Abstract
Background:
Triclosan, a widely-used antimicrobial in personal care products, has shown endocrine disrupting activity in experimental studies. However, there is limited evidence from epidemiologic studies on health effects.
Objective:
To examine the association between urinary triclosan concentrations and semen quality.
Methods:
A total of 262 men enrolled in the Environmental and Reproductive Health (EARTH) Study provided 581 paired urine and semen samples (2009–2017). Urinary triclosan concentrations were quantified and semen analysis was evaluated according to WHO guidelines. We used linear mixed regression models to estimate the associations between specific gravity-adjusted urinary triclosan concentrations with semen parameters, with a random intercept to account for multiple samples per man and adjusting for age, body mass index (BMI), smoking, physical activity, sexual abstinence time, and season and year of samples’ collection.
Results:
Men had a mean (standard deviation) age of 36.6 (5.24) years and BMI of 27.9 (5.94) kg/m2. Seventy four percent of the samples had detectable (>2.3 μg/L) concentrations. We did not observe significant dose response trends between SG-adjusted urinary triclosan concentrations and semen parameters. However, in the adjusted analysis, compared to men with non-detectable triclosan concentrations in the lowest quartile, those in the second, third, and fourth quartiles had −1.32% (95%CI: −2.04, −0.59), −0.91% (95%CI: −1.63, −0.18), and −0.46% (95%CI: −1.25, 0.33) lower percent normal morphology sperm, respectively. Similarly, a lower percentage of morphologically normal sperm was found among men with detectable triclosan concentrations, compared to men with non-detectable triclosan [−0.96% (95% CI: −1.57, −0.35)]. In sensitivity analyses, there was stronger negative associations on the percent morphologically normal sperm in the earlier time period due to the significant negative trend in detectable triclosan concentrations over time.
Conclusion:
Despite the lack of observed dose response relationship, we found consistent patterns of lower percent morphologically normal sperm for men with urinary triclosan in the 2nd or 3rd quartile compared to undetectable concentrations. This association was stronger for samples obtained prior to 2013 when triclosan was more often detectable in urine.
Keywords: triclosan, semen quality, endocrine disruptor, infertility, male
Introduction
Triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol), a potential developmental and reproductive toxicant (Gore et al., 2015), has been a multi-purpose biocide. It has been used for over forty years in personal care products (PCPs) such as toothpastes, mouth wash, lotions, shampoos, cosmetics, soaps, skin creams, and deodorants as well as textiles, such as bed cloths, sportswear, shoes, and carpets (Dann and Hontela, 2011; Fang et al., 2010; Singer et al., 2002; von der Ohe et al., 2012). Triclosan is also used as a plastic additive in toys, medical devices, and household, veterinary, and industrial products (Dann and Hontela, 2011; Fang et al., 2010). Routes of exposure are through dermal and mucosal contact with consumer products, and through ingestion of contaminated water or food (Calafat et al., 2008; DeSalva et al., 1989; Sandborgh-Englund et al., 2006). Due to its widespread use, the general population are ubiquitously exposed to triclosan; it has been detected in urine in approximately 75% of the 2003–2004 National Health and Nutrition Examination Survey (NHANES) participants (Calafat et al., 2008).
Triclosan has become a public and scientific concern due to its ubiquity in the environment (Halden, 2014). However, compared to other endocrine disrupting chemicals (EDCs), we know far less regarding the impact of triclosan as one of the “emerging EDCs of interest” (Gore et al., 2015). Von Der Ohe et al (von der Ohe et al., 2012) called triclosan the “forgotten priority substance” due to scarcity of the research studies. While non-human studies have shown sufficient evidence of its possible toxicity, surprisingly, very few studies have examined the association with reproductive outcomes in humans specifically on semen quality. Therefore, we examined whether urinary triclosan concentrations are associated with semen quality. We hypothesized that higher triclosan concentration in urine would be associated with poorer semen quality among men of reproductive age seeking care at a fertility center.
Methods
Study population
Study participants were men enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort started in 2004 at the Massachusetts General Hospital (MGH) Fertility Center to evaluate environmental and dietary determinants of fertility (Messerlian et al., 2018b). Men between 18 and 55 years who planned to use their own gametes at enrollment were eligible to participate in the study. Of the men approached, approximately 55% agreed to participate. This analysis included men who provided on the same day at least one spot urine sample for the measurement of triclosan concentrations and one semen sample for semen analysis. Between 2007 and 2017, 800 urine samples and 1006 semen samples were provided, including 618 urine and semen samples collected on the same day. We excluded azoospermic (n=5) samples and samples with incomplete semen quality data (n=32) to provide – paired urine-semen samples. The final sample included 262 men who provided 581 paired urine and semen samples (2009–2017) (Supplemental Figure 1). The EARTH study was approved by the Human Studies Institutional Review Boards of the Partners’ hospitals, the Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC). Participants signed an informed consent after the study procedures were explained by trained research study staff and all questions were answered.
Men self-reported demographics, data on lifestyle such as time spent in leisure, physical and sedentary activities assessed using a validated questionnaire (Wolf et al., 1994) and medical history such as history of reproductive diseases and surgeries. Infertility diagnosis was abstracted from electronic medical records. Trained study staff abstracted clinical information from medical records and measured their height and weight to calculate body mass index (BMI) (kg/m2) at the time of enrollment. Each man provided a urine sample and a semen sample at enrollment, and during each subsequent visit until the couple had a livebirth or stopped treatment at MGH.
Quantification of triclosan concentrations in urine
Men provided spot urine samples that were collected in a sterile, clean polypropylene specimen cup onsite at the MGH Fertility Center. Specific gravity (SG) was measured at room temperature and within several hours (typically within one hour) of the urine collection using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) that was calibrated with deionized water before each measurement. The urine was then divided into aliquots, frozen, and stored at −80 °C. Samples were shipped on dry ice overnight to the CDC where the aliquots were stored at or below −40 °C until analysis. The limit of detection (LOD) for triclosan ranged between 1 and 2.3 μg/L over time. Briefly, the analytical techniques for quantification of the urinary triclosan concentration involved enzymatic deconjugation of the triclosan conjugates followed by online solid-phase extraction, separation by high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry (Dwivedi et al., 2018; Zhou et al., 2014). In addition to study samples, each analytical run included low-concentration and high-concentration quality control urine samples and reagent blanks to assure the accuracy and reliability of the data.
We used SG to adjust triclosan concentrations for urinary dilution using the following formula: Pc = P[ (1.017 − 1)/(SG − 1)], where Pc is the SG-corrected Triclosan concentration (μg/L), P is the measured triclosan concentration (μg/L) of the urine sample, and 1.017 is the mean SG concentration in the study population (Nassan et al., 2019).
Semen quality measurement
Men provided the semen samples onsite at the MGH andrology laboratory by masturbation into a sterile plastic specimen cup. Participants were asked to abstain from ejaculation for 2–5 days before providing the specimen. Men reported the duration of ejaculation abstinence before providing the samples. All semen samples were analyzed using standardized protocols and quality control as described previously (Nassan et al., 2016). After collection and before analysis, the sample was liquefied at 37°C for 20 minutes. Ejaculate semen volume (mL) was measured using a graduated serological pipet. Sperm concentration (million/mL) and percent motile sperm were assessed using a computer-aided semen analyzer (CASA: 10HTM-IVOS, Hamilton-Thorne Research, Beverly, MA, USA). We also calculated the total sperm count (million/ejaculate) as semen volume × sperm concentration. Sperm morphology (percent normal sperm) was assessed on two slides per specimen (≥200 cells assessed per slide) via a microscope with an oil-immersion ×100 objective (Nikon, Tokyo, Japan). To classify men as having normal or below normal morphology, we used the Strict Kruger scoring criteria (Kruger et al., 1988). Andrologists at MGH regularly participated in internal and external quality control checks.
Statistical analysis
We categorized the SG-adjusted urinary triclosan concentration into quartiles where the lowest quartile included all concentrations that were below LOD (26% of the samples) and was used as the reference group. We calculated descriptive statistics for men’s baseline characteristics across quartiles of the SG-adjusted urinary triclosan concentration and tested for differences using Kruskal–Wallis tests for continuous variables and Pearson Chi-square tests (or Fisher exact test whenever appropriate) for categorical variables. We evaluated the distribution of semen parameters and we natural-log transformed ejaculate volume, sperm concentration, and total sperm count. We also calculated the intraclass correlation (ICC) for the urinary concentrations of triclosan. We used linear mixed effect models to examine the associations between SG-adjusted urinary triclosan concentration, in quartiles and as a binary variable (detectable vs not-detectable), with semen parameters and included a random intercept for each man to account for the correlation between the multiple semen samples per man. Results were presented as adjusted percent changes for the log-transformed outcomes and as absolute differences for the non-transformed outcomes across exposure categories. We selected potential confounders based on prior knowledge, descriptive statistics in the study population, and using directed acyclic graphs (DAGs) (Supplemental Figure 2) (Weng et al., 2009). The final model adjusted for age, sexual abstinence time, BMI, tobacco smoking, season and calendar year of sample collection, and moderate to vigorous physical activity. In the sperm motility models, we further adjusted for duration elapsed between semen sample collection and analysis.
We also conducted sensitivity analyses by dichotomizing the samples based on the median year (2009-2012 and 2013-2017). We conducted an additional analysis in which semen parameters were dichotomized using the WHO-2010 lower reference limits (WHO, 2010). In this analysis, we used generalized linear mixed models with a random intercept, binary distribution, and logit link adjusting for the same covariates as above. We assessed effect modification by age and BMI by adding an interaction term in the adjusted models. We conducted all statistical analyses using the Statistical Analysis System Software package SAS 9.4 (SAS Institute Inc., Cary NC) and we considered a two-sided p-value < 0.05 as statistically significant.
Results
Our analysis included 262 men who provided 581 urine and 581 semen samples collected on the same days. Of those men, 116 (44%) provided one pair of semen and urine samples, 73 (28%) provided two, 24 (9%) provided three, and 49 (19%) provided 4–8 pairs of samples. Among the 146 men who provided at least 2 samples, 45 (31%) men had at least one sample with detectable triclosan concentrations and at least one sample with non-detectable triclosan at another visit. The majority of those (35 men (78%)) had the earlier sample with detectable concentrations of triclosan. The mean (standard deviation, SD) age of the 262 men was 36.6 (5.24) years and BMI of 27.9 (5.94) kg/m2. The majority of the men were Caucasian (88%), did not currently smoke tobacco (97%), and 62% had a graduate degree. Most semen samples (94%) were analyzed within 30 minutes after specimen collection and men had sexual abstinence of 2–4 days for 57% of the samples, (Table 1). Men whose baseline urine sample was categorized in the highest triclosan quartile tended to have had more reproductive surgeries such as hernia repair. Otherwise, men had similar demographics over the quartiles of SG-adjusted urinary triclosan concentrations. The overall median (interquartile range, IQR) SG-adjusted urinary triclosan concentration was 7.16 (2.55, 40.2) μg/L. For samples with detectable triclosan concentrations, the median (IQR) SG-adjusted urinary triclosan concentration was 13.8 (4.57, 79.7) μg/L.
Table 1.
Demographics and semen sample collection characteristics across quartiles of SG-adjusted triclosan urinary concentrations (in μg/L) among 262 men (581 urine and 581 semen samples) participating in the EARTH Study.
| Quartile | Q1 | Q2 | Q3 | Q4 | Total | |
|---|---|---|---|---|---|---|
| Median (IQR) of SG-adjusted triclosan concentration, [range] | Non-detectable | 3.25 (2.32,4.57), [0.73, 6.63] | 13.8 (9.62,23.1), [6.80, 41.2] | 191 (79.7,388), [41.3, 2516] | 7.16 (2.55, 40.2) , [<LOD, 2517] | |
| Baseline characteristics for men, N (%) | N=63 (24) | N=58 (22) | N=62 (24) | N=79 (30) | N=262 (100) | P-value |
| Age (years) | 38.0 (6.11) | 36.9 (5.41) | 36.9 (5.41) | 35.7 (4.9) | 36.6 (5.24) | 0.10 |
| BMI, N (%) | 0.32 | |||||
| Underweight | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | |
| Normal weight | 18 (29) | 16 (28) | 16 (26) | 30 (38) | 80 (31) | |
| Overweight | 31 (49) | 23 (40) | 27 (44) | 35 (44) | 116 (44) | |
| Obese | 13 (21) | 19 (33) | 19 (30) | 14 (18) | 65 (25) | |
| Race, N (%) | 0.50 | |||||
| Caucasian | 57 (90) | 49 (84) | 52 (84) | 72 (91) | 230 (88) | |
| Black/African American | 2 (3) | 1 (2) | 4 (6) | 1 (1) | 8 (3) | |
| Asian | 4 (6) | 6 (10) | 3 (5) | 4 (5) | 17 (6) | |
| Native American/Alaska Native | 0 (0) | 2 (3) | 3 (5) | 2 (3) | 7 (3) | |
| Education, N (%) | 0.70 | |||||
| Below College | 6 (10) | 6 (10) | 5 (8) | 11 (14) | 28 (11) | |
| College or Graduate Degree | 57 (90) | 52 (90) | 57 (92) | 68 (86) | 234 (89) | |
| Reproductive diseases, N (%) | 9 (14) | 7 (12) | 9 (15) | 19 (24) | 44 (17) | 0.22 |
| Reproductive Surgeries, N (%) | 7 (11) | 1 (2) | 8 (13) | 14 (18) | 30 (11) | 0.02 |
| Smoking Status, N (%) | 0.16 | |||||
| Never | 46 (73) | 31 (53) | 43 (69) | 52 (66) | 172 (67) | |
| Former | 15 (24) | 25 (43) | 18 (29) | 21 (27) | 79 (30) | |
| Current | 2 (3) | 2 (3) | 1 (2) | 6 (8) | 11 (4) | |
| Moderate to vigorous physical activity, hrs/week | 6.25 (10.6) | 4.33 (12.9) | 5.80 (8.88) | 4.23 (5.66) | 5.11 (9.57) | 0.11 |
| Time-varying characteristics for semen samples, n (%) | 151 (26) | 143 (25) | 144 (25) | 143 (25) | 581 (100) | |
| Season of sample collection, April to September | 67 (44) | 54 (38) | 57 (40) | 64 (45) | 242 (42) | 0.54 |
| Sexual Abstinence Time | - | - | 0.77 | |||
| <2 days | 43 (28) | 51 (36) | 50 (35) | 50 (35) | 194 (33) | |
| 2 - 3 days | 72 (48) | 61 (43) | 60 (42) | 57 (40) | 250 (43) | |
| 3 - 4 days | 20 (13) | 18 (13) | 20 (14) | 26 (18) | 84 (14) | |
| >4 days | 16 (11) | 13 (9) | 14 (10) | 10 (7) | 53 (9) | |
| Time between sample collection and analysis | 0.32 | |||||
| ≤30 min | 142 (94) | 138 (97) | 133 (92) | 136 (95) | 549 (94) | |
| > 30 min | 7 (5) | 4 (3) | 4 (3) | 5 (4) | 20 (3) | |
| Missing | 2 (1) | 1 (1) | 7 (5) | 2 (1) | 12 (2) | |
N (%) is presented for categorical/binary variables and mean (standard deviation) is presented for continuous variables.
From Chi-square (or Fisher’s exact test when appropriate) for discrete variables and Kruskal-Wallis for continuous variables.
Reproductive diseases: Report of any of the following: varicocele testicular torsion, testicular injury, undescended testis, hernia, epididymitis, prostatitis & seminal vesicle infection.
Reproductive Surgeries: Report of any of the following: orchidopexy, varicocelectomy, hydrocelectomy, hernia repair, urethral repair, hypospadias repair, sympathectomy & bladder neck surgery.
Includes weight and aerobic exercise and sports.
Summation of the percentages may not add up to 100% due to approximations.
Abbreviations: N; number of participants, n; number of semen samples, EARTH; the Environment and Reproductive Health study, Q; quartile, IQR; interquartile range, SG; specific gravity, BMI; body mass index, hrs; hours, min; minutes.
The multivariable adjusted ICC of the SG-adjusted urinary triclosan concentrations was 0.68 (95% confidence interval (95% CI): 0.61, 0.74). Among all 581 semen samples, the median and IQR for sperm concentration was 51.2 (25.5, 97.0) million/mL, 121 (61.9, 233) million for sperm count, 47.0% (27.0%, 64.0%) for percent motile sperm, and 6.0% (4.0%, 9.0%) for percent morphologically normal sperm (Supplemental Table 1).
In the adjusted analysis, we did not observe any statistically significant dose response trend between SG-adjusted urinary triclosan concentrations and any of the semen parameters. However, compared to men with non-detectable triclosan concentrations (e.g., in the lowest quartile), those in the second, third, and fourth quartiles had −1.32% (95%CI: −2.04, −0.59), −0.91% (95%CI: −1.63, −0.18), and −0.46% (95%CI: −1.25, 0.33) lower percent normal morphology sperm, respectively (Figure 1.A and Table 2). We observed consistent patterns of lower sperm parameters among men who had detectable triclosan in urine, especially men whose urinary triclosan concentrations were in the second quartile compared to the lowest quartile, despite not being statistically significant (Table 2). When combining all detectable triclosan concentrations (quartiles 2, 3, and 4) together into one group, men with detectable triclosan in urine had a 0.96% lower (95% CI: −1.57, −0.35) % normal morphology sperm compared to men with no detectable triclosan (Figure 1.B, Supplemental Table 2). No association was observed of triclosan concentrations with sperm concentration, percent motile sperm, and percent progressive motile sperm. The results were essentially the same in the unadjusted analysis (Table 2).
Figure 1.
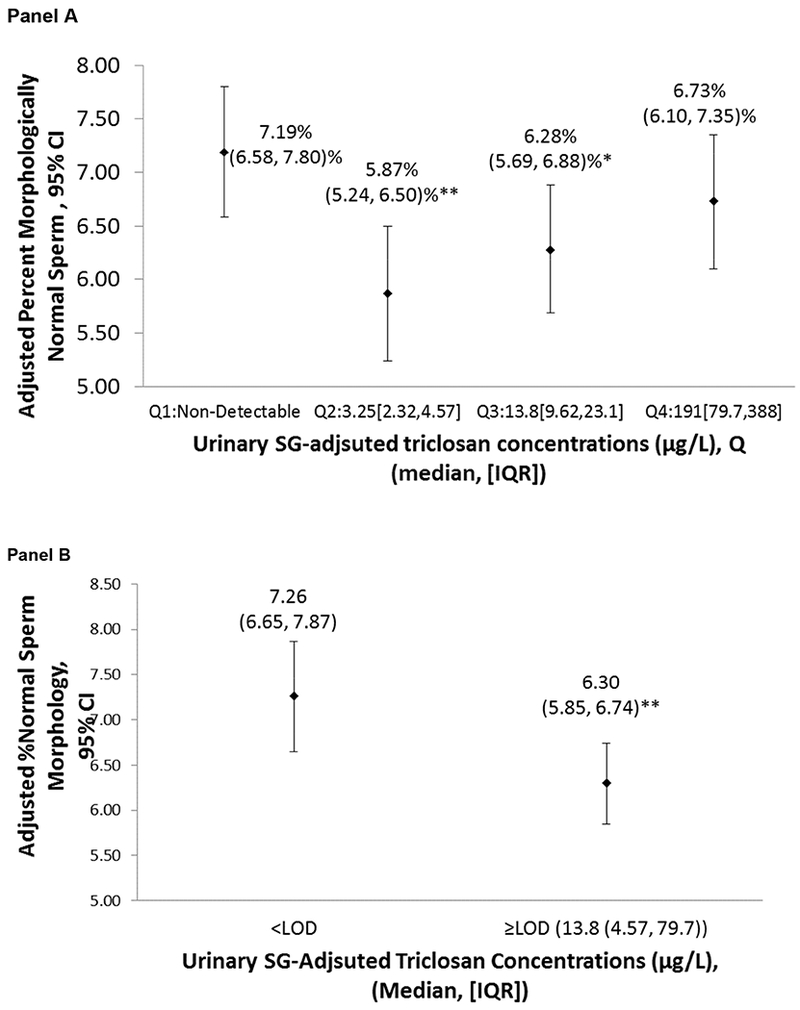
Adjusted means and 95% confidence intervals of % morphologically normal sperm associated with the SG-adjusted triclosan urinary concentrations among 262 men (581 semen samples) participating in the EARTH Study.
Adjusted marginal means were estimated using linear mixed models and a random intercept for each man for the semen quality parameters. The adjusted marginal means in each exposure category were adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variables.
The model adjusted for age (years, continuous), sexual abstinence time (days, categorical), body mass index (Kg/m2, continuous), tobacco smoking (yes/no), season (binary), moderate and vigorous physical activity (continuous), and calendar year (continuous).
Motility models were further adjusted for time elapsed between semen collection and analysis.
* p<0.05, ** p< 0.01 compared to < LOD.
Abbreviations: 95% CI; 95% confidence interval, N; number of men, n; number of samples, EARTH; the Environment and Reproductive Health study, Q; quartile, SG; specific gravity, LOD; limit of detection, IQR; interquartile range.
Table 2.
Associations between the SG-adjusted triclosan urinary concentrations and semen quality among 262 men (581 semen samples) participating in the EARTH Study.
| Men (N) / semen samples (n) | 63/151 | 58/143 | 62/144 | 79/143 | ||
|---|---|---|---|---|---|---|
| Median SG-adjusted urinary triclosan concentration (IQR), [range] | Q1: Non-detectable | Q2: 3.25 (2.32,4.57), [0.73, 6.63] | Q3: 13.8 (9.62,23.1), [6.80, 41.2] | Q4: 191 (79.7,388), [41.3, 2516] | ||
| Unadjusted Associations | P, trend | |||||
| Semen volume (mL) | Ref | −0.45 (−9.52, 9.53) | 5.28 (−4.36, 15.9) | −4.51 (−14.2, 6.28) | 0.11 | |
| Sperm concentration (million/mL) | Ref | −4.89 (−18.6, 11.1) | −4.14 (−18.0, 12.1) | −1.98 (−17.7, 16.8) | 0.87 | |
| Total sperm count (million) | Ref | −7.03 (−22.6, 11.7) | 0.42 (−16.4, 20.6) | −6.34 (−23.4, 14.5) | 0.55 | |
| Percent Motile Sperm (%) | Ref | −2.58 (−6.79,1.62) | −0.59 (−4.80,3.62) | 0.04 (−4.62,4.71) | 0.63 | |
| Percent Progressive Motile Sperm (%) | Ref | −1.04 (−3.77,1.70) | −0.21 (−2.96,2.53) | 0.10 (−2.93,3.14) | 0.72 | |
| Percent Morphologically Normal Sperm (%) | Ref | −1.30 (−2.01,−0.59)** | −0.88 (−1.59,−0.17)* | −0.46 (−1.23,0.31) | 0.44 | |
| Adjusted Associations | P, trend | |||||
| Semen volume (mL) | Ref | 0.28 (−8.58, 9.99) | 4.58 (−4.73, 14.8) | −5.50 (−14.9, 4.93) | 0.06 | |
| Sperm concentration (million/mL) | Ref | −4.40 (−18.2, 11.7) | −5.24 (−19.0, 10.9) | −2.40 (−18.3, 16.6) | 0.86 | |
| Total sperm count (million) | Ref | −5.84 (−21.3, 12.7) | −1.78 (−18.0, 17.7) | −8.13 (−24.8, 12.3) | 0.45 | |
| Percent Motile Sperm (%) | Ref | −2.29 (0.00,1.94) | −0.26 (0.00,4.02) | 0.89 (0.00,5.65) | 0.42 | |
| Percent Progressive Motile Sperm (%) | Ref | −0.85 (−3.62,1.91) | 0.23 (−2.56,3.03) | 0.92 (−2.18,4.02) | 0.42 | |
| Percent Morphologically Normal Sperm (%) | Ref | −1.32 (−2.04,−0.59)** | −0.91 (−1.63,−0.18)* | −0.46 (−1.25,0.33) | 0.41 | |
Abbreviations: 95% CI; 95% confidence interval, N; number of men, n; number of samples, EARTH; the Environment and Reproductive Health study, Q; quartile, SG; specific gravity, IQR; interquartile range, Ref; reference.
Adjusted associations were estimated using linear mixed models and a random intercept for each man for the semen quality parameters. The adjusted associations in each exposure category were adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variables.
The model adjusted for age (years, continuous), sexual abstinence time (days, categorical), body mass index (Kg/m2, continuous), tobacco smoking (yes/no), season (binary), moderate and vigorous physical activity (continuous), and calendar year (continuous). Motility models were further adjusted for time elapsed between semen collection and analysis.
Semen volume, sperm concentration and total sperm count are presented as % changes compared to the lowest quartile and the other parameters are presented as mean differences compared to the lowest quartile.
p<0.05,
p< 0.01 compared to lowest quartile.
There was a significant downward trend of the SG-adjusted urinary triclosan concentrations over years (Spearman correlation= −0.26, P-value < 0.001) (Supplemental Figure 3). In the samples provided earlier in the study (2009-2012), the proportion of samples with detectable triclosan concentrations was 79% as compared to 69% in the later period (2013-2017). Results of sperm morphology were similar in the earlier and the later years with consistently stronger negative associations in the earlier time, most probably due to the significant negative trend over time (Supplemental Table 3). Consistently, among men with non-detectable triclosan in urine, the adjusted proportion of men with percent normal morphology sperm that is below the WHO lower reference limits was 10.2% (95% CI: 5.40,18.3) compared to 20.8% (95%CI: 12.5,32.5) for men whose triclosan concentration were in the second quartile (P= 0.048) (Supplemental Table 4). In the analysis where we dichotomized triclosan concentrations (detectable vs non-detectable), the adjusted proportion of men with percent normal morphology sperm below the WHO lower reference limits was 9.90% (95% CI: 5.30,17.8) for men whose triclosan concentration were non-detectable, compared to 17.0 % (95%CI: 11.9,23.6) for men whose triclosan concentrations were detectable (P= 0.09) (Supplemental Table 5). There was no significant effect modification by age or BMI (data not shown).
Discussion
We did not observe any significant dose response trend between increasing SG-adjusted urinary triclosan concentrations and semen parameters. However, we did observe consistent patterns and some isolated findings of lower morphologically normal sperm for men with urinary triclosan in the 2nd or 3rd quartile compared to undetectable concentrations. We observed downward trend of triclosan urinary concentrations over time. Therefore, the observed negative association with sperm morphology was stronger in the earlier times where triclosan was more detectable in urine. The observed downward trend in our study population is consistent with the downward trend of urinary concentrations of triclosan in the U.S. general population (CDC, 2019). Perhaps this decline is related, at least in part, to the restriction that FDA issued on the use of triclosan in antiseptic wash products (FDA, 2016). Although this rule was not implemented until 2016, public concern started earlier and may have contributed to modified uses of the compound.
In the same cohort, we previously published that SG-adjusted urinary triclosan concentrations were highly correlated within couples suggesting similar exposure sources (Spearman correlation= 0.66) (Nassan et al., 2019). In addition, in women from the same cohort, higher SG-adjusted triclosan concentrations were associated with lower ovarian reserve as measured by antral follicle count (AFC) (−4%; 95% CI: −7, −1) with stronger effects among younger and leaner women (Minguez-Alarcon et al., 2017). Our results along with previous results from the same cohort indicate that urinary concentrations of triclosan were associated with lower fertility potentials in both partners. In addition, prenatal maternal triclosan concentrations were also associated with a 38 g decrease in the child’s birth weight (95% CI: −76, 0) among couples from the same cohort (Messerlian et al., 2018a).
Animal studies have found evidence between triclosan exposure with reproductive and developmental changes (Johnson et al., 2016). The mode of action of triclosan is still unclear. However, in-vitro studies have demonstrated that triclosan could bind with estrogen and androgen receptors (with low affinity) to act as an agonist, antagonist, or to result in no action (Witorsch, 2014). Triclosan exposure was also associated with adverse effects on the male reproductive system by disrupting steroidogenesis. In another in-vitro study, triclosan suppressed cyclic adenosine monophosphate synthesis in rodent Leydig cells leading to steroidogenesis disruption and thus decreased testosterone synthesis (Kumar et al., 2008). High doses of triclosan could inhibit testosterone synthesis but only recombinant human chorionic gonadotropin induced synthesis, while the basal testosterone production remained the same (Forgacs et al., 2012). Another in-vitro study in rodent Leydig cells has found that higher doses of triclosan lead to a significantly lower testicular weight and sex accessory tissues (Kumar et al., 2009). In addition, the authors observed downregulation of testicular steroidogenic acute regulatory protein, androgen receptor, and a decreased in-vitro activity of testicular steroidogenic enzymes. They also reported lower serum concentrations of testosterone, luteinizing hormone, and follicle stimulating hormone. All these findings were followed by lower semen production (Kumar et al., 2009). However, these results were not reproducible in two in-vivo studies in the same animal species (Axelstad et al., 2013; Zorrilla et al., 2008).
Very few epidemiological studies have examined the association between urinary triclosan concentrations and semen quality. Zamkowska et al (2018) conducted a recent overview of the current epidemiological evidence between environmental exposure to triclosan and semen quality (Zamkowska et al., 2018), and noted only three studies had been reported and showed inconsistent results (Chen et al., 2013; Den Hond et al., 2015; Zhu et al., 2016). They concluded that the lack of consistency was likely due to use of various methods of urinary triclosan quantification and different statistical methods. Therefore, because of the small number of studies and the inconsistent methods and thus the results, they recommended further studies were warranted.
Consistent with our results, in a fertility clinic, Zhu et al. (2016) found that urinary triclosan concentrations were negatively associated with the percent morphologically normal sperm, in addition to, sperm concentration, and sperm count, but only among men within the lowest tertile of urinary triclosan concentration (Zhu et al., 2016). They observed non-significant association between urinary triclosan and semen quality among all other men (Zhu et al., 2016). This finding is consistent with our study where the most significant results were between the second quartile compared to the lowest quartile. This may indicate non-linear dose response relation between triclosan exposure and sperm morphology. However, our results are not consistent with other studies. Chen et al. (2013) observed no relationship between exposure to triclosan and idiopathic male infertility (Chen et al., 2013). The findings reported by Chen et al. are consistent with those reported in a study conducted in Belgium. The authors of the latter study investigated the association between triclosan urinary concentrations and other chemicals among subfertile men (Den Hond et al., 2015). Den Hond et al. observed no relationship between triclosan urinary concentrations and sperm quality either (Den Hond et al., 2015). The discrepancy in the results could be because the urinary concentration of triclosan in our population (SG-adjusted median of the samples with detectable concentrations was 13.8 μg/L: IQR: (4.57, 79.7) μg/L) was higher than those reported previously in other studies conducted abroad (Chen et al., 2013; Den Hond et al., 2015; Zhu et al., 2016) with geometric means of 1.12 to 2.8 μg/L μg/L. This could be related to different uses in different countries (Chen et al., 2013; Den Hond et al., 2015). In addition, the lack of association observed in the aforementioned studies (with lower concentrations) could be due to the decline in triclosan concentrations we observed over time. On the other hand, in a more recent study not included in the review (Zamkowska et al., 2018), Smarr et al. (2018) has reported that triclosan urinary concentrations were associated with higher sperm count and total concentration based on a single spot urine sample, collected between 2005 and 2009 from men who were part of couples recruited upon discontinuing contraception for the purpose of the woman to become pregnant (Smarr et al., 2018). The inconsistency in results may be due, at least in part, to the nature of the study populations. In addition, the models were adjusted differently for urinary dilution (i.e., creatinine), race, age, and BMI without adjustment for abstinence time, tobacco smoking, season and calendar year of the sample, or physical activity that could have resulted in residual confounding. The urinary concentration of triclosan in our population was comparable to 17.6 (IQR: 4.42, 77.1) μg/L among men in Smarr et al (Smarr et al., 2018) as well as males in the U.S. general population (geometric means range between 9.95 to 14.8 μg/L for years 2009 to 2014) based on NHANES (CDC, 2019).
Our study has limitations including that because the study population was based in a fertility clinic, it may not be possible to generalize our findings to men from the general population. However, our results may be applicable to men seeking infertility treatment. Although the EARTH Study is a prospective study, the current analysis is cross-sectional and causality cannot be concluded. However, because we have repeated measures, relatively high ICC for the triclosan concentration over time, and covariate adjustment, these results add to the evidence of the association between triclosan and semen quality. Also, misclassification of triclosan exposure based on urinary triclosan concentrations from spot samples is possible because this chemical has a relatively short half-life (Calafat et al., 2008) and exposure to triclosan is likely to be episodic in nature. However, misclassification is less likely because ICC for the triclosan concentration was high over time and urine is the optimal medium for measuring non-persistent, semi-volatile environmental chemicals such as triclosan (Calafat et al., 2015).
Conclusion
In conclusion, among men of reproductive age attending a fertility center, we did not observe significant dose response trends between SG-adjusted urinary triclosan concentrations and semen parameters. However, we observed consistent patterns and some isolated findings of lower percent morphologically normal sperm for men with urinary triclosan in the 2nd or 3rd quartile compared to undetectable concentrations. We observed a downward trend of detectable triclosan urinary concentrations over time, and the associations of higher triclosan with decreased percent morphologically normal sperm were stronger for samples obtained prior to 2013, when triclosan was more often detectable in urine.
Supplementary Material
We did not observe a dose response relationship between triclosan and semen quality.
There were consistent patterns of lower percent morphologically normal sperm for men with urinary triclosan in the 2nd or 3rd quartile compared to undetectable concentrations.
This association was stronger for samples obtained prior to 2013 when triclosan was more often detectable in urine.
There was a significant downward trend of urinary triclosan concentrations over years.
Acknowledgments
The authors gratefully acknowledge the contributions of all members of the EARTH study team, specifically research nurse Myra G. Keller, senior research staff Patricia Morey, and the physicians and staff at the Massachusetts General Hospital fertility center. We acknowledge the technical assistance of X. Zhou, J. Tao, and the late Xiaoyun Ye (Centers for Disease Control and Prevention, Atlanta, Georgia) in measuring the urinary concentrations of triclosan. A special thank you is due to all of the study participants.
Funding
The project was financed by grants R01ES009718 and P30ES000002 from the National Institute of Environmental Health Sciences (NIEHS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors has any conflicts of interest to declare.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
References
- Axelstad M, et al. , 2013. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food and Chemical Toxicology. 59, 534–540. [DOI] [PubMed] [Google Scholar]
- Calafat AM, et al. , 2015. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ Health Perspect. 123, A166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, et al. , 2008. Urinary concentrations of triclosan in the U.S. population: 2003-2004. Environ Health Perspect. 116, 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention). National Report on Human Exposure to Environmental Chemicals. Vol. 2019 CDC, 2019. [Google Scholar]
- Chen M, et al. , 2013. Association of exposure to phenols and idiopathic male infertility. J Hazard Mater. 250-251, 115–21. [DOI] [PubMed] [Google Scholar]
- Dann AB, Hontela A, 2011. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 31, 285–311. [DOI] [PubMed] [Google Scholar]
- Den Hond E, et al. , 2015. Human exposure to endocrine disrupting chemicals and fertility: A case-control study in male subfertility patients. Environ Int. 84, 154–60. [DOI] [PubMed] [Google Scholar]
- DeSalva SJ, et al. , 1989. Triclosan: a safety profile. Am J Dent. 2 Spec No, 185–96. [PubMed] [Google Scholar]
- Dwivedi P, et al. , 2018. Impact of enzymatic hydrolysis on the quantification of total urinary concentrations of chemical biomarkers. Chemosphere. 199, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JL, et al. , 2010. Occurrence, efficacy, metabolism, and toxicity of triclosan. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 28, 147–71. [DOI] [PubMed] [Google Scholar]
- FDA USFDA, FDA issues final rule on safety and effectiveness of antibacterial soaps. 2016.
- Forgacs AL, et al. , 2012. BLTK1 Murine Leydig Cells: A Novel Steroidogenic Model for Evaluating the Effects of Reproductive and Developmental Toxicants. Toxicological Sciences. 127, 391–402. [DOI] [PubMed] [Google Scholar]
- Gore AC, et al. , 2015. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 36, E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden RU, 2014. On the need and speed of regulating triclosan and triclocarban in the United States. Environ Sci Technol. 48, 3603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, et al. , 2016. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environment International. 92-93, 716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger TF, et al. , 1988. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 49, 112–117. [DOI] [PubMed] [Google Scholar]
- Kumar V, et al. , 2008. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: Probable mechanism of action. Toxicology. 250, 124–131. [DOI] [PubMed] [Google Scholar]
- Kumar V, et al. , 2009. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reproductive Toxicology. 27, 177–185. [DOI] [PubMed] [Google Scholar]
- Messerlian C, et al. , 2018a. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environ Int. 114, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, et al. , 2018b. The Environment and Reproductive Health (EARTH) Study: A Prospective Preconception Cohort. Hum Reprod Open. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, et al. , 2017. Urinary triclosan concentrations and diminished ovarian reserve among women undergoing treatment in a fertility clinic. Fertil Steril. 108, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, et al. , 2016. A crossover-crossback prospective study of dibutyl-phthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environ Int. 95, 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, et al. , 2019. Correlation and temporal variability of urinary biomarkers of chemicals among couples: Implications for reproductive epidemiological studies. Environ Int. 123, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, et al. , 2006. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 69, 1861–73. [DOI] [PubMed] [Google Scholar]
- Singer H, et al. , 2002. Triclosan: Occurrence and Fate of a Widely Used Biocide in the Aquatic Environment: Field Measurements in Wastewater Treatment Plants, Surface Waters, and Lake Sediments. Environmental Science & Technology. 36, 4998–5004. [DOI] [PubMed] [Google Scholar]
- Smarr MM, et al. , 2018. Male urinary biomarkers of antimicrobial exposure and bi-directional associations with semen quality parameters. Reprod Toxicol. 77, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Ohe PC, et al. , 2012. Triclosan--the forgotten priority substance? Environ Sci Pollut Res Int. 19, 585–91. [DOI] [PubMed] [Google Scholar]
- Weng H-Y, et al. , 2009. Methods of Covariate Selection: Directed Acyclic Graphs and the Change-in-Estimate Procedure. American Journal of Epidemiology. 169, 1182–1190. [DOI] [PubMed] [Google Scholar]
- WHO, 2010. WHO laboratory manual for the Examination and processing of human semen. World Health Organization Department of Reproductive Health and Research, Geneva, Switzerland. [Google Scholar]
- Witorsch RJ, 2014. Critical analysis of endocrine disruptive activity of triclosan and its relevance to human exposure through the use of personal care products. Crit Rev Toxicol. 44, 535–55. [DOI] [PubMed] [Google Scholar]
- Wolf AM, et al. , 1994. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 23, 991–9. [DOI] [PubMed] [Google Scholar]
- Zamkowska D, et al. , 2018. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. Int J Occup Med Environ Health. 31, 377–414. [DOI] [PubMed] [Google Scholar]
- Zhou X, et al. , 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 944, 152–6. [DOI] [PubMed] [Google Scholar]
- Zhu W, et al. , 2016. Environmental Exposure to Triclosan and Semen Quality. Int J Environ Res Public Health. 13, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla LM, et al. , 2008. The Effects of Triclosan on Puberty and Thyroid Hormones in Male Wistar Rats. Toxicological Sciences. 107, 56–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


