellitus. BMC Public Health 2012,12:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chanthong P, Lapphra K, Saihongthong S, Sricharoenchai S, Wittawatmongkol O, Phongsamart W, et al. Echocardiography and carotid intima-media thickness among asymptomatic HIV-infected adolescents in Thailand. AIDS 2014,28:2071–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Janssens B, Van Damme W, Raleigh B, Gupta J, Khem S, Soy Ty K, et al. Offering integrated care for HIV/AIDS, diabetes and hypertension within chronic disease clinics in Cambodia. Bull World Health Organ 2007,85:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kagaruki GB, Mayige MT, Ngadaya ES, Kimaro GD, Kalinga AK, Kilale AM, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health 2014,14:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kayima J, Nankabirwa J, Sinabulya I, Nakibuuka J, Zhu X, Rahman M, et al. Determinants of hypertension in a young adult Ugandan population in epidemiological transition-the MEPI-CVD survey. BMC Public Health 2015,15:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khalsa A, Karim R, Mack WJ, Minkoff H, Cohen M, Young M, et al. Correlates of prevalent hypertension in a large cohort of HIV-infected women: Women’s Interagency HIV Study. AIDS 2007,21:2539–2541. [DOI] [PubMed] [Google Scholar]
- 120.Fourie CM, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis 2015,240:154–160. [DOI] [PubMed] [Google Scholar]
- 121.Krauskopf K, Van Natta ML, Danis RP, Gangaputra S, Ackatz L, Addessi A, et al. Correlates of hypertension in patients with AIDS in the era of highly active antiretroviral therapy. J Int Assoc Provid AIDS Care 2013,12:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwarisiima D, Balzer L, Heller D, Kotwani P, Chamie G, Clark T, et al. Population-Based Assessment of Hypertension Epidemiology and Risk Factors among HIV-Positive and General Populations in Rural Uganda. PLoS One 2016,11:e0156309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med 2014,12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Okello S, Asiimwe SB, Kanyesigye M, Muyindike WR, Boum Y 2nd, Mwebesa BB, et al. D-dimer levels and traditional risk factors are associated with incident hypertension among HIV-infected individuals initiating antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nduka CU, Stranges S, Bloomfield GS, Kimani PK, Achinge G, Malu AO, et al. A plausible causal link between antiretroviral therapy and increased blood pressure in a sub-Saharan African setting: A propensity score-matched analysis. Int J Cardiol 2016,220:400–407. [DOI] [PubMed] [Google Scholar]
- 126.Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One 2011,6:e22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Calmy A, Gayet-Ageron A, Montecucco F, Nguyen A, Mach F, Burger F, et al. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS 2009,23:929–939. [DOI] [PubMed] [Google Scholar]
- 128.Gaziano TA, Abrahams-Gessel S, Alam S, Alam D, Ali M, Bloomfield G, et al. Comparison of Nonblood-Based and Blood-Based Total CV Risk Scores in Global Populations. Glob Heart 2016,11:37–46 e32. [DOI] [PubMed] [Google Scholar]
- 129.Gaziano TA, Pandya A, Steyn K, Levitt N, Mollentze W, Joubert G, et al. Comparative assessment of absolute cardiovascular disease risk characterization from non-laboratory-based risk assessment in South African populations. BMC Med 2013,11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peer N, Lombard C, Steyn K, Gaziano T, Levitt N. Comparability of total cardiovascular disease risk estimates using laboratory and non-laboratory based assessments in urban-dwelling South Africans: the CRIBSA study. S Afr Med J 2014,104:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sritara P Twelve-year changes in vascular risk factors and their associations with mortality in a cohort of 3499 Thais: the Electricity Generating Authority of Thailand Study. International Journal of Epidemiology 2003,32:461–468. [DOI] [PubMed] [Google Scholar]
- 132.Krikke M, Hoogeveen RC, Hoepelman A, Visseren F, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med 2016,17:289–297. [DOI] [PubMed] [Google Scholar]
- 133.Longenecker CT, Eckard AR, McComsey GA. Statins to improve cardiovascular outcomes in treated HIV infection. Curr Opin Infect Dis 2016,29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gaziano TA, Abrahams-Gessel S, Denman CA, Montano CM, Khanam M, Puoane T, et al. An assessment of community health workers’ ability to screen for cardiovascular disease risk with a simple, non-invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: an observational study. Lancet Glob Health 2015,3:e556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee ES, Vedanthan R, Jeemon P, Kamano JH, Kudesia P, Rajan V, et al. Quality Improvement for Cardiovascular Disease Care in Low- and Middle-Income Countries: A Systematic Review. PLoS One 2016,11:e0157036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schoffelen AF, de Groot E, Tempelman HA, Visseren FL, Hoepelman AI, Barth RE. Carotid Intima Media Thickness in Mainly Female HIV-Infected Subjects in Rural South Africa: Association With Cardiovascular but Not HIV-Related Factors. Clin Infect Dis 2015,61:1606–1614. [DOI] [PubMed] [Google Scholar]
- 137.Vedanthan R, Kamano JH, Bloomfield GS, Manji I, Pastakia S, Kimaiyo SN. Engaging the Entire Care Cascade in Western Kenya: A Model to Achieve the Cardiovascular Disease Secondary Prevention Roadmap Goals. Glob Heart 2015,10:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta N, Bukhman G. Leveraging the lessons learned from HIV/AIDS for coordinated chronic care delivery in resource-poor settings. Healthc (Amst) 2015,3:215–220. [DOI] [PubMed] [Google Scholar]
- 139.Pastakia SD, Ali SM, Kamano JH, Akwanalo CO, Ndege SK, Buckwalter VL, et al. Screening for diabetes and hypertension in a rural low income setting in western Kenya utilizing home-based and community-based strategies. Global Health 2013,9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.UNAIDS. Fact Sheet 2015. In. Geneva, Switzerland; 2015. [Google Scholar]