Abstract
Background:
Neoadjuvant treatment of colorectal liver metastases has become increasingly common, and while effective, often renders small metastases difficult to visualize on intraoperative US. The objective of this study was to determine the utility of a 3D image-guidance system in patients with intraoperative sonographically-occult CRLM.
Methods:
50 patients with at least one CRLM ≤ 1.5 cm were enrolled in this prospective trial of an FDA-approved Explorer image-guidance system. If the tumor(s) seen on preoperative imaging were not identified with intraoperative US, Explorer was used to target the US examination to the involved area for a more focused assessment. The primary endpoint was the proportion of cases with sonographically-occult metastases identified using Explorer.
Results:
Forty-eight patients with preoperative scans within eight weeks of surgery were included for analysis. Forty-six patients were treated with preoperative chemotherapy (median 4 months, range 2–24 months). Overall, 22 sonographically-occult tumors in 14 patients were interrogated by Explorer, of which 15 tumors in 10 patients were located with image-guidance assistance. The only difference between patients with tumors not identified on US and those who did was the number of tumors (median 3 vs. 2, p = 0.018).
Conclusion:
3D image-guidance can assist in identifying small CRLM, particularly after treatment with chemotherapy.
Trial registration:
ClinicalTrials.gov, , https://clinicaltrials.gov/ct2/show/NCT02806037.
Introduction
Approximately 50% of patients with colorectal cancer will develop liver metastases.1 With improving surgical techniques and the use of downstaging neoadjuvant chemotherapy, as many as 20–30% of patients now have resectable disease.2 In modern series, 5-year survival after resection with curative intent is over 50%.3 Indications for resection continue to expand. In addition, adjuncts such as thermal and electrical ablation can expand the pool of patients who are candidates for resection by allowing for the effective oncologic treatment of tumors in a patient’s future liver remnant. The successful treatment of small lesions with either resection or ablation requires accurate preoperative planning and intraoperative localization with a combination of manual palpation and intraoperative ultrasound (US).
Modern chemotherapy has led to tumors shrinking in size or even “disappearing” radiologically or at exploratory laparotomy. However, this often does not represent true pathological complete response, and persistent microscopic disease maybe present in up to 80% of cases.4 In addition, steatosis, a feature of chemotherapy-associated liver injury, is associated with hyperechogenicity of the background liver parenchyma, which decreases the sensitivity of US to liver lesions.5,6 While there are multiple reports that describe the controversy around and approaches to “disappearing” liver metastases, there are few strategies to treat tumors that are visible on preoperative scans but occult on intraoperative US. Hence, the surgeon is often faced with the dilemma of how to proceed when a preoperatively identified liver metastasis is not found during exploratory surgery.
Failure to identify and treat areas of active disease represents a failure of surgical therapy, and leads to early disease progression. The first generation of image guidance systems has demonstrated the ability to accurately identify hepatic tumors in both open and laparoscopic surgery by intraoperatively coupling the patient’s anatomy as it appears in the operating room to preoperative imaging. Our hypothesis is that assistance with an image-guided surgery (IGS) system will improve intraoperative identification of colorectal liver metastases.
Methods
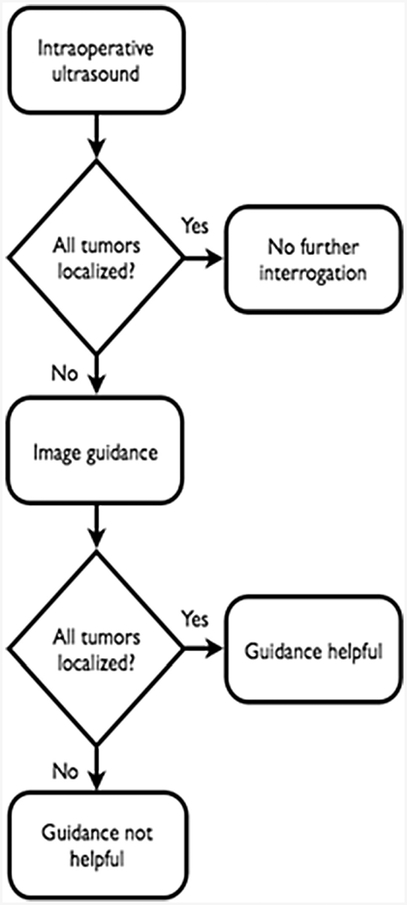
This single-arm prospective clinical trial of an FDA-approved Explorer system (Analogic, Inc., Boston, MA) was approved by the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board with an institutional waiver of informed consent and was in compliance with HIPAA. Patients were identified preoperatively. Inclusion criteria were one or more colorectal liver metastases, at least one of which was ≤1.5 cm in maximal diameter on preoperative cross-sectional imaging, a treatment plan including liver resection and/or ablation, and adequate preoperative CT or MR images. Radiologic review was performed by the operative surgeon and by the first author (TPK). The Explorer system was used as previously described.7 Briefly, preoperative imaging data were downloaded from the hospital Patient Archiving and Communication System. The liver, tumors, and vasculature were segmented from the portal-venous phase of a CT scan or an MRI. MRI may identify small and disappearing tumors compared to CT scan after a patient has been treated by chemotherapy. For this study this is irrelevant, as whichever radiologic modality was used and demonstrated a ≔1.5 cm tumor generated a model with that tumor that was used for intraoperative guidance. Next, three-dimensional (3D) models were constructed from the segmented imaging data, placing tumors visualized on a CT/MRI into the liver model. The system can be used laparoscopically or open, with no difference in intraoperative technique between the two modalities. Intraoperatively, the liver was first adequately mobilized and positioned to allow sufficient exposure and access to the lesions of interest. The Aloka Alpha 7 US and T probe were used to localize tumors identified on the preoperative scan. If all lesions seen on preoperative imaging were found, the Explorer system was not utilized (Fig. 1). If one or more lesions could not be found, then an optically-tracked sterile tool was used to swab the round ligament, the left and right inferior border of the liver, and the falciform ligament. The Explorer system matches these swabbed features with the preoperative model using a process called registration. A sterile optically-tracked rigid body was then attached to the US transducer such that the US data were overlaid on the CT scan and models (Fig. 2). Both of these optically-tracked tools are available for use in open as well as minimally-invasive liver surgery.8 The surgeon used the tracked US transducer to reattempt localization of the missing tumor. If subsequently found with US, the lesion was treated at the discretion of the surgeon (resection or ablation). If the liver needed to be repositioned during the use of the image guidance system, registration was performed again to ensure accuracy of the optical tracking of the probes. To assess the accuracy of the system, routine postoperative scans obtained as part of the standard of care were reviewed to confirm that the tumor in question was resected or ablated. There were no tumors located with image guidance alone that were not on preoperative imaging, as the image guidance model is constructed solely from the preoperative CT/MRI. True disappearing liver metastases, meaning those not visualized on preoperative CT/MRI could not be included in the model so were thus excluded from this study. Lesions visible in the preoperative model but not identified by guided US were not treated (i.e. the image guidance 3D model alone was not used to guide treatment).
Figure 1.
Trial schema
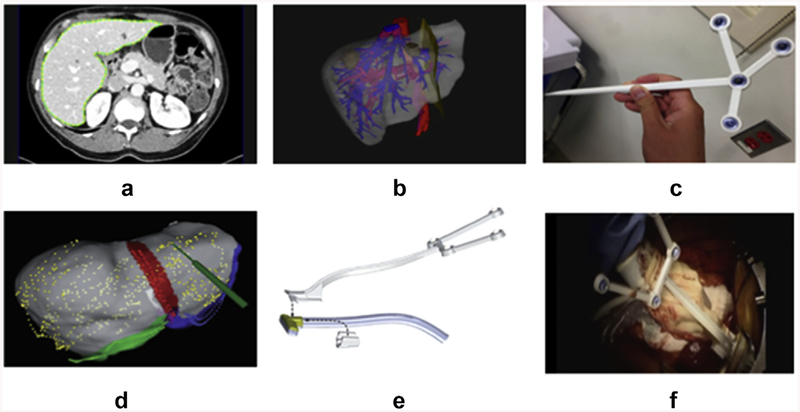
Figure 2.
Workflow for Explorer system: (a) image segmentation, (b) preoperative planning and model generation, (c) surgical tool tracking, (d) intraoperative rigid registration, (e) tracking of ultrasound transducer, and (f) interrogation of liver of liver surface with tracked ultrasound
The amount of time the surgeon spent using US before invoking the guidance system was recorded. The location, number, and size were recorded. Prospectively collected data included age, sex, BMI, date of preoperative imaging, date of surgery, prior treatment history, comorbidities, liver steatosis (as determined by MSK pathologist), clinical risk score,9 number, location, and size of metastases, and postoperative imaging. The primary endpoint of the study was the proportion of sonographically occult tumors subsequently found by the surgeon using the image guidance system. Secondary endpoints were the proportion of patients in whom image guidance was clinically helpful (as determined by surgeon) and the differences in patient and tumor characteristics between patients with sonographically occult tumors located using the guidance system and those with tumors that were not. Only patients with preoperative scans within eight weeks of surgery were included in this analysis.
The study sample size was 50, which allowed an estimation of the proportion of patients in which Explorer aided in the identification of tumor to within +/−14%. For example, if 25 of the 50 tumors were localized with guidance of Explorer system, the resulting 95% confidence interval with 50 patients was 43–57%.
Results
Patient demographics
From December 2013 through March 2016, 50 patients were enrolled. Two patients had preoperative scans dating more than eight weeks from the date of surgery and were excluded from the analysis. The remaining 48 patients were the focus of our analysis. The median age was 53 years (range 31–74 years) and 27 patients were male. The median clinical risk score (CRS) was 3 (range 1–4). The median time interval between the preoperative scan used in the study and the date of surgery was one month (range 0–2 months). Ten patients had liver steatosis based on preoperative imaging; no patients had known liver cirrhosis based on clinical history or preoperative evaluation. Nearly all patients (46/48) were treated with chemotherapy, with a median length of treatment of 4 months (range 2–24 months). This included seven patients who had been treated with hepatic arterial infusion (HAI) pump therapy with 5-fluoro-2-deoxyuridine (FUDR). The median number of liver tumors ≤1.5 cm in maximal diameter per patient on preoperative imaging was 2 (range 1–13). Patient demographics and presenting characteristics are summarized in Table 1.
Table 1.
Patient demographics and presenting characteristics
| Age (years), median (range) | 53 (31–74) |
| Sex | |
| Male | 27 |
| BMI,a median (range) | 27 (19–48) |
| CRSb | 3 (1–4) |
| Preoperative platelet count (×103/μL), median (range) | 186 (80–440) |
| Known steatosis | 10 |
| Known cirrhosis | 0 |
| Preoperative PVEc | 3 |
| Prior chemotherapy | 46 |
| Number of months, median (range) | 4 (2–24) |
| Preoperative imaging modality | |
| CTd | 40 |
| MRIe | 8 |
| Months between preoperative imaging and DOS,f median (range) | 1 (0–2) |
Body mass index.
Clinical risk score.
Portal vein embolization.
Computed tomography.
Magnetic resonance imaging.
Date of surgery.
Intraoperative data
Forty-six of the procedures were open. A total of 119 tumors were interrogated with US. The median number of tumors ≤1.5 cm per patient that underwent attempted localization with US was 2 (range 1–13). Median US time to identify tumors ≤1.5 cm was4.5 min (range 0.1–17.5 min) (Table 2). There was no difference in the number of tumors ≤1.5 cm per patient from preoperative imaging or that underwent attempted US localization between patients with preoperative CT compared with MRI. The primary endpoint was met in 14 patients who had one or more liver tumors ≤1.5 cm that were not located with US and the Explorer image guidance system was then utilized. There were a total of 22 tumors evaluated with Explorer system assistance in these 14 patients. Ten of these 14 patients had liver lesions that were subsequently located with image guidance assistance; in one additional patient, the Explorer system aided in confirming the absence of a liver lesion. In the other 4 patients, the tumors that remained undetectable with Explorer were all located in the posterior right liver (segments 6 and 7). There were no other differences in the tumor size or location relative to the capsule or vascular pedicles between these 4 patients and the 10 patients with tumors identified with Explorer guidance. As a result of utilizing the image guidance system, the surgical management plan was changed for 5 of the 14 patients; for 4 patients, the plane of resection was altered to include additional segments where lesions had identified with image guidance, and for 1 patient, an additional ablative procedure was performed. In the 5 patients with image guidance located tumors without a change in treatment plan, the tumors were already included in the planned resection.
Table 2.
Intraoperative and image guidance use details
| Median number of tumors ≤1.5 cma on preoperative imaging (range) | 2 (1−13) |
| Median number of tumors ≤1.5 cm found with USb alone (range) | 2 (0–9) |
| Median US time (min), median (range) | 4.5 (0.1–17.5) |
| Patients with tumors ≤1.5 cm not found with US alone, interrogated with IGSc | 14 |
| Patients with tumors located with IGS | 10/14 |
| Patients where IGS changed surgical management | 5/14 |
| IGS registration time (min), median (range) | 1.63 (0.8–4.4) |
| Operative approach | |
| Open | 46 |
| Laparoscopic | 2 |
| Type of surgery | |
| Major hepatic resection | 12 |
| Minor hepatic resection | 18 |
| Wedge hepatic resection | 15 |
| Hepatic arterial infusion pump insertion only | 3 |
Centimeter.
Ultrasound; min, minutes.
Image guidance system.
In all 10 patients who underwent either resection or ablation for tumors located with image guidance, the immediate postoperative scans demonstrated the lesions were all adequately treated, and were encompassed in either the resected liver or the ablation defect. At a median follow-up time of 6 months, there was no evidence of local recurrence in any of these patients. Additional intraoperative and image guidance use details are summarized in Table 2.
Characteristics of patients with tumors not found on US
A comparison of patients who had all tumors identified by US and those who did not revealed a difference in the number of tumors per patient on preoperative scans (Table 3). There was a median of 2 tumors (range 1–4), sized ≤1.5 cm, per patient in the group with all tumors identified compared to 3 tumors (range 1–13) per patient in the group with not all tumors identified (p = 0.018). The total US time per patient approached, but did not reach, statistical significance, with 3.8 min in patients with all tumors identified and 5.9 min in patients with a tumor not identified on US (p = 0.059). In patients with all tumors identified compared to patients with some tumors not found on US, there were no significant differences in BMI, clinical risk score, preoperative steatosis, or the percentage of patients treated with chemotherapy (Table 3).
Table 3.
Comparison of patients with all tumors found with ultrasound alone vs. not all tumors found with ultrasound alone
| Age (years), median (range) | 53 (31–74) | 53 (33–73) | 59 (31−74) | 0.510 |
| Sex | ||||
| Male | 27 | 18 | 9 | 0.471 |
| BMI,c median (range) | 27 (19–48) | 27.7 (19.7–48.1) | 25.4 (18.5–42.1) | 0.286 |
| CRS,d median (range) | 3 (1–4) | 3 (1–4) | 3 (2–3) | 0.175 |
| Preoperativee platelets (×103/μL), median (range) | 186 (80–440) | 195 (93–440) | 181 (80–236) | 0.208 |
| Known steatosis | 10 | 6 | 4 | 0.397 |
| Known cirrhosis | 0 | 0 | 0 | - |
| Neoadjuvant chemo | 46 | 32 | 14 | 0.354 |
| Number of months, median (range) | 4 (2–24) | 4 (2–24) | 4 (2–18) | 0.541 |
| Number of tumors on preop scan per pt,f median (range) | 3 (1–12) | 3 (1–9) | 4 (2–12) | 0.276 |
| Number of tumors on preop scan ≤1.5 cm per pt, median (range) | 2 (1–13) | 2 (1–4) | 3 (1–13) | 0.018 |
| Number of tumors not found with US per pt, median (range) | 0 (0–4) | 0 | 1 (1–4) | <0.001 |
| US time (min), median (range) | 4.5 (0.1–17.5) | 3.8 (0.1–17.5) | 5.9 (1.9–15.0) | 0.059 |
Patients.
Ultrasound.
Body mass index.
Clinical risk score.
Preoperative.
Patient.
Per tumor analysis
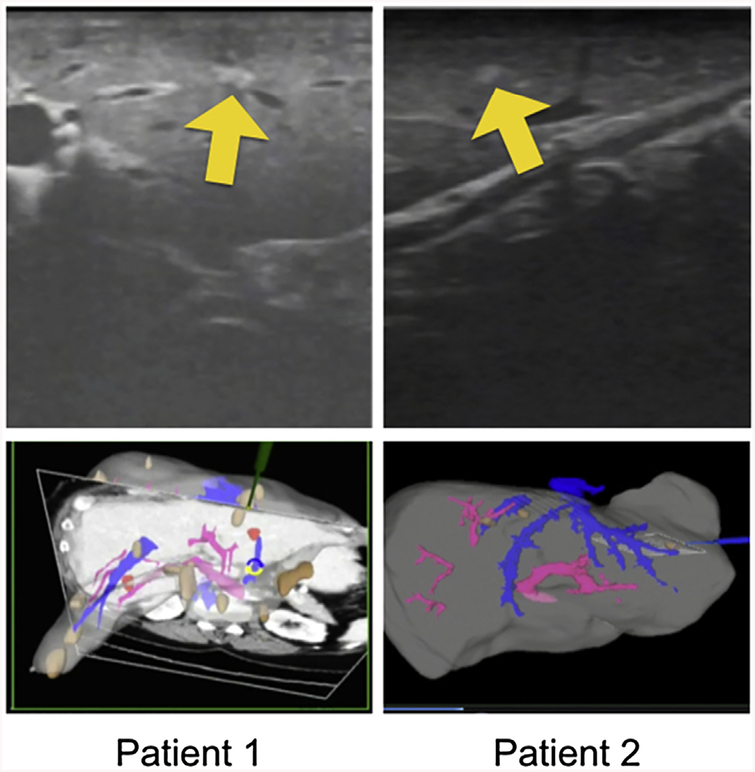
Fourteen patients with 22 tumors had tumors that were not identified on US and were interrogated with Explorer. Fifteen of these tumors in 10 patients were identified on tracked US with image guidance (Fig. 3). As Fig. 3 demonstrates, the tumors were visualized on US after Explorer guided the surgeon to this area of the liver. The surgeon was not, however, able to find the tumor prior to use of the guidance system. Seven tumors in four patients were not identified on US despite image guidance. When tumors that were located with Explorer guidance were compared to those that were not, there was no difference in subcapsular location, location relative to vascular pedicles or hepatic veins, or tumor size. Four of these seven tumors were included in the planned resection specimen and confirmed as metastatic colorectal cancer histologically and two were included in the planned ablation zone. One was followed on subsequent cross-sectional imaging and remained stable in short term follow-up.
Figure 3.
Explorer system in use for two patients: metastases initially occult with US interrogation but visible after localization with explorer
Discussion
The liver is an optimal organ for the development of image guidance due to its complex 3D anatomy and the effects of chemotherapy and steatosis on the capacity of intraoperative US to identify small tumors. The high quality of preoperative imaging with triple-phase CT and MR has rapidly outpaced the capabilities of intraoperative US. Neurosurgery is a field that has already benefited from the use of intraoperative image guidance because of an inherent advantage. The neurosurgery patient and, effectively, the brain can be fixed to the table, making the target “rigid,” so there is no motion artifact.10 Liver image guidance remains in its infancy because of the difficulties of deformation and motion.
There are two FDA-approved liver image guidance systems: the Explorer system and the CASCination system (Bern, Switzerland).11 The Explorer system relies on swabbing four anatomical regions to register the liver to the 3D model created from preoperative imaging and uses infrared tracking of instruments.12 Early studies demonstrated that in 8 liver resections, the Explorer system error for registration of target surface areas was 2–6 mm.13 Since these initial studies, the system has been used for laparoscopic procedures and ablation guidance.8 In our prior experience, we used tracked US in 50 cases over a 7-month period and in 6 cases (12%), the tracked US alone provided additional information that was required to perform the planned resection or ablation.7 These findings led to the current study with a focus specifically on identifying small colorectal cancer liver metastases.
As preoperative CT and MRI have improved, small lesions are identified with greater frequency, and it is common for surgeons to experience difficulty locating hepatic tumors in the operating room. While little has been published on this, there are reports of sonographically occult tumors during percutaneous interventions. In many parts of the world, US is used to guide percutaneous ablations due to its low cost, favorable safety profile, and lack of radiation. There are numerous studies from Europe and Asia that have described this difficulty. One series of 898 patients with small (<3 cm) hepatocellular carcinomas diagnosed on CT/MRI reported that 25% of tumors were not visible on US.14 Factors significantly associated with US occult tumors included tumor size, distance from the diaphragm, cirrhosis, and a macronodular liver. Interventional radiologists have developed multiple technologies to overcome the difficulty of relying on traditional b-mode US for percutaneous ablations. A series from South Korea reported on 44 patients with sono-graphically occult hepatic lesions using b-mode US.15 The mean tumor size was 1.8 cm. Using contrast-enhanced US, 38 of 44 tumors were identified and with biopsies proving accuracy. There are also systems that have been evaluated that use virtual navigation in patients who need percutaneous ablations and have tumors not visible with contrast-enhanced US. Mauri et al. have used an US-CT/MRI fusion guidance system to perform percutaneous ablations on 295 tumors not seen on contrast-enhanced US with a 90% success rate.16
There are limitations to the use of current liver image guidance systems. One difficulty is confirming accuracy. In a study evaluating the use of Explorer to guide minimally invasive liver ablations, 27 ablations were performed with both intraoperative US and Explorer guidance.17 There was a 5.5 mm +/−5.6 mm difference between the image guidance predicted positions and the intraoperative US image. Although this is not a direct measurement of accuracy, inaccuracy greater than 5–7 mm is clinically relevant. This is a limitation of the current system that is inherent to some locations of the liver. The current study removes this limitation of the image guidance system, as the guidance system is used to demonstrate a general area of liver with a tumor on preoperative imaging to the surgeon in order to have a more focused US examination. While surgeons traditionally do this using intrahepatic anatomic landmarks, the current study demonstrates this approach is not always accurate. Inaccuracy of even 1 cm using the Explorer system to localize a small tumor should have minimal effect on the ability of the surgeon to identify the tumor if it is indeed visible on guided US.
It is possible that future image guidance systems will combine additional technologies. There are mouse models, for example, that have demonstrated the use of indocyanine green for identification of liver tumors viewed with fluorescence goggles.18 Another approach uses electromagnetic tracking. One series reports a 93% first ablation attempt success rate using this system that tracks the tip of the ablation probe.19 Another technique is to place percutaneous coils into tumors prior to the use of chemotherapy. In one series of 46 patients with 57 poorly visible liver tumors, the use of intratumoral coils to guide ablations performed with unenhanced CT scans or percutaneous US was feasible.20 Image guidance surgery also has significant growth potential in the field oflaparoscopic liver surgery. With the added dexterity of laparoscopic equipment, tracking tools and ultra sound probes are able to reach the posterior and lateral aspects of the liver and avoid any interference from the patients’ ribs. While the present study only included two laparoscopic surgeries, it is our institutional practice to use the Explorer system routinely in high-risk cases, both open and laparoscopic, as the system works similarly in both scenarios.
There are several limitations to this study. First, there is no pathologic confirmation of ablated tumors. This is in line with our current practice in that tumors that have been identified as CRLM on preoperative imaging are not biopsied prior to resection or ablation. Pathologic correlation is more relevant for disappearing liver metastases, where there is no radiographic evidence of the tumor and a section of liver is empirically resected. A second limitation is that we did not use an ultrasonographer blinded to the results of the surgeons’ US examination. Instead we chose a real-world evaluation of this technology. The 5 surgeons whose patients were enrolled in this trial have all been in practice for more than 5 years in a center that performs over 300 liver resections annually, all of which are done using US. It is impossible to quantify the general ultrasonographic skills of this group, but we believe it is reasonable to conclude that this group has ultrasonographic skills that, at a minimum, are on par with most surgeons who perform liver resections. A third potential limitation is the short follow up time (6 months). This would be true if the outcomes for this trial were oncologic outcomes. Instead, the outcomes are technical - the utility of the Explorer device. So follow up time was not built into the study. A fourth limitation is the lack of contrast-enhanced US at our institution, as this method was not FDA-approved in the United States at the time of this study. One study from Japan described a series of 100 patients evaluated with preoperative CT, MRI, and contrast US and intraoperative US and contrast US.21 Only 67% of the patients were treated with chemotherapy and only 5% of known nodules were not identified with standard intraoperative US. In addition, steatosis is not described. It has been reported that contrast enhanced US is limited in patients with steatotic livers.22,23 The population in the Japanese series is different from ours. It is unclear whether this adjunct will improve the outcomes of ultrasonography in the setting of image guidance. In addition, contrast US is not performed with laparoscopy, whereas the Explorer system works with both open and laparoscopic surgery. Futures studies will determine if there is a synergistic role between these two modalities. Lastly, the image guidance system produced by Analogic, Inc. is no longer commercially available; however, a similar image-guidance system, CAS-ONE LIVER, produced by CAScination AG is available and FDA-approved for liver surgery. CAS-ONE costs $150,000 to $200,000 for purchase of the preoperative model-generating software and intraoperative system. It is common, however, for institutions to lease, instead of purchase, devices such as this. This system is also not specific to liver surgery and can be used for image guidance with other organs as well. There are limited recurring costs after purchase of the system as the tracking tools can be re-sterilized after each use.
Conclusions
Intraoperative imaging lags behind our capability to identify small tumors on preoperative imaging. While “disappearing liver metastases” are frequently discussed in the literature, metastases that are visible on preoperative imaging but are US occult in the operating room are not. As more and better chemotherapy is used and patient BMIs continue to rise, this problem will only grow. It is vital that intraoperative imaging capabilities catch up to the quality of preoperative imaging. Image guidance is the logical next step. This series is the first to prospectively demonstrate the ability of an image guidance system to help with the identification of colorectal cancer liver metastases and have a meaningful effect on intraoperative clinical decisions. This is an important first step toward increasing the research and implementation centered on image-guided liver surgery. Although the system clearly has room for development, this study proves, even with first-generation image guidance systems, that there is a clinical utility for guiding US to identify small colorectal cancer liver metastases after chemotherapy.
Source of funding
Analogic, Inc., reimbursed Drs. Kingham, Simpson, and Jarnagin for travel to a meeting with Analogic engineers in 2015. For the remaining authors, there are no disclosures. This study was funded in part by NIH/NCI grant R01 CA162477 and NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of interest
None to declare.
References
- 1.Steele G Jr., Ravikumar TS. (1989) Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 210:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D et al. (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657 [Discussion 65 7–65 8]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlik TM, Choti MA. (2007) Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 11:1057–1077. [DOI] [PubMed] [Google Scholar]
- 4.Benoist S, Brouquet A, Penna C, Julie C, El Hajjam M, Chagnon S et al. (2006) Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 24:3939–3945. [DOI] [PubMed] [Google Scholar]
- 5.Catalano O, Nunziata A, Lobianco R, Siani A. (2005) Real-time harmonic contrast material-specific US of focal liver lesions. Radiographics 25: 333–349. [DOI] [PubMed] [Google Scholar]
- 6.van Vledder MG, Torbenson MS, Pawlik TM, Boctor EM, Hamper UM, Olino K et al. (2010) The effect of steatosis on echogenicity of colorectal liver metastases on intraoperative ultrasonography. Arch Surg 145: 661–667. [DOI] [PubMed] [Google Scholar]
- 7.Kingham TP, Scherer MA, Neese BW, Clements LW, Stefansic JD, Jarnagin WR. (2012) Image-guided liver surgery: intraoperative projection of computed tomography images utilizing tracked ultrasound. HPB (Oxf) 14:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingham TP, Jayaraman S, Clements LW, Scherer MA, Stefansic JD, Jarnagin WR. (2013) Evolution of image-guided liver surgery: transition from open to laparoscopic procedures. J Gastrointest Surg 17: 1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230: 309–318 [Discussion 318–321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bale R, Widmann G, Jaschke W. (2011) Navigated open, laparoscopic, and percutaneous liver surgery. Minerva Chir 66:435–453. [PubMed] [Google Scholar]
- 11.Banz VM, Baechtold M, Weber S, Peterhans M, Inderbitzin D, Candinas D. (2014) Computer planned, image-guided combined resection and ablation for bilobar colorectal liver metastases. World J Gastroenterol 20:14992–14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements LW, Chapman WC, Dawant BM, Galloway RL Jr., Miga MI. (2008) Robust surface registration using salient anatomical features for image-guided liver surgery: algorithm and validation. Med Phys 35: 2528–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cash DM, Miga MI, Glasgow SC, Dawant BM, Clements LW, Cao Z et al. (2007) Concepts and preliminary data toward the realization of image-guided liver surgery. J Gastrointest Surg 11:844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim PN, Choi D, Rhim H, Rha SE, Hong HP, Lee J et al. (2012) Planning ultrasound for percutaneous radiofrequency ablation to treat small (</= 3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: a multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol 23:627–634. [DOI] [PubMed] [Google Scholar]
- 15.Yoon SH, Lee KH, Kim SY, Kim YH, Kim JH, Lee SH et al. (2010) Real time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol 20: 2047–2056. [DOI] [PubMed] [Google Scholar]
- 16.Mauri G, Cova L, De Beni S, Ierace T, Tondolo T, Cerri A et al. (2015) Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol 38:143–151. [DOI] [PubMed] [Google Scholar]
- 17.Hammill CW, Clements LW, Stefansic JD, W olf RF, Hansen PD, Gerber DA. (2014) Evaluation of a minimally invasive image-guided surgery system for hepatic ablation procedures. Surg Innov 21: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Akers WJ, Bauer AQ, Mondal S, Gullicksrud K, Sudlow GP et al. (2013) Intraoperative detection of liver tumors aided by a fluorescence goggle system and multimodal imaging. Analyst 138: 2254–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sindram D, Simo KA, Swan RZ, Razzaque S, Niemeyer DJ, Seshadri RM et al. (2015) Laparoscopic microwave ablation of human liver tumours using a novel three-dimensional magnetic guidance system. HPB (Oxf) 17:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farouil G, Deschamps F, Hakime A, de Baere T. (2014) Coil-assisted RFA of poorly visible liver tum ors: effectiveness and risk factors of local tum or progression. Cardiovasc Intervent Radiol 37: 716–722. [DOI] [PubMed] [Google Scholar]
- 21.Arita J, Ono Y, Takahashi M, Inoue Y, Takahashi Y, Matsueda K et al. (2015) Routine preoperative liver-specific magnetic resonance imaging does not exclude the necessity of contrast-enhanced intraoperative ultrasound in hepatic resection for colorectal liver metastasis. Ann Surg 262:1086–1091. [DOI] [PubMed] [Google Scholar]
- 22.Bartolotta TV, Midiri M, Scialpi M, Sciarrino E, Galia M, Lagalla R. (2004) Focal nodular hyperplasia in normal and fatty liver: a qualitative and quantitative evaluation with contrast-enhanced ultrasound. Eur Radiol 14:583–591. [DOI] [PubMed] [Google Scholar]
- 23.Shan QY, Chen LD, Zhou LY, Wang Z, Liu GJ, Huang Y et al. (2016) Focal lesions in fatty liver: if quantitative analysis facilitates the differentiation of atypical benign from malignant lesions. Sci Rep 6:18640. [DOI] [PMC free article] [PubMed] [Google Scholar]