Abstract
Objectives:
Post-imaging mathematical prediction models (MPMs) provide guidance for the management of solid pulmonary nodules by providing a lung cancer risk score from demographic and radiologists-indicated imaging characteristics. We hypothesized calibrating the MPM risk-score threshold to a local study cohort would result in improved performance over the original recommended MPM-thresholds. We compared the pre- and post-calibration performance of four MPM models and determined if improvement in MPM prediction occurs as nodules are imaged longitudinally.
Materials and Methods:
A common cohort of 317 individuals with computed tomography detected, solid nodules (80 malignant, 237 benign) were used to evaluate the MPM performance. We created a web-based application for this study that allows others to easily calibrate thresholds and analyze the performance of MPMs on their local cohort. 30 patients with repeated imaging was tested for improved performance longitudinally.
Results:
Using calibrated thresholds, Mayo Clinic and Brock University (BU) MPMs performed the best (AUC= 0.63, 0.61) compared to the Veteran’s Affairs (0.51) and Peking University (0.55). Only the BU had consensus with the original MPM-threshold, the other calibrated thresholds improved MPM accuracy. No significant improvements in accuracy were found longitudinally between time-points.
Conclusions:
Calibration to a common cohort can select the best performing MPM for your institution. Without calibration, BU has the most stable performance in solid nodules ≥8mm but has only moderate potential to refine subjects into appropriate work-up. Application of MPM is recommended only at initial evaluation as no increase in accuracy was achieved over time.
Keywords: Risk Assessment; lung neoplasms; tomography, x-ray computed; logistic models; area under curve
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide[1]. Computed tomography (CT) imaging is used to characterize lung nodules. Size-based guidelines exist to provide clinicians with criteria to assess the potential malignancy of pulmonary nodules including Lung-RADs Assessment Categories, American College of Chest Physicians Clinical Practice Guidelines, and Fleischner Society Follow-Up Guidelines[2–4]. However, these have the potential to misclassify small malignant nodules and large benign nodules leading to suboptimal treatment plans[5–7]. This is particularly true of first encounters, or ‘de novo’ nodules, which often fall into CT surveillance recommendations without access to growth information.
Pre-imaging lung cancer risk models have been produced which seek to stratify the individual’s benefit from screening thereby reducing unnecessary radiation exposure on subjects with limited benefit from CT imaging[8]. To better characterize imaging-detected nodules, post-imaging mathematical prediction models (MPMs) developed using multivariate logistic regressions of known lung cancer risk factors including family history, demographics, and radiologist-defined imaging characteristics to provide a malignancy risk stratification after an imaging encounter[9–13]. MPMs have been utilized on an ad hoc basis by clinicians seeking standardized input from evidence-based models. However, recently, an MPM was incorporated into the British Thoracic Society’s (BTS) Guidelines for Nodule Follow-up following an initial size-based stratification of risk (grade C recommendation) indicating a growing interest in the increased use of MPMs for day-to-day management of pulmonary nodule subjects[14].
This study compares four previously published post-imaging MPMs: the Mayo Clinic model (MC)[9], the U.S. Department of Veterans Affairs model (VA)[10], the Peking University model (PU)[12], and the Brock University model (BU)[11], on a large cohort of trial subjects and a longitudinal cohort of retrospective clinical subjects. As these MPMs were developed using different imaging parameters (clinical chest radiographs[9,12], clinical CT scans[10,12], or lung cancer screening CT scans[11]), different proportions of malignant cases (MC: 35%; VA: 54%; PU: 61%; BU 6%), and variable size distributions (mean size malignant/benign; MC: 17.8mm/11.6mm; VA: 18.9mm/14.8mm; PU: 21.3mm/17.2mm; BU: 15.7mm/4.1mm), we expected significant cohort dependence to be seen when each MPM was applied to an independent dataset.
While several studies have attempted to compare the accuracy of various post-imaging MPMs, they have reported performance (sensitivity, specificity) based on optimized cutoff points for their unique study cohorts as opposed to the recommended thresholds associated witha given MPM[15–17]. These studies reported that independent cohort-optimized thresholds can vary greatly from the MPM- thresholds and adjustments to the cut-off used affects sensitivity and specificity values[17]. This presence a lack of clarity in the appropriate cutoff point for a given MPM to be applied in the clinical context[18]. Here, we evaluate the current clinical usefulness of MPMs using the recommended thresholds and compare the performance to our study-optimized cut-offs.
Materials and Methods
Study Cohorts
As mentioned previously, the MPMs investigated here have been built and tested in diverse datasets. For this study, two cohorts of subjects with pulmonary nodules were investigated: a research cohort and a longitudinal clinical cohort (Table 1).
Table 1:
Subject and nodule demographics of study cohorts.
| Cohort | Demographics | Malignant | Benign |
|---|---|---|---|
| Research | Number of Subjects | 80 | 237 |
| Age (years) (Mean, Range) |
64.0 (41–87) |
62.1 (40–86) |
|
| Sex | 54F : 26M | 113F : 124M | |
| Pack-years (Mean, Range) |
40.7 (0–80) |
15.8 (0–50) |
|
| Nodule Size (Mean, Range) |
16.3mm (4–30mm) |
9.2mm (4–30mm) |
|
| < 6mm | 5 | 59 | |
| ≥ 6mm to < 8mm | 6 | 56 | |
| ≥ 8mm to < 15mm | 27 | 92 | |
| ≥ 15 mm | 42 | 30 | |
| LDCT screening eligible (Yes: No) |
48:32 | 85:152 | |
| Longitudinal Clinical | Subjects | 16 | 14 |
| Age (years) (Mean, Range) |
46.5 (23–64) |
61.1 (40–74) |
|
| Sex | 9F : 7M | 10F : 4M | |
| Pack-years (Mean, Range) |
21.2 (0–50) |
14.2 (0–25) |
|
| Nodule Size (Mean, Range) |
18.9mm (3–48mm) |
13.3mm (3–29mm) |
Definition of abbreviations: F = female, M = male, LDCT = low-dose computed tomography
: LDCT screening eligibility criteria based on age between 55 and 80, and ≥ 30 pack years who currently smoke or have quit within the past 15 years.
Research Trial Cohort
The research cohort consisted of 317 subjects (80 malignant, 237 benign) retrospectively included from two separate prospective trials collecting high-resolution CT scans (217 COPDGene[19], 100 INHALE[20]). Data was collected with informed consent by the parent studies and use of the de-identified data for our study was approved by the University of Iowa Institutional Review Board. While neither study was specifically aligned with the recommendations for screening lung cancer, both had de-novo nodules encountered during imaging. The primary goal of the COPDGene study was to find underlying genetic factors of chronic obstructive pulmonary disorder (COPD), however, as COPD is a known co-morbidity of lung cancer, an ongoing ancillary study was established to track participants with malignant nodules. The primary goal of the INHALE study was to evaluate lung cancer risk with measures of COPD from spirometry and imaging. Demographic and historical information was collected from participants in these trials and radiologist reports were generated to include descriptions of nodule findings. For the COPDGene study, diagnosis for each subject was either confirmed malignant (pathology) or confirmed benign (pathology, resolution, and/or 2-year stability). The INHALE study confirmed malignancy through histological confirmation accessed through the Detroit area Surveillance, Epidemiology, and End Results registry and benign cases were selected to match size characteristics. Further information about these studies is included in the Supplementary Information.
Longitudinal Clinical Cohort
The longitudinal clinical cohort was included as a proof of concept on MPM prediction performance improvement over time and repeated imaging. The cohort consisted of 30 subjects (16 malignant, 14 benign) with 92 clinical CT scans (Table 1). With Institutional Review Board approval, the medical records of University of Iowa Hospitals and Clinics patients with nodules indicated were retrospectively reviewed. Medical histories and radiological reports were reviewed for the following inclusion criteria: (1) a solid pulmonary nodule in repeated CT imaging and (2) confirmed malignant (pathology) or confirmed benign (pathology, resolution, and/or 2-year stability). For this assessment, we compared the performance of MPM predictions at (a) the initial (incidental) imaging encounter on which the pulmonary nodule was identified (TP_I), (b) the final imaging encounter before diagnosis (TP_F), and (c) across all the imaging encounters between detection and diagnosis.
Mathematical Prediction Models
Four MPMs were assessed: the Mayo Clinic model (MC)[9], the U.S. Department of Veterans Affairs model (VA)[10], the Peking University model (PU)[12], and the Brock University model (BU)[11]. Pertinent risk variables were manually extracted from subject records and a risk score was calculated for each CT scan (Table 2); detailed descriptions of the MPM equations and variable descriptions is provided in the Supplementary Information. Unless the radiological report specifically indicated the presence of calcification, spiculation, or the absence of a border, nodules were considered non-calcified, non-spiculated, and smooth bordered. The MC and VA models discussed stratified risk into three tiers based on malignancy probability value[21,22]. The PU model specified two categories split by a single probability cutoff value[12]. Similarly, the BU model is incorporated into the British Thoracic Society (BTS) Guidelines for nodules ≥ 8mm in diameter with a single threshold splitting routine follow-up and additional work-up [14,23].
Table 2:
Tabular form of mathematical prediction model’s (MPMs) base equations. Risk variables are categorized into demographical (subject reported) and radiological (clinician reported) factors. Units are coded in clinical terms; for use in the equation(s), sex (F=1,M=0) and presence (Y=1,N=0) are numerically coded. To obtain a prediction value for a given MPM, multiply each coeffiecnt by the subject’s risk variable value and take the summation with the base intercept/offset. The resulting number is the x in the logistic equation: êx/((1 + êx)) = risk prediction. For example, performing the VA MPM prediction for a 62-year-old, never-smoker, with a 10mm nodule would yield x = (62*0.0779 + 0*2.061 + 0*0.0567 + 10*0.112 – 8.404) = −2.454; plugging into the logistic equation would yield a risk prediction = 0.079.
| Risk variable | Units | MPM Coefficient | ||||
|---|---|---|---|---|---|---|
| MC | VA | PU | BU | |||
| Demographical | Age | Years | 0.0391 | 0.0779 | 0.07 | 0.0287 |
| Sex | F/M | 0.6011 | ||||
| Ever Smoker | Y/N | 0.7917 | 2.061 | |||
| Time of smoking cessation | Years | 0.0567 | ||||
| Cancer history | Y/N | 1.3388 | ||||
| Family history of cancer | Y/N | 1.267 | ||||
| Family history of lung cancer | Y/N | 0.2961 | ||||
| Radiological | Emphysema | Y/N | 0.2953 | |||
| Upper lobe | Y/N | 0.7838 | 0.6581 | |||
| Diametera | MM | 0.1274 | 0.1 12 | 0.0676 | −5.3854a | |
| Spiculation | Y/N | 1.0407 | 0.736 | 0.7729 | ||
| Smooth Border | Y/N | −1.408 | ||||
| Calcification | Y/N | −1.615 | ||||
| Nodule type | Solid : Y/N | 0 | ||||
| Part Solid: Y/N | 0.377 | |||||
| Non-Solid: Y/N | −0.1276 | |||||
| Nodule count | Count | −0.0824 | ||||
| Base Intercept/Offset | −6.872 | −8.404 | −4.496 | 0.2761 | ||
Definition of abbreviations: MPM – mathematical prediction model; MC – Mayo Clinic; VA – Veteran’s Affairs; BU – Brock University; PU – Peking University; F – Female; M – Male; Y – Presence; N – Absence.
In the BU model, nodule size is defined by (diameter in millimeters/10)^−0.5
Statistical Analysis
MPMs raw prediction performance was assessed using area-under the receiver-operator characteristic curve (ROC-AUC) with 95% confidence intervals. Measures of sensitivity and specificity were calculated from Youden’s J statistic optimal threshold, a common method for determining the best cutoff point, which maximizes the balance of sensitivity and specificity [24]. The stability of the Youden thresholds was assessed using median absolute deviation (MAD) below 0.05 on sub-set sizes between 50 and 250 subjects using 41,000 naïve bootstrapping trials sampling without replacement. As our research cohort contained class imbalance (more benign than malignant nodules), the area-under the precision-recall curve (PRAUC) was also assessed to provide a more robust analysis of performance; similar to ROCAUC, PR-AUC is optimal at 1.0 and evaluates the distribution of separation between classes [25]. ROC-AUC and PR-AUC are not suitable assessment measurements when the predictions are discrete categories, as they are in the MPM-recommended thresholds. Instead we assessed the performance by recommendation-induced misclassification of nodule or delay in ground-truth diagnosis. MPM-recommended categories were binarized into benign-tagged (‘low-risk’ or ‘watchful waiting’) and malignant-tagged (‘high-risk’ or recommended immediate additional work-up). Statistical differences between MPM classifications (inter-MPM and intra-MPM) were analyzed using McNemar and Delong tests [26].
In conjunction with this paper, we have developed an easy to use application which allows independent researchers and clinicians to perform the analysis detailed in this study using their local population, including exploring calibrated thresholds and comparison of MPM performance. The application is hosted at: https://www.i-clic.uihc.uiowa.edu/resources/sieren/mpm/.
Results
Research Cohort Comparison of MPMs
Calibrated Thresholds Equalize Performance Among MPMs
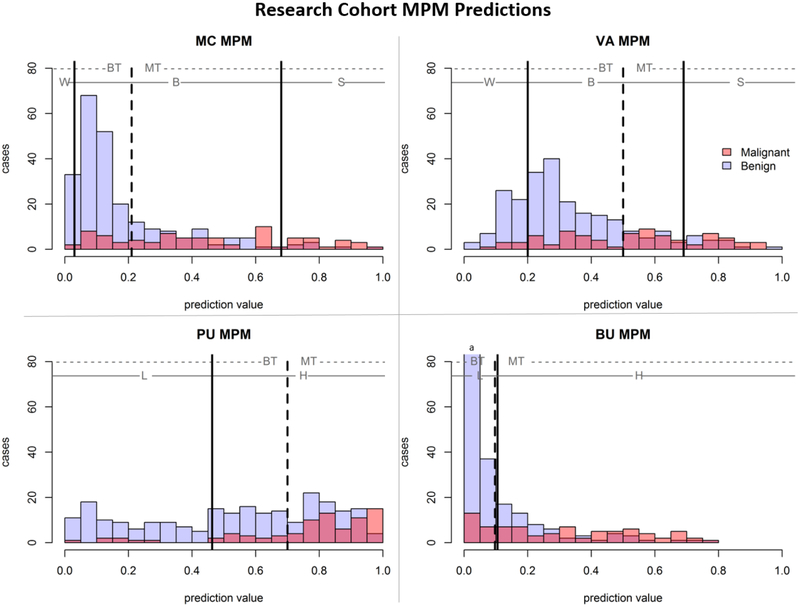
The four models (MC, VA, PU, BU) were applied to the research cohort (N = 317, 80 malignant, 237 benign) yielding four risk scores (one per MPM) per subject which were compared with the nodule’s known diagnosis (Figure 1, solid line). The optimal AUC-cutoff (Figure 1, dashed line) was derived for each of the models. The MC (AUC: 0.63) and BU (AUC: 0.61) MPMs achieved the best separation between classes on this cohort compared to PU (AUC: 0.55) and VA (AUC: 0.51) MPMs. The MC and BU MPMs were both statistically significantly better than the VA MPM (p = 0.02); all other pairwise comparisons of significance yielded p-values above the assigned alpha (0.05). No MPM significantly outperformed all others, revealing relative similarity in their calibrated discriminatory capability between malignant and benign nodules. Testing the Youden threshold stability (MAD < 0.05) at different calibration set sizes demonstrated stability at 100 subjects for three MPMS (MC, BU, PU) and stability at 145 subjects for all four MPMs (see Supplemental Information, Figure A1).
Figure 1 –
Histograms of MPM predictions split based on true nodule classification. Solid lines indicate MPM-derived thresholds with MPM-assigned categories of watchful-waiting (W), biopsy (B), surgery (S), low-risk (L), or high-risk (H). The dashed line indicates cohort AUC-derived threshold for optimal separation of classes, with cases to the left of the line assigned ‘benign-tagged’ and cases to the right of the line assigned ‘malignant-tagged’.
Calibrated Thresholds Out-perform the Original Recommended Thresholds in Work-up Categorization
The impact of risk stratification based on the calibrated threshold (Table 3) and MPM-associated categories (Table 4) were applied to the predictions (Figure 1). Using the MPM-associated categories, up to 25% of the malignant lesions would have been assigned low-risk, while 25.3% to 97.5% of benign lesions would have been recommended for further work-up. The BU MPM was the only model to have agreement between the Youden-optimized calibrated threshold (0.10) and the MPM-associated guidelines (0.10) for the full cohort; however, in nodules ≥ 15mm the Youden optimized threshold was much higher (0.32). Furthermore, McNemar’s comparison between the optimal and recommended thresholds demonstrated significant difference between the classification accuracy of three of the MPMs (MC, VA, PU) with p <0.001, indicating that calibration to the local dataset improves discriminative prowess over original MPM-associated risk categorizations. As the BU Youden optimal threshold was nearly identical to the recommended, there was no statistical significance p=0.99, this stability indicates the BU MPM-associated thresholds were already well calibrated for this cohort.
Table 3:
Performance measures using cohort-derived optimized Youden thresholds (Figure 1, dashed lines). Refer to Supplemental Table A.1 for complete size-based breakdown.
| MPM | Nodule Size | Optimized threshold performance and recommendation for treatment/ nodule evaluation | |
|---|---|---|---|
| MC | All | < 21% Low Risk | ≥ 21% High Risk |
| 19M: 180B | 61M: 57B | ||
| 24% malignant wait | 24% benign immediate work-up | ||
| ≥ 8mm to < 15mm | 8M: 67B | 19M: 25B | |
| 30% malignant wait | 30% benign immediate work-up | ||
| ≥ 15mm | 2M: 2B | 40M: 28B | |
| 5% malignant wait | 93% benign immediate work-up | ||
| VA | All | < 50% Low Risk | ≥ 50% High Risk |
| 34M: 197B | 46M: 40B | ||
| 43% malignant wait | 17% benign immediate work-up | ||
| ≥ 8mm to < 15mm | 16M: 75B | 11M: 17B | |
| 59% malignant wait | 18% benign immediate work-up | ||
| ≥ 15mm | 7M: 8B | 35M: 22B | |
| 17% malignant wait | 73% benign immediate work-up | ||
| BU | All | < 10% Low Risk | ≥ 10% High Risk |
| 19M: 178B | 61M: 59B | ||
| 24% malignant wait | 25% benign extra procedures | ||
| ≥ 8mm to < 15mm | 9M: 61B | 18M: 31B | |
| 33% malignant wait | 34% benign immediate work-up | ||
| ≥ 15mm | 0M: 1B | 42M: 29B | |
| 0% malignant wait | 97% benign immediate work-up | ||
| PU | All | < 70% Low Risk | ≥ 70% High Risk |
| 18M: 154B | 62M: 83B | ||
| 22% malignant wait | 35% benign immediate work-up | ||
| ≥ 8mm to < 15 | 9M: 56B | 18M: 36B | |
| 33% malignant wait | 39% benign immediate work-up | ||
| ≥ 15mm | 6M: 18B | 36M: 12B | |
| 14% malignant wait | 39% benign immediate work-up | ||
Definition of abbreviations: MPM – mathematical prediction model; MC – Mayo Clinic; VA – Veteran’s Affairs; BU – Brock University; PU – Peking University; M – malignant; B - benign
Table 4:
MPM assigned categories breakdown of nodule risk prediction. Refer to Supplemental Table A.2 for complete size-based breakdown.
| MPM | Nodule Size | Malignancy probability and associated clinical guidelines for treatment/ nodule evaluation | ||
|---|---|---|---|---|
| MC | < 3% Watchful waiting | 3–68% Needle biopsy | > 68% Surgery | |
| All | 0M: 6B | 61M: 225B | 19M: 6B | |
| 0% malignant wait | 98% benign immediate work-up | |||
| ≥ 8mm to < 15mm | 0M: 0B | 26M: 91B | 1M: 1B | |
| 0% malignant wait | 100% benign immediate work-up | |||
| ≥ 15mm | 0M: 0B | 24M: 24B | 18M: 6B | |
| 0% malignant wait | 100% benign immediate work-up | |||
| VA | < 20% Watchful waiting | 20–69% Needle biopsy | > 69%Surgery | |
| All | 7M: 58B | 51M: 163B | 22M: 16B | |
| 9% malignant wait | 76% benign immediate work-up | |||
| ≥ 8mm to < 15mm | 1M: 13B | 25M: 75B | 1M: 4B | |
| 4% malignant wait | 86% benign immediate work-up | |||
| ≥ 15mm | 3M: 0B | 18M: 18B | 21M: 12B | |
| 7% malignant waits | 100% benign immediate work-up | |||
| BU | < 10% Low risk | ≥ 10% Higher Risk | ||
| All | 20M: 177B | 60M: 60B | ||
| 25% malignant wait | 25% benign extra procedures | |||
| ≥ 8mm to < 15mm | 9M: 61B | 18M: 31B | ||
| 33% malignant wait | 34% benign immediate work-up | |||
| ≥ 15mm | 0M: 1B | 42M: 29B | ||
| 0% malignant wait | 97% benign immediate work-up | |||
| PU |
< 46.3% Nodule considered benign |
≥ 46.3% Nodule considered malignant |
||
| All | 8M: 87B | 72M: 150B | ||
| 10% malignant wait | 63% benign immediate work-up | |||
| ≥ 8mm to < 15mm | 3M: 33B | 24M: 59B | ||
| 7% malignant wait | 64% benign immediate work-up | |||
| > 15mm | 3M: 4B | 39M: 26B | ||
| 7% malignant wait | 87% benign immediate work-up | |||
Definition of abbreviations: MPM – mathematical prediction model; MC – Mayo Clinic; VA – Veteran’s Affairs; BU – Brock University; PU – Peking University; M – malignant; B - benign
Comparison to Fleischner Size-based Clinical Management Recommendations
The Fleischner Guidelines for Management of Incidental Pulmonary Nodules Detected on CT indicates that solid pulmonary nodules have a differential follow-up using three size-based thresholds (<6mm; 6–8mm; >8mm). Table A.4 in the Supplemental Materials shows the breakdown for these categories and the clinical consequences of the follow-up recommendations. To compare the Fleischner to the calibrated MPMs, the size-threshold of ≥8mm was used for ‘high-risk’ prediction and <8mm for ‘low-risk’ prediction. McNemar’s analysis demonstrated that the Youden calibrated predictions for all four MPMs was statistically superior (p < 0.01) than the Fleischner predictions.
Calibrated Thresholds Improve Specificity in Nodules ≥8mm
Size is a common variable among the MPMs and is prominent in current management guidelines. An accurate MPM risk assessment would be most clinically interesting and powerful on the nodules ≥8mm to <15mm at baseline with 5–15% probability of malignancy in Lung-RADS – in this study, 119 nodules (27 malignant, 92 benign). The best compromising MPM at this size category was the PU model, which using MPM-associated thresholds achieved 97% sensitivity but only 36% specificity; applying Youden optimal threshold achieved 67% sensitivity and improved specificity to 61% (Tables 3–4). Using the MPM-associated threshold, VA model would have only missed one malignant nodule, but at the cost of 79 benign nodules undergoing biopsy (75 cases) or surgery (4 cases); the optimized threshold improved VA MPM specificity for the nodules between 8–15mm to 82%. The MC model was the only MPM to completely reduce wait-time on malignant lesions sending 26 to biopsy and 1 to surgery; however, all benign lesions would have also been assigned to biopsy (91 cases) or surgery (1 case); here, applying optimized thresholds significantly improves specificity to 70% with sensitivity of 70%. In considering nodules between 8 and 15 mm in diameter, the MPM-associated recommendation thresholds for work-up have little benefit in tradeoff between sensitivity and specificity. Applying optimized thresholds improves specificity at the cost of some sensitivity.
Size-Exclusion Prior to MPM in BTS Guidelines Appropriate
The BU model is unique as it has been incorporated into the BTS guidelines for management of nodules; per BTS decision flowchart, only nodules ≥ 8mm are to be assessed with the BU MPM[27]. Tables 3–4 demonstrates the BU accuracy for that size-based subset. On our cohort, following the BTS exclusion of nodules < 8mm in diameter would have meant 11 malignant and 115 benign nodules would not be assessed with the BU due to size-exclusion. Applying the BU to the size-excluded, no malignant and 9 benign nodules are labeled ‘high risk’ by the BU MPM. Of the11 malignant size-excluded nodules, one is recommended to be ‘discharged’, four are recommended for a 1-year follow-up CT, and six are recommended for a 3-month CT -indicating the need for more sophisticated discrimination techniques geared towards small nodules. The BTS recommendation to not include BU prediction on small nodules is appropriate, and as the BU threshold did not change with calibration, the recommended decision of 10% risk (0.1 prediction value) is well founded.
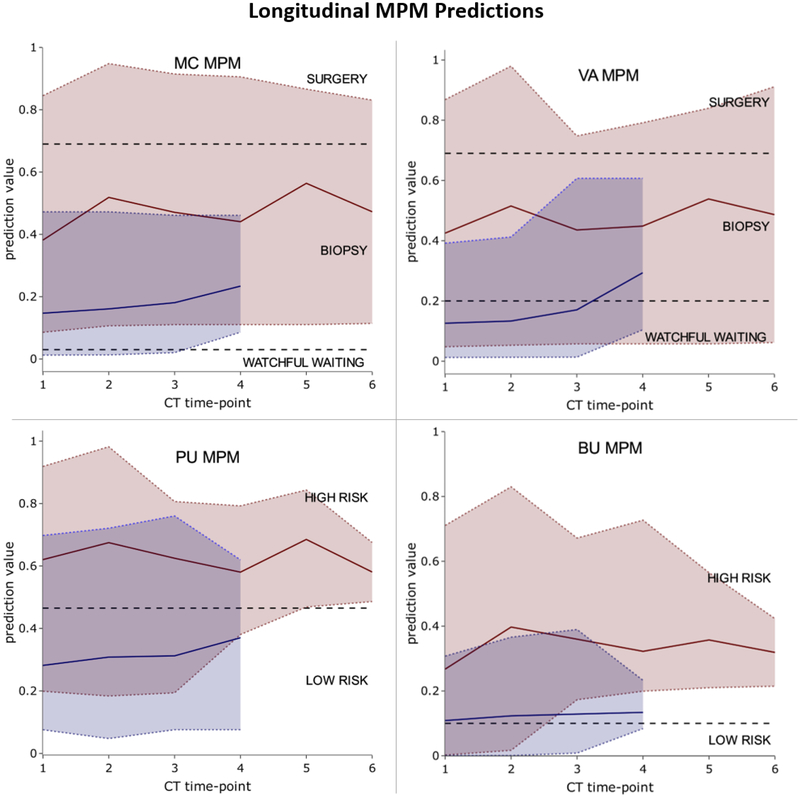
Longitudinal Cohort
We investigated the improvement in MPM performance over repeated imaging time-point on a clinical, longitudinal dataset of nodules imaged up to 6 times (average 3.1±1.1) prior to diagnosis (Figure 2). The average number of days between sequential patient imaging encounters was 214 days (±338 days) with malignant nodules tending to have a slightly longer time between scans (218 days ±368) compared to benign nodules (197 days±305).
Figure 2 –
MPM prediction value over CT number on longitudinal cohort. The range in prediction values for malignant (red) and benign (blue) are shown with minimum and maximum values indicated by dashed colored lines. The average prediction value for the two classes is shown with the solid colored lines. Black dashed lines indicate MPM-derived thresholds.
The VA model was the only MPM to also decrease the percentage of benign nodules at TP_F that were categorized as high risk. The TP_I AUCs (MC: 0.62–0.96; VA: 0.65–0.96; BU: 0.51–0.90; PU: 0.70–0.98) were consistently higher than the TP_F AUCs in three of the MPMs (MC: 0.56–0.94; VA: 0.34–0.78; BU: 0.53–0.92; PU: 0.44–0.88). McNemar’s p-value between TP_I and TP_F showed no statistical significance between MPM predictions at TP_I and TP_F (MC: 0.76, VA: 0.08, BU: 0.91, PU: 0.18), indicating no improvement to MPM risk assessment closer to diagnosis. This data suggests that MPM risk should not be incorporated into longitudinal evaluation of detected pulmonary nodules.
Discussion
We have applied four post-imaging MPMs to a large cohort of trial subjects and to a longitudinal cohort of clinical subjects. To our knowledge, this is the first study to compare MPMs by both the MPM-associated categories and AUC-derived (calibrated) classifications and to observe of MPM stability over longitudinal scans.
Recent alignment of size-based recommendations indicates that nodules ≥ 8mm in maximum diameter are at a heightened risk of malignancy[2,3,14]. Hammer et al. investigated eight risk calculators on a cohort of 86 nodules (59 malignant), showing a consistent under-estimation of malignancy risk. Here, we have a smaller proportion (25%) of malignancies in our cohort, yet our results concur with the assessment that care needs to be taken when assessing larger nodules (≥8mm) with these MPMs [15]. The applied BU model on the ≥8mm sub-cohort also demonstrated an under-estimation of true malignancy risk with an over-estimation of risk on benign nodules. Given average nodule size in the MPM development cohorts was larger than 8mm, it would be likely that the development-cohorts size bias would lead to more large benign nodules being tagged as suspicious.
Chung et al. recently validated the BU model on two large clinical cohorts showing that while the full model achieved AUCs of 0.901–0.911, the AUC-derived optimal threshold was 1.8–4% lower than the recommended BTS guidelines; this is a difference of 4–9% in sensitivity[23]. However, that study contained a significant size-bias between benign and malignant cases. While nodule diameter is not a variable in the BU model, the BTS flow-diagram applies the BU model only to nodules ≥8mm diameter (≥300mm3 volume). Here we have applied the BU model in the manner recommended by BTS and demonstrated than all 11 below the size-stratified malignant nodules had a BU less than the threshold 10%. In practice, these malignant lesions would have remained untreated for at least 3 months before additional imaging.
While the BTS closely followed the original BU model study for this risk threshold, many independent surveys of MPMs have relied solely on the threshold derived from their cohort’s AUC optimum[15–17]. Here we have displayed both the AUC-derived threshold from our cohort as well as the MPM-derived thresholds. When using our cohort-derived optimal cutoff point, MPM specificity was higher (65.0–83.0%) than through using the MPM-derived assigned categories (2.5%−74.7%), but MPM sensitivity was lower (58.0–78%) compared to MPM assigned categories (75.0%−100%). Based on MPM assigned categories, only the MC model would have detected 100% of malignancies at the imaging time point, but this is at the cost of requiring biopsy/surgery for all benign lesions. It is important to note that some studies have reported high AUCs of MPMs in their independent cohorts, but these studies have looked solely at the AUC-derived thresholds to assess MPM performance[16,18].
Our study has several limitations. First, the mean nodule size of the cohorts was smaller than those used to develop the MPMs. As nodule size was a common variable among the MC, VA, and PU MPMs, this could have affected the prediction results. Second, the MPMs investigated here use subject-provided demographic/historical information and radiologist-described image characteristics, both of which can suffer from subjective variability and completeness. Radiologist variability is more easily investigated and has been shown to be different between radiologists as well as within a radiologist on so-called “coffee-break” reads in which a period of time is placed between repeated analysis[28,29]. While to a certain extent, the variability is built into the risk models in the development dataset, the modeling of noisy data is likely different between the development cohort and the user-end radiologist. Maiga et al. compared the MC model with clinician assigned risk from qualitative statements of cancer risk, showing that the current trend of qualitative risk statements for malignancy are highly variable and recommend a standardized scale for clinicians to follow[30]. Recent advances in CT including dose reduction techniques and reconstruction algorithms, have the potential to affect signal-to-noise ratio within the scan, thereby a potential source of variation that could affect both radiologist/reader efficiency and consistency. We do believe some of this variation is already contained within the development of the MPMs given the diverse (often clinical) datasets on which they were developed. Interesting to this point, the Mayo Clinic model (chest radiographs) performs on par with the Brock University model (low dose CT). Our cohort included only solid nodules, further studies are required to determine if MPM performance is affected when used on cohort of sub-solid tumors. Our research cohort consisted of 25.2% malignant cases and longitudinal clinical cohort 53.3% malignant cases; the MPMs compared here were developed on cohorts of subjects with difference malignancy rates (MC: 35%; VA: 54%; BU 6%; PU: 61%). We have included the PR-AUC measure to further describe the discrimination ability of MPMs in cohorts with disproportionate numbers of malignant and benign cases.
With the move towards digitized healthcare reporting and standardization of care, computer-based risk models have a natural place in the decision pipeline. There is a benefit to adding fully-automated, non-subjective systems with high performance to supplement radiologist reads with additional risk assessments. Efforts to develop tools which do not incur user subjectivity have been previously described; Mehta et al. compared the MC MPM with three multi-variate models developed with volumetric features extracted from semi-automatic (single click) segmentation of the nodule[17]. Machine learning for the assessment of lung cancer risk have been further developed to reduce extraction variability[31–37].
The number of lung nodules detected is set to increase with increased access to screening and clinical CT scanning. To make the screening and detection power of CT efficient and safe in practice, there is a great need for better informed decision making. Given proper assessment and application, post-imaging risk models have the potential to improve decision making processes. While standardization and wide-spread usage of these automated techniques has yet to happen, MPMs are being utilized in clinics today. This paper has demonstrated the need for clarification in malignancy thresholds reported and demonstrated the cohort dependence built into these MPMs. We thereby recommend if an MPM is to be utilized for newly detected pulmonary nodules, that it is first calibrated with a retrospectively collected dataset (≥100 subjects) from the utilizing intuition to ensure a locally optimal threshold value. We have developed an easy to use web-based application to assist institutions in performing MPM calibration and comparison of performance metrics between models. The application allows MPM discriminative power to be assessed using either ROC-AUC (balanced cohort) or PR-AUC (unbalanced cohort) measures and provides sensitivity and specificity. The lack of improvement in risk prediction from these MPMs over time suggests caution in the utility of these tools during surveillance stage of clinical management.
Supplementary Material
Key points:
Post-imaging lung cancer risk mathematical predication models (MPMs) perform poorly on local populations without calibration
An application is provided to facilitate calibration to new study cohorts: the Mayo Clinic model, the U.S. Department of Veteran’s Affairs model, the Brock University model, and the Peking University model
No significant improvement in risk prediction occurred in nodules with repeated imaging sessions, indicating potential value of risk prediction application is limited to the initial evaluation
Acknowledgements
We thank Kimberly Sprenger, Debra O’Connel-Moore, Mark Escher, Patrick Thalken, and Kimberly Schroeder for technical assistance.
Funding
This work was supported in part by Grant IRG-77-004-34 from the American Cancer Society, administered through the Holden Comprehensive Cancer Center at the University of Iowa. The COPDGene Study was supported by NHLBI U01 HL089897 and U01 HL089856. The COPDGene study (NCT00608764) is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee comprised of AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion. The INHALE study was supported by Award Number R01CA141769 and P30CA022453 from the National Cancer Institute, Health and Human Services Award HHSN26120130011I and the Herrick Foundation.
List of Abbreviations
- AUC
area under the curve
- BTS
British Thoracic Society
- BU
Brock University model
- COPD
chronic obstructive pulmonary disorder
- CT
computed tomography
- MAD
median absolute deviation
- MC
Mayo Clinic model
- MPMs
mathematical prediction models
- PR
precision recall
- PU
Peking University model
- ROC
receiver-operator characteristic
- TP_1
initial imaging encounter on which the pulmonary nodule was identified
- TP_F
final imaging encounter before pulmonary nodule diagnosis
- VA
U.S. Department of Veterans Affairs model
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Guarantor:
The scientific guarantor of this publication is Dr Jessica C. Sieren.
Conflict of Interest:
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and Biometry:
The first and last authors, as biomedical engineers, have experience with biostatistics methods. No complex statistical methods were necessary for this paper.
Informed Consent:
The University of Iowa Institutional Review Board has approved this study (IRB 201603824). Informed consent was obtained from the research cohort participants through the parent studies; COPDgene and INHALE (including the approval of collected data for expanded research questions beyond the parent study purpose). For the retrospective clinical cohort, written informed consent was waived by the Institutional Review Board.
Ethical Approval:
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap:
40 subjects from study subjects or cohorts have been previously reported by our lab in a machine learning approach development:
30 Dilger SK, Uthoff J, Judisch A et al (2015) Improved pulmonary nodule classification utilizing quantitative lung parenchyma features. J Med Imaging (Bellingham) 2:041004
This study includes a small subset of data from larger/extensive studies, which have been reported in the literature:
19 Regan EA, Hokanson JE, Murphy JR et al (2010) Genetic epidemiology of COPD (COPDGene) study design. Copd 7:32–43
20 Schwartz AG, Lusk CM, Wenzlaff AS et al (2016) Risk of Lung Cancer Associated with COPD Phenotype Based on Quantitative Image Analysis. Cancer Epidemiol Biomarkers Prev 25:1341–1347.
- Retrospective
- observational
- performed at one institution
References
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30 [DOI] [PubMed] [Google Scholar]
- 2.MacMahon H, Naidich DP, Goo JM et al. (2017) Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 284:228–243 [DOI] [PubMed] [Google Scholar]
- 3.American College of Radiology (2014). Lung CT Screening Reporting and Data System (Lung-RADS ). Available via: https://wwwacrorg/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. Accessed 12/12/2018 [Google Scholar]
- 4.Gould MK, Donington J, Lynch WR et al. (2013) Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e93S–e120S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta HJ, Mohammed TL, Jantz MA (2017) The American College of Radiology Lung Imaging Reporting and Data System: Potential Drawbacks and Need for Revision. Chest 151:539–543 [DOI] [PubMed] [Google Scholar]
- 6.Pinsky PF, Gierada DS, Black W et al. (2015) Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 162:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Riel SJ, Ciompi F, Jacobs C et al. (2017) Malignancy risk estimation of screen-detected nodules at baseline CT: comparison of the PanCan model, Lung-RADS and NCCN guidelines. Eur Radiol 27:4019–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray EP, Teare MD, Stevens J, Archer R (2016) Risk Prediction Models for Lung Cancer: A Systematic Review. Clin Lung Cancer 17:95–106 [DOI] [PubMed] [Google Scholar]
- 9.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES (1997) The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 157:849–855 [PubMed] [Google Scholar]
- 10.Gould MK, Ananth L, Barnett PG, Veterans Affairs SCSG (2007) A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 131:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McWilliams A, Tammemagi MC, Mayo JR et al. (2013) Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 369:910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Wang J (2012) A mathematical model for predicting malignancy of solitary pulmonary nodules. World J Surg 36:830–835 [DOI] [PubMed] [Google Scholar]
- 13.Herder GJ, van Tinteren H, Golding RP et al. (2005) Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 128:2490–2496 [DOI] [PubMed] [Google Scholar]
- 14.Baldwin DR, Callister ME (2015) The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 70:794–798 [DOI] [PubMed] [Google Scholar]
- 15.Hammer MM, Nachiappan AC, Barbosa EJM Jr. (2018) Limited Utility of Pulmonary Nodule Risk Calculators for Managing Large Nodules. Curr Probl Diagn Radiol 47:23–27 [DOI] [PubMed] [Google Scholar]
- 16.Al-Ameri A, Malhotra P, Thygesen H et al. (2015) Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 89:27–30 [DOI] [PubMed] [Google Scholar]
- 17.Mehta HJ, Ravenel JG, Shaftman SR et al. (2014) The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest 145:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perandini S, Soardi GA, Motton M, Montemezzi S (2015) Critique of Al-Ameri et al. (2015) - Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 90:118–119 [DOI] [PubMed] [Google Scholar]
- 19.Regan EA, Hokanson JE, Murphy JR et al. (2010) Genetic epidemiology of COPD (COPDGene) study design. Copd 7:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz AG, Lusk CM, Wenzlaff AS et al. (2016) Risk of Lung Cancer Associated with COPD Phenotype Based on Quantitative Image Analysis. Cancer Epidemiol Biomarkers Prev 25:1341–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swensen SJ, Silverstein MD, Edell ES et al. (1999) Solitary pulmonary nodules: clinical prediction model versus physicians. Mayo Clin Proc 74:319–329 [DOI] [PubMed] [Google Scholar]
- 22.Cummings SR, Lillington GA, Richard RJ (1986) Managing solitary pulmonary nodules. The choice of strategy is a “close call”. Am Rev Respir Dis 134:453–460 [DOI] [PubMed] [Google Scholar]
- 23.Chung K, Mets OM, Gerke PK et al. (2018) Brock malignancy risk calculator for pulmonary nodules: validation outside a lung cancer screening population. Thorax 73:857–863 [DOI] [PubMed] [Google Scholar]
- 24.Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35 [DOI] [PubMed] [Google Scholar]
- 25.Jesse D, Goadrich M (2006) The Relationship between Precision-Recall and ROC Curves. Proceedings of the 23rd International Conference on Machine Learning, Pittsburgh, 25–29 June 2006, 233–240 [Google Scholar]
- 26.McNemar Q (1947) Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 12:153–157 [DOI] [PubMed] [Google Scholar]
- 27.Callister ME, Baldwin DR, Akram AR et al. (2015) British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 70 Suppl 2:ii1–ii54 [DOI] [PubMed] [Google Scholar]
- 28.McNitt-Gray MF, Kim GH, Zhao B et al. (2015) Determining the Variability of Lesion Size Measurements from CT Patient Data Sets Acquired under “No Change” Conditions. Transl Oncol 8:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin H, Huang C, Wang W, Luo J, Yang X, Liu Y (2017) Measuring Interobserver Disagreement in Rating Diagnostic Characteristics of Pulmonary Nodule Using the Lung Imaging Database Consortium and Image Database Resource Initiative. Acad Radiol 24:401–410 [DOI] [PubMed] [Google Scholar]
- 30.Maiga AW, Deppen SA, Massion PP et al. (2018) Communication About the Probability of Cancer in Indeterminate Pulmonary Nodules. JAMA Surg 153:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dilger SK, Uthoff J, Judisch A et al. (2015) Improved pulmonary nodule classification utilizing quantitative lung parenchyma features. J Med Imaging (Bellingham) 2:041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhara AK, Mukhopadhyay S, Dutta A, Garg M, Khandelwal N (2016) A Combination of Shape and Texture Features for Classification of Pulmonary Nodules in Lung CT Images. J Digit Imaging 29:466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira JR, Oliveira MC, de Azevedo-Marques PM (2017) Characterization of Pulmonary Nodules Based on Features of Margin Sharpness and Texture. J Digit Imaging 31:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nibali A, He Z, Wollersheim D (2017) Pulmonary nodule classification with deep residual networks. Int J Comput Assist Radiol Surg 12:1799–1808 [DOI] [PubMed] [Google Scholar]
- 35.Sun W, Zheng B, Qian W (2017) Automatic feature learning using multichannel ROI based on deep structured algorithms for computerized lung cancer diagnosis. Comput Biol Med 89:530–539 [DOI] [PubMed] [Google Scholar]
- 36.Way TW, Sahiner B, Chan HP et al. (2009) Computer-aided diagnosis of pulmonary nodules on CT scans: improvement of classification performance with nodule surface features. Med Phys 36:3086–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, Tan Y, Hua Y, Wang M, Zhang G, Zhang J (2010) Feature selection and performance evaluation of support vector machine (SVM)-based classifier for differentiating benign and malignant pulmonary nodules by computed tomography. J Digit Imaging 23:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.