Abstract
Introduction:
Hepatitis B virus (HBV) infection is hyperendemic in Cameroon, and health care workers (HCWs) are at high-risk of infection. We aimed to assess prevalence, risk factors and vaccine coverage of HBV infection among HCWs in Cameroon.
Methods:
We conducted a cross-sectional study in 16 hospitals across all regions of Cameroon. HCWs were tested for HBV using rapid diagnostic tests (RDT). We collected data on socio-demographics and HBV vaccination status. We estimated prevalence of HBV and used Poisson regression models with robust standard errors to model the prevalence ratios of HBV positivity between covariates.
Results:
We enrolled 1,824 of 1,836 eligible HCWs (97.5%). The mean age was 34 (SD: 10) years, 65.3% (n=1787) were women, and 11.4% (n=1747) had three or more doses of the HBV vaccine. Overall, we found a HBV prevalence of 8.7% (95% CI: 5.2 – 14.3%). Patient transporters had the highest crude prevalence (14.3%; 95%CI: 5.4–32.9%), whereas medical doctors had the lowest (3.2%; 95%CI: 0.8%−12.1%). The Far North Region had the highest prevalence of HBV (24.0%; 95%CI: 18.3%−30.8%). HBV prevalence decreased with increasing doses of the HBV vaccine (10.3% for no doses vs 3.5% for three or more doses; P<0.001).
Conclusion:
Approximately 1 in 12 HCWs in Cameroon have evidence of HBV infection, yet fewer than 1 in 6 have been fully vaccinated. Our results illustrate the urgent need to scale up systematic HBV screening and targeted vaccination of HCWs in the region.
Keywords: Hepatitis B, prevalence, health care workers, vaccine efficacy, Cameroon
More than 2 billion people have been infected with Hepatitis B virus (HBV) globally, and 240 million are chronic carriers of the disease.1,2Africa is considered to be endemic for HBV; and the disease is hyperendemic in Cameroon where prevalence has been estimated at 11.9%. 3,4 Health care workers (HCWs) are one of the most vulnerable groups to HBV infection from occupational exposures, with up to four times greater risk of contracting the infection than the general population.5 Although Cameroon introduced the anti-HBV vaccine in their expanded program of immunization in 2005, this program does not cover most HCWs as they were largely born before 2005 6. Moreover, there is a lack of awareness of HBV knowledge and practices among HCWs in sub-Saharan Africa, which further contributes to their risk, and that of their patients.2 Indeed, despite the established elevated risks of HBV infection in hospital settings, documented vaccination rates in small regional studies among HCWs in Cameroon remain low.6
However, to our knowledge, no study has rigorously estimated the prevalence of HBV infection among HCWs across all regions of Cameroon.7 Therefore, we conducted a Hepatitis B surface antigen (HBsAg) seroprevalence survey in a large sample of HCWs across all ten regions of Cameroon. We also assessed the vaccination status and correlates of HBV infection among this highly vulnerable group to identify areas for prevention, intervention, and populations to target with them.
METHODS:
Study design and setting
This was a hospital based, cross-sectional study, conducted between July and August 2016. Sixteen national and regional hospitals across the 10 regions of Cameroon were selected.
Sampling procedures, data and sample collection
All HCWs in selected hospitals were informed and invited for HBsAg testing. Testing stations were set up in each hospital ward. All active healthcare personnel, older than 18 years, working as HCWs were eligible for inclusion in this study. We aimed to recruit a total of 2,000 HCWs based on the number of tests available for this study. We obtained national proportions of HCWs per region from a registry published in 2015.8 We then applied these national proportions to our sample size to obtain the self-weighted target number of HCWs per region (Table 1). Consenting participants completed a structured questionnaire to collect data on sociodemographic characteristics, HBV exposure risks, HBV vaccination, and prevention methods. Whole blood was collected from each participant by venipuncture into ehtylenediaminetriacetic acid and serum separator tubes. HBsAg was tested using the SelexOn lateral flow assays (SelexOn, Infopia, Seoul, Korea) on 100µL of whole blood according to manufacturer instructions.
Table 1:
Sample distribution
| Region | Targeted number of participants |
|---|---|
| Centre | 555 |
| Littoral | 296 |
| Far North | 218 |
| West | 246 |
| North West | 151 |
| South West | 188 |
| South | 75 |
| East | 87 |
| Adamawa | 69 |
| North | 115 |
| Total | 2000 |
Statistical analysis
Data were entered into Microsoft Excel (Microsoft, Redmond, Washington). Statistical analyses were performed using Stata version 13 (StataCorp, College Station, TX). Data was described using count (percent) and chi-squared testing was used to test associations between categorical variables. We used Poisson regression with robust standard errors to assess the independent associations between HBV prevalence and covariates, including region, vaccination status, sex, age, and profession. Vaccine efficacy was calculated as 1- [prevalence among participants with ≥3 doses of HBV vaccine (i.e. fully vaccinated)/prevalence among participants with 0 doses of vaccine] to estimate the efficacy of HBV vaccination.
Ethics Statement
This study was approved by the National Ethics Committee of Cameroon. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. All participants signed informed consent.
RESULTS
A total of 1,824 participants were enrolled in this study. The mean age was 34 (SD:10) years. Women represented 65.3% (1167/1787) of this population. We tested 1,790 for HBsAg. Nurses were the most represented profession (35.2%; 626/1779), whereas medical doctors made up the smallest proportion of the sample (3.5%; 62/1779). Nearly two thirds of the participants had less than five years of experience. We found that 23% (403/1754) of the participants had received at least one dose of the vaccine, while only 11.4% (200/1747) completed the recommended three shots vaccination series (P<0.001).
The overall prevalence of HBV was 8.7% (156/1790 95%CI: 5.2 – 14.3%). Table 2 summarizes HBV prevalence by key demographic characteristics. The prevalence was higher in men than in women [13.0% (81/620; 95% CI: 10.6 – 16.0%) vs 6.3% (74/1167; 95% CI: 5.1 – 7.9%), P<0.001]. HBV prevalence varied significantly (P<0.001) across regions ranging from 5.1% (9/175; 95%CI: 2.7 −9.6%) in the Southwest region to 24.0% (43/179; 95%CI: 18.3 −30.8%) in the Far North Region.
Table 2:
HBV prevalence rate by category
| Variable | n | HBsAg+ | Prevalence, in % (95% CI) |
P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 620 | 81 | 13.0 (10.6 – 16.0) | <0.0001 |
| Female | 1167 | 74 | 6.3 (5.1 – 7.9) | |
| Age | ||||
| <=25 | 451 | 44 | 9.7 (7.3– 12.9) | 0.28 |
| 26–39 | 818 | 75 | 9.2 (7.4 – 11.4) | |
| >=40 | 521 | 37 | 7.1 (5.2 – 9.7) | |
| Region | ||||
| Adamaoua | 69 | 7 | 10.1 (4.9 – 19.9) | <0.0001 |
| Centre | 425 | 23 | 5.4 (3.6 – 8.0) | |
| Est | 85 | 4 | 4.7 (1.7 – 11.9) | |
| Far North | 179 | 43 | 24.0 (18.3 – 30.8) | |
| Littoral | 273 | 17 | 6.2 (3.9 – 9.8) | |
| Nord | 194 | 23 | 11.8 (8 – 17.2) | |
| North West | 139 | 11 | 8.0 (4.4 – 13.7) | |
| West | 176 | 10 | 5.7 (3 – 10.2) | |
| South | 75 | 9 | 12.0 (6.3 – 21.6) | |
| South West | 175 | 9 | 5.1 (2.7 – 9.6) | |
| Prior Vaccination | ||||
| No | 1351 | 140 | 10.3 (8.8 – 12.1) | <0.0001 |
| Yes | 403 | 14 | 3.5 (2.0 – 5.8) | |
| Vaccination doses | ||||
| 0 | 1351 | 140 | 10.3 (8.8– 12.0) | <0.0001 |
| 1 | 101 | 6 | 6.0 (2.7–12.7) | |
| 2 | 95 | 4 | 4.2 (1.6–10.8) | |
| 3 | 185 | 3 | 1.5 (0.5–4.8) | |
| >3 | 15 | 0 | 0.0 | |
| Profession | ||||
| Medical Doctor | 62 | 2 | 3.2 (0.8–12.1) | 0.19 |
| Nurse | 626 | 50 | 8.0 (6.1–10.4) | |
| Intern | 535 | 52 | 9.7 (7.5–12.5) | |
| Laboratory Technician | 110 | 4 | 3.6 (1.4–9.3) | |
| Patient-transporter | 28 | 4 | 14.3 (5.4–32.9) | |
| Cleaning Agent | 132 | 13 | 9.8 (5.8–16.3) | |
| Others | 286 | 27 | 9.4 (6.5–13.4) | |
| Working Experience, in years | ||||
| <=1 | 582 | 55 | 9.4 (7.3 – 12.1) | 0.34 |
| 2–5 | 469 | 45 | 9.6 (7.2 – 12.6) | |
| 6–10 | 333 | 29 | 8.7 (6.1 – 12.3) | |
| >10 | 387 | 25 | 6.4 (4.4 – 9.4) |
HCWs who were vaccinated had a significantly lower prevalence of HBV than those who were not vaccinated [3.5% (14/403; 95%CI: 2.0 −5.8%) vs 10.3% (140/1351; 95%CI: 8.8 −12.1%); P<0.001]. We also found that the prevalence decreased with increasing number of vaccine doses (figure 1). Overall vaccine efficacy against HBV infection was 85% (95%CI: 55–95%). Furthermore, we observed that HCWs that were professionally trained (medical doctors, nurses, laboratory technicians) had a higher vaccination coverage than ancillary staff (patient transporters, sanitation staff, others) (26.2% (350/1337; 95%CI: 23.9 – 28.6%) vs 14.4% (64/445; 95%CI: 11.4 – 17.9%); P<0.001, Table 3). Although the difference in prevalence by profession was not significant (P=0.19), we observed that medical doctors and laboratory technicians had the lowest crude prevalence of HBV 3.2% (2/62; 95%CI: 0.8–12.1%) and 3.6% (4/110; 95%CI: 1.4–9.3%) respectively.
Figure 1:
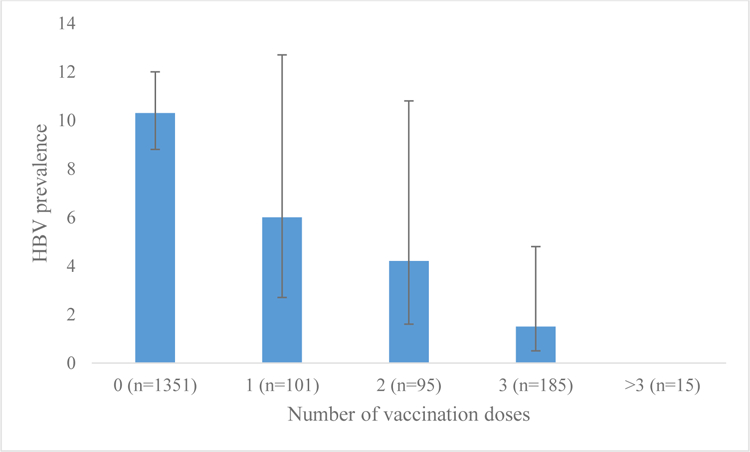
HBV vaccination doses vs HBV prevalence
Table 3:
Vaccination coverage for professional vs ancillary HCWs
| HCWs Categories | n | Number of vaccinated HCWs |
Percentage of vaccinated HCWs(95%CI) |
P-value |
|---|---|---|---|---|
| Professional staff (Medical Doctors, Nurses, Laboratory Technicians) |
1337 | 350 | 26.2 (23.9 – 28.6) | <0.0001 |
| Ancillary staff (Patient transporters, Cleaning Agents, Others) |
445 | 64 | 14.4 (11.4 – 17.9) |
Adjusted risk factors for HBV infection
Using a Poisson regression model with robust standard errors (Table 4) we found that participants in the Far North region had nearly three times the prevalence (APR 2.8; 95%CI 2.0 −4.0%; P<0.001) of participants from other regions of Cameroon. We also found that non-vaccinated HCWs had 2.7 times the prevalence of vaccinated participants (95%CI: 1.6–4.6%, P<0.001). Women had 40% lower prevalence of HBV compared to men (APR=0.6; 95% CI 0.4–0.9%; P<0.001).
Table 4:
Risk factors for contracting HBV
| Variables | Adjusted prevalence ratio |
P-value | 95% CI |
|---|---|---|---|
| Region (ref=all other regions) | |||
| Extreme North | 2.8 | <0.001 | 2.0 – 4.0 |
| Vaccination status (ref=vaccinated) | |||
| Non-vaccinated | 2.7 | <0.001 | 1.6 – 4.6 |
| Sexe (ref=male) | |||
| Female | 0.6 | 0.004 | 0.4 – 0.9 |
| Age (ref<=25) | |||
| 26–39 | 1.2 | 0.41 | 0.8 – 1.7 |
| >=40 | 0.9 | 0.56 | 0.5 – 1.4 |
| Profession (ref=professionals) | |||
| Ancillay | 1.2 | 0.37 | 0.8 – 1.7 |
DISCUSSION
In this national seroprevalence study in Cameroon, we found an alarmingly high HBsAg prevalence of 8.7% among HCWs. HBV infection was particularly common in men, in non-vaccinated staff, and in the Far North region of Cameroon. These data corroborate others from tertiary hospitals in Tanzania and Uganda, which also found remarkably high prevalence of HBsAg positivity in HCWs, at 7.0% and 8.1% respectively.2,5 Our findings are also comparable to estimates in other high-risk groups in Cameroon. Frambo et al reported a prevalence of 9.7% among pregnant women, and Noubiap et al reported a prevalence of 10.1% in blood donors.9,10 Despite the importance of the problem, most studies on HCWs are done on small samples sizes. A recent systematic review of the seroprevalence of HBV infection in Cameroon found only four relevant studies on HCWs, and none of them covered all regions of the country.7 Our study is the first to our knowledge conducted across all the regions of Cameroon, and illustrates an important and readily addressable public health problem in the region.
Our results also reinforce both the value and unmet need of vaccination for HCWs in Cameroon. Less than one in four HCWs in our sample had received any immunizations against HBV, of whom only approximately 1 in 10 had received the three recommended doses. Importantly, only 12.1 % of the HbsAg negative participants were fully vaccinated – indicating a significant opportunity for disease prevention. Although professionally trained personnel tended to be more likely to be vaccinated than ancillary staff (26.2% vs 14.4%), all groups remained well below target, indicating the necessity to establish vaccination programs in this high risk population. Not surprisingly, we found that HBV prevalence significantly decreased with increasing number of HBV vaccine doses, and estimated a vaccine efficacy of 85%. This result is similar to CDC estimates which indicate that the vaccine is 80% to 100% effective in preventing infection or clinical hepatitis in those who receive the complete vaccine series.11 Our findings reinforce the effectiveness of the complete vaccination schedule and the value of completing in high risk populations.
Several studies have demonstrated high rates of naturally acquired immunity ranging from 60–90% among adults in endemic countries, particularly in sub-Saharan Africa where infections occur perinatally or during childhood. 12 Ideally, targeted vaccination programs that identify individuals who are HBsAg positive and those who are HBcAb negative would be more efficient. As in many resources limited countries, the cost of determining anti-HBs titers is higher than the cost of the HBV vaccination series. Specifically in Cameroon, the cost of vaccination can be as low as 3.50 USD while determining anti-HBc titers can cost up to17 USD13,14. Because vaccination remains the primary means of prevention against HBV infection, and because it has clear secondary public health benefits in HCWs, vaccination of this population should be prioritized. WHO has specified that HCWs require additional attention for HBV screening and vaccination.15 However, our results highlight that these recommendations are not yet well implemented in low and middle income countries such as Cameroon.
Our results also demonstrated that HCWs from the Far North have the greatest burden of HBV. We hypothesize that this is explained by the fact that the Far North is located in a zone of political instability and conflict, and is likely to be contributing to both decreased rates of HBV screening and vaccination.16 The risk of contracting HBV in resource-limited settings and areas of conflict has been demonstrated to be significantly higher due to a combinations of factors including: poor vaccine coverage, poor educational attainment, inadequate prevention and screening strategies, frequent iatrogenic transmission, and limited access to treatment.17 Consequently, additional resources and attention is likely to be needed in the Far North region in Cameroon and similarly conflict-affected regions.
Finally, our results should prompt more formal consideration of access to HBV treatment programs in the region. Although additional testing is needed to risk stratify those with a positive HBsAg test, our study highlights an important need to decentralize HBV treatment in Cameroon to minimize the substantial long term morbidity associated with untreated HBV infection.
Limitations:
Although this study was conducted across all regions of Cameroon, our sample might not be representative of the national HCWs distribution because we used convenience sampling. As recommended by the WHO, we used a RDT to test for HBsAg. However, the use of a RDT might underestimate the prevalence of HBV as compared to a reference testing methods such as enzyme linked immunoabsorbent assays.18 Information about vaccination status and doses were self-reported and might be subject to recall bias, which can affect estimation of vaccine efficacy. Furthermore, our estimate of vaccine efficacy does not take into consideration the timing of vaccination in relation to the timing of infection as the data was collected retrospectively.
In conclusion, we found a considerable burden of HBV infection among HCWs in Cameroon, particularly among men, ancillary staff and residents of the unstable Far North region. Moreover, the vaccination coverage was alarmingly low among this high-risk population, highlighting the need and opportunity for testing and vaccination strategies specifically designed for HCWs in the region. Such programs could have far reaching public health benefits both for our HCWs and the patients they serve.
ACKNOWLEDGEMENTS:
We thank S. Ewale Bah for his contribution in planning the implementation of this study. We are grateful to J. Mendouga, S. Njimo, P. Ndoh, D. Mougoue, W. Tenlep, M. Soua, A. Osteng, H. Nanda, B. Djonga, R. Sandjong who collected samples in hospitals across the country, and to the Regional Hospitals management for facilitating the study. We are indebted to the HCWs who participated in the study. We thank Infopia, Korea who donated the Selexon RDT and cartridges that were used to test for HBV. We thank Médecins Sans Frontières and Laboratoire du Lac for the logistic support they provided. This study was funded by the Ministry of Public Health of Cameroon. The sponsor had no role in the design, data collection, analysis, interpretation, or writing of this paper, nor in the decision to submit for publication.
Statement of financial support: The study was sponsored and coordinated by the Ministry of Public Health of Cameroon.
List of abbreviations in the order of appearance:
- HBV
Hepatitis B Virus
- HCWs
Health Care Workers
- RDT
Rapid Diagnostic Tests
- HBsAg
Hepatitis B Surface Antigen
- HBcAb
Hepatitis B Core Antibody
Footnotes
Conflicts of Interest declaration for all authors: The authors declare no conflict of interest.
REFERENCES:
- 1.WHO. WHO | Hepatitis B WHO; http://www.who.int/mediacentre/factsheets/fs204/en/. Published 2017. Accessed October 16, 2017. [Google Scholar]
- 2.Mueller A, Stoetter L, Kalluvya S, et al. Prevalence of hepatitis B virus infection among health care workers in a tertiary hospital in Tanzania. BMC Infect Dis 2015;15:386. doi: 10.1186/s12879-015-1129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zampino R, Boemio A, Sagnelli C, et al. hepatitis B virus burden in developing countries 2015 Advances in Hepatitis B virus. World J Gastroenterol 2015;21(42):11941–11953. doi: 10.3748/wjg.v21.i42.11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NJOUOM R, FONTANET A. Épidémiologie Des Hépatites Virales B, C, et Delta Au Cameroun: Analyse Des Échantillons de L’enquête Démographique de Santé 2011; 2011. http://anrs-cameroun.org/anrs-12289. Accessed October 16, 2017.
- 5.Ziraba AK, Bwogi J, Namale A, Wainaina CW, Mayanja-Kizza H. Sero-prevalence and risk factors for hepatitis B virus infection among health care workers in a tertiary hospital in Uganda. BMC Infect Dis 2010;10. doi: 10.1186/1471-2334-10-191. [DOI] [PMC free article] [PubMed]
- 6.Fritzsche C, Becker F, Hemmer CJ, et al. Hepatitis B and C: neglected diseases among health care workers in Cameroon. Trans R Soc Trop Med Hyg 2013;107:158–164. doi: 10.1093/trstmh/trs087. [DOI] [PubMed] [Google Scholar]
- 7.Bigna JJ, Amougou MA, Asangbeh SL, et al. Seroprevalence of hepatitis B virus infection in Cameroon: a systematic review and meta-analysis. BMJ Open 2017;7(6):e015298. doi: 10.1136/bmjopen-2016-015298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tandi TE, Cho Y, Akam AJ-C, et al. Cameroon public health sector: shortage and inequalities in geographic distribution of health personnel. Int J Equity Health 2015;14(1):43. doi: 10.1186/s12939-015-0172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frambo AA, Atashili J, Fon P, Ndumbe P. Prevalence of HBsAg and knowledge about hepatitis B in pregnancy in the Buea Health District, Cameroon: a cross-sectional study. BMC Res Notes 2014;7(1):394. doi: 10.1186/1756-0500-7-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noubiap JJN, Joko WYA, Nansseu JRN, Tene UG, Siaka C. Sero-epidemiology of human immunodeficiency virus, hepatitis B and C viruses, and syphilis infections among first-time blood donors in Edéa, Cameroon. Int J Infect Dis 2013;17(10):e832–e837. doi: 10.1016/j.ijid.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Pinkbook | Hepatitis B | Epidemiology of Vaccine Preventable Diseases | CDC. https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html. Accessed March 1, 2018. [Google Scholar]
- 12.Pellissier G, Yazdanpanah Y, Adehossi E, et al. Is Universal HBV Vaccination of Healthcare Workers a Relevant Strategy in Developing Endemic Countries? The Case of a University Hospital in Niger. Bisser S, ed. PLoS One 2012;7(9):e44442. doi: 10.1371/journal.pone.0044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centre Pasteur du Cameroun. http://www.pasteur-yaounde.org/files/catalogue2016.pdf. Accessed June 21, 2018.
- 14.Cameroun : Seulement 2000 Fcfa pour se vacciner contre l’hépatite B - Actu Cameroun https://actucameroun.com/2017/08/15/cameroun-seulement-2000-fcfa-pour-se-vacciner-contre-lhepatite-b/. Accessed June 21, 2018.
- 15.World Health Organization. Global Hepatitis Programme. Guidelines for the prevention, care, and treatment of persons with chronic hepatitis B infection 2015;(March):134 http://www.worldcat.org/title/guidelines-for-the-prevention-care-and-treatment-of-persons-with-chronic-hepatitis-b-infection/oclc/907556882&referer=brief_results. [PubMed]
- 16.Noubiap JJN, Nansseu JRN, Ndoula ST, Bigna JJR, Jingi AM, Fokom-Domgue J. Prevalence, infectivity and correlates of hepatitis B virus infection among pregnant women in a rural district of the Far North Region of Cameroon. BMC Public Health 2015;15:454. doi: 10.1186/s12889-015-1806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol 2013;8(4):371–380. doi: 10.2217/fvl.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kukar Neetu; Garg Ravinder; Maharishi R; Syal Neha; Arora Harkiran; Handa A. ELISA versus Rapid test kits for screening of HIV and Hepatitis B and Hepatitis C among Blood donors in a tertiary care hospital. Sch J Appl Med Sci 2017;(5 (3A)):727–729. doi: 10.21276/sjams.2017.5.3.9. [DOI]


