Abstract
Background
The relapse rate of alcohol dependence (AD) after detoxification is high, but few studies have investigated the clinical predictors of relapse after hospitalized detoxification in real-world clinical practice, especially among Chinese patients.
Methods
This longitudinal cohort study followed up 122 AD patients who were discharged from January 1, 2016 to January 30, 2018 from their most recent hospitalization for detoxification. These patients were interviewed by telephone from May 20, 2017, to June 30, 2018, at least 6 months after discharge. During the interview, the relapse were assessed by using a revised Chinese version of the Alcohol Use Disorder Identification Test. Candidate predictors, such as therapeutic modalities during hospitalization and at discharge, medical history data related to alcohol use, and demographic information, were obtained from the medical records in the hospital information system.
Results
During the 6–24 months (with a median of 9 months) follow-up period, the relapse rate was 53.3%. Individuals with a college education level and those who had not been treated with the brief comprehensive cognitive-motivational-behavioural intervention (CCMBI) were more likely than their counterparts to relapse after hospitalized detoxification, and their adjusted HRs (95% CIs) were 1.85 (1.09, 3.16) and 2.00 (1.16, 3.46), respectively. The CCMBI use predicted a reduction in the relapse rate by approximately one-fifth.
Conclusion
Undergoing the CCMBI during detoxification hospitalization and having less than a college-level education could predict a reduced risk of AD relapse. These findings provide useful information both for further clinical research and for real-world practice.
Keywords: Alcohol dependence, Relapse, Real-world study, Psychotherapy, Pharmaceutical treatment
Introduction
Excessive alcohol consumption not only contributes to functional impairment and decreased well-being of individuals but also results in a large public health burden worldwide. At the global level in 2016, alcohol consumption accounted for 5.3% (approximately 3 million) of all deaths worldwide and 5.1% of disability-adjusted life years (DALYs) lost (World Health Organization, 2018). One of the key reasons is that excessive drinking often leads to alcohol dependence (AD), which, as a chronic recurrent encephalopathy, has a disease course marked by repeated relapse. A recent systematic review gave the estimated current and lifetime prevalence of AD in China as 2.2% and 3.7%, respectively (Cheng et al., 2015). Follow-up studies have shown that the relapse rates of AD during the first 6 months after inpatient or outpatient treatment ranged from 42.9% to 60% in Western patient groups, and the rate of lapse (resumption of alcohol use) in the first 12 months after hospitalized detoxification was 100% in a group of Chinese AD patients (Addolorato et al., 2007; Moos & Moos, 2006; Weisner, Matzger & Kaskutas, 2003; Wojnar et al., 2009; Gao et al., 2018).
Many effective treatments for AD, both pharmaceutical and psychological, have been available in real-world addiction treatment settings. Pharmaceutical maintenance medications, such as disulfiram, acamprosate, naltrexone, nalmefene and topiramate, have been proven to have a mild-to-moderate effect in reducing relapse (Goh & Morgan, 2017; Li et al., 2017). Compared to pharmaceutical treatments, the acceptability of psychotherapy is better due to medication side effects concerns. There has been supportive evidence for the effectiveness of several psychotherapy modalities, including motivational interviewing (MI), cue exposure treatment, various cognitive-behavioral treatments, and brief interventions as effective psycho-social modalities in the treatment of alcohol problems (Martin & Rehm, 2012; Mellentin et al., 2016). There is also increasing evidence on the better relapse-prevention effect of a combination of pharmacotherapy and psychosocial therapy than pharmaceutical mono-therapy (Assanangkornchai & Srisurapanont, 2007; Connor, Haber & Hall, 2016). In China, some researchers also investigated the efficacy of electro-acupuncture aversion therapy for AD and found that this treatment effectively reduces the lapse rate (Jin, Li & Yang, 2006; Zhang, Zhang & Fan, 2014).
However, the real-world clinical application of the above-mentioned effective treatments for AD is still limited. First, the aforementioned treatment modalities were derived from randomized controlled trials (RCTs) with questionable external validity, because their subjects were highly selective and conditions were highly controlled, rather different from real-world clinical practice (Kennedy-Martin et al., 2015; Rothwell, 2005). Second, although some of the aforementioned pharmaceutical treatments have been recommended to prevent alcoholism relapse by the Chinese Clinical Guideline of Diagnosis and Treatment for Alcohol Use Related Disorders (Li et al., 2017), it still remains unclear whether they are applicable to Chinese AD patients. Specifically, most of the supporting evidence for these preventive medications has come from studies among Western patient groups and most of these medications are not readily available in China (i.e., disulfiram, acamprosate, and nalmefene). In addition, in China, many psychiatrists might use an integrated package of multiple evidence-based psychotherapy modalities to treat AD, but the literature contains little research on the studies of such comprehensive psychotherapeutic packages.
To fulfill these needs, the present study was set out to investigate alcohol use disorder relapse after hospitalized detoxification and its association with treatment modalities during hospitalized detoxification and at discharge in a Chinese patient group with AD.
Methods
This real-world longitudinal cohort study was approved by the ethics committee of West China Hospital of Sichuan University in 2016 (No. 22), and verbal informed consent was obtained from each participant via telephone.
Participants
This study followed up 122 alcohol-dependent patients who were discharged from January 1, 2016, to January 30, 2018, from their most recent hospitalization for detoxification in the Psychological Comprehensive Ward of the Mental Health Center, West China Hospital of Sichuan University, China. Each patient had a primary clinical diagnosis of a behavioural or mental disorder due to use of alcohol (coded F10.2-F10.5 in ICD-10) and met the diagnostic criteria for AD (coded F10.2 in ICD-10). Patients with intellectual disability, head trauma, any illicit substance abuse or dependence, or severe physical disease were excluded.
Measurements
A case report form edited by the research group was used to collect the baseline data, including demographic information, alcohol resumption characteristics, and therapeutic modalities during hospitalization and at discharge.
Baseline measurements
Information about demographic information (including age and education), the hospital length of stay (LOS) days and data related to alcohol use (including age of first drink, years of alcohol use, and a past history of hospitalization for alcohol detoxification) was collected from inpatient medical records.
This study was concerned especially with whether brain atrophy was associated with relapse, as there is sufficient evidence to indicate that brain atrophy is the most specific and common brain pathology found by clinical neuroimaging scans (Dupuy & Chanraud, 2016). Given that brain structure pathologies are common among alcoholic patients seeking hospitalized detoxification (Bjork, Grant & Hommer, 2003), all 122 participants in the present study had undergone structural neuroimaging using clinical magnetic resonance imaging (MRI) or computed tomography (CT). Thus the presence of brain atrophy was based on the result of MRI or CT at admission of each patient. Smoking status was determined by self-reporting. Participants who had never used nicotine or quitted for more than three months were considered non-smokers; the remaining patients were considered smokers.
During hospitalized detoxification, all participants received pharmacological therapies for symptomatic and supportive treatment. Among these pharmacological therapies, this study was interested in the use of psychotropic medications including benzodiazepines, antidepressants, antipsychotics, and antiepileptics, which were taken by a substantial part of the participants, not only during hospitalization but also after discharge. Previous studies have indicated that many comorbid mental/brain conditions (disorders or symptoms) such as depression, anxiety, psychoses, insomnia, and epilepsy play important roles in the development of alcoholism as either risks or outcomes, and pharmaceutical intervention for these conditions might be effective for alcoholism relapse prevention (Agabio, Trogu & Pani, 2018; Klimkiewicz et al., 2015; Li et al., 2017). Among the aforementioned medications identified by RCTs as “effective” medications for relapse prevention, only topiramate was used by a substantial portion of the participants because it was the only one readily available in the hospital. Thus, topiramate was the only specific individual medication investigated in this study.
In addition to routine psychological supportive intervention and relevant health education for all alcoholic inpatients, the inpatient ward also provided a voluntary basis brief comprehensive cognitive-motivational-behavioural intervention (CCMBI) package service for AD patients. This study thus investigated whether the patients had received CCMBI service as a psychotherapeutic variable to predict AD relapse.
Outcomes
During the follow-up interview, all participants were asked “Have you ever drunk alcohol again since the latest discharge from the detoxification hospitalization?” Subjects who replied “yes” were further interviewed for their time (months) of the first re-drink and alcohol consumption of the most severe month after discharge using a revised Chinese version of the Alcohol Use Disorder Identification Test (AUDIT). The original Chinese version of AUDIT consists of 10 questions (total score range from 0 to 40) to estimate the severity of alcohol consumption in the preceding year (Babor, Higgins-Biddle & Robaina, 2014), different language versions (including the Chinese version) have been validated worldwide (Li et al., 2003). The timeframe of original Chinese AUDIT was revised from “twelve-month” to “one-month” for this study. Accordingly, this study defined a total AUDIT score ≥8 as relapse of alcohol use disorder (Babor, Higgins-Biddle & Robaina, 2014; Li et al., 2003). Time to re-drink was considered as the start point of relapse. The length (in months) of the interval between the last discharge and the follow-up interview (IDF) was also collected.
Follow-up interview procedure
The follow-up interview for each participant was administered by an experienced psychiatric nurse and a psychiatry resident, both of whom were trained to ensure that the interview was standardized. They interviewed all 122 participants over telephone from May 20, 2017, to June 30, 2018, that is, at least 6 months after their discharge. All participants were given general health education to encourage them to remain abstinence or come to further clinical assessment for their relapse at the follow-up telephone interview.
Statistical analysis
The rates and means (their 95% confidence intervals (95% CIs)) of baseline variables and outcomes were estimated using descriptive statistics. A univariate Cox proportional hazards regression model was implemented to assess the association between covariates and the probability of events (relapse) among individuals with AD. To determine the independent relapse predictors of AD, we performed multivariable Cox regression using probable predictors defined based on a two-tailed alpha level of 0.15 on univariate analysis as independent variables, as in a previous study (Boschloo et al., 2012). All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 22.0 for Windows), and final results were evaluated based on a two-tailed alpha level of 0.05.
Results
Descriptive demographic and clinical features of participants
All patients were males. The age of the sample ranged from 25 to 73 years and was 44.6 (95% CI [42.8, 46.4]) years on average. Accordingly, the quartile groups aged 25–36 years, 37–44 years, 45–53 years, and 54–73 years included 30 (24.6%), 34 (27.9%), 36 (29.5%), and 22 (18.0%) participants, respectively. Their median duration of past alcohol use was 20 years. Their average LOS was 15.4 (95% CI [14.2, 16.5]) days, and their lengths of IDF ranged from 6 to 24 months with a median number of 9 months. The descriptive statistics of other clinical features were listed in Table 1.
Table 1. The relapsea rate (%) after hospitalized alcohol dependence detoxification and its associations with demographic and clinical features.
| Sample ratio (%) | Relapse rate (95% CI)b | Univariable model | Multivariable model | ||
|---|---|---|---|---|---|
| X2c | HRd(95% CI)b | aHRe(95% CI)b | |||
| Total (n = 122) | 100 | 53.28 (44.30, 62.26) | – | – | |
| Age group (years) | – | 0.63 | – | – | |
| 25–36 | 24.59 | 56.67 (37.85, 75.49) | 1 | – | |
| 37–44 | 27.87 | 50.00 (32.29, 67.71) | 0.90 (0.46, 1.77) | – | |
| 45–53 | 29.51 | 58.33 (41.42, 75.25) | 1.00 (0.53, 1.90) | – | |
| 54–73 | 18.03 | 45.45 (22.86, 68.05) | 0.76 (0.35, 1.66) | – | |
| Education level | – | 3.42** | |||
| Lower than college | 62.30 | 47.37 (35.88, 58.85) | 1 | 1 | |
| College | 37.70 | 63.04 (48.55, 77.54) | 1.59(0.97,2.60)** | 1.85 (1.09, 3.16)* | |
| Years of alcohol use | – | 0.12 | |||
| Up to 20 years | 50.00 | 50.82 (37.91, 63.73) | 1 | – | |
| More than 20 years | 50.00 | 55.74 (42.91, 68.56) | 1.09(0.67,1.78) | – | |
| Smoker | – | 0.00 | |||
| Yes | 89.34 | 55.05 (45.56, 64.53) | 1.03(0.47,2.25) | – | |
| No | 10.66 | 38.46 (7.86, 69.06) | 1 | – | |
| First-time hospitalization for alcohol detoxification | – | 4.00* | |||
| Yes | 57.38 | 45.71 (33.75, 57.68) | 1 | 1 | |
| No | 42.63 | 63.46 (49.94, 77.00) | 1.64 (1.01, 2.67)* | 1.41 (0.85, 2.33) | |
| Brain atrophy | – | 0.78 | |||
| Yes | 30.33 | 45.95 (29.10, 62.79) | 0.78 (0.45, 1.36) | – | |
| No | 69.67 | 56.47 (45.71, 67.23) | 1 | – | |
| Treated with CCMBIf | – | 4.24* | |||
| Yes | 47.54 | 43.10 (29.97, 56.24) | 1 | 1 | |
| No | 52.46 | 62.50 (50.31, 74.69) | 1.69 (1.02, 2.78)* | 2.00 (1.16, 3.46)* | |
| Discharge with BDZg | – | 0.74 | |||
| Yes | 45.90 | 57.14(43.77,70.52) | 1 | – | |
| No | 54.10 | 50.00 (37.61, 62.39) | 0.81 (0.50, 1.31) | – | |
| Discharge with antipsychotics | – | 2.32** | |||
| Yes | 66.39 | 48.15 (37.03, 59.27) | 1 | 1 | |
| No | 33.61 | 63.41 (48.02, 78.81) | 1.47 (0.89, 2.41)** | 1.43 (0.85, 2.40) | |
| Discharge with antidepressants | – | 0.26 | |||
| Yes | 26.23 | 50.00 (31.68, 68.32) | 1 | – | |
| No | 73.77 | 54.44 (43.96, 64.93) | 1.16 (0.66, 2.04) | – | |
| Discharge with topiramate | – | 0.09 | |||
| Yes | 53.28 | 53.85 (41.40, 66.29) | 1 | – | |
| No | 46.72 | 52.63 (39.27, 66.00) | 0.93 (0.57, 1.51) | – | |
| Discharge with antiepileptics | – | 0.21 | |||
| Yes | 83.61 | 51.96 (42.10, 61.82) | 1 | – | |
| No | 16.39 | 60.00 (36.48, 83.52) | 1.16 (0.62, 2.17) | – | |
Notes.
Relapse: defined using the revised Chinese version of Alcohol Use Disorder Identification Test (AUDIT), assessing the alcohol consumption in the highest-severity month; the cutoff is ≥8.
95% CI, 95% confidence interval.
Based on Cox proportional hazards regression analysis.
HR, Hazard ratio based on univariate Cox proportional hazards regression analysis.
aHR, Adjusted hazard ratio based on multivariable Cox proportional hazards regression analysis; factors with P < 0.15 in the univariate analyses were entered into the statistical model.
Comprehensive cognitive-motivational-behavioural intervention (CCMBI): CCMBI was developed primarily based on the WHO manual for brief intervention for hazardous and harmful drinking, and added components of motivational interviewing, cue exposure, and aversion therapy.
BDZ: Benzodiazepines.
P < 0.05.
P < 0.15.
Rates of relapse
According to the follow-up interview, 53.3% (95% CI [44.3%, 62.3%]) of the participants were relapsed. The time from discharge to relapse ranged from 1 to 21 months, with a median of 2 months. The rates of relapse of each patient group by each categorical and dichotomous variable are listed in Table 1.
Identification of predictors of relapse
Among the variables in univariate Cox regression models to predict relapse (including LOS, the length of IDF and other variables listed in Table 1), the experience of repeated hospitalization for alcohol detoxification (p = 0.048), having been treated with the CCMBI (p = 0.042), education level (p = 0.067) and being discharged with antipsychotics (p = 0.130) were identified as probable predictors.
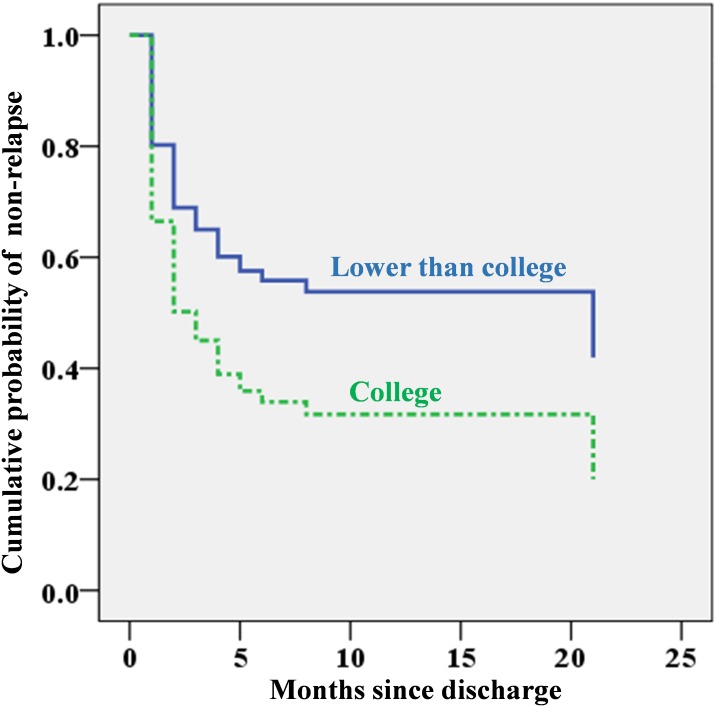
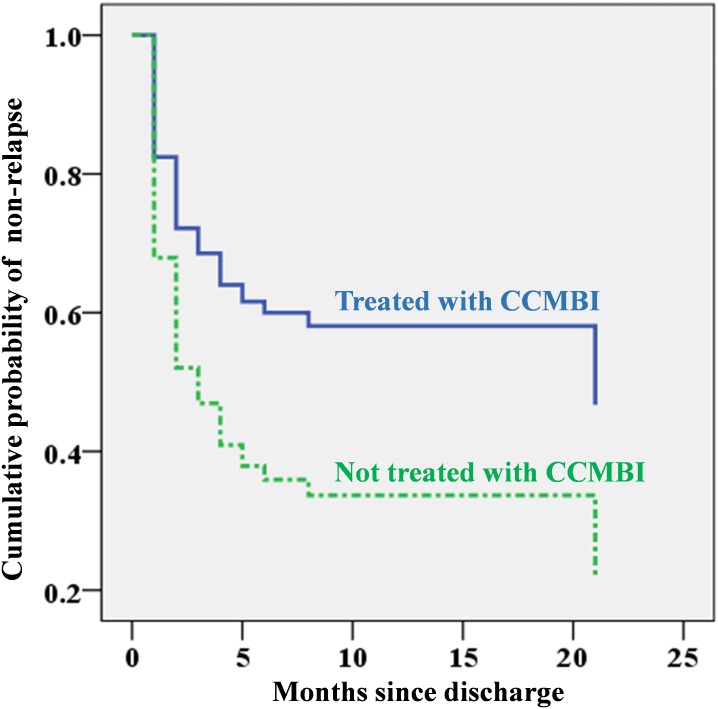
The multivariable Cox regression model to further explore the prediction property of these four probable predictors identified education level and CCMBI use as independent predictors. Individuals with a college education level and those who had not been treated with the CCMBI were more likely than their counterparts to relapse after hospitalized detoxification, and their adjusted HRs (95% CIs) were 1.85 (1.09, 3.16) and 2.00 (1.16, 3.46), respectively. The CCMBI use was associated with a reduction in relapse by approximately one-fifth in this study. The independent predictive effects of education level and CCMBI for relapse are depicted in Figs. 1 and 2.
Figure 1. Cumulative probability of non-relapse since discharge by education level based on Cox regression analysis (using whether the patient had previously been hospitalized for alcohol detoxification, use of the CCMBI, and discharge with antipsychotics as covariates).
Figure 2. Cumulative probability of non-relapse since discharge by whether the combined cognitive-motivational-behavioural intervention (CCMBI) was applied during hospitalization for alcohol detoxification based on Cox regression analysis (using education category, whether the patient had previously been hospitalized for alcohol detoxification, and discharge with antipsychotics as covariates).
Discussion
To our knowledge, this study is the first of its kind to explore real-world therapeutic predictors of alcoholism relapse, including both pharmaceutical and psychological predictors, during the months following hospitalized detoxification (at least six months of follow-up) in more than 100 patients. By doing so, this study found that the rate of alcohol use disorder relapse (53.3%) after detoxification in China was comparable to the international rates. The relapse rate found in this study was in the range of relapse rates of treated AD (from 42.9% to 60%) reported in different studies (Addolorato et al., 2007; Moos & Moos, 2006; Weisner, Matzger & Kaskutas, 2003; Wojnar et al., 2009).
The most exciting finding of this study was that ongoing CCMBI during detoxification hospitalization was a statistically significant predictor for less relapse of alcoholism. The CCMBI was associated with a reduction in relapse by approximately one-fifth and resulted in a HR of 2.00 in this study. A partial explanation for this effect size may be that the treatment itself was an integration of multiple components of effective psychotherapies, including components of the WHO manual for brief intervention for hazardous and harmful drinking, MI, cue exposure, and aversion therapy (Li et al., 2017; Miller & Rollnick, 2012; McQueen, Ballinger & Howe, 2017; Penberthy et al., 2011). However, these results should be interpreted with caution because patients who consent to receive the CCMBI in real-world clinical practice may have stronger motivation to accept and comply with treatment—and may consequently have a better prognosis—than their counterparts (Bauer, Strik & Moggi, 2014). Additional strictly designed RCT studies are needed to test the real therapeutic efficacy of the CCMBI. Nonetheless, integrating the findings of this study with the previously documented evidence that psychotherapy improved the prognosis of AD (Martin & Rehm, 2012; Mellentin et al., 2016; Jin, Li & Yang, 2006; Zhang, Zhang & Fan, 2014), the CCMBI is worthy of recommendation for use in routine real-world clinical practice.
A surprising finding of this study is that college-level education, compared to a lower education level, was a predictor for relapse. Previous studies usually reported that a lower education level was associated with a higher relapse rate (Bottlender & Soyka, 2005; Moos & Moos, 2006), supporting a popular interpretation wherein individuals with a lower education level have poorer awareness or knowledge of alcohol-related health problems (Moos & Moos, 2006). However, Boschloo et al. (2012) have found that a high education level was a significant risk factor for the persistence of AD. The reasons for a higher education level being associated with relapse of AD or persistence of AD are complex but could be at least partly explained as follows: First, assuming that individuals with a higher education level may have already had greater awareness or knowledge of alcohol-related health problems, they might thus receive less benefit from health education, which is considered as an important component of therapeutic intervention for AD (WHO, 2001). Second, the aetiology of AD in patients with a higher education level might be attributable in larger part to factors other than poor health awareness or knowledge, such as stress, family history of AD, and genes that could decrease the response to treatment or prevention. Last but not least, the social and cultural circumstances in China made alcoholic beverages a popular element of social occasions, especially for highly educated groups, and patients with higher education may have more “social burdens and pressure” to drink or be exposed to more cues related to alcohol use (Zhao, 2006).
Unexpectedly, this study did not find any investigated pharmaceutical interventions that were significantly associated with relapse of AD, even in the case of topiramate, whose effectiveness in reducing AD has been repeatedly identified by published research (Blodgett et al., 2014; Goh & Morgan, 2017). However, the findings regarding these medications’ lack of preventive effectiveness against AD relapse need to be interpreted carefully due to the following relevant limitations of this real-world study: First, this study analysed medications prescribed at discharge but did not assess patients’ compliance with the prescriptions. Second, most investigated medications in this study were used to address or prevent comorbid mental disorders or symptoms, which themselves might influence the prognosis of AD (Schellekens et al., 2015; Suter, Strik & Moggi, 2011). This study, however, failed to control such confounding effects due to the lack of systemic, standardized, or structured measurements for comorbid disorders or symptoms in real-word clinical practice. From this point of view, more measurement-based clinical practice would improve the quality of real-world clinical research. In addition, the sample size of this study is insufficient to generate statistical significance for predictors with small effect size.
This study has several other limitations in addition to the ones mentioned above. As the participants were AD patients who had experienced hospitalized detoxification, this study may have been be biased towards severe cases, causing the probability of relapse to be overestimated (Cohen, 1984). Because patients were recruited from only one hospital, the study might lead to selection bias. Although measurements of AD relapse through telephone interviews have been validated and used in some previous studies (Glass et al., 2017; Moos & Moos, 2006), they could be further validated in this Chinese patient group with the assistance of laboratory test indicators, such as serum ethanol concentration and carbohydrate-deficient transferrin (CDT) (Bortolotti et al., 2018). As the participants were all males, the findings of this study should not be generalized to female patients, although a recent review indicated that the relapse rate of AD was similar between genders (Petit et al., 2017).
Conclusion
The aforementioned limitations notwithstanding, this study found that the brief CCMBI during detoxification hospitalization and an education level lower than college predicted a reduced risk of AD relapse. These findings provide useful information both for further clinical research and for real-world practice.
Supplemental Information
Acknowledgments
The authors wish to thank the participants in this study for their cooperation.
Funding Statement
This work was supported by a grant from the National Scientific and Technical Fund of China (grant No. 81571305), the Department of Science and Technology of Sichuan provincial government (grant No. 2019YFS0153), and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yu-Jie Tao and Wan-Jun Guo conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Li Hu conceived and designed the experiments, performed the experiments, approved the final draft.
Ying He, Bing-Rong Cao, Juan Chen, Ying-Hua Ye, Ting Chen, Jia-Jun Xu, Jing Li, Ya-Jing Meng and Tao Li performed the experiments.
Xia Yang performed the experiments, contributed reagents/materials/analysis tools.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the Ethics Committee of the West China Hospital, Sichuan University in 2016 (No. 22).
Data Availability
The following information was supplied regarding data availability:
Data is available as Supplemental File.
References
- Addolorato et al. (2007).Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D’Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. The Lancet. 2007;370:1915–1922. doi: 10.1016/s0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Agabio, Trogu & Pani (2018).Agabio R, Trogu E, Pani PP. The cochrane library. Hoboken: John Wiley & Sons, Ltd; 2018. Antidepressants for the treatment of people with co-occurring depression and alcohol dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assanangkornchai & Srisurapanont (2007).Assanangkornchai S, Srisurapanont M. The treatment of alcohol dependence. Current Opinion in Psychiatry. 2007;20:222–227. doi: 10.1097/YCO.0b013e3280fa837d. [DOI] [PubMed] [Google Scholar]
- Babor, Higgins-Biddle & Robaina (2014).Babor TF, Higgins-Biddle JC, Robaina K. The alcohol use disorders identification test, adapted for use in the United States: a guide for primary care practitioners. Geneva: World Health Organization; 2014. [Google Scholar]
- Bauer, Strik & Moggi (2014).Bauer S, Strik W, Moggi F. Motivation as a predictor of drinking outcomes after residential treatment programs for alcohol dependence. Journal of Addiction Medicine. 2014;8:137–142. doi: 10.1097/ADM.0000000000000013. [DOI] [PubMed] [Google Scholar]
- Bjork, Grant & Hommer (2003).Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. The American Journal of Psychiatry. 2003;160:2038–2045. doi: 10.1176/appi.ajp.160.11.2038. [DOI] [PubMed] [Google Scholar]
- Blodgett et al. (2014).Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcoholism, Clinical and Experimental Research. 2014;38:1481–1488. doi: 10.1111/acer.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti et al. (2018).Bortolotti F, Sorio D, Bertaso A, Tagliaro F. Analytical and diagnostic aspects of carbohydrate deficient transferrin (CDT): a critical review over years 2007–2017. Journal of Pharmaceutical and Biomedical Analysis. 2018;147:2–12. doi: 10.1016/j.jpba.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Boschloo et al. (2012).Boschloo L, Vogelzangs N, Van den Brink W, Smit JH, Beekman AT, Penninx BW. Predictors of the 2-year recurrence and persistence of alcohol dependence. Addiction. 2012;107:1639–1640. doi: 10.1111/j.1360-0443.2012.03860. [DOI] [PubMed] [Google Scholar]
- Bottlender & Soyka (2005).Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: predictors of outcome 6 months after treatment. European Addiction Research. 2005;11:132–137. doi: 10.1159/000085548. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2015).Cheng HG, Deng F, Xiong W, Phillips MR. Prevalence of alcohol use disorders in mainland China: a systematic review. Addiction. 2015;110:761–774. doi: 10.1111/add.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen (1984).Cohen PCJ. The clinician’s illusion. Archives of General Psychiatry. 1984;41:1178–1182. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- Connor, Haber & Hall (2016).Connor JP, Haber PS, Hall WD. Alcohol use disorders. The Lancet. 2016;387:988–998. doi: 10.1016/s0140-6736(15)00122-1. [DOI] [PubMed] [Google Scholar]
- Dupuy & Chanraud (2016).Dupuy M, Chanraud S. Imaging the addicted brain: alcohol. International Review Neurobiology. 2016;129:1–31. doi: 10.1016/bs.irn.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2018).Gao Z, Wang Z, Cao B-r, He Y, Xu R-j, Li J. Determinants of drinking relapse after treatment in patients with alcohol dependence in Sichuan Province. Journal of Sichuan University (Med Sci Edi) 2018;49:264–270. [PubMed] [Google Scholar]
- Glass et al. (2017).Glass JE, McKay JR, Gustafson DH, Kornfield R, Rathouz PJ, McTavish FM, Atwood AK, Isham A, Quanbeck A, Shah D. Treatment seeking as a mechanism of change in a randomized controlled trial of a mobile health intervention to support recovery from alcohol use disorders. Journal of Substance Abuse Treatment. 2017;77:57–66. doi: 10.1016/j.jsat.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh & Morgan (2017).Goh ET, Morgan M. Review article: pharmacotherapy for alcohol dependence—the why, the what and the wherefore. Alimentary Pharmacology and Therapeutics. 2017;45:865–882. doi: 10.1111/apt.13965. [DOI] [PubMed] [Google Scholar]
- Jin, Li & Yang (2006).Jin M, Li J, Yang YC. Evaluation of the clinical effect of electro-acupuncture aversion therapy of alcohol-dependence. Chinese Journal of Clinical Rehabilitation. 2006;10:18–20. [Google Scholar]
- Kennedy-Martin et al. (2015).Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16(495) doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimkiewicz et al. (2015).Klimkiewicz A, Klimkiewicz J, Jakubczyk A, Kieres-Salomoński I, Wojnar M. Comorbidity of alcohol dependence with other psychiatric disorders. Part II. Pathogenesis and treatment. Psychiatria Polska. 2015;49:277–294. doi: 10.12740/PP/26071. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li J, Hao W, Li J, Guo W-J, Sun H-Q, Wang C-S, Wang X-Y, Zhao M, Zhou X-B. Clinical guidelines for the diagnosis and treatment of alcohol use-related disorders. People’s Medical Publishing House Co. Ltd; Beijing: 2017. [Google Scholar]
- Li et al. (2003).Li B, Shen Y-C, Zhang B-Q, Zhen X-H, Wang X-G. The test of AUDIT in China. Chinese Mental Health Journal. 2003;17:1–3. [Google Scholar]
- Martin & Rehm (2012).Martin GW, Rehm J. The effectiveness of psychosocial modalities in the treatment of alcohol problems in adults: a review of the evidence. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2012;57:350–358. doi: 10.1177/070674371205700604. [DOI] [PubMed] [Google Scholar]
- McQueen, Ballinger & Howe (2017).McQueen JM, Ballinger C, Howe TE. Factors associated with alcohol reduction in harmful and hazardous drinkers following alcohol brief intervention in Scotland: a qualitative enquiry. BMC Health Services Research. 2017;17:181. doi: 10.1186/s12913-017-2093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellentin et al. (2016).Mellentin AI, Nielsen B, Nielsen AS, Yu F, Stenager E. A randomized controlled study of exposure therapy as aftercare for alcohol use disorder: study protocol. BMC Psychiatry. 2016;16:112. doi: 10.1186/s12888-016-0795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller & Rollnick (2012).Miller WR, Rollnick S. Motivational interviewing: preparing people for change. Second Edition Guilford Press; New York: 2012. [Google Scholar]
- Moos & Moos (2006).Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy et al. (2011).Penberthy JK, Hook JN, Vaughan MD, Davis DE, Wagley JN, Diclemente CC, Johnson BA. Impact of motivational changes on drinking outcomes in pharmacobehavioral treatment for alcohol dependence. Alcoholism, Clinical and Experimental Research. 2011;35:1694–1704. doi: 10.1111/j.1530-0277.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- Petit et al. (2017).Petit G, Luminet O, Cordovil de Sousa Uva M, Monhonval P, Leclercq S, Spilliaert Q, Zammit F, Maurage P, De Timary P. Gender differences in affects and craving in alcohol-dependence: a study during alcohol detoxification. Alcoholism, Clinical and Experimental Research. 2017;41:421–431. doi: 10.1111/acer.13292. [DOI] [PubMed] [Google Scholar]
- Rothwell (2005).Rothwell PM. External validity of randomised controlled trials: to whom do the results of this trial apply? The Lancet. 2005;365:82–93. doi: 10.1016/s0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- Schellekens et al. (2015).Schellekens AF, De Jong CA, Buitelaar JK, Verkes RJ. Co-morbid anxiety disorders predict early relapse after inpatient alcohol treatment. European Psychiatry. 2015;30:128–136. doi: 10.1016/j.eurpsy.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Suter, Strik & Moggi (2011).Suter M, Strik W, Moggi F. Depressive symptoms as a predictor of alcohol relapse after residential treatment programs for alcohol use disorder. Journal of Substance Abuse Treatment. 2011;41:225–232. doi: 10.1016/j.jsat.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Weisner, Matzger & Kaskutas (2003).Weisner C, Matzger H, Kaskutas LA. How important is treatment? One-year outcomes of treated and untreated alcohol-dependent individuals. Addiction. 2003;98:901–911. doi: 10.1046/j.1360-0443.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- Wojnar et al. (2009).Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Sliwerska E, Burmeister M. Association between Val66Met Brain-Derived Neurotrophic Factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcoholism, Clinical and Experimental Research. 2009;33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2001).World Health Organization (WHO) Geneva: World Health Organizationhttp://www.who.int/iris/handle/10665/67210 Brief intervention for hazardous and harmful drinking: a manual for use in primary care/Thomas F. Babor, John C. Higgins-Biddle. 2001
- World Health Organization (2018).World Health Organization (WHO) Geneva: World Health Organizationhttps://www.who.int/substance_abuse/publications/global_alcohol_report/en/ Global status report on alcohol and health 2018. 2018
- Zhang, Zhang & Fan (2014).Zhang Q-F, Zhang L, Fan Y-J. Clinical efficacy of electric acupuncture aversion therapy in treatment of alcohol dependence. Medical Journal of Chinese People’s Health. 2014;26:13–15. [Google Scholar]
- Zhao (2006).Zhao R-G. The evolvement of ancient traditional drinking concept and the times’character of Chinese drinking. Dietetic Culture Research. 2006;1:59–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Data is available as Supplemental File.