Primary sclerosing cholangitis (PSC) is characterized by progressive fibrosis around intrahepatic and extrahepatic bile ducts that can progress to cirrhosis and liver failure. There are no regulatory drugs approved for PSC, and liver transplantation is the only effective therapy.(1) Although the concentric accumulation of periductular fibrosis suggests the biliary epithelial cell (cholangiocyte) plays an integral role in disease initiation and/or progression, multiple factors from the gut, like microbes and their molecular products, endogenous metabolites like bile acids, and primed immune cells, have been hypothesized to play a significant role in the hepatobiliary injury and adverse clinical sequelae in PSC. Several lines of evidence suggest a role for gut dysbiosis in the pathogenesis of PSC. Principle among these is that more than 70% of patients with PSC also have inflammatory bowel disease (IBD),(1) typically ulcerative colitis (UC), a chronic intestinal disorder associated with dysbiosis and intestinal epithelial barrier defects.(2) The significance of the association between PSC and UC remains unknown. A recent publication by Nakamoto et al. interrogates a possible link between PSC/UC-associated gut microbial dysbiosis, expansion of potentially pathologic symbionts (pathobionts), and immunological consequences with the pathophysiology of PSC.(3) Using an impressive combination of approaches, including mice recolonized with human fecal samples, microbial sequencing, bacterial culture, and organoid cell culture techniques, they implicate a subset of PSC/UC-associated microorganisms as central mediators of bacterial translocation, immune regulation, and disease progression (Fig. 1).
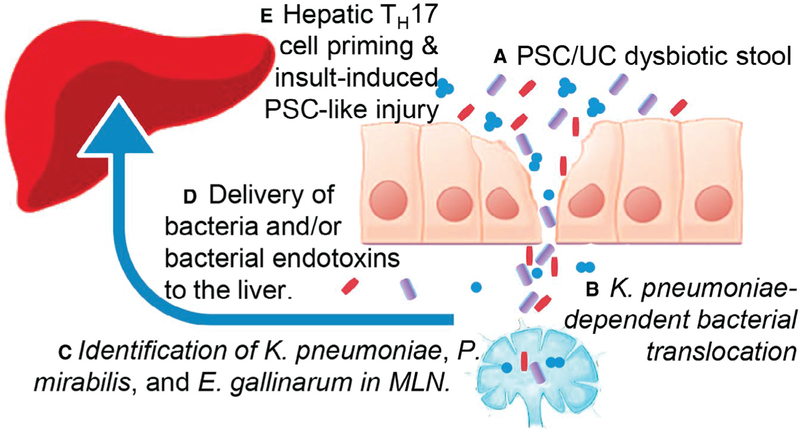
FIG. 1.
Using mice recolonized with human fecal samples from PSC/UC (A), UC, or healthy control patients and a monolayer culture system of human intestinal organoids, Nakamoto et al., found that the pathobiont, Klebsiella pneumoniae, promoted bacterial translocation via intestinal epithelial cell barrier dysfunction (B). They further identified three bacterial species that translocated to the mesenteric lymph nodes (MLN) of recolonized mice (C). The bacteria and/or bacterial endotoxins from these three microbes were delivered to the liver (D) and cooperatively promoted TH17 priming (E). In the TH17 primed liver, a second trigger, such as the experimentally induced injury with DDC or taurocholic acid, promoted severe hepatobiliary injury.
Initially, germ-free (GF) mice were recolonized with fecal samples derived from patients with PSC/UC (PSCUC mice), UC only (UC mice), or healthy controls (HC; HC mice). T-cell characterization revealed that hepatic TH17 primed cells were observed solely in a subset of the PSCUC mice. Additionally, the PSCUC mice exhibited increased expression of hepatic inflammatory markers; however, no histological or serological changes were observed in the liver. To further explore the role of the gut microbial composition in liver injury, the groups of recolonized mice were placed on a diet containing 3,5-dicarbethoxy-1, 4-dihydrocollidine (DDC), a model of periductular fibrosis and inflammation resembling PSC. They found that the PSCUC mice exhibited increased hepatic TH17 T cells, periductular fibrosis, and elevated serological markers of liver injury compared with GF or HC mice. These data were consistent with unique PSC/UC-associated gut microbes that promote bacterial translocation, TH17 priming, and hepatobiliary injury, yet also suggest that while the microbial profile may prime the hepatic environment for pathological processes, an injurious stimulus (i.e., DDC) promotes hepatobiliary disease manifestation.
The authors next attempted to grow bacteria from the livers, mesenteric lymph nodes (MLNs), and spleens from the recolonized mice. Bacteria were exclusively grown from the MLNs of PSCUC mice, and 16s sequencing identified Klebsiella pneumoniae, Proteus mirabilis, and Enterococcus gallinarum. Mice colonized with only these three bacterial species (3-mix mice) showed a strong TH17 response in both the colon and liver, and the bacteria efficiently translocated to the MLN. Mono-colonization or dual-colonization of GF mice was less efficient in TH17 induction, suggesting that the three bacteria cooperatively promoted a TH17 response. Moreover, selective inhibition of crial species that translocated from the gut to the MLN, promoted a hepatic TH17 immune response, and recapitulated the severe DDC-induced disease observed in PSCUC mice.
Athough the three bacteria cooperatively promoted a robust TH17 response, mono-colonization of K. pneumoniae promoted bacterial translocation through the intestinal epithelia. Of note, increased abundance of the family Enterobacteriaceae, including K. pneumonia, in primary biliary cholangitis has been implicated in the metagenomics-based identification of the inferred pathway “bacterial invasion of epithelia,”(4) which suggests that bacterial translocation may represent a common pathological process in establishing a hepatic environment primed for hepatobiliary pathology. To explore further the role of K. pneumoniae in bacterial translocation, the authors used a monolayer culture of intestinal organoids. K. pneumoniae isolates induced epithelial pore structures (i.e., structures that deteriorate epithelial barrier function). The pore-forming ability was strain-specific, as 4 of 11 K. pneumoniae isolates failed to form intestinal epithelial pores. Moreover, gnotobiotic mice in which a non-pore-forming K. pneumoniae was substituted for a pore-forming K. pneumoniae strain suggested that the pore-forming ability of K. pneumoniae was central to initiating the TH17 response and promoting liver injury in the DDC-fed mouse model. Of interest, the severe hepatobiliary injury and elevated TH17 response in PSCUC mice was also observed in the taurocholic acid–feeding model of hepatobiliary injury. These results reinforce the pathological contributions of the microorganisms and highlight a potential role of bile acids in microorganismassociated hepatobiliary disease. Finally, to determine whether antibiotic treatment modulates liver immune responses, PSCUC mice were treated with either metronidazole or vancomycin. Metronidazole and vancomycin have shown promising results in uncontrolled studies as a therapy for PSC (especially vancomycin) and possess antibacterial properties against K. pneumoniae and E. gallinarum, respectively. These PSCUC mice showed a robust TH17 hepatic immune response that was significantly reduced with either antibiotic. Given that K. pneumonia should not be affected by vancomycin, yet was essential for pore formation in their in vitro model, it was proposed that the decrease in TH17 in vancomycin-treated mice suggested the presence of additional pore-forming microbes targeted by vancomycin, revealing the complexity of both the gut microbiota and the immune response.
Thus, the major findings of this interesting work were the identification of pathobionts associated with PSC/UC that promote epithelial barrier dysfunction, the inflammatory Th17 T-cell response, and worsened disease outcomes in mouse models of biliary disease. These studies provide a mechanistic link between gut microbes and PSC/UC, and importantly, provide preclinical evidence for targeted microbial therapeutics for PSC. The interpretation of this work needs to be viewed from the perspective of the other known features that contribute to PSC pathogenesis. Genome-wide association studies have revealed more than 20 risk genes, including those implicated in immunity (e.g., human leukocyte antigen).(1) However, the non-Mendelian inheritance of this disease supports that environmental exposures are a predominant risk. Microbes represent one of many environmental exposures that may influence PSC initiation or progression. The concentric periductular fibrosis also implicates the cholangiocyte as a central contributor to the pathogenesis of PSC. Indeed, cholangiocytes sense and respond to direct pathogen infection (e.g., Cryptosporidium parvum) as well as microbially derived molecules (e.g., lipopolysaccharide).(5) Additionally, the gut microbiota of PSC patients is distinct from IBD patients and healthy controls(6); however, the relationship between the gut microbiota and the liver is bidirectional. For example, bile can shape intestinal microbiota composition, whereas microbes and their metabolites provided to the liver via the portal circulation or directly from retrograde migration into the biliary tree influence hepatic function. Therefore, bile from a cholestatic liver should modify gut microbial composition; hence, the cause versus effect nature of hepatobiliary injury and dysbiosis remains obscure. Further support for a role of intestinal microbiota in PSC comes from studies performed in GF mice. In the bile acid toxicity model (Abcb4−/−), an aggravation of bile duct disease was observed in the GF compared with conventionally raised mice.(7) In contrast, the immune-driven NOD.c3c4 model(8) and the model presented by Nakamoto et al. suggests that the lack of microbes has a protective effect on bile duct disease progression. These differences highlight the complexity of the relationship between the gut and liver and require further clarification. Although validation of the identified microorganisms and further mechanistic explorations of hepatic TH17 differentiation and how these cells influence PSC is needed, the work is a major step forward in understanding the link between the gut and hepatobiliary pathology. Moreover, the study demonstrates that by digging deeper into the data, we can move beyond observational and correlative microbiome studies and perform hypothesis-driven microbiome research.
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1).Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016;375:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365: 1713–1725. [DOI] [PubMed] [Google Scholar]
- 3).Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol 2019;4:492–503. [DOI] [PubMed] [Google Scholar]
- 4).Tang R, Wei Y, Li Y, Chen W, Chen H, Wang Q, et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 2018;67:534–541. [DOI] [PubMed] [Google Scholar]
- 5).Chen XM, O’Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol 2008;86:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Karlsen TH. Primary sclerosing cholangitis: 50 years of a gut-liver relationship and still no love? Gut 2016;65:1579–1581. [DOI] [PubMed] [Google Scholar]
- 7).Tabibian JH, O’Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology 2016;63:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Schrumpf E, Kummen M, Valestrand L, Greiner TU, Holm K, Arulampalam V, et al. The gut microbiota contributes to a mouse model of spontaneous bile duct inflammation. J Hepatol 2017;66:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]