Abstract
Purpose:
To investigate the relationship of osteocalcin (OC), a marker of bone formation, and C-telopeptide of type I collagen (CTX), a marker of bone resorption, with long-term incidence of hip fracture in older women.
Methods:
We included 1,680 women from the population-based Cardiovascular Health Study (mean [SD] age 74.5 [5.0] years). The longitudinal association of both markers with incidence of hip fracture was examined using multivariable Cox proportional hazards models.
Results:
During a median follow-up of 12.3 years, 288 incident hip fractures occurred. Linear spline analysis did not demonstrate an association between OC levels and incident hip fracture. By contrast, increasing levels of CTX up to the middle-upper range were associated with a significantly greater risk of hip fracture (HR=1.52 per SD increment, 95% CI=1.10–2.09), while further increases were associated with a marginally non-significant lower risk (HR=0.80 per SD increment, 95% CI=0.63–1.01), after full adjustment for potential confounders. In analyses of quartiles, CTX exhibited a similar inverted U-shaped relationship with incident fracture after adjustment, with a significant association observed only for the comparison of Quartile 3 to Quartile 1 (HR=1.63, 95% CI=1.10–2.43). In a subset with available measures, both OC and CTX were inversely associated with bone mineral density of the hip.
Conclusion:
CTX, but not OC, levels were associated with incident hip fracture in post-menopausal women, a relationship characterized by an inverted U-shape. These findings highlight the complex relationship of bone turnover markers with hip fracture risk.
Keywords: bone turnover markers, osteoporosis, hip fracture risk, postmenopausal women
Mini abstract:
The relationship of osteocalcin (OC) and C-telopeptide of type I collagen (CTX) with long-term incidence of hip fracture was examined in 1,680 post-menopausal women from a population-based study. CTX, but not OC, levels were associated with incident hip fracture in these participants, a relationship characterized by an inverted U-shape.
Introduction
Fragility fractures are a major health problem in older adults, especially women [1]. Among such osteoporotic fractures, those involving the hip are of greatest consequence owing to their associated disability, mortality, and costs [2]. Although dual-energy X-ray absorptiometry (DXA) determination of bone mineral density (BMD) is widely used in clinical practice to stratify fracture risk [3], this approach is insensitive [4], Measurement of circulating bone turnover markers (BTMs) allows evaluation of the underlying processes driving bone loss, namely, low formation, increased resorption, or both [5]. However, available studies examining the relationship of BTMs with incident fracture have yielded inconsistent results [6]. Hence, BTMs are currently used clinically to monitor treatment effects and adherence to antiresorptive and anabolic drugs, but they are not recommended clinically for prediction of fracture risk [7].
BTMs are subject to considerable pre-analytical variability, such as fasting or non-fasting specimen collection and assay preparation, for which most existing studies have not been designed to control [6]. This has led to calls for standardization of patient preparation and specimen handling in studies evaluating BTMs for fracture risk assessment [8]. Among BTMs, osteocalcin is a highly osteoblast-specific marker of bone formation, while C-terminal cross-linking telopeptide of type I collagen (CTX), though less specific for bone, has been identified by expert panels as the most promising measure of bone resorption [9, 10].
The relationships of osteocalcin and CTX with fracture incidence among women have been evaluated in multiple longitudinal studies [11–22]. Earlier studies have reported both positive and null associations with fracture risk, but they have involved varying fracture types and locations and, importantly, most have not included morning fasting specimens or complete adjustment for confounding factors. A meta-analysis of such earlier studies reported a positive association for the bone formation marker procollagen type I N-terminal propeptide (PINP) and for CTX with all fractures in women, but not for hip fractures alone [23]. In a more recent study of post-menopausal women that overcame limitations of specimen preparation and insufficient adjustment for clinical covariates, neither PINP nor CTX was associated with incident hip fracture, but confidence bounds were broad [24].
We undertook measurement of OC and CTX among female participants of the population-based Cardiovascular Health Study (CHS) in a project designed to investigate the independent associations of these BTMs with geriatric outcomes – incident hip fracture, diabetes and clinically significant aortic stenosis. Such evaluations called for careful adjustment for a range of factors apt to influence bone metabolism [25] and potentially confound the associations of interest. Here we focus on the relationships of OC and CTX with incident hip fracture, our primary outcome, and with hip BMD in the subset of participants who underwent DXA scanning as our secondary outcome. We tested the hypothesis that high levels of CTX, reflecting higher bone resorption, and, reactively, OC [25] would be associated with increased hip fracture risk.
Materials and Methods
Study population
CHS is a population-based longitudinal study of community-dwelling, ambulatory adults 65 to 100 years old. Eligible participants were sampled from Medicare-eligibility lists at four U.S. field centers, as previously described [26]. Briefly, participant eligibility required age ≥65 years, expectation to live in the area for 3 years after recruitment, absence of active cancer treatment, and the ability to provide consent. The rate of participation refusal was 38.6%. An original cohort of 5,201 mostly white participants was recruited in 1989–90, followed by a supplemental cohort of 687 predominantly African-American individuals in 1992–93. In-person examinations were performed annually through 1998–99 and, in a subset, again in 2005–06. Telephone interviews were conducted semiannually from 1989 to 1999 and biannually thereafter. Participants underwent standardized health assessments for demographic data, medical history, lifestyle habits, medication inventory, and diagnostic and laboratory testing [27, 28]. All field centers and the CHS Coordinating Center received institutional review board approval for the study and participants gave informed consent.
Our ancillary study included 1,760 women who completed the 1992–93 exam and had never-thawed fasting serum available that was drawn during this exam. For the present analysis, we excluded 15 participants with prevalent hip fracture and 65 on oral glucocorticoids or vitamin K antagonists, leaving 1,680 participants.
Bone turnover markers
Serum specimens were collected after an 8-hour overnight fast during a morning visit and stored at −80°C until measurement in 2017 at the CHS Core Laboratory (Laboratory for Clinical Biochemistry Research, University of Vermont) [29]. Total OC was measured using electrochemiluminescent immunoanalysis with validated automated methods (Roche Diagnostics, Indianapolis, IN). The interassay coefficient of variation (CV) at the CHS Core Laboratory ranged between 5.0–7.6%. CTX was measured using a Roche beta-CrossLaps assay (Roche Diagnostics, Indianapolis, IN). The interassay (CV) ranged between 4.2–6.75%.
Hip fractures
The primary outcome of this study was time to first hospitalization for hip fracture, identified using International Classification of Diseases, Ninth Revision, (ICD-9) codes from hospitalization records that were prospectively gathered from all CHS participants every 6 months. Hip fracture was defined as inpatient ICD-9 code 820.xx at any position. Follow-up for incident hip fracture was complete and extended from the 1992–93 visit until incident hip fracture, death, or June 30, 2013, whichever was earliest. By the end of follow-up, 1374 participants died and 306 were alive.
Bone mineral density
During the 1994–95 examination, participants at two of the four field centers underwent DXA using array beam mode QDR 2000 or 2000+ bone densitometers (Hologic, Inc., Bedford, MA) according to a standardized protocol [30]. All scans were interpreted blindly and monitored for quality control by a core laboratory, as previously described [30]. The secondary outcome measures of interest were BMD of the total hip and femoral neck, each with a CV <0.75%, which were available in 425 participants (mean age 73.8 [4.3] years) included in the current study sample.
Covariates
All covariates were collected at the 1992–93 exam, unless otherwise noted. Estimated glomerular filtration rate (eGFR) was calculated based on serum cystatin C using a validated formula [31]. Physical activity was measured in kcal/week using published methods [27]. Prevalent diabetes mellitus was defined by fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL or treatment with hypoglycemic medication. Excessive alcohol use was defined as >7 drinks/week. Prevalent cardiovascular disease (CVD) included coronary heart disease (CHD), congestive heart failure (CHF), claudication, atrial fibrillation, and stroke or transient ischemic attack (TIA), ascertained at enrollment for the original (1989–90) and supplemental (1992–93) cohorts, and the intervening period for the original cohort, as previously described [27, 32, 33]. Activities of daily living (ADLs), history of falls and mobility impairment were ascertained by questionnaire [34]. Limitation in ADLs was defined as difficulty in ≥1 of the following ADLs: bathing, dressing, eating, getting in and out of bed or chair, walking around home, and using the toilet [35]. Mobility impairment was defined as difficulty in walking 1/2 mile or walking up 10 steps. Cognitive impairment was defined as a Modified Mini-Mental State Examination (3MSE) score <80 [36]. Frailty was defined as a clinical syndrome in which three or more of the following were present: unintentional weight loss (≥10 lbs. in past year), self-reported exhaustion, weakness (grip strength), slow walking speed, and low physical activity [34].
Statistical analysis
Distributions of OC and CTX levels were assessed by visual inspection, and were deemed to be approximately normal. The shape of the relationship between each biomarker and the primary outcome was evaluated in Cox models with penalized cubic splines. To eliminate the influence of extreme values in these spline analyses, such values were winsorized at the 99th percentile of the distribution of both biomarkers. Owing to departure from linearity in the relationship of CTX, and to a lesser extent, OC, with incident hip fracture, we modeled the association for each marker using two linear splines with a knot chosen at the point of change in the hazard ratio. These associations are presented for continuous levels (per SD increment) of the BTMs for values below and above the inflection point identified by penalized cubic splines after full adjustment. In addition, we evaluated the associations of OC and CTX with the primary outcome by quartiles of each BTM. Confounders were a priori selected for adjustment in sequential models based on prior associations or known biological mechanisms. Model 1 included demographic variables along with location and timing of examination, namely, age, race, field center and season. In Model 2, we additionally included social and lifestyle factors, specifically education, body mass index (BMI), physical activity, smoking status, alcohol consumption, and estrogen replacement therapy. In Model 3, we added cardiovascular and metabolic risk factors, and prevalent CVD: systolic blood pressure, treatment with anti-hypertensive medications, diabetes, calcium supplementation, low density lipoprotein cholesterol (LDLc), high density lipoprotein cholesterol (HDLc), triglycerides, eGFR, C-reactive protein (CRP), and prevalent coronary CHD, CHF, claudication, atrial fibrillation, stroke and TIA. In the fully adjusted model (Model 4), we added measures of functional and cognitive decline, including limitation in ADLs, fall history and cognitive impairment. Owing to substantial missing data, we examined the impact of further adjusting for 25-hydroxyvitamin D, calcium, phosphate and parathyroid hormone (PTH) levels in a separate exploratory model. Adjustment for BMD was not performed because this measure was only available in a small subset of the study sample. In separate sensitivity analyses, we excluded participants taking vitamin D, calcitriol and bisphosphonates; African American participants [37]; and those taking thiazide diuretics. In our Cox models, the proportionality assumption was tested using Schoenfeld residuals, which revealed no violations. In a secondary analysis, we assessed the quasi-cross-sectional associations of BTMs with BMD measured 2 years later. These relationships were modeled using multiple linear regression in the subgroup with available BMD measurements and adjusted for the same potential confounders as above. All analyses were performed using R Statistical Software, version 3.5.1. (Vienna, Austria). A two-tailed p<0.05 was used to define statistical significance.
Results
Characteristics of the study cohort and relations to BTM levels
The mean (SD) age of the study sample was 74.5 (5.0) years. Fifteen percent were African American. There was a strong correlation between serum OC and CTX (Pearson’s r=0.80, p<0.001). The distributions of the main sociodemographic, clinical and laboratory characteristics at baseline are shown in Table 1 according to quartiles of circulating BTM levels. Participants with higher concentrations of serum OC and CTX were older, had higher systolic blood pressure and LDLc levels, and were more likely to have a history of CHD, CHF and stroke/TIA. Those with higher concentrations of the BTMs were also less likely to be African American or to have ever smoked; had lower BMI, less glucose dysregulation, lower HDLc, triglycerides, CRP and eGFR; reported less physical activity, and were less likely to report estrogen replacement therapy, calcium supplementation, and thiazide diuretic use. Blood samples with higher concentrations of OC were more likely to have been collected in the spring, while those with higher levels of CTX were more likely to have been collected in the summer.
Table 1.
Main demographic, behavioral and clinical characteristics at baseline by quartiles of bone turnover markers
| OC | CTX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles | Quartile 1 n=421 |
Quartile 2 n=420 |
Quartile 3 n=419 |
Quartile 4 n=420 |
p | Quartile 1 n=422 |
Quartile 2 n=420 |
Quartile 3 n=416 |
Quartile 4 n=422 |
p |
| BTM range, ng/mL | ≤17.0 | 17.1–23.0 | 23.1–30.0 | >30.0 | ≤0.23 | 0.24–0.37 | 0.38–0.52 | >0.52 | ||
| Demographic/Timing | ||||||||||
| Age, years | 73.4±4.4 | 74.4±4.9 | 74.3±5.0 | 76.0±5.5 | <0.001 | 73.2±4.4 | 74.7±4.9 | 74.5±4.9 | 75.7±5.5 | <0.001 |
| Black, n (%) | 79 (18.8) | 68 (16.2) | 69 (16.5) | 41 (9.8) | 0.002 | 70 (16.6) | 63 (15.0) | 62 (14.9) | 62 (14.7) | 0.864 |
| Season, n (%) | 0.061 | 0.305 | ||||||||
| Summer | 79 (18.8) | 88 (21.0) | 77 (18.4) | 83 (19.8) | 70 (16.6) | 77 (18.3) | 81 (19.5) | 99 (23.5) | ||
| Fall | 100 (23.8) | 116 (27.6) | 102 (24.3) | 81 (19.3) | 95 (23.5) | 104 (24.8) | 101 (24.3) | 99 (23.5) | ||
| Winter | 112 (22.6) | 103 (24.5) | 106 (25.3) | 97 (23.1) | 121 (28.7) | 98 (23.3) | 99 (23.8) | 100 (23.7) | ||
| Spring | 130 (30.9) | 113 (26.9) | 134 (32.0) | 159 (37.9) | 136 (32.2) | 141 (33.6) | 135 (32.5) | 124 (29.4) | ||
| Social/Lifestyle | ||||||||||
| Education ≤grade 11, n (%) | 121 (28.7) | 119 (28.5) | 121 (29.0) | 121 (28.9) | 0.356 | 118 (28.0) | 120 (28.8) | 101 (24.3) | 143 (34.0) | 0.104 |
| BMI, kg/m2 | 27.8±5.1 | 27.3±5.0 | 26.7±5.0 | 25.5±4.8 | <0.001 | 27.6±4.9 | 27.2±5.0 | 26.7±5.2 | 25.7±4.9 | <0.001 |
| Physical activity, kcal/week | 810 (293–1888) |
803 (270–1755) |
767 (240–1658) |
697 (223–1474) |
0.264 | 808 (270–1890) |
769 (270–1739) |
783 (266–1665) |
718 (158–1470) |
0.221 |
| Smoking, n (%) | 0.023 | 0.090 | ||||||||
| Never | 214 (51.9) | 240 (57.8) | 242 (59.5) | 258 (63.1) | 220 (52.8) | 246 (60.3) | 236 (58.1) | 252 (61.2) | ||
| Former | 148 (35.9) | 144 (34.7) | 128 (31.4) | 113 (27.6) | 149 (35.7) | 134 (32.8) | 131 (32.3) | 119 (48.3) | ||
| Current | 50 (12.1) | 31 (7.5) | 37 (9.1) | 38 (9.3) | 48 (11.5) | 28 (6.9) | 39 (9.6) | 41 (10.0) | ||
| Alcohol >7 drinks/ week | 34 (8.1) | 24 (5.7) | 25 (6.0) | 22 (5.2) | 0.330 | 27 (6.4) | 29 (6.9) | 23 (5.5) | 26 (6.2) | 0.874 |
| ERT, n (%) | 134 (31.8) | 45 (10.7) | 35 (8.4) | 13 (3.1) | <0.001 | 148 (35.1) | 47 (11.2) | 23 (5.5) | 9 (2.1) | <0.001 |
| Cardiovascular/Metabolic | ||||||||||
| Systolic BP, mmHg | 136±21 | 137±22 | 137±23 | 139±22 | 0.142 | 136±20 | 137±21 | 137±23 | 139±23 | 0.038 |
| Anti-hypertensive Rx, n (%) | 224 (53.2) | 233 (55.5) | 191 (45.6) | 206 (49.0) | 0.020 | 230 (54.5) | 221 (52.6) | 185 (44.5) | 218 (51.7) | 0.021 |
| Thiazides, n (%) | 124 (29.5) | 92 (21.9) | 58 (13.8) | 65 (15.5) | <0.001 | 128 (30.3) | 90 (21.4) | 68 (16.3) | 53 (12.6) | <0.001 |
| Calcium Rx, n (%) | 130 (30.9) | 131 (31.2) | 101 (24.1) | 75 (17.9) | <0.001 | 139 (32.9) | 120 (28.6) | 106 (25.5) | 72 (17.1) | <0.001 |
| Diabetes, n (%) | 98 (23.3) | 63 (15.0) | 43 (10.3) | 35 (8.3) | <0.001 | 90 (21.3) | 65 (15.5) | 36 (8.7) | 48 (11.4) | <0.001 |
| LDLc, mg/dL | 125±35 | 134±32 | 134±32 | 135±35 | <0.001 | 126±34 | 134±33 | 135±32 | 132±35 | 0.041 |
| HDLc, mg/dL | 60±17 | 56±14 | 57±14 | 56±14 | 0.003 | 60±16 | 57±15 | 57±14 | 55±13 | <0.001 |
| Triglycerides, mg/dL | 134 (102–198) |
128 (87–179) |
120 (92–164) |
126 (93–166) |
<0.001 | 139 (102–198) |
134 (93–190) |
118 (89–165) |
123 (85–161) |
<0.001 |
| eGFR, ml/min/1.73m2 | 80.4±18.1 | 77.0±18.1 | 75.3±17.6 | 69.2±19.6 | <0.001 | 80.1±18.7 | 76.1±17.7 | 75.3±17.9 | 70.4±19.5 | <0.001 |
| CRP, mg/L | 4.3 (1.8–8.8) |
3.2 (1.4–6.0) |
2.2 (1.1–5.2) |
1.7 (0.8–4.0) |
<0.001 | 4.3 (1.8–8.6) |
2.7 (1.2–5.8) |
2.5 (1.1–5.5) |
1.8 (1.0–4.5) |
<0.001 |
| Prevalent CHD, n (%) | 59 (14.0) | 81 (19.3) | 81 (19.3) | 94 (22.4) | 0.018 | 65 (15.4) | 73 (17.4) | 68 (16.3) | 109 (25.9) | <0.001 |
| Prevalent CHF, n (%) | 11 (2.6) | 12 (2.9) | 20 (4.8) | 31 (7.4) | 0.002 | 8 (1.9) | 20 (4.8) | 16 (3.8) | 30 (7.1) | 0.003 |
| Prevalent stroke/TIA, n (%) | 18 (4.3) | 20 (4.8) | 23 (5.5) | 24 (5.7) | 0.765 | 13 (3.1) | 21 (5.0) | 23 (5.5) | 28 (6.6) | 0.121 |
Prevalent claudication, 26 (1.5%), Prevalent AF, 25 (1.5%), AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; BTM, bone turnover marker; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate based on cystatin C (CKD-EPI); ERT, estrogen replacement therapy; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol; TIA, transient ischemic attack; Rx, treatment.
Table 2 gives the distributions by BTM quartiles of mineral metabolism, physical function and cognitive measures at baseline, and of DXA-determined BMD. Frequency of ADL impairment and proportion of participants with history of fall increased, while 3MSE score declined with increasing quartiles of serum OC and CTX. Furthermore, 25-hydroxyvitamin D levels and BMD at the total hip and femoral neck declined, while PTH increased, with rising quartiles of both BTMs.
Table 2.
Measures of physical function, cognition, mineral metabolism, and bone mineral density by BTM quartiles
| OC | CTX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles | Quartile 1 n=421 |
Quartile 2 n=420 |
Quartile 3 n=419 |
Quartile 4 n=420 |
p | Quartile 1 n=422 |
Quartile 2 n=420 |
Quartile 3 n=416 |
Quartile 4 n=422 |
p |
| BTM range, ng/mL | ≤17.0 | 17.1–23.0 | 23.1–30.0 | >30.0 | ≤0.23 | 0.24–0.37 | 0.38–0.52 | >0.52 | ||
| Functional/Cognitive | ||||||||||
| ADL impairment, n (%) | 39 (9.3) | 33 (7.9) | 47 (11.2) | 52 (12.4) | 0.131 | 37 (8.8) | 26 (6.2) | 45 (10.8) | 63 (14.9) | <0.001 |
| Mobility impairment, n (%) | 136 (32.9) | 124 (30.0) | 115 (28.3) | 119 (29.4) | 0.523 | 129 (30.9) | 115 (28.0) | 114 (28.2) | 136 (33.3) | 0.312 |
| Fall in previous year, n (%) | 64 (15.2) | 78 (18.6) | 71 (16.9) | 95 (22.6) | 0.035 | 53 (12.6) | 75 (17.9) | 73 (17.5) | 107 (25.4) | <0.001 |
| Frailty score | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.052 | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.002 |
| 3MSE score <80, n (%) | 25 (5.9) | 38 (9.0) | 37 (8.8) | 45 (10.7) | 0.096 | 24 (5.7) | 31 (7.4) | 37 (8.9) | 53 (12.6) | 0.003 |
| Mineral Metabolism | ||||||||||
| Calcium, mg/dL* | 9.5±0.4 | 9.5±0.3 | 9.5±0.4 | 9.6±0.4 | 0.007 | 9.5±0.4 | 9.5±0.4 | 9.5±0.3 | 9.5±0.4 | 0.349 |
| Phosphate, mg/dL* | 3.6±0.5 | 3.7±0.4 | 3.7±0.4 | 3.8±0.5 | <0.001 | 3.6±0.5 | 3.7±0.4 | 3.7±0.4 | 3.9±0.5 | <0.001 |
| PTH, mg/dL* | 45 (34–59) | 52 (40–65) | 54 (39–70) | 58 (45–81) | <0.001 | 47 (35–62) | 52 (40–65) | 53 (43–70) | 57 (41–78) | <0.001 |
| Vitamin D, mg/dL* | 26.4±16.3 | 24.4±10.4 | 23.0±8.7 | 22.9±9.6 | <0.001 | 27.0±16.6 | 23.7±9.9 | 22.8±9.5 | 23.0±8.8 | <0.001 |
| DXA Outcome Measures | ||||||||||
| BMD total hip, g/cm2 † | 0.81±0.16 | 0.76±0.13 | 0.73±0.13 | 0.70±0.13 | <0.001 | 0.80±0.16 | 0.76±0.13 | 0.72±0.13 | 0.71±0.13 | <0.001 |
| BMD femoral neck, g/cm2 † | 0.69±0.15 | 0.65±0.11 | 0.63±0.11 | 0.60±0.12 | <0.001 | 0.68±0.14 | 0.66±0.13 | 0.62±0.11 | 0.61±0.11 | <0.001 |
missing in at least n=467 (n=474 for vitamin D);
available in n=425 participants;
BMD, bone mineral density; BTM, bone turnover marker; PTH, parathyroid hormone.
BTMs and incident fracture risk
During median follow-up of 12.3 (IQR 7.3–18.5, maximum 22.1) years, a total of 288 participants suffered a hip fracture (13.75 cases per 1000 person-years). Table 3 shows the unadjusted incidence of hip fracture by quartiles of OC and CTX. The lowest fracture rates were observed for the lowest quartiles of serum OC and CTX compared with their upper three quartiles.
Table 3.
Incidence and adjusted hazard ratios for hip fracture by levels of serum OC and CTX
| Incident fracture (n) | Unadjusted incidence per 1000 PY (95% CI) | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Osteocalcin | ||||||
| Quartile 1 (≤17.0 ng/mL) | 62 | 10.99 (8.57–14.09) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Quartile 2 (>17.0–23.0 ng/mL) | 78 | 14.94 (11.97–18.65) | 1.27 (0.91–1.77) | 1.32 (0.91–1.90) | 1.34 (0.91–1.98) | 1.36 (0.92–2.02) |
| Quartile 3 (>23.0–30.0 ng/mL) | 72 | 13.50 (10.72–17.01) | 1.12 (0.80–1.58) | 1.16 (0.80–1.68) | 1.23 (0.82–1.85) | 1.23 (0.82–1.84) |
| Quartile 4 (>30.0 ng/mL) | 76 | 16.02 (12.79–20.05) | 1.20 (0.85–1.69) | 1.16 (0.79–1.70) | 1.30 (0.86–1.97) | 1.30 (0.86–1.98) |
| Per SD* increment (<19.3 ng/mL) | 1.54 (0.89–2.66) | 1.54 (0.84–2.85) | 1.70 (0.90–3.21) | 1.72 (0.91–3.25) | ||
| Per SD* increment (≥19.3 ng/mL) | 0.96 (0.82–1.12) | 0.94 (0.80–1.11) | 0.98 (0.82–1.16) | 0.97 (0.82–1.15) | ||
| CTX (ng/mL) | ||||||
| Quartile 1 (≤0.23 ng/mL) | 60 | 10.61 (8.24–13.67) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| Quartile 2 (>0.23–0.37 ng/mL) | 75 | 13.97 (11.14–17.52) | 1.16 (0.83–1.64) | 1.22 (0.84–1.76) | 1.33 (0.90–1.97) | 1.39 (0.93–2.06) |
| Quartile 3 (>0.37–0.52 ng/mL) | 82 | 16.12 (12.99–20.02) | 1.42 (1.01–1.98) | 1.43 (0.99–2.07) | 1.64 (1.11–2.43) | 1.63 (1.10–2.43) |
| Quartile 4 (>0.52 ng/mL) | 71 | 14.69 (11.64–18.54) | 1.18 (0.83–1.68) | 1.11 (0.75–1.64) | 1.33 (0.87–2.02) | 1.33 (0.87–2.04) |
| Per SD† increment (<0.43 ng/mL) | 1.34 (1.03–1.76) | 1.36 (1.01–1.83) | 1.53 (1.11–2.10) | 1.52 (1.10–2.09) | ||
| Per SD† increment (≥0.43 ng/mL) | 0.86 (0.69–1.06) | 0.79 (0.63–0.99) | 0.81 (0.64–1.03) | 0.80 (0.63–1.01) |
CI, confidence interval; PY, person years.
SD=10.75 ng/mL
SD=0.23 ng/mL
Model 1: adjusted for age, race, field center and season.
Model 2 additionally adjusted for BMI, education, smoking status, alcohol consumption, physical activity, estrogen replacement therapy.
Model 3 additionally adjusted for systolic blood pressure, anti-hypertensive therapy, diabetes, calcium supplementation, LDLc, HDLc, triglycerides, prevalent CHD, prevalent CHF, prevalent claudication, prevalent AF, prevalent stroke/TIA, eGFR, and C-reactive protein.
Model 4 additionally adjusted for ADL impairment, cognitive impairment and fall history.
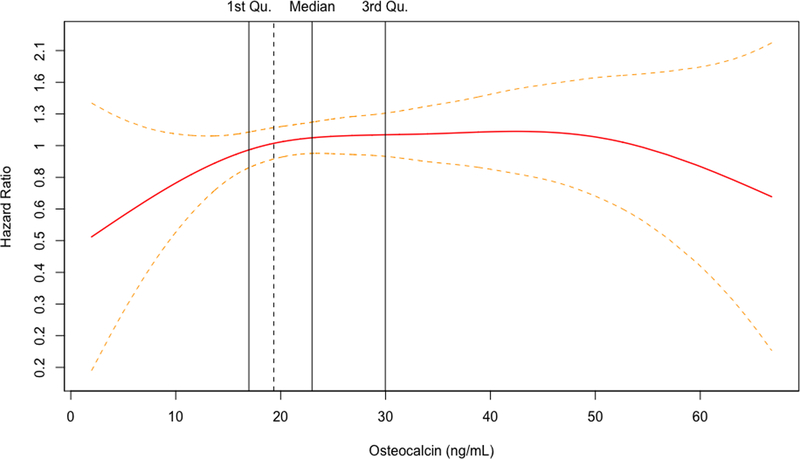
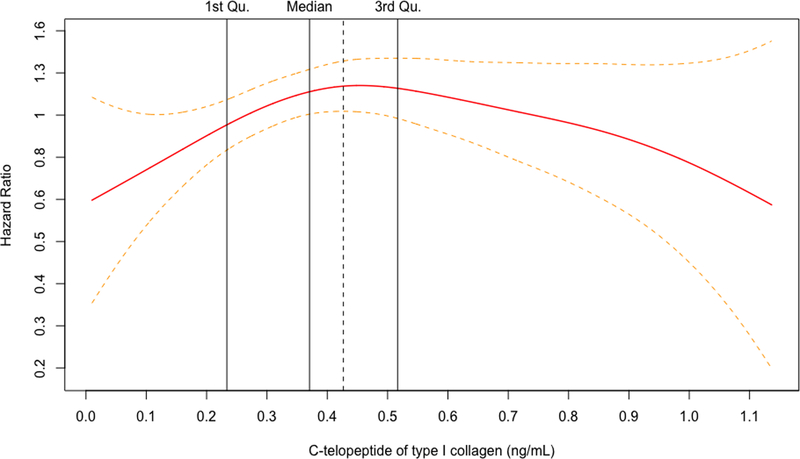
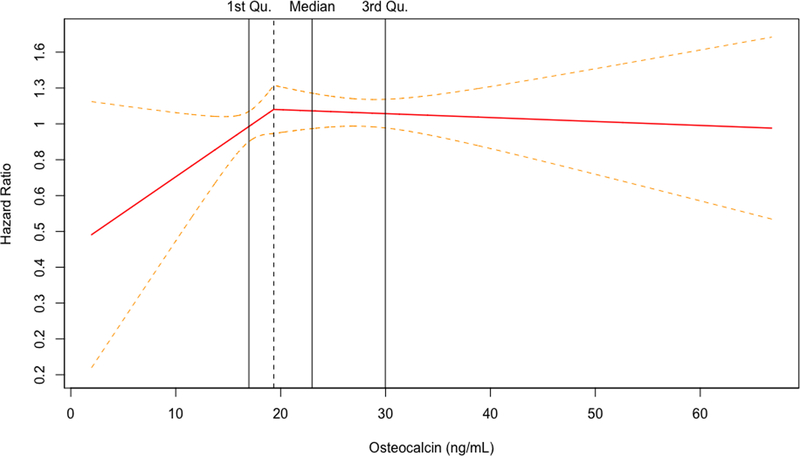
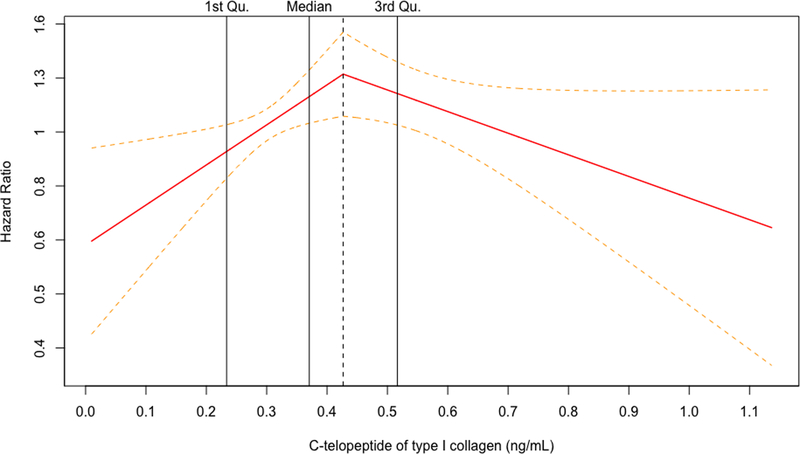
For both biomarkers, the penalized cubic splines plots revealed departures from a monotonic linear relationship in their adjusted relationships with hip fracture (Figure 1A and 1B). The relationship with incident hip fracture for OC appeared to show an increasing slope at the lower end of its concentration before leveling off early within the second quartile and seeming to show a decrease at the upper end of its concentration. In turn, CTX displayed an inverted U-shape relationship with hip fracture, with risk increasing from its lowest concentration and peaking within the third quartile, before decreasing with rising concentrations to the upper end of its distribution. Accordingly, the associations were modeled using linear splines below and above the inflection points on the corresponding cubic spline curves for each BTM (Figure 1C and 1D), as well as by comparison of increasing quartiles to the lowest quartile (Table 3).
Figure 1.
Penalized cubic splines plot for OC (A) and CTX (B) winsorized at the 99% percentile. Linear splines plot for OC (C) and CTX (D) winsorized at the 99% percentile. Models are adjusted for covariates in Model 4.
For OC, there were no significant associations with incident hip fracture at any level of adjustment in analyses of linear splines below or above the inflection point of 19.3 ng/mL (corresponding to the 35th percentile of OC’s distribution) or when modeled by quartiles, as shown in Table 3. For CTX, there was a significant positive association with incident hip fracture up to the inflection point of 0.43 ng/mL (corresponding to the 62nd percentile of CTX distribution) that became slightly stronger at with greater adjustment (Table 3). In the fully adjusted model (Model 4), every SD increment in CTX concentration up to the inflection point (among n=1035 participants) was associated with a 52% (10 to 109%) greater hazard of hip fracture (p=0.011). At CTX concentrations beyond this inflection point (involving n=645 participants), there was a significant inverse association with hip fracture after adjustment for demographic and behavioral risk factors (Model 2). Additional adjustment for clinical variables rendered the association marginally non-significant, such that every SD increment in CTX was associated with a 20% (−1% to 37%) lower hazard of hip fracture (p=0.060). As also detailed in Table 3, comparison of quartiles showed a significantly increased risk of hip fracture for the third, but not the second or fourth, quartile as compared to the first quartile. Specifically, comparison of quartile 3 versus quartile 1 showed a 63% (10 to 143%) greater hazard for the primary outcome in the fully adjusted model (p=0.016), a risk estimate that was modestly stronger than in the minimally adjusted model.
In sensitivity analyses that separately excluded women taking vitamin D, calcitriol and bisphosphonates; thiazide diuretics; and African Americans, the relationships between OC and CTX and incident hip fracture were not materially different (data not shown).
BTM and BMD
Assessment of the relationships of serum OC and CTX with BMD showed no apparent departures from linearity (plots not shown). As presented in Table 4, there were significant inverse associations for both OC and CTX with BMD at the total hip and femoral neck after minimal adjustment. These persisted after full adjustment for behavioral and clinical covariates, such that each SD increment in OC was associated with 0.021 g/m2 lower total hip BMD and 0.017 g/m2 lower femoral neck BMD; and each SD increment in CTX was associated 0.19 g/m2 lower total hip BMD and 0.015 g/m2 lower femoral neck BMD.
Table 4.
Relationship of serum OC and CTX with BMD at the total hip and femoral neck
| Total Hip | Femoral Neck | |||||
|---|---|---|---|---|---|---|
| Beta per SD increment | 95% C.I. | p | Beta per SD increment | 95% C.I. | p | |
| OC | ||||||
| Model 1 | −0.025 | −0.038, −0.012 | <0.001 | −0.022 | −0.034, −0.011 | <0.001 |
| Model 2 | −0.018 | −0.030, −0.006 | 0.003 | −0.016 | −0.026, −0.005 | 0.004 |
| Model 3 | −0.020 | −0.033, −0.008 | 0.002 | −0.016 | −0.027, −0.005 | 0.006 |
| Model 4 | −0.021 | −0.033, −0.008 | 0.002 | −0.017 | −0.028, −0.005 | 0.004 |
| CTX | ||||||
| Model 1 | −0.025 | −0.037, −0.014 | <0.001 | −0.021 | −0.032, −0.011 | <0.001 |
| Model 2 | −0.018 | −0.029, −0.007 | 0.001 | −0.015 | −0.025, −0.005 | 0.003 |
| Model 3 | −0.019 | −0.031, −0.007 | 0.001 | −0.014 | −0.024, −0.004 | 0.009 |
| Model 4 | −0.019 | −0.031, −0.007 | 0.002 | −0.015 | −0.025, −0.004 | 0.006 |
C.I., confidence interval.
Model 1: adjusted for age, race, field center and season.
Model 2 additionally adjusted for BMI, education, smoking status, alcohol consumption, physical activity, estrogen replacement therapy.
Model 3 additionally adjusted for systolic blood pressure, anti-hypertensive therapy, diabetes, calcium supplementation, LDLc, HDLc, triglycerides, prevalent CHD, prevalent CHF, prevalent claudication, prevalent AF, prevalent stroke/TIA, eGFR, C-reactive protein.
Model 4 additionally adjusted for ADL impairment, cognitive impairment and fall history.
Discussion
Main results
The present study examined the relationship of two important serum biomarkers of bone turnover with incident hip fracture in a large sample of older postmenopausal women. We found no association between OC and incident hip fracture but did observe an inverted U-shaped association for CTX. Rising concentrations of CTX to the high-middle range of its distribution were associated with a significant and moderately increased risk of hip fracture, while further increments in concentration were associated with a more modest lower risk of this outcome that was marginally non-significant. Sensitivity analyses restricting the sample to white women and excluding participants on bone-influencing medications did not meaningfully alter these associations. By contrast, both OC and CTX showed significant but modest inverse associations with hip BMD after similar adjustment in a subset of participants who underwent DXA.
Previous studies
A host of longitudinal studies have examined BTMs in relation to future fracture risk in men and women [11–22, 24, 38–41]. While several have reported significant associations of BTMs with incident fracture, even independent of BMD [12–15, 19, 38, 40], many have failed to detect such associations [16, 20, 21]. These inconsistent results are difficult to interpret on account of differences in age and sex of study participants, the diverse fracture types considered, the varying and frequently incomplete level of adjustment for potential confounders, and for a large majority, lack of appropriate sample collection procedures. Among available BTMs, international expert panels have identified PINP and CTX as the most promising measures of bone formation and resorption, respectively, recommending them as reference markers for observational and interventional studies [9, 10]. But consensus has emerged that proper assessment of the role of BTMs for fracture prediction must control for factors contributing to major pre-analytical variability of such biomarkers, with particular attention to morning collection of fasting blood samples [8].
Here we chose osteocalcin as our marker of bone formation, reflecting our study’s focus on bone health and glucose dysregulation [29], while selecting CTX as the optimal marker for bone resorption. Several longitudinal studies have assessed the relationship of osteocalcin specifically with incident hip fracture in women [18–20]. None found a significant association, but specimen collection was either non-fasting [19, 20] or unspecified [18], and the numbers of events were small to moderate (n=33 to 120 hip fractures). A more recent study of older adults that did undertake fasting blood collection also failed to detect an association for osteocalcin in post-menopausal women, but there were only 55 incident hip fracture cases among female participants [22].
As relates to CTX, a meta-analysis of earlier studies reported a significant association with any fracture, but not hip fracture, in women [23]. Such studies did not perform fasting blood collection and undertook limited adjustment for covariates [15, 19, 20]. Two more recent prospective studies evaluating the relationship between serum CTX, measured in morning fasting specimens, and hip fracture in postmenopausal women also failed to detect a significant association for CTX [22, 24]. The first [22] was underpowered, however, while the second [24] – a nested study with 400 cases and 400 controls – had substantial missingness for covariates in the fully adjusted model (n=608 cases and controls examined). The latter study reported fully adjusted risk estimates for interquartile comparisons that had wide confidence intervals (quartile 3 vs. 1: OR=1.53, 95% CI=0.82, 2.85; quartile 4 vs. 1: OR=1.25, 95% CI =0.68–2.30).
Our findings for osteocalcin are consistent with previous results [16, 18–20], and accord with prior scientific statements designating PINP over osteocalcin as the preferred biomarker of bone formation [9, 10]. The results for CTX stand out because they reveal a non-linear relationship with incident hip fracture. This inverted U-shaped association has not been previously reported although, as noted, a majority of previous studies have lacked power to investigate the functional form of CTX’s relationship with hip fracture. Interestingly, in the aforementioned recent nested case-control study,[24] the reported analysis of CTX quartiles showed a higher risk estimate for hip fracture for quartile 3 than quartile 4. The same study also assessed for, and did not find, non-linear associations using generalized additive models, but corresponding plots were not presented.
Interpretation of study findings
As CTX reflects osteoclastic digestion of the major protein in bone, collagen, and correlates with histomorphometric indices of bone resorption, we anticipated fracture risk to be greatest in those with the highest CTX. The reasons why this was not the case are unclear, but several possibilities merit consideration. As in previous studies [42], both CTX and OC were linearly inversely related to hip BMD in the subset of participants who underwent DXA. It is well documented, however, that fall risk is a more important determinant of hip fracture incidence than bone metabolism per se [43]. But both ADL difficulty and history of previous fall, like cognitive impairment, were positively associated with CTX (as with OC) and highest in its upper quartile, and adjustment for these factors did not alter the CTX-hip fracture association. Cardiovascular and kidney disease were likewise most prevalent in the upper quartile of the two BTMs. Such comorbidities have also been linked to an increased risk of fracture [44], yet adjustment for such factors modestly increased the risk estimates for both quartile 3 and quartile 4. Hence, one possibility for the declining hip fracture risk as concentrations of CTX rose to their highest levels is that lower physical activity by individuals with the comorbidities enriched in the upper quartile of CTX may have outweighed their otherwise higher susceptibility for fracture. Indeed, participants in the highest quartile of CTX had the lowest level of physical activity, yet residual or unmeasured confounding could explain the lower risk at these upper CTX concentrations.
Furthermore, BTMs are dynamic and influenced by recent events including fractures, immobility and short-term changes in exercise, diet, weight and some medications [7]. Thus, it is possible that the measured level of resorption represents such recent events rather than the chronic status of skeletal health in some of our participants. Such factors could have also contributed to the failure to detect a dose-dependent relationship between CTX and hip fracture. We assessed the robustness of the relationship by excluding participants taking key medications affecting mineral metabolism, but such sensitivity analyses did not meaningfully alter the association between CTX and risk of hip fracture.
Additionally, participants with the highest bone resorption and lowest BMD may have compensatory mechanisms that offset hip fracture risk by positively affecting other bone qualities that maintain bone strength, such as bone size and geometry. In older adults, there is evidence to suggest periosteal bone expansion counterbalances the high bone resorption and endocortical expansion that characterizes age-related bone loss [45], a mechanism that may be relevant in other age groups as well [46]. In one study, adolescents with a genetic predisposition to higher bone resorption had greater bone size despite lower BMD, suggesting higher bone resorption is offset by greater periosteal expansion [46]. Information on bone geometry is lacking in our cohort.
Implications
The present investigation is the largest to date to assess both OC and CTX in relation to incident hip fracture in post-menopausal women. Strengths of the study include its sample size and power, which allowed assessment of the functional form and provide insights into the non-linear relationship observed here; the use of fasting blood specimens; and extensive adjustment for potential confounders. Laboratory analysis of bone turnover markers for this ancillary study was performed on never-previously-thawed serum specimens stored at −80°C after collection under standardized conditions, thereby keeping biomarker degradation to a minimum. As such, the current findings yield important insights regarding the associations of these two major BTMs. In regard to total OC, the current study’s failure to detect a significant association with incident hip fracture, notwithstanding OC’s close correlation with CTX, adds support to the view that this marker of bone formation is of limited value for hip fracture prediction. As relates to CTX, the reverse U-shaped association reflects the multiple factors that influence this foremost bone resorption marker’s relationship with hip fracture risk, highlighting the challenge of using CTX and other BTMs for prediction of this outcome. While it remains to be determined whether the documented association of CTX with hip fracture is independent of BMD, the modest inverse relationship observed between CTX and BMD does suggest that the marker may be of value for hip fracture prediction. This will require further testing in appropriately powered studies, but our findings make clear that careful consideration of clinical and functional risk factors will be necessary for adequate characterization of this relationship and corresponding evaluation of risk prediction models.
Limitations
Our study has several limitations. Measures that could have provided further information on bone metabolism such as BMD and biochemical calciotropic factors (vitamin D, PTH, etc.) were only available in a small subset of participants and we therefore could not include them in our final adjusted models. Moreover, BMD measures were available 2 years after BTM determination. Nonetheless, we did observe the anticipated relationships of BTMs with vitamin D, PTH and, notwithstanding the later time point, BMD in these subsets. Measurements of PINP, the recommended marker of bone formation, were not available. Furthermore, we only have information regarding the occurrence of hip fracture and not vertebral or other types of non-vertebral fractures. It is possible that we may have found stronger or monotonic relationships between BTMs and fracture risk with inclusion of other types of fracture or with a focus on fracture at more metabolically active skeletal sites. Moreover, fracture cases were identified by ICD-9 codes and were not adjudicated, which could have led to misclassification. We also do not have any information regarding history of low trauma or fragility fracture. Since fracture risk is higher among those with a prior fragility fracture, this too could have influenced our results.
Conclusions
Among older women, CTX exhibited an inverted U-shaped association with incident hip fracture after adjustment for potential confounders, with risk peaking at the mid-upper range of the marker’s distribution, but no significant association was demonstrated for osteocalcin. Further work is necessary to understand this non-linear association, whether it is independent of BMD, and if CTX can be harnessed to improve prediction of hip and other fracture types.
Acknowledgements
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. D.M. was supported by The Glorney-Raisbeck Fellowship Program, Corlette Glorney Foundation and The New York Academy of Medicine. J.R.K. was supported by K24HL135413 from the NHLBI.
Footnotes
Disclosures: Jorge R. Kizer reports stock ownership in Amgen, Bristol-Myers Squibb, Gilead Sciences, Merck, Johnson and Johnson, and Pfizer. Daniele Massera, Shuo Xu, Marcella D. Walker, Rodrigo J. Valderrábano, Kenneth J. Mukamal, Joachim H. Ix, David S. Siscovick, Russell P. Tracy, John A. Robbins, Mary L. Biggs and Xiaonan Xue declare that they have no conflict of interest.
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–79511176917 [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Black DM, Nevitt MC, et al. (1990) Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA 263:665–668 [PubMed] [Google Scholar]
- 4.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR, Osteoporotic Fractures Research G (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18:1947–1954 [DOI] [PubMed] [Google Scholar]
- 5.Hannon R, Blumsohn A, Naylor K, Eastell R (1998) Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res 13:1124–1133 [DOI] [PubMed] [Google Scholar]
- 6.Vilaca T, Gossiel F, Eastell R (2017) Bone Turnover Markers: Use in Fracture Prediction. J Clin Densitom 20:346–352 [DOI] [PubMed] [Google Scholar]
- 7.Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA (2018) DIAGNOSIS OF ENDOCRINE DISEASE: Bone turnover markers: are they clinically useful? Eur J Endocrinol 178:R19–R31 [DOI] [PubMed] [Google Scholar]
- 8.Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET, National Bone Health Alliance Bone Turnover Marker P (2017) Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 28:2541–2556 [DOI] [PubMed] [Google Scholar]
- 9.Vasikaran S, Eastell R, Bruyere O, et al. (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22:391–420 [DOI] [PubMed] [Google Scholar]
- 10.Bauer D, Krege J, Lane N, et al. (2012) National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int 23:2425–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szulc P, Chapuy MC, Meunier PJ, Delmas PD (1996) Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture: a three year follow-up study. Bone 18:487–488 [DOI] [PubMed] [Google Scholar]
- 12.Akesson K, Ljunghall S, Jonsson B, Sernbo I, Johnell O, Gardsell P, Obrant KJ (1995) Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women. J Bone Miner Res 10:1823–1829 [DOI] [PubMed] [Google Scholar]
- 13.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11:1531–1538 [DOI] [PubMed] [Google Scholar]
- 14.Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K, Delmas PD (1997) Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab 82:719–724 [DOI] [PubMed] [Google Scholar]
- 15.Chapurlat RD, Garnero P, Breart G, Meunier PJ, Delmas PD (2000) Serum type I collagen breakdown product (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS study. Bone 27:283–286 [DOI] [PubMed] [Google Scholar]
- 16.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536 [DOI] [PubMed] [Google Scholar]
- 17.Luukinen H, Kakonen SM, Pettersson K, Koski K, Laippala P, Lovgren T, Kivela SL, Vaananen HK (2000) Strong prediction of fractures among older adults by the ratio of carboxylated to total serum osteocalcin. J Bone Miner Res 15:2473–2478 [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ 3rd, Crowson CS, O’Fallon WM, Wahner HW, Riggs BL (2003) Relative contributions of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. J Bone Miner Res 18:312–318 [DOI] [PubMed] [Google Scholar]
- 19.Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, Pettersson K, Vaananen HK, Akesson K, Obrant KJ (2004) Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res 19:386–393 [DOI] [PubMed] [Google Scholar]
- 20.Dobnig H, Piswanger-Solkner JC, Obermayer-Pietsch B, Tiran A, Strele A, Maier E, Maritschnegg P, Riedmuller G, Brueck C, Fahrleitner-Pammer A (2007) Hip and nonvertebral fracture prediction in nursing home patients: role of bone ultrasound and bone marker measurements. J Clin Endocrinol Metab 92:1678–1686 [DOI] [PubMed] [Google Scholar]
- 21.Ivaska KK, Gerdhem P, Vaananen HK, Akesson K, Obrant KJ (2010) Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res 25:393–403 [DOI] [PubMed] [Google Scholar]
- 22.Marques EA, Gudnason V, Lang T, Sigurdsson G, Sigurdsson S, Aspelund T, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris TB (2016) Association of bone turnover markers with volumetric bone loss, periosteal apposition, and fracture risk in older men and women: the AGES-Reykjavik longitudinal study. Osteoporos Int 27:3485–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson H, Oden A, Kanis JA, McCloskey EV, Morris HA, Cooper C, Vasikaran S, Turnover I-IJWGoSoBMoB (2014) A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int 94:560–567 [DOI] [PubMed] [Google Scholar]
- 24.Crandall CJ, Vasan S, LaCroix A, LeBoff MS, Cauley JA, Robbins JA, Jackson RD, Bauer DC (2018) Bone Turnover Markers Are Not Associated With Hip Fracture Risk: A Case-Control Study in the Women’s Health Initiative. J Bone Miner Res 33:1199–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khosla S (2013) Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci 68:1226–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright P, et al. (1991) The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1:263–276 [DOI] [PubMed] [Google Scholar]
- 27.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S (1995) Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 5:278–285 [DOI] [PubMed] [Google Scholar]
- 28.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP (1995) Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 41:264–270 [PubMed] [Google Scholar]
- 29.Massera D, Biggs ML, Walker MD, et al. (2018) Biochemical Markers of Bone Turnover and Risk of Incident Diabetes in Older Women: The Cardiovascular Health Study. Diabetes Care 41:1901–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T (2001) The association of bone mineral density and depression in an older population. J Am Geriatr Soc 49:732–736 [DOI] [PubMed] [Google Scholar]
- 31.Stevens LA, Coresh J, Schmid CH, et al. (2008) Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J (1995) Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol 5:270–277 [DOI] [PubMed] [Google Scholar]
- 33.Karas MG, Yee LM, Biggs ML, et al. (2016) Measures of Body Size and Composition and Risk of Incident Atrial Fibrillation in Older People: The Cardiovascular Health Study. Am J Epidemiol 183:998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried LP, Tangen CM, Walston J, et al. (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–156 [DOI] [PubMed] [Google Scholar]
- 35.Fitti JE, Kovar MG (1987) The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1 1–115 [PubMed] [Google Scholar]
- 36.Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R (1998) Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 29:388–398 [DOI] [PubMed] [Google Scholar]
- 37.Parisien M, Cosman F, Morgan D, et al. (1997) Histomorphometric assessment of bone mass, structure, and remodeling: a comparison between healthy black and white premenopausal women. J Bone Miner Res 12:948–957 [DOI] [PubMed] [Google Scholar]
- 38.Ross PD, Kress BC, Parson RE, Wasnich RD, Armour KA, Mizrahi IA (2000) Serum bone alkaline phosphatase and calcaneus bone density predict fractures: a prospective study. Osteoporos Int 11:76–82 [DOI] [PubMed] [Google Scholar]
- 39.Meier C, Nguyen TV, Center JR, Seibel MJ, Eisman JA (2005) Bone resorption and osteoporotic fractures in elderly men: the dubbo osteoporosis epidemiology study. J Bone Miner Res 20:579–587 [DOI] [PubMed] [Google Scholar]
- 40.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD (2005) Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res 20:1813–1819 [DOI] [PubMed] [Google Scholar]
- 41.Bauer DC, Garnero P, Harrison SL, Cauley JA, Eastell R, Ensrud KE, Orwoll E, Osteoporotic Fractures in Men Research G (2009) Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. J Bone Miner Res 24:2032–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11:337–349 [DOI] [PubMed] [Google Scholar]
- 43.Greenspan SL, Myers ER, Maitland LA, Resnick NM, Hayes WC (1994) Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA 271:128–133 [PubMed] [Google Scholar]
- 44.Carbone L, Buzkova P, Fink HA, Lee JS, Chen Z, Ahmed A, Parashar S, Robbins JR (2010) Hip fractures and heart failure: findings from the Cardiovascular Health Study. Eur Heart J 31:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK (2003) Bone loss and bone size after menopause. N Engl J Med 349:327–334 [DOI] [PubMed] [Google Scholar]
- 46.Kemp JP, Sayers A, Paternoster L, et al. (2014) Does bone resorption stimulate periosteal expansion? A cross-sectional analysis of beta-C-telopeptides of type I collagen (CTX), genetic markers of the RANKL pathway, and periosteal circumference as measured by pQCT. J Bone Miner Res 29:1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]