Abstract
Well water is the primary drinking source of nearly a quarter of North Carolina residents. Many communities across the state have been concerned about their well water quality and inorganic contamination. The “Well Empowered” study worked alongside a community in Stokes County, North Carolina to measure toxic metals in their well water as well as provide and test ZeroWater® filter pitchers in homes with arsenic (As) or lead (Pb) contamination. Multiple water samples, including a First Draw sample from the kitchen tap and a sample taken directly from the well, were collected from 39 homes in Stokes County. The samples were analyzed for 17 different inorganic contaminants, including As, boron (B), Pb, and manganese (Mn), using inductively coupled plasma mass spectrometry (ICP-MS). High concentrations of Pb along with copper (Cu), cadmium (Cd), and zinc (Zn) were only found in the First Draw sample and therefore are likely originating in the home plumbing system while As, iron (Fe), and Mn were consistent across all samples and therefore are present in the groundwater. However, the low concentrations of B (< 100 parts per billion (ppb)) make it unlikely that the source of As and Mn contamination was coal ash-derived. Out of the 39 homes, four had As levels exceeding the federal standard of 10 ppb and an additional two exceeded the Pb standard of 15 ppb. These homes were provided with a ZeroWater® filter pitcher and a water sample was taken pre- and post-filtration. The ZeroWater® filter removed 99% of As and Pb from the water, dropping the levels well below the drinking water standard levels. These ZeroWater® filter pitchers, while not a permanent solution are a low-cost option for homeowners experiencing As or Pb contamination.
Keywords: Well water, Water filters, Inorganics, Toxic Metals, Water quality
1. 1Introduction
Over 15 million households in the United States (U.S.) rely on private wells as their drinking water source. In contrast to public drinking water systems, these systems are not federally regulated and therefore are not routinely monitored for chemical or microbial contaminants. Instead, the cost and responsibility of testing the water and assuring well safety falls on individual households. There is a risk that well owners do not test their wells as frequently as they should, if at all, and are unaware of the quality of their water. A 2009 U.S. Geological Survey (USGS) evaluation of 1,389 domestic wells found that 23% of the sampled wells contained one or more contaminants, mostly inorganic chemicals, that exceeded either the U.S. Environmental Protection Agency (USEPA) Maximum Contaminant Levels (MCLs) or the USGS Health-Based Screening Levels (HBSLs) (Desimone et al., 2009). A wide variety of contaminants of concern have been detected in private wells, including toxic metals, such as arsenic (As), in several aquifers throughout the U.S. (Buschmann et al., 2008; Mukherjee et al., 2006; Sanders et al., 2012). The occurrence of contaminants, as well as the lack of testing by homeowners, makes private well water an area of concern for public health.
In North Carolina (NC), around 2.4 million (24%) residents use domestic wells as their source of drinking water (Dieter, 2018). Sanders et al. previously geocoded and spatially mapped As levels in 63,856 NC wells tested from 1998 – 2010, which were publicly available from the NC Department of Health and Human Services State Laboratory, and found that approximately 700 measurements exceeded the USEPA’s MCL of 10 μg/L for As, with the highest concentration measuring 806 μg/L (Sanders et al., 2012). Ingestion of As in drinking water is associated with heart disease, neurological abnormalities, as well as skin, lung, kidney, and bladder cancer (National Research Council (U.S.). Subcommittee on Arsenic in Drinking Water., 2001). Arsenic is not the only concern for contamination in NC. For example, manganese (Mn) has been shown to co-occur with As in well water in NC and is also associated with adverse health outcomes (Bouchard et al., 2011; Sanders et al., 2014; Spangler and Spangler, 2009; Wasserman et al., 2006). Moreover, other studies have found elevated levels of radium and radon (Vinson et al., 2009) and hexavalent chromium (Vengosh et al., 2016) in groundwater throughout NC.
The occurrence of contaminants in well water in NC, and elsewhere, can be derived from multiple contamination sources including (1) direct anthropogenic sources from human and animal wastes (i.e. bacteria, nitrate), or salts and metals from industry-derived contamination, including via leaking coal ash pits (Harkness et al., 2016); (2) anthropogenically-induced metals such as lead and zinc originating from corrosion in water pipes; and (3) naturally occurring contamination related directly to the composition of the aquifer rocks and soil (Gillispie et al., 2016; Vengosh et al., 2016; Vinson et al., 2009). Given the elevated levels of As in coal ash contaminated water (Harkness et al., 2016; Ruhl et al., 2012) and the known occurrence of other metals in coal ash, such as boron (B), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), mercury (Hg), and selenium (Se) (2012; Swaine, 1992), communities across NC have raised concerns about the potential of well contamination from coal ash-derived chemicals. Boron is considered a leachable coal ash contaminant and therefore can be used to identify coal ash contaminated water (Harkness et al., 2016; Ruhl et al., 2010). High concentrations of both B (>100 parts per billion (ppb)) and strontium (Sr) ( >150 ppb) as well as their distinct isotopic signature in coal ash relative to naturally occurring B and Sr in groundwater allows for identifying coal ash contamination in water (Harkness et al., 2016; Ruhl et al., 2014).
Among the solutions for toxic metals removal from drinking water is reverse osmotic treatment. Yet, for many low-income populations, reverse osmotic treatment is cost-prohibitive (Flanagan et al., 2016). Recently, laboratory tests have identified tabletop filters, ZeroWater® filters, that remove As (Barnaby et al., 2017), but to date, few studies have deployed these filters in communities. In addition to As removal, ZeroWater® filter pitchers are certified by NSF International to remove Cr, Pb, and Hg. These filters are also a low-cost option, pitchers range from $20-$40 depending on the size, and replacement filters are less than $15 each. In this manuscript, we report results from the “Well Empowered” study, a community-driven initiative sponsored by UNC Superfund Research Program (2018a). Working alongside community residents in Stokes county who were concerned about coal ash contamination, a three-phase project was developed, comprising of (1) a survey on well perceptions and testing behavior, (2) measurement of levels of toxic metals in drinking water, and (3) a pilot-scale implementation of filtration when toxic metals contamination was identified. The results presented in this manuscript represent phases two and three of the study.
2. Materials and Methods
2.1. Participant Recruitment and Ethics Statement
Private well owners and users who resided in the Walnut Cove and Belews Creek communities in Stokes County, NC were invited to participate in a survey about perceptions of well water quality and well testing. The survey was online and open to the public for a six month period with a total of 101 respondents. In the survey, 90 participants indicated they would be interested in having their well water tested. Of these, a subset were identified as proximate to potential industrial sources of toxic metals, such as coal ash impoundments. Participants (n=51) were contacted via telephone and invited to have their water tested for metals and 40 residents (78%) consented to participate in the study. Written and informed consent was provided at the time of sample collection. The Institutional Review Board at the University of North Carolina at Chapel Hill approved all procedures (IRB #16–1840).
2.2. Water Sampling
An in-home sampling date was scheduled with each of the 40 participants. A few days prior to the sampling date, a first draw sampling kit was mailed to each of the participants. The first-draw sampling kits included: a 1 L cubitainer to collect the water, a set of gloves, and an instruction sheet. Study participants were asked to stop using their water after 10 P.M. the night before the scheduled collection day. They were subsequently instructed to collect cold water from their kitchen faucet first thing in the morning before using water anywhere else in their house to capture a first draw sample (First Draw). This sample was collected into the 1 L cubitainer while the participant was wearing gloves and then stored in the participant’s refrigerator until the researchers collected the samples later that day.
At each home, three additional water samples were collected by researchers: a sample from the kitchen faucet when the researchers arrived (Second), a sample directly from the well head once the well had been purged (Well), and another from the kitchen faucet after the well had been purged (Third). All samples were collected into a 1 L cubitainer by gloved researchers. In order to get a sample of the groundwater, rather than water that had been sitting in the well pump or water lines, the water was purged from the well prior to the collection of the Well and Third samples. USGS well purging methods were followed (2006). Water samples were transported back to the University of North Carolina at Chapel Hill on ice and stored at 4°C until time of analysis.
2.3. Water Analysis
The water samples were analyzed for antimony (Sb), As, B, Cd, Cr, cobalt (Co), Cu, iron (Fe), Pb, Mn, nickel (Ni), Se, Sr, tin (Sn), uranium (U), vanadium (V), and zinc (Zn). All water samples were analyzed following EPA method 6020B on an Agilent 7500cx inductively coupled plasma mass spectrometer (ICP-MS) (Santa Claire, CA). The ICP-MS is fitted with an octopole collision/reaction cell (ORC). The ORC was operated in hydrogen, helium, and no gas modes to remove polyatomic interferences. Scans consisted of three points per mass, an integration time of 100 to 300 msec and five passes.
Samples were diluted to 2% with Plasma Pure® nitric acid (SPC Science, Montreal, CA). A combined eight-point calibration curve 0, 0.010, 0.025, 0.050, 0.100, 0.500, 1, 5, 10, and 25 ppb was prepared in 2% nitric acid for all elements. An extended curve of 50, 100, 250, 500, 1000 ppb was prepared for Mn, Fe, Cu, Zn, and Sr. Samples were stored at 20°C without further processing. The internal standards bismuth (Bi), indium (In), lithium (Li), scandium (Sc), and tellurium (Te) were used to correct for plasma instability and matrix effects.
Quality control included blanks, replicate sample analysis, laboratory standards, and a National Institute of Standards and Technology (NIST) traceable certified reference material, CRM-TMDW (High Purity Standards, Charleston, SC.) The mean percent + standard deviation (% + SD) recovery for the CRM was 103 ± 4.8%. B and Sb are not included in the CRM. Recoveries for these elements is based on laboratory standards and are 103% and 107 % respectively.
2.4. Water Standards
The federal government regulates the concentration of a variety of contaminants in public drinking water systems, including As, Cd, Cu, Pb, Sb, Se, and U. The EPA sets Maximum Contaminant Levels (MCLs) for the majority of these metals, which is an enforceable standard. Cu and Pb are regulated by a treatment technique (TT) action level by the EPA, which requires public water systems to immediately treat their system if more than 10% of tap water samples in the system exceed the action level. In addition to these federal regulations, the state of North Carolina also regulates B, Cr, Co, Fe, Mn, Ni, Sn, V and Zn under the Department of Environmental Quality (DEQ). The 15A NCAC 02L Groundwater Quality Standards are the maximum allowable concentrations of contaminants in groundwater that may be tolerated without creating a threat to public health or that would otherwise render the groundwater unsuitable for use as a drinking water source. 15A NCAC 02L Groundwater Quality Standards apply to B, Cr, Fe, Mn, and Zn. These standards include the following criteria: a concentration protective of the non-cancer or systemic effects of a contaminant, a concentration which corresponds to an incremental lifetime cancer risk of one-in-a-million, a taste threshold limit value, an odor threshold limit value, the National Drinking Water Maximum Contaminant Level, or the National Secondary Drinking Water Standard. Co, Sn, and V are regulated via an Interim Maximum Allowable Concentrations (IMACs), which is applied to chemicals that do not have established groundwater standards. IMACs represent a temporary standard set by DEQ. The relevant standards utilized in this analysis are summarized in Table 1.
Table 1.
Federal and NC state drinking water standards
| Contaminant | Standard (ppb) |
|---|---|
|
U.S. EPA Maximum Contaminant Level | |
| Antimony (Sb) | 6 |
| Arsenic (As) | 10 |
| Cadmium (Cd) | 2 |
| Selenium (Se) | 50 |
| Uranium (U) | 30 |
|
U.S. EPA Treatment Technique Action Level | |
| Copper (Cu) | 1300 |
| Lead (Pb) | 15 |
|
NC DEQ 15A NCAC 02L Groundwater Quality Standard | |
| Boron (B) | 700 |
| Total Chromium (Cr) | 10 |
| Iron (Fe) | 300 |
| Manganese (Mn) | 50 |
| Nickel (Ni) | 100 |
| Zinc (Zn) | 2000 |
|
NC DEQ 15A NCAC 02L Groundwater Quality Standard Interim Maximum Allowable Concentration | |
| Cobalt (Co) | 1 |
| Tin (Sn) | 1000 |
| Vanadium (V) | 0.3 |
2.5. Reporting Results
Participants were mailed a packet that included the concentrations of metals in each sample collected from their homes. Samples were compared to the US EPA MCLs for As, Cd, Sb, Se, and U and the US EPA TT Action Levels for Cu and Pb. Additionally, B, total Cr, Fe, Mn, Ni, and Zn were compared to the NCDEQ 15A NCAC 02L Groundwater Quality Standards. Co, Sn, and V were compared to the NC DEQ 15A NCAC 02L Groundwater Quality Standard IMACs. Sr, while measured in our analysis, is not regulated under NC or federal law. Any sample that exceeded any of these regulated standards was highlighted in their report and participants were given information about the potential health effects of the identified metals.
2.6. Filter Distribution and Testing
Participants that exceeded the EPA MCL of 10 ppb for As or the EPA TT Action Level of 15 ppb for Pb were offered a ZeroWater® filter pitcher for water treatment. The six participants who qualified for these filter pitchers were contacted via phone and offered a pitcher. Pitchers were distributed to all six homes. When the pitchers were distributed, a sample was collected from the participant’s kitchen faucet. These samples (Pre-filter) were collected to the same protocols performed during the first sampling trip. Following collection of the Pre-filter sample, the ZeroWater® pitcher was filled with water from the kitchen tap and allowed to filter. A second sample was collected from the pitcher filtrate (Post-filter). These samples were transferred back to UNC, stored, and analyzed as described above. Participants were sent their results both pre- and post-filtration to communicate whether the ZeroWater® filter pitchers were an effective treatment for their drinking water.
3. Results
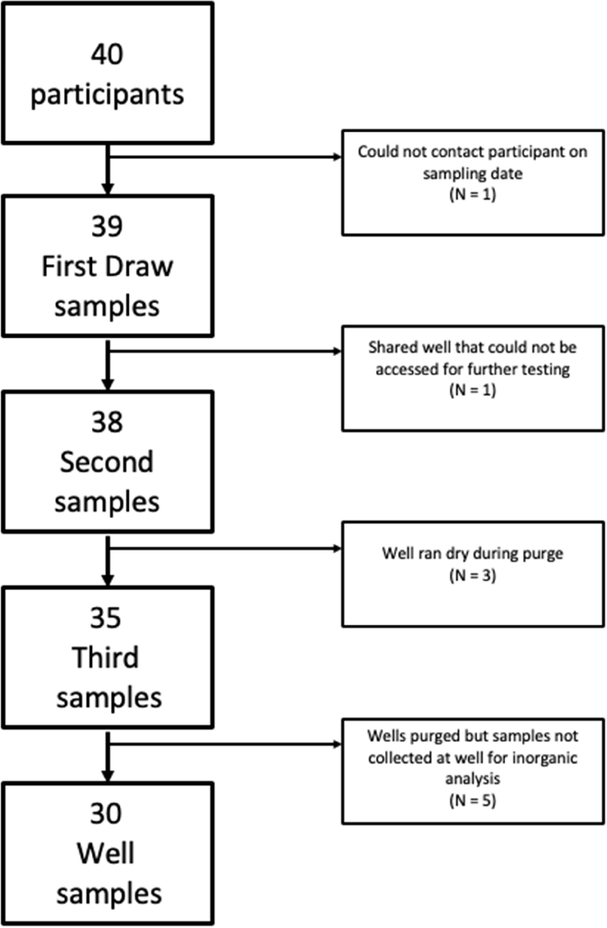
Of the 40 participants recruited, 39 homes were sampled for well water quality. For the demographic assessment a survey was distributed and filled out by the head of household (HOH) in each home, one survey was completed by the property landlord and not the HOH and was excluded (n=38; Figure 1). Sample demographics for the HOHs whose water was sampled are detailed in Table 2. Briefly, the HOHs in this study were predominantly male (66%), with ages ranging from 43 to 82 yrs. They were predominantly White (66%), had attended at least some college (63%), and earned less than $60,000 per year (55%). Just over half of the HOHs had private health insurance (51%) and most lived in their current home for more than 10 years (84%).
Figure 1.
Flow chart of how many of each sample type were collected
Table 2.
Demographics of the study participants for the Well Empowered Study (N=38)
| Number of participants N (%) | |
|---|---|
|
Gender | |
| Female | 13 (34) |
| Male | 25 (66) |
|
Age | |
| 30–49 | 2 (5) |
| 50–64 | 17 (45) |
| 65+ | 19 (50) |
|
Race | |
| White | 25 (66) |
| Black/African American | 11 (29) |
| White and Black/African American | 2 (5) |
|
Education | |
| < High School | 4 (11) |
| High School degree | 17 (45) |
| College degree | 12 (32) |
| Advanced degree | 3 (8) |
| Other | 2 (5) |
| Income | |
| Under $19,999 | 9 (24) |
| $20,000–59,999 | 12 (32) |
| $60,000–99,999 | 10 (26) |
| $100,000 or more | 3 (8) |
| Missing | 4 (11) |
| Insurance Type | |
| Public | 7 (18) |
| Private | 20 (51) |
| Both | 5 (13) |
| None | 4 (10) |
| Missing | 3 (8) |
| Length of time living at address | |
| Less than 10 years | 6 (16) |
| More than 10 years | 32 (84) |
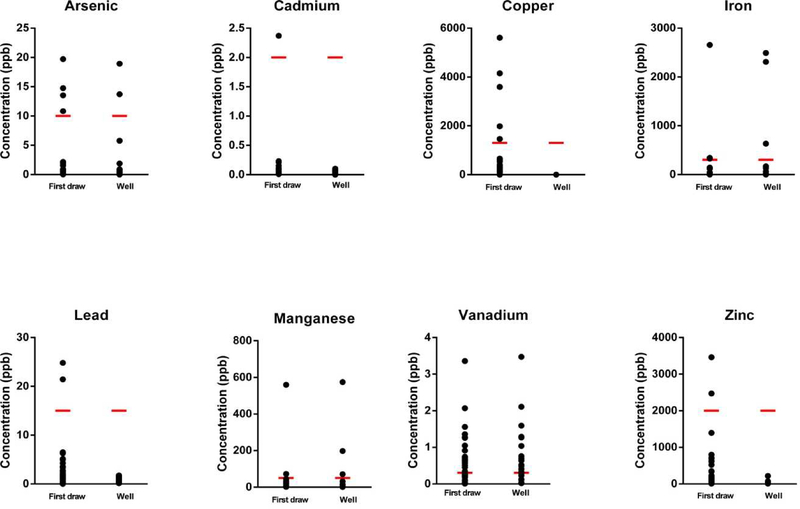
As detailed, a total of four water samples were collected from study participants. Specifically study participants collected water from their kitchen faucets first thing in the morning to represent the First Draw. When the researchers arrived for sampling an additional sample was collected from the kitchen faucet (Second), a sample directly from the well head once the well had been purged (Well), and another from the kitchen faucet after the well had been purged (Third). The levels of metals measured in the First Draw, collected by the participant, and the Well sample, following purging, are reported (Table 3; Figure 2). The Well sample indicates what chemicals are found in the groundwater while the First Draw sample conveys what the homeowners are being exposed to as well as what contaminants are leaching from the plumbing system. In the First Draw, exceedances of the EPA MCL for As (n = 4) and Cd (n = 1), the EPA TT for Cu (n = 6) and Pb (n = 2), and the NCAC 02L Groundwater Standard for Fe (n = 3), Mn (n = 2), and Zn (n = 3) were detected. Notably, the NCAC 02L IMAC for V was exceeded in the First Draw sample for 20 houses (51%). Overall, fewer exceedances were detected in the Well samples. Specifically, there were exceedances of the EPA MCL for As (n = 2), the NCAC 02L Groundwater Standard for Fe (n = 3) and Mn (n = 3), and the NCAC 02L IMAC for Co (n = 1) and V (n = 18). No exceedances were detected in any of the water samples for total Cr, Ni, Se, and Sb (data not shown). While Sr is not regulated, levels ranged from (1.4–1795.50 ppb) were detected in well samples. In addition, B concentrations did not exceed the NCAC 02L Groundwater Standard of 700 ppb and were relatively low, ranging from 0.97 ppb to 17.95 ppb in the Well samples and from 1.30 ppb to 24.02 ppb in the First Draws (Table 3). Results for Second and Third tap samples can be found in Supplemental Table 1.
Table 3:
Levels of inorganic contaminants in the First Draw and Well samples of Well Empowered participants (N=39).
| Contaminant | First Draw (N = 39) | Well (N = 30) | ||||
|---|---|---|---|---|---|---|
| N Detect (%) | Median (Range) | # Exceed (%) | N Detect (%) | Median (Range) | # Exceed(%) | |
| Arsenica | 39 (100%) | 0.22 (0.02, 19.72) | 4 (10%) | 30 (100%) | 0.18 (0.01, 18.93) | 2 (7%) |
| Boronc | 39 (100%) | 2.25 (1.30, 24.02) | 0 (0%) | 30 (100%) | 2.26 (0.97, 17.95) | 0 (0%) |
| Cadmiuma | 33 (85%) | 0.03 (<LOD, 2.37) | 1 (3%) | 24 (80%) | 0.01 (<LOD, 0.10) | 0 (0%) |
| Chromiumc | 37 (95%) | 0.12 (<LOD, 1.83) | 0 (0%) | 29 (97%) | 0.24 (<LOD, 4.19) | 0 (0%) |
| Cobaltd | 33 (85%) | 0.02 (<LOD - 0.17) | 0 (0%) | 21 (70%) | 0.01 (<LOD, 1.01) | 1 (3%) |
| Copperb | 39 (100%) | 136.63 (0.66, 12,748.31) | 6 (15%) | 29 (97%) | 3.87 (<LOD, 20.35) | 0 (0%) |
| Ironc | 39 (100%) | 8.23 (0.09, 2,656.48) | 3 (8%) | 30 (100%) | 6.03 (0.26, 2,490.50) | 3 (10%) |
| Leadb | 39 (100%) | 1.57 (0.02, 24.80) | 2 (5%) | 29 (97%) | 0.44 (<LOD, 1.73) | 0 (0%) |
| Manganesec | 39 (100%) | 1.32 (0.06, 559.77) | 2 (5%) | 30 (100%) | 2.45 (0.09, 574.27) | 3 (10%) |
| Strontiume | 39 (100%) | 57.54 (0.04, 355.43) | N/A | 30 (100%) | 68.76 (1.41, 795.50) | N/A |
| Vanadiumd | 34 (87%) | 0.30 (<LOD, 3.36) | 20 (51%) | 27 (90%) | 0.52 (<LOD, 3.47) | 18 (60%) |
| Zincc | 39 (100%) | 169.54 (8.32, 3,462.79) | 3 (8%) | 30 (100%) | 18.66 (7.54, 217.42) | 0 (0%) |
USEPA MCL
USEPA TT Action Level
NCDEQ 15A NCAC 02L Groundwater Quality Standard
NCDEQ 15A NCAC 02L Groundwater Quality Standard IMAC
Not regulated
Figure 2.
Distribution of metals levels in First Draw and Well samples. The red lines indicate the federal or NC state standard for each metal (Table 1).
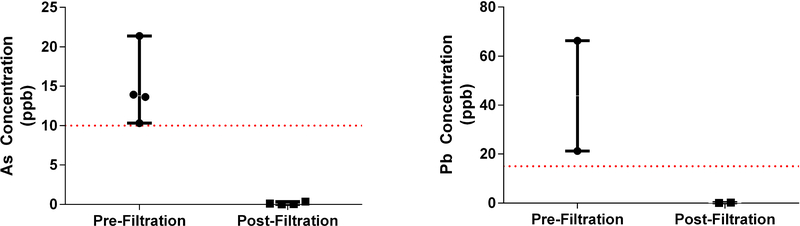
The four homes that exceeded the EPA MCL of 10 ppb for As and the two homes that exceeded the EPA TT of 15 ppb for Pb were provided with ZeroWater® filter pitchers, and the concentrations of As and Pb in the water were tested pre- and post-filtration. In all six homes, the pre-filtration concentrations were above the EPA standard and the ZeroWater® filter was effective in removing the metals to a concentration well below the standard (Table 4; Figure 3). In the case of As, the average percent removal for the four homes was 99.0%. The highest starting As concentration in a home was 21.39 ppb, greater than twice the EPA MCL. The ZeroWater® filter pitcher reduced the level to 0.06 ppb, a 99.7% removal. In the case of Pb, the highest starting concentration in the home was 66.30 ppb, more than four times greater than the EPA TT. This level was reduced to 0.10 ppb with the ZeroWater® filter pitcher, a 99.9% reduction. The average percent removal for Pb in the two homes was 99.4%.
Table 4.
Effectiveness of ZeroWater® filters to remove arsenic and lead.
| EPA standard (ppb) | Pre-Filtration Concentration (ppb) | Post-Filtration Concentration (ppb) | % Removal | Average % Removal | |
|---|---|---|---|---|---|
| Arsenic | 10 | 21.39 | 0.06 | 99.73 | 98.98 |
| 10.33 | 0.11 | 98.92 | |||
| 13.93 | 0.01 | 99.96 | |||
| 13.64 | 0.37 | 97.30 | |||
| Lead | 15 | 66.30 | 0.10 | 99.85 | 99.37 |
| 21.26 | 0.24 | 98.89 |
Figure 3.
Pre- and post-filtration As and Pb levels. The dotted red lines indicate the EPA standards for arsenic (10 ppb) and lead (15 ppb) in drinking water.
4. Discussion
In this study, we set out to ascertain well water quality for a community in Stokes County, North Carolina with concerns over potential toxic metals contamination in private wells from a nearby coal ash pit. The results of drinking water testing for inorganic contaminants using ICP-MS found exceedances of the relevant standards for As, Cd, Co, Cu, Fe, Pb, Mn, V, and Zn. This study includes results from a pilot-study to remove As and Pb from drinking water that lays the foundation for a state-wide initiative. Notably, we report that As and Pb could be removed to levels below the EPA MCL and EPA TT using a ZeroWater® filter pitcher, representing a low-cost solution for homeowners facing contamination with these toxic metals.
These results demonstrate some differences between contaminants identified in the First Draw sample, which was collected by the homeowner directly from the kitchen tap, and the Well sample. Interestingly, Cu, Cd, Pb, and Zn exceedances were only found in the First Draw sample. Because these contaminants exceeded standards in the First Draw sample and not the Well sample, these data suggest that they originate in the water system rather than the underlying aquifer. The remaining contaminants, As, Fe, and Mn, were stable across each of the samples These results confirm As and Mn contamination in private wells in North Carolina (Sanders et al., 2012). Importantly, given the published relationship between high levels of both B (> 100 ppb) and Sr (> 150 ppm) from coal ash seepage samples (Harkness et al., 2016), the low B concentrations (0.97 – 17.95 ppb) in well water in the present study make it unlikely that the As and Mn contamination are a result of coal ash leakage. Instead, the occurrence of relatively elevated As and Mn could be derived from geogenic sources. This is consistent with the geographic location of Stokes County within the Carolina Slate Belt where high frequency of elevated As and Mn concentrations in groundwater has been detected (Gillispie et al., 2016; Reid, 2010).
The NCAC 02L IMAC for V (0.3 ppb) was exceeded in over half of the First Draw and Well samples. Specifically, 20 (51%) and 18 (62%) of the First Draw and Well samples, respectively, exceeded the NCAC 02L IMAC of 0.3 ppb. V is not regulated in drinking water at the federal level nor is it regulated by other states. There is currently very little information regarding the health effects of low levels of V exposure in drinking water, and the scientific justification for the current IMAC has not been thoroughly described by NCDEQ. Notably, other states, including California and Florida, have advisory drinking water or groundwater levels for V at much higher levels (50 ppb and above) than the NCAC 02L IMAC (2004; 2018b). In a recent government memorandum to the NC Environmental Review Committee and the Joint Legislative Oversight Committee on Health and Human Services, NCDEQ indicated that the agency would recommend an updated IMAC of 20 ppb, instead of 0.3 ppb (Young, 2016). Under those updated guidelines, there would be no exceedances for V in this study. However, as of now there still have been no changes to the V IMAC.
Previous research demonstrated that ZeroWater® filters were effective in removing As from water in the laboratory setting as well as from two well samples in New Hampshire (Barnaby et al., 2017). In the present study, these filters were tested as a potential treatment method for the study participants. The filters are certified by NSF International to remove Cr, Pb, and Hg. Based on these findings, ZeroWater® filter pitchers were distributed to the six study participants whose water exceeded standards for As or Pb. The filters removed 99% of both As and Pb and, following filtration, the water was well below the EPA MCL for As of 10 ppb and EPA TT for Pb of 15 ppb. While pitcher filtration is not a permanent solution, this option is much more cost effective than commercially available reverse osmosis or ion-exchange treatment systems. Our findings demonstrate that ZeroWater® filters are a low-cost solution for homeowners facing low levels of As and Pb contamination.
5. Conclusion
In summary, the results of the present study indicated that most of the homes (90%) sampled in Stokes County exceeded at least one of the federal or state standards for drinking water or groundwater quality. The majority of these homes exceeded the IMAC for V, although the IMAC is a temporary state standard and the health implications of low V exposure are unknown. Some inorganic contaminants, such as Cu, Cd, Pb, and Zn, only exceeded regulatory limits in First Draw samples and not Well samples, indicating they were likely coming from leaching of the plumbing system. This study is not without limitations. The sample size was modest and the results may not be generalizable to other areas of NC. Thus, future research should aim to include a larger study population. In addition, the effectiveness of the ZeroWater® filters in removing inorganic metals could depend on a variety of factors including pH, oxygen-reduction potential, and the temperature of the water as well as usage of the filter. Thus, it is possible that under different conditions or over time the results may differ. Importantly, given the lack of required monitoring of private wells, researchers should continue to assess levels of contaminants and their various potential sources for public health protection. A noteworthy finding of the present study was the effectiveness of the ZeroWater® filters in removing both As and Pb to levels well below the EPA regulatory limits. These results demonstrate that ZeroWater® filters are a cost-effective means of removing As and Pb from drinking water.
Supplementary Material
Highlights.
Lead, copper, cadmium, and zinc contamination likely originated from pipes
Arsenic, iron, and manganese contamination was present in groundwater
Low concentrations of boron suggest geogenic contamination
ZeroWater® filters reduced arsenic and lead levels from well water samples
ZeroWater® filters are a low-cost treatment option for arsenic and lead contamination
Acknowledgments
Funding Information
This work was supported by the National Institutes of Health [grant numbers: T32ES007018, P42ES005948].
Footnotes
Antimony (Sb), Arsenic (As), Bismuth (Bi), Boron (B), Cadmium (Cd), Chromium (Cr), Cobalt (Co), Copper (Cu), Department of Environmental Quality (Department of Environmental Quality), Head of household (HOH), Health-Based Screening Level (HBSL), Indium (In), Inductively coupled plasma mass spectrometry (ICP-MS), Interim Maximum Allowable Concentration (IMAC), Iron (Fe), Lead (Pb), Lithium (Li), Manganese (Mn), Maximum Contaminant Level (MCL), Mercury (Hg), National Institute of Standards and Technology (NIST), Nickel (Ni), North Carolina (NC), parts per billion (ppb), Scandium (Sc), Selenium (Se), Strontium (Sr), Tellurium (Te), Tin (Sn), Treatment technique (TT), United States (U.S.), Uranium (U), U.S. Environmental Protection Agency (USEPA), U.S. Geological Survey (USGS), Vanadium (V), Zinc (Zn)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Private Drinking Water Wells. In: U. S. E. P. Agency, (Ed.). [Google Scholar]
- Risk Explantion Frequently Asked Questions. In: N. C. D. o. E. Quality, (Ed.). [Google Scholar]
- Vanadium: Health Effects In: A. o. T. S. a. D. Registry, (Ed.). [Google Scholar]
- Ground Water Standards and Guidance Concentrations used in Watershed Assessments In: F. D. o. E. Protection, (Ed.), Tallahassee, Florida: 2004. [Google Scholar]
- Collection of water samples In: U. S. G. Survey, (Ed.), U.S. Geological Survey Techniques of Water-Resources Investigations, 2006. [Google Scholar]
- Coal Ash Toxicity. Electric Power Research Institute, 2012. [Google Scholar]
- Building capacity for Well Empowered communities: Preventing exposure to toxic metals in private wells. 2018a.
- Drinking Water Notification Levels and Response Levels: An Overview In: C. S. W. R. C. Board, (Ed.), 2018b. [Google Scholar]
- Barnaby R, et al. , 2017. Effectiveness of table top water pitcher filters to remove arsenic from drinking water. Environ Res. 158, 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, et al. , 2011. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 119, 138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann J, et al. , 2008. Contamination of drinking water resources in the Mekong delta floodplains: arsenic and other trace metals pose serious health risks to population. Environ Int. 34, 756–64. [DOI] [PubMed] [Google Scholar]
- Desimone LA, et al. , 2009. Quality of water from domestic wells in principal aquifers of the United States, 1991–2004 : overview of major findings. U.S. Dept. of the Interior, U.S. Geological Survey, Reston, Va. [Google Scholar]
- Dieter CA, Maupin MA, Caldwell RR, Harris MA, Ivahnenko TI, Lovelace JK, Barber NL, and Linsey KS, Estimated use of water in the United States in 2015 In: U. S. G. Survey, (Ed.), Vol. 1441, U.S. Geological Survey Circular, 2018. [Google Scholar]
- Flanagan SV, et al. , 2016. Arsenic in private well water part 3 of 3: Socioeconomic vulnerability to exposure in Maine and New Jersey. Sci Total Environ. 562, 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillispie EC, et al. , 2016. Soil Weathering as an Engine for Manganese Contamination of Well Water. Environ Sci Technol. 50, 9963–71. [DOI] [PubMed] [Google Scholar]
- Harkness JS, et al. , 2016. Evidence for Coal Ash Ponds Leaking in the Southeastern United States. Environmental Science & Technology. 50, 6583–6592. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, et al. , 2006. Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr. 24, 142–63. [PubMed] [Google Scholar]
- National Research Council (U.S.). Subcommittee on Arsenic in Drinking Water., 2001. Arsenic in drinking water : 2001 update. National Academy Press, Washington, DC. [Google Scholar]
- Reid JC, Haven WT, Eudy DD, Milosh RM, and Stafford EG, 2010. Arsenic in grounwater in the North Carolina Eastern slate belt (Esb): Nash and Halifax counties, North Carolina. 47, 117–122. [Google Scholar]
- Ruhl L, et al. , 2010. Environmental impacts of the coal ash spill in Kingston, Tennessee: an 18-month survey. Environ Sci Technol. 44, 9272–8. [DOI] [PubMed] [Google Scholar]
- Ruhl L, et al. , 2012. The Impact of Coal Combustion Residue Effluent on Water Resources: A North Carolina Example. Environmental Science & Technology. 46, 12226–12233. [DOI] [PubMed] [Google Scholar]
- Ruhl LS, et al. , 2014. Boron and strontium isotopic characterization of coal combustion residuals: validation of new environmental tracers. Environ Sci Technol. 48, 14790–8. [DOI] [PubMed] [Google Scholar]
- Sanders AP, et al. , 2014. Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: a semi-ecologic study. BMC Public Health. 14, 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, et al. , 2012. Arsenic in North Carolina: public health implications. Environ Int. 38, 10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler AH, Spangler JG, 2009. Groundwater manganese and infant mortality rate by county in North Carolina: an ecological analysis. Ecohealth. 6, 596–600. [DOI] [PubMed] [Google Scholar]
- Swaine DJ, 1992. Guest editorial: Environmental aspects of trace elements in coal. Environ Geochem Health. 14, 2. [DOI] [PubMed] [Google Scholar]
- Vengosh A, et al. , 2016. Origin of Hexavalent Chromium in Drinking Water Wells from the Piedmont Aquifers of North Carolina. Environmental Science & Technology Letters. 3, 409–414. [Google Scholar]
- Vinson DS, et al. , 2009. Relationships between radium and radon occurrence and hydrochemistry in fresh groundwater from fractured crystalline rocks, North Carolina (USA). Chemical Geology. 260, 159–171. [Google Scholar]
- Wasserman GA, et al. , 2006. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 114, 124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M, Final Report on the Study of Standards and Health Screening Levels for Hexavalent Chromium and Vanadium. In: N. C. D. o. E. Quality, (Ed.), 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.