Abstract
Background:
Emerging evidence suggests airborne metals may be associated with breast cancer risk. However, breast cancer is heterogenous and associations with heavy metals vary by subtype. Heavy metals possess both carcinogenic and xenoestrogenic properties which may be related to different tumor etiologies. Therefore, we tested for etiologic heterogeneity, using a case-series approach, to determine whether associations between residential airborne metal concentrations and breast cancer differed by tumor subtype.
Methods:
Between 2005–2008, we enrolled incident breast cancer cases into the Breast Cancer Care in Chicago study. Tumor estrogen and progesterone receptors status was determined by medical record abstraction and confirmed immunohistochemically (N=696; 147 ER/PR-negative). The 2002 USEPA’s National Air Toxics Assessment census-tract estimates of metal concentrations (antimony, arsenic, beryllium, cadmium, chromium, cobalt, lead, manganese, mercury, nickel and selenium) were matched to participants’ residences of the same year. Adjusted logistic regression models were used to examine whether the airborne heavy metal associations differed by tumor ER/PR status. Principal component analysis was performed to assess associations by metal co-exposures.
Results:
Comparing the highest and lowest quintiles, higher concentrations of antimony (odds ratio[OR]: 1.8, 95% confidence interval[CI]: 0.9, 3.7, P-trend: 0.05), cadmium (OR: 2.3, 95% CI: 1.2, 4.4, P-trend: 0.04) and cobalt (OR: 2.0, 95% CI: 0.9, 4.4, P-trend: 0.04) were associated with ER/PR-negative breast cancer. Mixture analysis using principal components suggested co-exposures to multiple airborne heavy metals may drive associations with tumor receptor status.
Conclusions:
Among women diagnosed with breast cancer, metallic air pollutants were associated with increased odds of developing ER/PR-negative breast cancer.
Keywords: Breast cancer, Air pollution, Heavy metals, ER/PR status, NATA
1. Introduction
Air pollution affects large populations and may be associated with the development of breast cancer [1–7]. Metallic components of air pollution are of interest due to their long half-lives and ability to accumulate in breast tissue [8]. Emerging evidence from large, prospective cohorts suggest that airborne metals may be related to breast cancer risk. For instance, associations have been reported for antimony, arsenic, cadmium, cobalt, mercury and lead [9, 10]. These studies suggest airborne metals may affect breast cancer risk in a subtype-specific manner: Liu et al. (2015) found arsenic and cadmium exposures increased only estrogen and progesterone receptor (ER/PR)-negative breast cancer risk; White et al. (2019) reported no differential risks for cadmium and lead, but found antimony, cobalt and mercury were associated with elevated estrogen receptor (ER)-positive risk.
Findings from experimental studies suggest that heavy metals possess both xenoestrogenic and carcinogenic properties, offering a potential hypothesis for the subtype-specific associations. Arsenic, beryllium, cadmium, chromium, lead, mercury and nickel have been reported to inhibit DNA damage repair proteins by damaging zinc finger motifs and may alter DNA damage repair pathways via generation of free radicals [11–15]. These hormone-independent, biological effects may be more strongly related to development of hormone receptor negative breast cancer [16–19]. Other metals, including antimony, arsenic, cadmium, cobalt, manganese, and selenium, are reported to mimic estradiol by binding to and activating estrogen receptor-alpha [20–22]. Xenoestrogenic exposures may be more strongly, but not exclusively, associated with the development of hormone receptor positive breast cancer [23, 24]. As some metals possess both carcinogenic and xenoestrogenic properties (i.e. arsenic, cadmium), it is possible they may influence breast cancer risk via multiple biological pathways.
Based on the broad biological effects of metals, we aimed to test whether exposures to airborne metals prior to cancer diagnosis are differentially associated with tumor ER/PR status. To accomplish this, we used a case-series design to test for etiologic heterogeneity [25, 26]; in instances where modest etiologic heterogeneity exists, the case-series approach has been demonstrated to improve statistical power [27]. We hypothesize that metals with carcinogenic properties would be associated with ER/PR-negative breast cancer and metals with xenoestrogenic properties would be associated with ER/PR-positive breast cancer. Based on prior literature, for metals that possessed both, we hypothesized relationships would be stronger for ER/PR-negative disease [3].
2. Materials and Methods
2.1. Study population
Breast Cancer Care in Chicago (BCCC) is a population-based study of recently diagnosed breast cancer patients from the Chicagoland area. The study population has been described in detail elsewhere [28]. Briefly, women were eligible if diagnosed with a first primary in situ or invasive breast cancer between 2005 and 2008; between 30 and 79 years old at diagnosis; self-identified as non-Latina (nL) White, nL Black, or Latina; and resided in the metropolitan Chicago area at time of diagnosis. Overall, 989 women were enrolled and completed an interview on social, demographic, and healthcare-related factors. The protocol for this study was approved by the University of Illinois at Chicago Institutional Review Board and all study participants gave written informed consent.
2.2. Tumor subtype
Breast tumor ER/PR status was determined via medical record abstraction. Overall, 696 (70% of the full sample) women had information on tumor ER and PR status. Women were classified as ER/PR-positive if either estrogen or progesterone receptors were present and classified as ER/PR-negative if both receptor types were absent (double negative). Of the 696 women, 222 (32%) women consented to retrieval of clinical breast tissue samples where ER/PR status was confirmed by immunohistochemical analysis. For the 222 women with histologically confirmed subtype, we observed very high concordance (> 99%) with the tumor subtype listed in their medical records.
2.3. Residential airborne heavy metal concentrations
To estimate residential, airborne heavy metal concentrations, we combined participant’s 2002 residential address with census-tract level, ambient air concentration data from 2002 using the U.S. Environmental Protection Agency’s National Air Toxics Assessment (NATA). Use of the 2002 NATA allowed us to examine airborne metal concentrations between three to six years prior to breast cancer diagnosis. The process for air toxics concentration estimates was as follows: emission data were compiled from countrywide anthropogenic sources in the National Emissions Inventory and were prepared for use as model inputs. Ambient concentrations of air toxics at the census tract level were estimated using the Assessment System for Population Exposure Nationwide (ASPEN) dispersion model for area and mobile source emissions and the Human Exposure Model (HEM) dispersion model to assess stationary source emissions [29]. Total ambient concentrations, including those of 11 heavy metals (antimony, arsenic, beryllium, cadmium, chromium, cobalt, lead, manganese, mercury, nickel, and selenium) were calculated by combining all exposure sources with background concentrations. The NATA estimates reflect Ambient, airborne concentrations of heavy metals and do not account for exposures which may occur indoors, due to smoking behaviors, or via other routes (i.e., dermal or ingestion).
2.4. Individual and neighborhood level characteristics
Upon enrollment in the BCCC study, information on age at diagnosis, race/ethnicity, education, household income, body mass index (BMI), age at first birth, number of live births, and menopausal status were collected via questionnaire.
We constructed established neighborhood-level metrics of socioeconomic affluence and disadvantage, defined at the participant’s census tract, using data from the 2000 United States Census [28, 30, 31]. Briefly, census tract affluence was defined as the weighted contribution of the proportions of families with income of $75,000 or more, adults with college education or more, and civilian labor force employed in professional and managerial occupations, whereas census tract disadvantage was measured by combining the proportions of families with incomes below the poverty line, families receiving public assistance, persons unemployed, and female-headed households with children [30, 31]. Both variables weighted the relevant input variables equally. We standardized census tract affluence and disadvantage so their sum had a mean of zero with a standard deviation of one. Z-scores were used to determine low (< −1), intermediate (−1 ≤ z ≤ 1), and high (> 1) levels of neighborhood affluence and disadvantage.
2.5. Statistical analysis
We first examined participant characteristics stratified by ER/PR status. For the airborne heavy metal concentrations, we explored distributions and examined pairwise Spearman correlations (rs). We further examined airborne metal concentrations by race/ethnicity and other participant characteristics. Individual heavy metal concentrations were categorized into quintiles and modeled using logistic regression to estimate odds ratios and 95% confidence intervals with respect to ER/PR-negative breast cancer. P-values for linear trends were calculated by including the heavy metal quintiles as ordinal variables. Significant associations from case-series studies of etiologic heterogeneity can be interpreted as an indication for distinct causal mechanisms for breast cancer subtypes, or a different strength of effect via the same mechanism [25].
Based on prior studies in this population, we adjusted our models for predictors of ER/PR status [28]. Model covariates included were: age at diagnosis (years); race/ethnicity (nL White, nL Black, Latina); BMI (kg/m2; categorized as underweight and normal, < 25; overweight, 25–30; obese, 30+); individual and neighborhood-level socioeconomic factors (education, income, neighborhood affluence and disadvantage); and reproductive factors (age at first birth, number of live births, and menopausal status). To examine the potential impact of multiple exposures simultaneously, we performed a principal component analysis of the eleven airborne heavy metals. We estimated components if their eigenvalues were greater than one and examined metal loading factors into each component. Unlike other mixture approaches, principle component analysis is unconstrained and does not rely on associations with the outcome to define mixtures [32]. Statistical significance was defined as a two-sided P-value ≤ 0.05. Participants were excluded from the present analysis if they were missing residence in 2002 (n= 106), breast cancer ER/PR status (n= 187) or any of the covariate data (n= 24); thus, the final analytic sample size was 672 (68% of total sample) (Supplemental Figure 1). All analyses were conducted using Stata version 15 (Stata Corp., College Station, TX).
3. Results
Table 1 presents the sample population characteristics by tumor hormone receptor status. Women diagnosed with ER/PR-positive tumors tended to be older at diagnosis, have fewer live births and later ages at first birth. nL Black women were more likely to be diagnosed with ER/PR-negative tumors; nL White women were more likely to be diagnosed with ER/PR-positive tumors. Obese women and women with lower socioeconomic status (lower educational attainment, annual income, and census tract affluence, and higher census tract disadvantage) were also more likely to have an ER/PR-negative diagnosis. The odds of ER/PR-negative disease were similar for pre- and postmenopausal women. Participants’ age at diagnosis, education level, household income, age at first birth and number of live births were only weakly correlated with the ambient airborne metal concentrations (−0.2 < rs < 0.2) (Supplemental Table 1). Conversely, race/ethnicity was related to the airborne metals: nL Black women generally resided in census tracts with higher median concentrations of antimony, cadmium, lead, and manganese; nL White women resided in census tracts with higher median concentrations of arsenic, beryllium, chromium, cobalt, and nickel (Supplemental Figure 2). Although 187 women were missing ER/PR status, this missingness was not related to any of the airborne metal concentrations (Supplemental Table 2).
Table 1.
Participant characteristics at diagnosis (n= 696)
| Tumor receptor status | ||
|---|---|---|
| Characteristic | ER/PR-positive No. (%) |
ER/PR-negative No. (%) |
| Total | 549 (79) | 147(21) |
| Age, mean yrs. (SD) | 56.0(11.3) | 53.7(10.6) |
| Education, mean yrs. (SD) | 13.5 (2.9) | 12.7 (2.6) |
| Annual income, mean $/thousand (SD) | 65.0 (54.2) | 42.0(40.1) |
| Parity, mean live births (SD) | 2.1 (2.0) | 2.5(1.8) |
| Age at first birth, mean yrs. (SD) | 27.7 (8.8) | 23.8 (8.0) |
| Race/ethnicity | ||
| nL White | 253(88) | 35(12) |
| nL Black | 199 (70) | 87 (30) |
| Latina | 97 (79) | 25(21) |
| Body Mass Index (kg/m2) | ||
| Underweight/Normal (< 24.9) | 177(83) | 37(17) |
| Overweight (25–30) | 177(82) | 38(18) |
| Obese (30+) | 192 (73) | 72 (27) |
| Missing | 3 | 0 |
| Census tract affluence | ||
| Low | 28 (72) | 11 (28) |
| Intermediate | 392(77) | 118(23) |
| High | 129 (88) | 17(12) |
| Missing | 0 | 1 |
| Census tract disadvantage | ||
| Low | 102(90) | 11(10) |
| Intermediate | 351 (78) | 100(22) |
| High | 93 (73) | 35 (27) |
| Missing | 0 | 1 |
| Menopause status | ||
| Premenopausal | 106(80) | 27 (20) |
| Postmenopausal | 441 (79) | 119(21) |
| Missing | 2 | 1 |
Women with missing continuous covariate information: age, 1 ER/PR-positive; education, 2 ER/PR-positive; income, 14 ER/PR-positive, 3 ER/PR-negative; age first live birth, 1 ER/PR-positive.
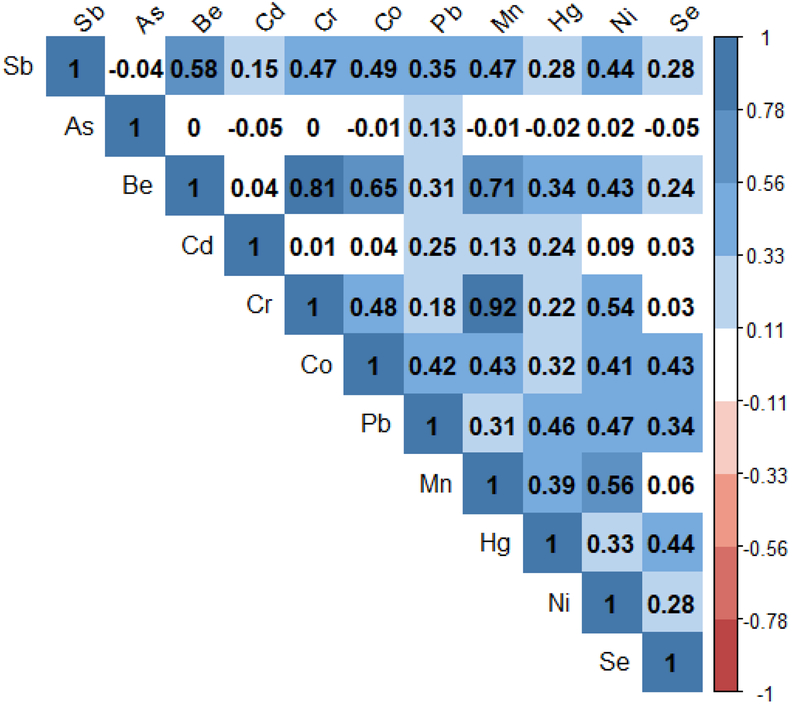
The Spearman’s correlations of the heavy metal air concentrations are displayed in Figure 1. Generally, the correlations were positive reflecting increasing airborne concentrations of one metal was associated with increasing airborne concentrations of others, suggesting co-exposure is common. For instance, antimony was positively correlated with the other estimated air concentrations (rs ranging from 0.15 to 0.58) except for arsenic (rs= −0.05). Arsenic showed weak correlations with all other metals. Notably strong correlation coefficients were observed for chromium and beryllium (rs= 0.81) and manganese (rs= 0.92), as well as beryllium and manganese (rs= 0.71). Distributions of the heavy metal air concentrations are presented in Supplemental Table 3. The highest median airborne heavy metal concentrations were observed for lead (median= 4.6 ng/m3, interquartile range (IQR)= 4.2–5.1), manganese (median= 3.5 ng/m3, IQR= 2.7–4.4) and nickel (median= 1.1 ng/m3, IQR= 1.0–1.4).
Figure 1.
Spearman correlation matrix for the eleven airborne heavy metal concentrations (Abbreviations: Sb, antimony; As, arsenic; Be, beryllium; Cd, cadmium; Cr, chromium; Co, cobalt; Pb, lead; Mn, manganese; Hg, mercury; Ni, nickel; Se, selenium).
Associations between residential airborne heavy metal concentrations with odds of ER/PR-negative breast cancer are shown in Table 2. Comparing the highest and lowest quintiles, we found elevated odds of ER/PR-negative tumors for antimony (OR= 1.8, 95% CI: 0.9, 3.7), cadmium (OR= 2.3, 95% CI: 1.2, 4.4), cobalt (OR= 2.0, 95% CI: 0.9, 4.4) and manganese (OR= 2.1, 95% CI: 0.9, 4.8). We observed significant increasing trends for increasing concentrations of antimony (P-trend= 0.05), cadmium (P-trend= 0.04), and cobalt (P-trend= 0.04). Although we did not find evidence of linear trends for airborne lead and manganese concentrations with ER/PR status (lead P-trend= 0.16; manganese P-trend= 0.13), association point estimates suggested that women residing in census tracts with moderate to high concentrations were at increased odds of an ER/PR-negative breast cancer.
Table 2.
Airborne metal concentrations (ng/m3), odds ratios (OR) and 95% confidence intervals (CIs) for the relationship between residential airborne heavy metals quintiles and ER/PR-negative tumor receptor status (n= 672)
| Ql | Q2 | Q3 | Q4 | Q5 | P-trend | ||
|---|---|---|---|---|---|---|---|
| Antimony | Conc. (ng/m3) | <0.02 | 0.02–0.03 | 0.03–0.04 | 0.04–0.06 | 0.06+ | |
| OR (95% CI) | Ref. | 1.3 (0.7, 2.5) | 1.7 (0.8, 3.3) | 2.1 (1.1, 4.1) | 1.8 (0.9, 3.7) | 0.05 | |
| Arsenic | Cone. (ng/m3) | <0.82 | 0.83–0.96 | 0.97–1.19 | 1.20–1.74 | 1.75+ | |
| OR (95% CI) | Ref. | 0.9 (0.5, 1.7) | 0.8 (0.4, 1.5) | 1.3 (0.7, 2.5) | 0.8 (0.5, 1.5) | 0.89 | |
| Beryllium | Conc. (ng/m3) | <0.05 | 0.05–0.06 | 0.06–0.06 | 0.06–0.09 | 0.09+ | |
| OR (95% CI) | Ref. | 0.5 (0.3, 0.9) | 0.5 (0.3, 1.0) | 0.8 (0.4, 1.5) | 0.7 (0.3, 1.5) | 0.53 | |
| Cadmium | Conc. (ng/m3) | <0.23 | 0.23–0.32 | 0.33–0.54 | 0.54–0.90 | 0.91+ | |
| OR (95% CI) | Ref. | 1.5 (0.8, 2.9) | 1.5 (0.8, 2.9) | 1.1 (0.5, 2.2) | 2.3 (1.2, 4.4) | 0.04 | |
| Chromium | Conc. (ng/m3) | <0.65 | 0.66–0.72 | 0.73–0.89 | 0.90–1.64 | 1.65+ | |
| OR (95% CI) | Ref. | 1.1 (0.6, 2.1) | 1.3 (0.7, 2.4) | 1.2 (0.7, 2.3) | 1.0 (0.5, 2.1) | 0.83 | |
| Cobalt | Conc. (ng/m3) | < 0.010 | 0.010–0.014 | 0.014–0.017 | 0.017–0.024 | 0.024+ | |
| OR (95% CI) | Ref. | 1.4 (0.7, 2.8) | 2.2 (1.2, 4.2) | 1.8 (0.9, 3.4) | 2.0 (0.9, 4.4) | 0.04 | |
| Lead | Conc. (ng/m3) | <4.0 | 4.1–4.4 | 4.4–4.7 | 4.7–5.3 | 5.3+ | |
| OR (95% CI) | Ref. | 1.4 (0.7, 2.8) | 1.9 (0.9, 3.9) | 1.4 (0.7, 2.9) | 1.4 (0.7, 2.9) | 0.16 | |
| Manganese | Conc. (ng/m3) | <2.4 | 2.5–3.2 | 3.2–3.8 | 3.8–4.8 | 4.8+ | |
| OR (95% CI) | Ref. | 2.8 (1.3, 5.8) | 2.7 (1.3, 5.9) | 3.4 (1.5, 7.7) | 2.1 (0.9, 4.8) | 0.13 | |
| Mercury | Conc. (ng/m3) | 0.068 | 0.069–0.077 | 0.077–0.084 | 0.085–0.103 | 0.104+ | |
| OR (95% CI) | Ref. | 1.0 (0.5, 2.0) | 1.0 (0.5, 1.9) | 1.9 (1.0, 3.5) | 1.0 (0.5, 2.1) | 0.23 | |
| Nickel | Conc. (ng/m3) | <0.93 | 0.94–1.08 | 1.08–1.19 | 1.20–1.46 | 1.47+ | |
| OR (95% CI) | Ref. | 1.0 (0.5, 2.0) | 0.8 (0.4, 1.6) | 0.9 (0.4, 1.8) | 1.7 (0.8, 3.4) | 0.26 | |
| Selenium | Conc. (ng/m3) | <0.09 | 0.09–0.11 | 0.11–0.16 | 0.16–0.20 | 0.20+ | |
| OR (95% CI) | Ref. | 1.2 (0.6,2.2) | 1.1 (0.6, 2.0) | 1.9 (1.0, 3.6) | 1.1 (0.6, 2.0) | 0.35 |
Models adjusted for age, race/ethnicity, education, BMI, income, census tract affluence and disadvantage, and reproductive factors.
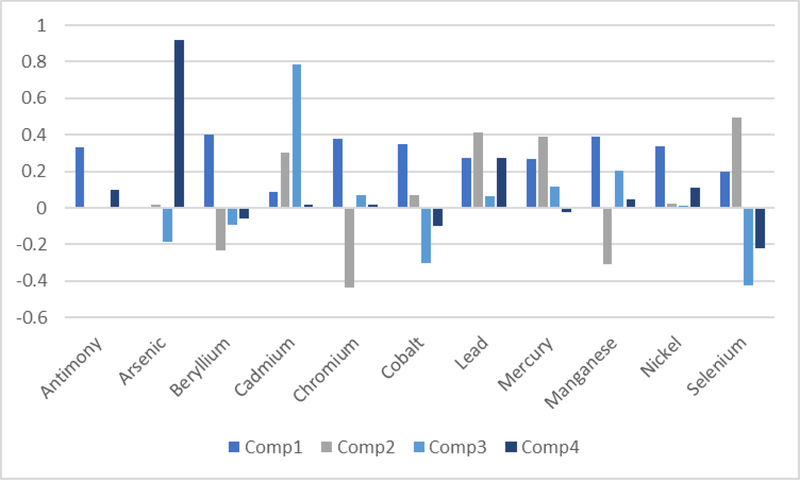
Figure 2 displays the loading factors for the eleven heavy metals into the top four principal components. The first component comprised most of the metals equally, with the exceptions of arsenic and cadmium; this was the only mixture that showed associations with increasing odds of ER/PR-negative breast cancer (Table 3). The second component was mainly comprised of lead, mercury and selenium and generally was not associated with breast cancer ER/PR status. The third and fourth components were primarily comprised of cadmium and arsenic, respectively, and again showed mostly null associations with breast cancer receptor status.
Figure 2.
Loading factors for the eleven airborne heavy metals into the top four principal components.
Table 3.
Odds ratios (OR) and 95% confidence intervals (CIs) for the relationship between residential airborne heavy metals mixture quintiles and ER/PR-negative tumor receptor status (n= 672)
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | |
|---|---|---|---|---|---|---|
| Mixture 1 | Ref. | 2.0(1.0,4.0) | 1.5(0.8,3.1) | 2.0(1.0,4.1) | 2.3(1.1,4.8) | 0.06 |
| Mixture 2 | Ref. | 1.2(0.6,2.4) | 1.9(0.9,3.7) | 1.2(0.6,2.5) | 1.7(0.9,3.3) | 0.19 |
| Mixture 3 | Ref. | 0.9(0.5,1.6) | 0.8(0.4,1.5) | 1.0(0.5, 1.8) | 1.3(0.7,2.3) | 0.42 |
| Mixture 4 | Ref. | 1.1(0.6,2.1) | 1.0(0.5,2.0) | 0.7(0.4, 1.4) | 1.1 (0.6,2.2) | 0.85 |
Models adjusted for age, race/ethnicity, education, BMI, income, census tract affluence and disadvantage, and reproductive factors.
4. Discussion
Using a case-series approach to examine the etiologic heterogeneity of breast cancer subtypes, we found, after adjustment for individual and neighborhood-level factors, that increasing residential airborne concentrations of cadmium, antimony, and cobalt were associated with higher odds of ER/PR-negative breast cancer. Suggestive associations with ER/PR-negative breast cancer were observed for manganese and lead. Based on studies linking airborne concentrations of these metals with breast cancer risk [9, 10], we find evidence for distinct causal mechanisms by tumor subtype. For the seven metals that possess carcinogenic properties and hypothesized to be associated with ER/PR-negative disease, only cadmium was associated with greater odds of ER/PR-negative disease. None of the metals previously shown to be xenoestrogenic were associated with greater odds of ER/PR-positive disease and, contrary to our expectations, both antimony and cobalt were associated with higher odds of ER/PR-negative breast cancer. Mixture analysis using a principal component approach suggested that the observed associations were likely not driven by a single airborne metal, rather co-exposures to multiple metallic airborne components may be related to ER/PR-negative breast cancer.
Cadmium is the most studied metal in relation to breast cancer and prior research suggests higher airborne exposures may increase breast cancer risk [9, 10]. Among women diagnosed with breast cancer, we found increasing airborne concentrations of cadmium were associated with higher odds of ER/PR-negative breast cancer. Our findings are supported by Lui et al.’s (2015) prospective cohort study that observed higher cadmium concentrations were associated with incident ER/PR-negative breast cancer [9]. As studies suggest cadmium has both xenoestrogenic and carcinogenic properties, it is possible that cadmium may be mechanistically related to both ER/PR-positive and ER/PR-negative breast cancers [13, 22]. The stronger association for ER/PR-negative breast cancer identified using the single metal model suggested that cadmium’s carcinogenic properties, rather than xenoestrogenic, may have a stronger influence on breast cancer development. Interestingly, in our mixture analysis, cadmium loaded heavily into the third component, but this component was not related to tumor ER/PR status. This may be due to the negative loading of cobalt into the same component; cobalt, like cadmium, appeared to increase the odds of ER/PR-negative tumors. As these metals loaded into the component with opposing strengths, it is possible they canceled out their individual associations with breast cancer subtype.
Based on earlier studies that suggest antimony may be xenoestrogenic and related to breast cancer risk [10, 22, 33, 34], we hypothesized that higher airborne concentrations would be associated with ER/PR-positive breast cancer. Contrary to this, we found higher concentrations were associated with prevalent ER/PR-negative cancer. Antimony is relatively understudied compared to other heavy metals and reported associations in the breast cancer literature are mixed. In the only prospective study, White et al. (2019) observed moderate, census-tract airborne concentrations were associated with increased ER-positive risk, while the highest concentrations were associated with lower ER-negative risk [10]. In case-control studies using exposure biomarkers, the association is more established: compared to cancer-free controls, breast cancer cases are reported to have higher concentrations of antimony in both their plasma and hair [33, 34]. Notably, there is limited evidence that antimony is genotoxic in mammals and may inhibit DNA damage repair enzymes [35–38]. These characteristics may influence ER/PR-negative breast cancer risk and potentially support our observation that, among women diagnosed with breast cancer, higher airborne concentrations may increase odds of ER/PR-negative disease.
We also identified an association between higher airborne concentrations of cobalt and greater odds of ER/PR-negative breast cancer. This observation was discordant with our hypothesis that cobalt would be more strongly associated with ER/PR-positive breast cancer due to its estrogen-mimicking properties. Although White et al. (2019) observed higher ER-positive breast cancer risk for women with moderate airborne concentrations, our findings suggest that cobalt’s xenoestrogenic effects may not drive associations with breast cancer risk. Interestingly, co-exposure to cobalt and lead is associated with both DNA single strand breaks and inhibition of DNA damage repair mechanisms, which could increase ER/PR-negative breast cancer risk [39–41]. Moreover, higher concentrations of airborne cobalt and lead are associated with greater mammographic density [42], a potential risk factor for ER-negative breast cancer [43]. In the present study, cobalt was moderately correlated with lead (rs= 0.42), suggesting a possible mixture effect which may potentially explain the observed association with ER/PR-negative breast cancer. As most of the airborne metal concentrations had similar loading fractions into the first principal component, our mixture analysis provided additional support that co-exposures to airborne heavy metals, including cobalt and lead, may drive associations with tumor characteristics.
Although this study identified suggestive relationships between residential, airborne heavy metal concentrations and odds of ER/PR-negative breast cancer, it is subject to limitations. Most importantly, we had to exclude a portion of women due to missing residential history (n= 106; 11% of total sample). However, compared to excluded women, those included in the final sample had a similar risk of being diagnosed with ER/PR-positive breast cancer (79% of women included vs. 76% of women excluded). As inclusion in the final study population was not associated with tumor ER/PR status, it is unlikely this missing information would bias our association estimates. Similarly, 187 women (20%) were missing information on tumor ER/PR status; however, ER/PR status missingness was not associated with any of the airborne metal concentrations, thus any bias introduced based on selection into the final study population on these criteria is likely to be minimal. The BCCC study population was from a single geographic region potentially limiting the generalizability and variability in exposures. Nonetheless, 416 unique census tracts were represented across the 696 participants, and according to estimates from NATA there was considerable variation in airborne heavy metal concentrations within the Chicagoland area. We also relied on 2002 NATA estimates; while these data reflect airborne metal concentrations three to six years before diagnosis in our population, breast cancer has a long induction and latent period. Thus, future analyses might consider other potential exposure windows to identify associations between airborne metals and breast cancer. Although we accounted for both individual and neighborhood-level factors related to race and socioeconomic status, there exists the potential for residual confounding by other environmental stressors. Also, as we used NATA to estimate concentrations of airborne metallic components, our analysis does not address other potential sources of metal exposures, including from water or diet.
This study builds upon the limited literature linking airborne metallic components with breast cancer subtype formation [3, 10]. Although larger studies have investigated the relationship between airborne metals and breast cancer risk, this is the first study specifically designed to test for etiologic heterogeneity. White et al. (2019) did explore etiologic heterogeneity as a secondary analysis, but compared to that study, our participants were far more racially and ethnically diverse and, perhaps as a result, lived in census tracts with higher airborne metal concentrations. Moreover, the proportion of ER/PR-negative cases in our analysis was higher (21%) compared to theirs (14%), giving our investigation improved power to detect etiologic heterogeneity. Another strength is this study is only the second to address the potential influence of airborne metal mixtures. By applying principal component analysis, we used an unsupervised mixture approach based entirely on the correlational structure of the airborne metal concentrations [44]. Conversely, the weighted quantile sum approach applied in an earlier study was designed to detect the ‘bad actors,’ which may be an inappropriate approach with highly correlated components, and when mixtures, rather than the individual pollutants, are the true cause of disease development [45]. We found, based on the moderate positive correlations between the airborne metal concentrations and our mixture analysis, that the observed associations with tumor characteristics are not likely driven by a single metal. Finally, although a latency period for breast cancer has not been established, we assessed airborne heavy metals between three to six years prior to a diagnosis and observed evidence for etiologic heterogeneity, suggesting this time-period may reflect an important window of susceptibility for breast tumor subtype formation.
5. Conclusions
Airborne heavy metals have broad carcinogenic and xenoestrogenic properties relevant to breast cancer. We found that higher concentrations of cadmium, antimony and cobalt were associated with greater odds of ER/PR-negative breast cancer and observed evidence that metal mixtures may be important. Additional studies of heavy metal ambient airborne concentrations in other geographic regions of the U.S. are required to confirm our findings. Moreover, additional epidemiologic studies using biomarkers of chronic inhalation exposure and a cohort approach may be required to disentangle the subtype-specific effects of metals in relation to breast cancer risk. However, our study demonstrates, on the population-level, that airborne metallic components may have greater influence on the development of ER/PR-negative, rather than ER/PR-positive, breast cancer.
Supplementary Material
Highlights.
Evidence suggests airborne metals are related to subtype-specific breast cancer risk
We tested for etiologic heterogeneity of metallic air pollutants and tumor subtype
Sb, Cd and Co were more strongly associated with ER/PR-negative breast cancer
Mixture analysis suggests that co-exposures to multiple metals is important
Airborne metals may be more strongly associated with aggressive breast cancer subtypes
Source of Funding:
The Breast Cancer Care in Chicago study is supported by the National Institute of Health (NIH P50 2CA106743). J. Kresovich received additional support from the National Institute for Occupational Safety and Health (NIOSH T42 OH008672–10) and the National Cancer Institute Cancer Education and Career Development Program (NIH R25 CA057699)
Abbreviations:
- BMI
Body Mass Index
- BCCC
Breast Cancer Care in Chicago
- ER/PR
Estrogen and Progesterone Receptor
- NATA
National Air Toxics Assessment
- nL
non-Latina
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests: None declared
IRB Approval: The protocol for this study was approved by the University of Illinois at Chicago Institutional Review Board and all study participants gave written, informed consent to participate in the study.
References
- 1.Reding KW, Young MT, Szpiro AA, Han CJ, DeRoo LA, Weinberg C, Kaufman JD, Sandler DP: Breast Cancer Risk in Relation to Ambient Air Pollution Exposure at Residences in the Sister Study Cohort. Cancer Epidemiol Biomarkers Prev 2015, 24(12):1907–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei Y, Davis J, Bina WF: Ambient air pollution is associated with the increased incidence of breast cancer in US. Int J Environ Health Res 2012, 22(1):12–21. [DOI] [PubMed] [Google Scholar]
- 3.Garcia E, Hurley S, Nelson DO, Hertz A, Reynolds P: Hazardous air pollutants and breast cancer risk in California teachers: a cohort study. Environ Health 2015, 14(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg MS, Labrèche F, Weichenthal S, Lavigne E, Valois MF, Hatzopoulou M, Van Ryswyk K, Shekarrizfard M, Villeneuve PJ, Crouse D et al. : The association between the incidence of postmenopausal breast cancer and concentrations at street-level of nitrogen dioxide and ultrafine particles. Environ Res 2017, 158:7–15. [DOI] [PubMed] [Google Scholar]
- 5.Andersen ZJ, Stafoggia M, Weinmayr G, Pedersen M, Galassi C, Jørgensen JT, Oudin A, Forsberg B, Olsson D, Oftedal B et al. : Long-Term Exposure to Ambient Air Pollution and Incidence of Postmenopausal Breast Cancer in 15 European Cohorts within the ESCAPE Project. Environ Health Perspect 2017, 125(10):107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shmuel S, White AJ, Sandler DP: Residential exposure to vehicular traffic-related air pollution during childhood and breast cancer risk. Environ Res 2017, 159:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White AJ, Bradshaw PT, Hamra GB: Air pollution and Breast Cancer: A Review. Current Epidemiology Reports 2018, 5:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ionescu JG, Novotny J, Stejskal V, Lätsch A, Blaurock-Busch E, Eisenmann-Klein M: Increased levels of transition metals in breast cancer tissue. Neuro Endocrinol Lett 2006, 27 Suppl 1:36–39. [PubMed] [Google Scholar]
- 9.Liu R, Nelson DO, Hurley S, Hertz A, Reynolds P: Residential exposure to estrogen disrupting hazardous air pollutants and breast cancer risk: the California Teachers Study. Epidemiology 2015, 26(3):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White AJ, O’Brien KM, Niehoff NM, Carroll R, Sandler DP: Metallic Air Pollutants and Breast Cancer Risk in a Nationwide Cohort Study. Epidemiology 2019, 30(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiseman H, Halliwell B: Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996, 313 ( Pt 1):17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyersmann D, Hartwig A: Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 2008, 82(8):493–512. [DOI] [PubMed] [Google Scholar]
- 13.Morales ME, Derbes RS, Ade CM, Ortego JC, Stark J, Deininger PL, Roy-Engel AM: Heavy Metal Exposure Influences Double Strand Break DNA Repair Outcomes. PLoS One 2016, 11(3):e0151367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witkiewicz-Kucharczyk A, Bal W: Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol Lett 2006, 162(1):29–42. [DOI] [PubMed] [Google Scholar]
- 15.Hartwig A, Asmuss M, Ehleben I, Herzer U, Kostelac D, Pelzer A, Schwerdtle T, Bürkle A: Interference by toxic metal ions with DNA repair processes and cell cycle control: molecular mechanisms. Environ Health Perspect 2002, 110 Suppl 5:797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, Stern HM, Ge L, O’Brien C, Haydu L, Honchell CD, Haverty PM, Peters BA, Wu TD, Amler LC et al. : Genetic alterations and oncogenic pathways associated with breast cancer subtypes. Mol Cancer Res 2009, 7(4):511–522. [DOI] [PubMed] [Google Scholar]
- 17.Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B, Kalva S, Potter J, Tran TV, Chen J et al. : Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 2014, 16(6):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L et al. : Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012, 486(7403):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guler G, Himmetoglu C, Jimenez RE, Geyer SM, Wang WP, Costinean S, Pilarski RT, Morrison C, Suren D, Liu J et al. : Aberrant expression of DNA damage response proteins is associated with breast cancer subtype and clinical features. Breast Cancer Res Treat 2011, 129(2):421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB: Metals and breast cancer. J Mammary Gland Biol Neoplasia 2013, 18(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin MB, Reiter R, Pham T, Avellanet YR, Camara J, Lahm M, Pentecost E, Pratap K, Gilmore BA, Divekar S et al. : Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology 2003, 144(6):2425–2436. [DOI] [PubMed] [Google Scholar]
- 22.Darbre PD: Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol 2006, 26(3):191–197. [DOI] [PubMed] [Google Scholar]
- 23.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME: Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev 2004, 13(10):1558–1568. [PubMed] [Google Scholar]
- 24.Dey S, Soliman AS, Merajver SD: Xenoestrogens may be the cause of high and increasing rates of hormone receptor positive breast cancer in the world. Med Hypotheses 2009, 72(6):652–656. [DOI] [PubMed] [Google Scholar]
- 25.Begg CB, Zhang ZF: Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev 1994, 3(2):173–175. [PubMed] [Google Scholar]
- 26.Begg CB, Zabor EC, Bernstein JL, Bernstein L, Press MF, Seshan VE: A conceptual and methodological framework for investigating etiologic heterogeneity. Stat Med 2013, 32(29):5039–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Zabor EC: Detecting and exploiting etiologic heterogeneity in epidemiologic studies. Am J Epidemiol 2012, 176(6):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauscher GH, Campbell RT, Wiley EL, Hoskins K, Stolley MR, Warnecke RB: Mediation of Racial and Ethnic Disparities in Estrogen/Progesterone Receptor-Negative Breast Cancer by Socioeconomic Position and Reproductive Factors. Am J Epidemiol 2016, 183(10):884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum AS, Axelrad DA, Woodruff TJ, Wei YH, Ligocki MP, Cohen JP: National estimates of outdoor air toxics concentrations. J Air Waste Manag Assoc 1999, 49(10):1138–1152. [DOI] [PubMed] [Google Scholar]
- 30.Sampson RJ, Morenoff JD, Earls F: Beyond social capital: Spatial dynamics of collective efficacy for children. Am Sociol Rev 1999, 64(5):633–660. [Google Scholar]
- 31.Browning CR, Wallace D, Feinberg SL, Cagney KA: Neighborhood social processes, physical conditions, and disaster-related mortality: The case of the 1995 Chicago heat wave. Am Sociol Rev 2006, 71(4):661–678. [Google Scholar]
- 32.Czarnota J, Gennings C, Wheeler DC: Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform 2015, 14(Suppl 2):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotsopoulos J, Sukiennicki G, Muszyńska M, Gackowski D, Kąklewski K, Durda K, Jaworska K, Huzarski T, Gronwald J, Byrski T et al. : Plasma micronutrients, trace elements, and breast cancer in BRCA1 mutation carriers: an exploratory study. Cancer Causes Control 2012, 23(7):1065–1074. [DOI] [PubMed] [Google Scholar]
- 34.Benderli Cihan Y, Sözen S, Oztürk Yıldırım S: Trace elements and heavy metals in hair of stage III breast cancer patients. Biol Trace Elem Res 2011, 144(1–3):360–379. [DOI] [PubMed] [Google Scholar]
- 35.De Boeck M, Kirsch-Volders M, Lison D: Cobalt and antimony: genotoxicity and carcinogenicity. Mutat Res 2003, 533(1–2):135–152. [DOI] [PubMed] [Google Scholar]
- 36.Asakura K, Satoh H, Chiba M, Okamoto M, Serizawa K, Nakano M, Omae K: Genotoxicity studies of heavy metals: lead, bismuth, indium, silver and antimony. J Occup Health 2009, 51(6):498–512. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Sato H, Kubota Y, Utsumi H, Bedford JS, Okayasu R: Inhibition of DNA-double strand break repair by antimony compounds. Toxicology 2002, 180(3):249–256. [DOI] [PubMed] [Google Scholar]
- 38.Schaumlöffel N, Gebel T: Heterogeneity of the DNA damage provoked by antimony and arsenic. Mutagenesis 1998, 13(3):281–286. [DOI] [PubMed] [Google Scholar]
- 39.Hengstler JG, Bolm-Audorff U, Faldum A, Janssen K, Reifenrath M, Götte W, Jung D, Mayer-Popken O, Fuchs J, Gebhard S et al. : Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 2003, 24(1):63–73. [DOI] [PubMed] [Google Scholar]
- 40.Chacón RD, Costanzo MV: Triple-negative breast cancer. Breast Cancer Res 2010, 12 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis JD, Lin SY: DNA damage and breast cancer. World J Clin Oncol 2011, 2(9):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White AJ, Weinberg CR, O’Meara ES, Sandler DP, Sprague BL: Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res 2019, 21(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K: Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev 2004, 13(12):2090–2095. [PubMed] [Google Scholar]
- 44.Pearson K: On lines and planes of closest fit to systems of points in space. Philos Mag 1901, 2(7–12):559–572. [Google Scholar]
- 45.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P: Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agr Biol Envir St 2015, 20(1):100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.