Abstract
Objective:
CD4 count decline often triggers antiretroviral regimen switches in resource limited settings, even when viral load testing is available. We therefore compared CD4 failure and CD4 trends in patients with viremia with or without antiretroviral resistance.
Methods:
We conducted a retrospective cohort study investigating the association of HIV drug resistance with CD4 failure or CD4 trends in patients on first-line antiretroviral regimens during viremia.
Patients with viremia (HIV RNA > 1000 copies/mL) from two HIV treatment programs in South Africa (n=350) were included. We investigated the association of M184V and NNRTI resistance with World Health Organization immunological failure criteria and CD4 count trends, using chi-square tests and linear mixed models.
Results:
Fewer patients with the M184V mutation reached immunologic failure criteria than those without: 51/151(34%) versus 90/199 (45%) (p=0.03). Similarly, 79 of 220 (36%) patients, who had major NNRTI resistance, had immunological failure compared to 62 of 130 (48%) without (Chi square p= 0.03). The CD4 count decline among patients with the M184V mutation was 2.5 cells/mm3/year compared to 14 cells/mm3/year for those without M184V (p=0.1) but the difference in CD4 count decline with and without NNRTI resistance was marginal.
Conclusion:
Our data suggest that CD4 count monitoring may lead to inappropriate delayed therapy switches for patients with HIV drug resistance. Conversely, patients with viremia but no drug resistance are more likely to have a CD4 count decline and, thus, may be more likely to be switched to a second-line regimen.
Keywords: First-line antiretroviral therapy, immunologic failure criteria, HIV-1 viral load testing, HIV-1 drug resistance, adherence, M184V, Major NNRTI drug resistance mutations
Introduction
Antiretroviral therapy (ART) dramatically reduces mortality from HIV-associated disease (1,2) by suppressing HIV replication and allowing for CD4 count recovery. In order for ART to fully suppress HIV replication, high levels of adherence to pill taking is required(3). Failure to adhere to pill taking may lead to viral replication with CD4 decline and, eventually, HIV related illnesses. Intermittent adherence may also lead to the accumulation of HIV drug resistance mutations, preventing future virologic suppression even with subsequent improved adherence(4). Thus a primary goal of clinical and laboratory monitoring of ART is to detect viremia early in order to achieve virologic suppression through improved adherence without a regimen switch(5–7) or, in cases of sustained viremia, prompt switch to an alternative regimen. Unlike high income settings, HIV drug resistance testing is not routinely available to aid regimen switch decisions in most resource limited settings. Therefore laboratory monitoring relies on a combination of CD4 count testing and HIV RNA or CD4 count alone. In the absence of drug resistance testing, determining which patients with viremia need improved adherence and which patients need a regimen switch is often unclear and leaves considerable room for clinician discretion. Switching in the absence of drug resistance may leave the patient without clear additional alternatives and may fail to address underlying adherence issues (8). Delaying switch in the presence of drug resistance may lead to further emergence of resistance mutations compromising future regimen choices. Standardized algorithms have been promoted to optimize ART delivery from a public health approach. Specifically, World Health Organization guidelines recommend a regimen switch when the HIV RNA level remains >1000 copies/mL for over two months despite adherence interventions (9). The South African National Guidelines add if “adherence issues addressed” (10). In clinical practice some patients remain on the same ART regimen despite multiple assays demonstrating HIV RNA levels >1000 copies/mL (5,11,12) with regimen switch occurring only after a substantial decline in CD4 count (11,13,14)
As HIV RNA testing is expanded in areas which previously have only had access to CD4 count monitoring (9) clinicians need to learn the distinct roles of HIV RNA and CD4 count testing. Thus, we sought to test the hypothesis that among patients with viremia, CD4 count decline would be less in patients with HIV drug resistance than in patients without drug resistance (and likely very poor adherence). We hypothesized that the detection of M184V compared to no detectable M184V would be associated with less immunologic failure and a less steep CD4 count decline during viremia. This hypothesis is based on the rapid loss of the M184V mutation in the absence of selective pressure from taking antiretroviral agents.
Methods
Inclusion of patients and patient data
We combined data from two routine clinical HIV care programs in South Africa: the Tygerberg Hospital and the Aurum Institute workplace and community HIV care programs. These programs have provided HIV care starting in 2002 and have data on over 20,000 patients receiving ART (15,16) Patients included from the Tygerberg program were those with first-line ART treatment failure and HIV drug resistance testing as part of a study investigating its role in clinical management (17). Patients included from the Aurum program were those who had initiated ART and had follow-up HIV RNA monitoring results and had viremia and HIV drug resistance testing on stored plasma specimens as part of resistance surveillance or clinical research (5,18). Due to limited resources not all patients with viremia had HIV drug resistance testing in either program. Of the 23,215 patients, 4,674 (20%) had at least one HIV RNA >1000 copies/mL; 2,258 (10%) had two or more consecutive HIV RNA values of >1000 copies/mL, and 5,064 (22%) met WHO CD4 count failure criteria. In this convenience sample of 350 patients with HIV drug resistance results there was no consideration of CD4 count in selection for resistance testing. Resistance testing was performed either when clinical resources were available or on randomly selected (by random number generation) stored specimens among patients with viremia and available stored specimens. ART regimens in the Aurum programs were more likely to be a combination of zidovudine, lamivudine, and efavirenz compared to stavudine, lamivudine, and nevirapine in the Tygerberg program. Patients in both programs were started on ART based on CD4 count and clinical illness guidelines and received similar routine HIV RNA and CD4 count assays and general clinical management. HIV RNA monitoring and CD4 count testing occurred approximately every 6 months, according to guidelines in place at the time. Local laboratory information system databases and cohort clinic records were queried to obtain applicable patients’ CD4 count, HIV RNA, and ART regimen data, and the resultant patient data sets were merged with genotypic HIV drug resistance data. Human subject research approval for this retrospective analysis was obtained from the Johns Hopkins University Institutional Review Board, the University of KwaZulu-Natal Biomedical Research Ethics Committee, and the Stellenbosch University Health Research Ethics Committee.
Laboratory assays
HIV RNA was assayed with the Amplicor HIV-1 Monitor Test (Roche Diagnostics) or the NucliSens EasyQ HIV-1 assay (bioMerieux, Boxtel, Netherlands). HIV drug resistance genotyping was performed by PCR and Sanger sequencing using in-house methods (5,20).
Data analysis
We defined HIV viremia as HIV RNA >1000 copies/mL while on first-line therapy after an initial drop >1 log10 copies/mL from the pre-ART level. Persistent viremia was defined as HIV viremia >1000 copies/mL detected on at least two consecutive HIV RNA assays while on first-line therapy. WHO CD4 failure criteria were a fall of CD4 count to below the pre-ART value or persistent CD4 levels <100 cells/mm3 after >12 months of ART(9). Resistance mutations were defined based on the IAS-USA 2008 resistance guide(19).
Population characteristics were summarized using medians with interquartile ranges (IQR) or proportions. Resistance and CD4 count failure definition or change was assessed with chi-square testing or linear mixed models. The date of failure for linear mixed models was the date of a viral load of >1000 copies/mL after initial suppression to <400 copies/mL. We included presence of either the M184V mutation or any NNRTI resistance and HIV RNA level as fixed effects and cohort as a random effect. Assessment of the M184V mutation and NNRTI resistance were selected prior to data analysis. The M184V mutation was selected because its presence suggests continued selective pressure from partial or complete ART adherence. We selected any NNRTI mutation because this is the most common class of resistance mutations during first-line drug failure in our setting. Data were analysed using STATA 13 (STATA Corporation, College Station, TX)
Results
Cohort characteristics
We included 350 patients with HIV drug resistance testing, 166 (47%) were men, the median age was 37 years (IQR: 31, 43), the median CD4 count at ART initiation was 119 cells/mm3 (IQR: 68, 200). All participants had at least one HIV RNA >1000 copies/mL; 186/350 (53%) had persistent viremia while 141/350 (40%) met the WHO CD4 count failure criteria (Table).
Table:
Characteristics of 350 patients who had HIV drug resistance testing
| Category | Aurum | Tygerberg |
|---|---|---|
| Number (Percentage) or Median (interquartile range) |
Number (Percentage) or Median (interquartile range) |
|
| Cohort n | 184 | 166 |
| Sex | ||
| Male | 119 (65) | 47 (28) |
| Female | 65 (35) | 119 (72) |
| Age, median | 38 (33, 45) | 35 (29, 41) |
| CD4 count at ART initiation | 129 (64, 223) | 116 (68, 174) |
| Single HIV RNA >1000 | 184 (100) | 166 (100) |
| 2 consecutive VL>1000 (WHO virologic failure definition) | 88 (48) | 99 (60) |
| WHO immunologic failure criteria | 103 (56) | 38 (23) |
| Duration of ART at time of resistance testing, months | 20 (11, 31) | 14 (9.2, 24) |
| ART regimen at resistance testing | ||
| NRTI + lamivudine | ||
| AZT | 93 (51) | 26 (16) |
| D4T | 53 (29) | 138 (83) |
| TDF | 36 (20) | 2 (1.2) |
| NNRTI | ||
| Efavirenz | 131 (71) | 80 (48) |
| Nevirapine | 53 (29) | 87 (52) |
The median time from ART initiation to resistance testing was 17 months (IQR: 10, 29) and the median time from first HIV RNA >1000 copies/mL to resistance testing was 0 months (IQR: 0, 3.3); 308 (88%) of HIV drug resistance tests came from the first visit with an HIV RNA >1000 copies/mL; 151 (43%) had the M184V mutation while 220 (63%) had any NNRTI resistance, 117 (33%) of whom had the K103N mutation; 14 (4%) had thymidine analogue mutations (TAMs).
Associations of CD4 count changes with virologic failure and HIV drug resistance
When associating HIV drug resistance with immunologic failure among the 350 patients, only 51 of 151(34%), with the M184V mutation reached immunologic failure criteria compared to 90 of 199 (45%) without M184V (Chi square p=0.03). Similarly, 79 of 220 (36%) with a major NNRTI resistance mutation had immunological failure compared to 62 of 130 (48%) without NNRTI resistance (Chi square p= 0.03). There was no association between obtaining the resistance test at either the first detected viremia (164 patients) or a subsequent point during sustained viremia (186 patients) and HIV drug resistance (Chi square p=0.4 for M184V and p=0.6 for NNRTI mutations).
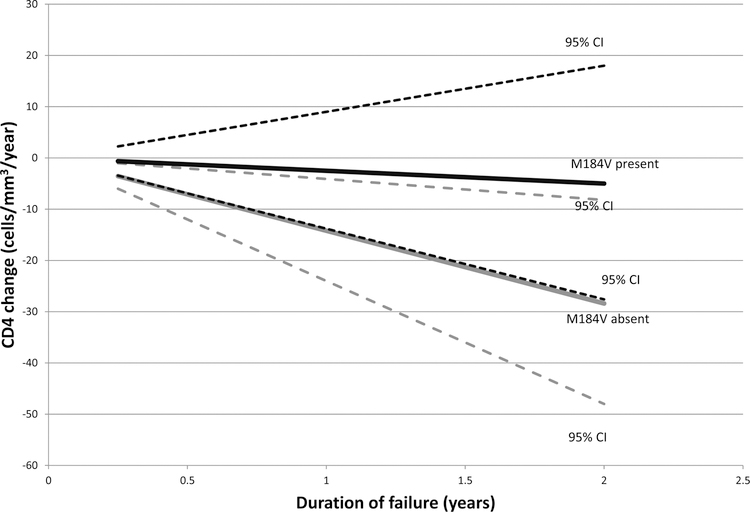
During periods of persistent viremia (186 patients) CD4 count declined by 10 cells/mm3/year (95% CI: −14, −5.7) compared to increasing by 80 cells/mm3/year (95% CI: 78, 82) during periods of suppression (HIV RNA <50 copies/mL). Among those with M184V and viremia the mean decline in CD4 count was 2.5 cells/mm3/year (95% CI: −14, 9.0) compared to a decline of 14 cells/mm3/year (95% CI: −24, −4.1) in those without M184V (p-value for difference 0.1; Figure). The difference in CD4 count decline with and without NNRTI resistance was not significant: a mean CD4 count decline of 7.9 cells/mm3/year (95% CI: −16, 0.66) with NNRTI resistance compared to a decline of 12 cells/mm3/year (95% CI: −27, 2.8) without NNRTI resistance (p-value for difference, 0.6).
Figure: CD4 count decline by presence of the M184V mutation and virologic failure (HIV RNA >1000 c/mL).
The slopes of CD4 decline and 95% confidence intervals in patients with or without the M184V mutation, in patients with persistent virologic failure, are shown. Black lines: M184V present; Grey lines: M184V absent. Broken lines indicate the upper and lower 95% confidence intervals.
Discussion
We have identified an important relationship between the presence of HIV drug resistance mutations and a lower chance of meeting immunologic failure criteria during viremia and a trend toward a slower CD4 count decline. In the absence of routinely available HIV drug resistance testing, switch decisions in the setting of viremia may incorporate CD4 count, particularly a more rapid CD4 count decline. This approach may misclassify patients in terms of the need for a regimen switch. Indeed, the current tendency in primary health clinics in South Africa of switching therapy only after evidence of immune decline in patients with viremia (11) may invert the optimal switch decision. Whereas patients with viremia and CD4 count decline are less likely to have failure as a result of HIV drug resistance and may need adherence support to achieve suppression patients with viremia and relatively preserved CD4 counts may have a higher probability of HIV drug resistance and may benefit from a regimen switch. Moreover without timely switching, these viremic patients could fuel onward transmitted resistance, compromising programmatic first-line regimen success (21).
CD4 count changes are only moderately predictive of persistent viremia (22–25). However a better gold standard may be whether CD4 count criteria accurately predict the presence of HIV drug resistance, the group that needs regimen change for therapeutic failure. When considering this standard, CD4 count is an even worse predictor. Indeed those with CD4 failure criteria are less likely to have resistance. While those who may most benefit from a regimen switch are less likely to meet CD4 failure criteria.
Our study has the advantage of being a natural experiment based in real-world practice. However it also has several limitations. Because it is based on clinical cohorts the precise timing that patients came for laboratory testing (or missed appointments) varies. In addition, because it is from routine clinical care, we lacked sophisticated types of adherence measures such as electronic pill bottles. Finally, we obtained drug resistance testing on a convenience sample that may not reflect the prevalence of HIV drug resistance within the cohorts. No test decisions were made based on CD4 count changes, thus there is no systematic bias regarding our study objectives from this approach.
Conclusion:
We believe that HIV RNA monitoring is the optimal approach for monitoring ART. However when combined with CD4 count decline, as has been observed to occur when the switch decision is largely up to clinician discretion, the predictive value is diminished. Eliminating CD4 count monitoring, during ART therapy, after patients with low baseline CD4 counts have reached a count of 200 cells/mm3 or above could be considered where HIV RNA testing is available, as this would avoid having management decisions being lead astray by inappropriate interpretation of CD4 count trends.
Acknowledgements:
All authors: CJH, JM and GvZ planned the investigation and wrote the manuscript draft. CJH conducted the statistical analysis. All authors approved the final manuscript.
Funding: CJH is partially supported by NIAID AI083099. JM is partially supported by R01MH105134 and GVZ is partially supported by R01MH105134 and U01CA200441.
Footnotes
Disclosures: All authors report no conflicts of interest with this work.
References:
- 1.UNAIDS. Getting to Zero: 2011–2015 Strategy. Joint United Nations Programme on HIV/AIDS [Internet] 2010. [cited 2015 Mar 17]. Available from: http://www.unaids.org/sites/default/files/sub_landing/files/JC2034_UNAIDS_Strategy_en.pdf
- 2.Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013. January;10(4):e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima VD, Bangsberg DR, Harrigan PR, Deeks SG, Yip B, Hogg RS, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr 2010. December;55(4):460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, Perry S, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS 2003. September 5;17(13):1925–32. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann CJ, Charalambous S, Sim J, Ledwaba J, Schwikkard G, Chaisson RE, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis 2009. December 15;49(12):1928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orrell C, Harling G, Lawn SD, Kaplan R, McNally M, Bekker L-G, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther 2007. January;12(1):83–8. [PubMed] [Google Scholar]
- 7.Chang LW, Harris J, Humphreys E. Optimal monitoring strategies for guiding when to switch first-line antiretroviral therapy regimens for treatment failure in adults and adolescents living with HIV in low-resource settings. Cochrane database Syst Rev 2010. January;(4):CD008494. [DOI] [PubMed]
- 8.Hoffmann CJ, Charalambous S, Grant AD, Morris L, Churchyard GJ, Chaisson RE. Durable HIV RNA resuppression after virologic failure while remaining on a first-line regimen: a cohort study. Trop Med Int Health 2014. February;19(2):236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Technical and operational considerations for implementing HIV viral load testing [Internet] World Health Organization; 2014. [cited 2015 Mar 16]. Available from: http://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/ [Google Scholar]
- 10.South African Department of Health. National consolidated guidelines for the prevention of mother to child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults [Internet] 2014. [cited 2015 Mar 16]. Available from: http://www.health.gov.za/docs/Policies/2014/HIV_Guidelines_Jan2015-final_edits-YP.pdf
- 11.Johnston V, Fielding KL, Charalambous S, Churchyard G, Phillips A, Grant AD. Outcomes following virological failure and predictors of switching to second-line antiretroviral therapy in a South African treatment program. J Acquir Immune Defic Syndr 2012. November 1;61(3):370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MP, Cutsem G Van, Giddy J, Maskew M, Keiser O, Prozesky H, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr 2012. August 1;60(4):428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimmel AD, Weinstein MC, Anglaret X, Goldie SJ, Losina E, Yazdanpanah Y, et al. Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr 2010. July;54(3):258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keebler D, Revill P, Braithwaite S, Phillips A, Blaser N, Borquez A, et al. Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Glob Heal 2014. January;2(1):e35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charalambous S, Grant AD, Innes C, Hoffmann CJ, Dowdeswell R, Pienaar J, et al. Association of isoniazid preventive therapy with lower early mortality in individuals on antiretroviral therapy in a workplace programme. AIDS 2010. November;24 Suppl 5:S5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Innes C, Hamilton R, Hoffmann CJ, Hippner P, Fielding K, Grant AD, et al. A novel HIV treatment model using private practitioners in South Africa. Sex Transm Infect. 2012. March;88(2):136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Zyl GU, van der Merwe L, Claassen M, Zeier M, Preiser W. Antiretroviral resistance patterns and factors associated with resistance in adult patients failing NNRTI-based regimens in the Western Cape, South Africa. J Med Virol 2011. October;83(10):1764–9. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann CJ, Ledwaba J, Li J-F, Johnston V, Hunt G, Fielding KL, et al. Resistance to tenofovir-based regimens during treatment failure of subtype C HIV-1 in South Africa. Antivir Ther 2013. January;18(7):915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson VA, Brun-Vezinet F, Clotet B, Gunthard HF, Kuritzkes DR, Pillay D, et al. Update of the Drug Resistance Mutations in HIV-1. Top HIV Med 2008. December;16(5):138–45. [PubMed] [Google Scholar]
- 20.Van Zyl GU, Claassen M, Engelbrecht S, Laten JD, Cotton MF, Theron GB, et al. Zidovudine with nevirapine for the prevention of HIV mother-to-child transmission reduces nevirapine resistance in mothers from the Western Cape, South Africa. J Med Virol 2008. June;80(6):942–6. [DOI] [PubMed] [Google Scholar]
- 21.Kantor R, Smeaton L, Vardhanabhuti S, Hudelson SE, Wallis CL, Tripathy S, et al. Pretreatment HIV Drug Resistance and HIV-1 Subtype C Are Independently Associated With Virologic Failure: Results From the Multinational PEARLS (ACTG A5175) Clinical Trial. Clin Infect Dis 2015. May;60(10):1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS 2008. October 1;22(15):1971–7. [DOI] [PubMed] [Google Scholar]
- 23.Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankalé J-L, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis 2011. December;53(12):1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantor R, Diero L, Delong A, Kamle L, Muyonga S, Mambo F, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource-limited settings. Clin Infect Dis 2009. August 1;49(3):454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallabhaneni S, Chandy S, Heylen E, Ekstrand ML. Evaluation of WHO immunologic criteria for treatment failure: implications for detection of virologic failure, evolution of drug resistance and choice of second-line therapy in India. J Int AIDS Soc 2013. January;16:18449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseinipour MC, van Oosterhout JJG, Weigel R, Phiri S, Kamwendo D, Parkin N, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS 2009. June 1;23(9):1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marconi VC, Sunpath H, Lu Z, Gordon M, Koranteng-Apeagyei K, Hampton J, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis 2008. May 15;46(10):1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]