Abstract
The zebrafish kidney regenerates after injury by development of new nephrons from resident adult kidney stem cells. Although adult kidney progenitor cells have been characterized by transplantation and single cell RNA seq, signals that stimulate new nephron formation are not known. Here we demonstrate that fibroblast growth factors and FGF signaling is rapidly induced after kidney injury and that FGF signaling is required for recruitment of progenitor cells to sites of new nephron formation. Chemical or dominant negative blockade of Fgfr1 prevented formation of nephron progenitor cell aggregates after injury and during kidney development. Implantation of FGF soaked beads induced local aggregation of lhx1a:EGFP+ kidney progenitor cells. Our results reveal a previously unexplored role for FGF signaling in recruitment of renal progenitors to sites of new nephron formation and suggest a role for FGF signaling in maintaining cell adhesion and cell polarity in newly forming kidney epithelia.
Keywords: Kidney, regeneration, adult kidney stem cell, nephron, fibroblast growth factor
Introduction
During growth or following acute injury, fish generate new kidney nephrons from a latent stem cell pool to expand or regenerate kidney function (Diep et al., 2011; Kamei et al., 2019; Reimschuessel, 2001; Zhou et al., 2010). During regeneration, new nephrons are integrated into the kidney structure by connecting to existing tubules in a process which replicates mesonephric development (Diep et al., 2011; McCampbell et al., 2015). The lhx1a:EGFP transgene marks kidney stem cells that have been shown to develop into nephrons after serial transplantation (Diep et al., 2011). In addition, a population of putative quiescent adult kidney stem cells has been identified in single cell RNA seq experiments based on a shared gene expression profile with mammalian metanephric nephron progenitor cells (Tang et al., 2017). After injury, these cells are stimulated to expand in number, migrate, and aggregate as small domed clusters on existing tubules to form nephron precursor structures (Diep et al., 2011; Kamei et al., 2019). New nephron aggregates form specifically on existing distal tubule nephron segments, implying growth and development signals are spatially restricted during kidney regeneration (Diep et al., 2011; Kamei et al., 2019). Recently we reported that Wnt signaling plays a major role in patterning new nephron aggregates and driving cell proliferation in extending nephrons (Kamei et al., 2019) however the identity of early acting signals that recruit progenitor cells to sites of new nephron formation are unknown.
In this report, we show that multiple fibroblast growth factors (FGFs) are induced following acute kidney injury and that they are expressed specifically in tubular epithelial cells. We show that injury induced FGF signaling is required for nephrogenic aggregate formation and maintenance of aggregate cell-cell adhesion. FGFs are also sufficient to act as stem cell chemotactic factors in uninjured kidneys. Our results suggest that injury-induced FGF signaling provides a spatial, chemotactic cue to organize kidney stem cell-derived new nephrons at sites of integration and function with the kidney tubular architecture.
Results
FGF signaling is induced in response to acute kidney injury
Acute kidney injury by gentamicin injection in adult zebrafish induced the synchronous formation of many new nephrons marked by lhx1a expression at 7 days post-injury (dpi) (figure 1F,G). To assess the potential involvement of FGF / receptor tyrosine kinase (RTK) signaling in kidney regeneration we assayed expression of the FGFR / RTK response gene dusp6, a MAP kinase phosphatase, under kidney injury conditions. dusp6 was not expressed in the naïve adult fish kidney (Figure 1H), but was expressed in the nascent nephrogenic aggregates at 7 dpi, similar to lhx1a (Figure 1I), suggesting a role for FGF signaling during kidney regeneration. In histological sections, un-injured fish kidney showed no specific dusp6 expression by in situ hybridization (Figure 1J). By 3 days post injury, dusp6 was broadly expressed throughout the kidney and was enriched in a subset of the gentamicin-injured renal tubules (Figure 1K). At 5 dpi dusp6 expression was restricted to single cells and cell clusters in the kidney mesenchyme adjacent to injured tubules (Figure 1L). By 7 dpi the expression of dusp6 was further restricted to cell clusters (Figure 1M), identified as new nephron aggregates by co-immunolabelling with anti-Dusp6 and lhx1a:EGFP (Supplemental figure 1A–C). The specific FGFR1 inhibitor PD166866 reduced dusp6 expression to sham-injured levels, confirming dusp6 in situ labelling reflects localized FGF signaling (Supplemental figure 1D). dusp6 expression after kidney injury suggests an early widespread activation of FGF signaling is followed by a stronger, more focal signaling in aggregating nephron progenitor cells and new nephron aggregates.
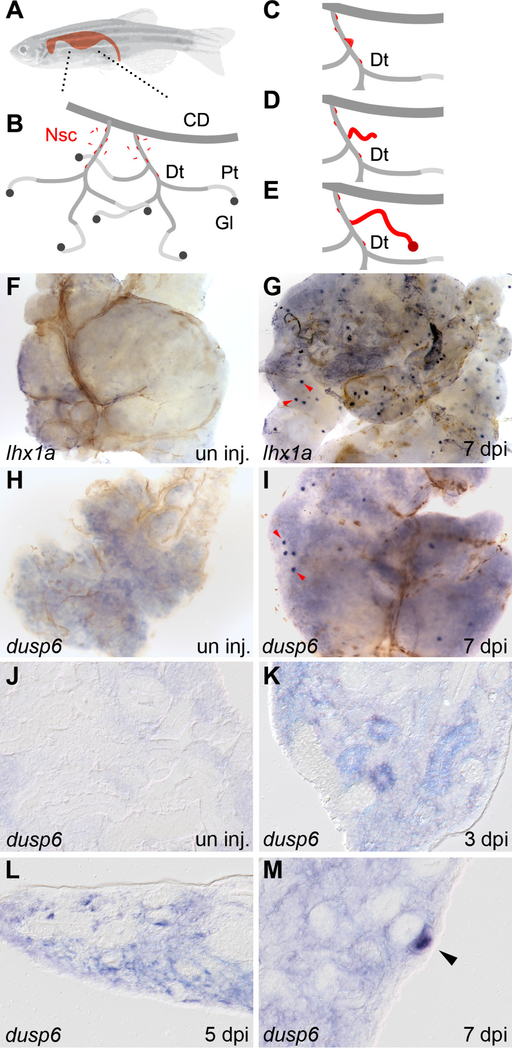
Figure 1: lhx1a and dusp6 expression marks new nephron formation in the regenerating adult zebrafish kidney.
(A) An adult zebrafish with the shape of the kidney highlighted. (B) Diagram of the organization of the adult kidney. Nephrogenic stem cells (Nsc) are colored red and appear in the interstitium and along the renal tubules. Segments of the kidney tubular structure are: CD, collecting duct; Dt, distal tubule; Pt, proximal tubule, Gl, glomerulus. (C-E) Diagram of stem cell based nephrogenesis. Stem cells accumulate on the Dt segment and form aggregates of cells (C). The aggregate elongates into a tubular epithelium and connects to the Dt (D). The new nephron fully differentiates to form a functional nephron (E). (F) Whole mount in situ hybridization of control uninjured adult kidneys show no expression of lhx1a. (G) Seven days after gentamicin injury lhx1a was induced in aggregates of nephrogenic cells, red arrowheads. (H) dusp6 was not expressed in controls but was induced in nephrogenic cells and cell aggregates at 7 dpi, red arrowheads (I). Scale Bars F-I are 500μm. (J) In histological sections, dusp6 was not expressed in uninjured control kidneys. J-M empty arrowheads indicate dusp6 negative tubules (K) dusp6 was induced by kidney injury in the interstitium and some renal tubules at 3 dpi, in aggregating single cells adjacent to kidney tubules at 5 dpi (L), and highly expressed in nephrogenic aggregates at 7 dpi (M). In K-M, representative dusp6 expressing structures are indicated with solid arrowheads. Scale Bars J-M are 50μm.
Multiple FGF ligands and receptors are expressed following kidney injury
To identify FGFs and FGF receptors involved in kidney regeneration responses we conducted an RT-PCR screen following acute kidney injury (Figure 2A). The FGF ligands fgf3, fgf4, fgf5, and fgf10a were upregulated as early as 3 days post-injury as were the FGF receptors fgfr1 and fgfr4 (Figure 2A). Interestingly, the fgfrl1 gene, regulator of kidney tubulogenesis and cell adhesion (Trueb et al., 2013; Yang et al., 2016), was transiently down-regulated following injury (Figure 2A). Other fgf and fgfr genes were either not detectable in kidney RNA samples or showed only minor changes in expression after injury (Supplemental figure 2A–C). Nephrogenic aggregates in regenerating zebrafish kidneys initially form adjacent to distal and collecting segments of renal tubules add nana (Diep et al., 2011; Kamei et al., 2015). To assess how Fgf ligand expression relates spatially to newly forming nephrons we assayed expression of the two most abundantly induced Fgf ligands, fgf4 and fgf10a, by in situ hybridization. fgf4 and fgf10a expression was concentrated in collecting ducts at 3 dpi (Figure 2B,C). fgf4 and fgf10a mRNA was localized to the apical cytoplasmic domain (Figure 2B,C) as has been observed for other actively translated epithelial mRNAs (Kamei et al., 2019; Moor et al., 2017). To show that the in situ pattern for fgf4 and fgf10a was not due to an “edge effect” and confirm the source of fgf4 and fgf10a was pre-existing kidney epithelial tubules, we separated kidney tissue into intact tubule and interstitial single cell fractions and then assayed fgf ligand expression by qRT-PCR. Cell fractions from sham-injured kidneys showed no expression of fgf ligands (Figure 2D,E), consistent with prior analyses of whole kidneys (Figure 2A). Similarly, interstitial single cell fractions showed no expression of fgf ligand mRNA either before or three days after injury (Figure 2D,E). However, tubule fractions from 3 dpi kidneys showed a 12.8-fold enrichment of fgf4 and a 8.6-fold induction of fgf10a expression (Figure 2D,E) compared to sham uninjured samples, confirming in situ hybridization results and identifying pre-existing tubules as the source of Fgf ligands induced by injury. The results suggest that Fgf ligand expression may provide a spatial cue for the formation new nephrons at sites of future attachment to the kidney collecting system.
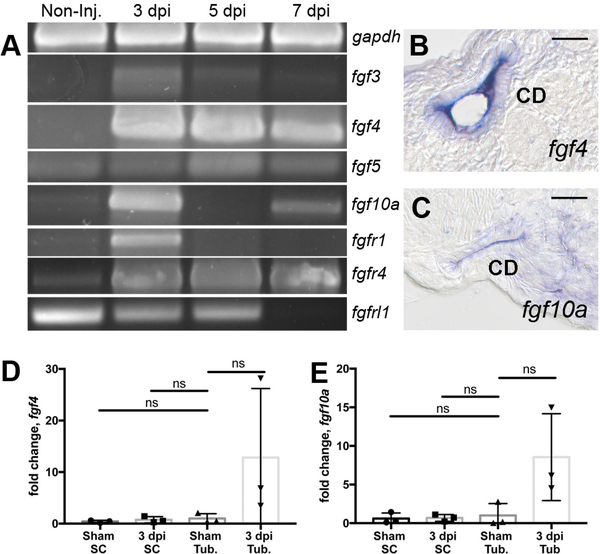
Figure 2: RT-PCR analysis identifies injury modulated FGF signaling molecules.
(A) RTPCR assays revealed that fgf3, fgf4, fgf5, fgf10a and the receptors fgfr1, fgfr4 were induced postinjury while the kinase domain deficient fgfrl1 gene exhibited reduced expression after injury. Histological sections of whole mount in situ hybridization 7 dpi injured kidneys show fgf4 (B) and fgf10a (C) were expressed in the collecting duct epithelium. (B-C) Scale bars are 20μm In kidney cell fractionation experiments, fgf4 (D) and fgf10 (E) were induced in isolated tubules and not in single interstitial cells at 3dpi compared to sham injured kidneys. qPCR represents 3 biological replicates. Comparisons were made by Kruskal-Wallis test. ns indicates not-significant. Data are expressed as mean ± SD.
FGF signaling is required for the formation of nephrogenic cell aggregates
To determine whether FGF signaling was required for kidney regeneration, we blocked the FGF signaling pathway both pharmacologically and genetically by inactivating the Fgfr1 receptor. In control regenerating kidneys, lhx1a+ nephrogenic aggregates were present throughout the kidney at 7 dpi (Figure 3A). Pharmacological inhibition of Fgfr1 signaling using the compound PD166866 resulted in a nearly complete failure to form lhx1a+ nephrogenic aggregates (Figure 3B) with the number of lhx1a+ aggregates reduced from an average of 77.9 per kidney to less than 2.4 (p = 0.01) per kidney at 7 dpi (Figure 3C). By qPCR, acute injury induced lhx1a expression 9.0-fold (p = 0.02) relative to sham-injured kidneys (Figure 3D). Treatment with PD166866 reduced lhx1a RNA expression to the level of sham-injured kidneys (Figure 3D). Genetic perturbation of FGF signaling was performed using the Tg(hsp70l:dnfgfr1-EGFP) zebrafish line, which expresses a dominant negative Fgfr1 receptor when heat shocked (Lee et al., 2005). Consistent with chemical inhibition of FGF signaling, dominant negative repression of Fgfr signaling blocked the appearance of lhx1a+ nephrogenic aggregates normally induced by AKI (Figures 3 E, F). The severity of the loss of FGF signaling was equivalent to pharmacological inhibition, with the average number of nephrogenic aggregates reduced from 9.5 in injured kidneys to 0.2 (p = 0.03) in injured and heat shocked Tg(hsp70l:dnfgfr1-EGFP) 7dpi kidneys (Figure 3G). To determine whether inhibition of FGF signaling was actually preventing aggregate formation as opposed to blocking expression of lhx1a, we sectioned injured kidneys for histological analysis. Nephrogenic aggregates typically appear as dome-shaped basophilic cell clusters abutting kidney tubules (Supplemental figure 3A). In multiple Fgfr signaling inhibited kidneys we found no morphological evidence of nephrogenic aggregates (Supplemental figure 3B). The results suggest that Fgf signaling is not simply required to induce lhx1a gene expression in new nephrons but rather is required for the formation of nephrogenic aggregates as a prelude to nephron regeneration.
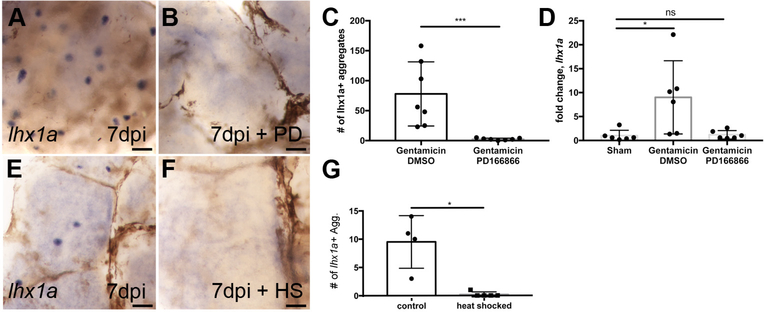
Figure 3: Inhibition of Fgf signaling prevents new nephron development.
(A) Injured zebrafish generate lhx1a+ nephrogenic aggregates at 7 dpi by whole mount in situ hybridization. (B) Treatment with the FGFR1 inhibitor PD166866 blocks the appearance of lhx1a+ nephrogenic aggregates in whole mount kidneys. (C) Quantification of lhx1a+ nephrogenic aggregates in multiple whole mount kidneys demonstrates PD166866 completely blocks kidney regeneration. N=7 fish per condition. Error bars indicate ±SD. p=0.01 by Mann-Whitney T-test. (D) qRTPCR quantitation of lhx1a expression shows PD166866 treatment blocks lhx1a expression post-injury. N=6 fish per condition. Error bars indicate ±SD. * indicates p=0.02 by Kruskal-Wallis test. (E) Control non-heat shocked Tg(hsp70:dnfgfr1) transgenic fish show lhx1a+ new nephron aggregates 7 days following gentamicin injury. (F) Heat shock induction of the dominant-negative Fgfr1 receptor blocked the development of lhx1a+ new nephron aggregates. (G) Quantification of lhx1a+ nephrogenic aggregates in multiple whole mount kidneys demonstrates that expression of the dominant-negative Fgfr1 receptor completely blocks kidney regeneration. Differences in injury responses (number of nephrogenic aggregates in A vs E) reflect strain dependent differences in sensitivity to gentamicin injury. N=4 control and N=5 heat shocked fish. Error bars indicate ±SD. p=0.03 by Mann-Whitney T-test. In panels A,B,E,F scale bars are 100μm.
FGF signaling is required for progenitor cell condensation at sites of nephron formation
The absence of nephrogenic aggregates in Fgfr inhibited kidneys suggested that progenitor cells may be lost or may fail to aggregate in the absence of FGF signals. To visualize the response of nephrogenic progenitor cells to Fgfr inhibition, we imaged lhx1a:EGFP+ cells after blocking Fgf signaling during different time windows of regeneration. Inhibition of Fgf signaling from 3 to 5 dpi followed by imaging at 5 dpi delayed the formation of condensed lhx1a:EGFP+ cell aggregates and caused the persistence of lamellipodia on clusters of lhx1a:EGFP+ cells (Figure 4 A, B). To better resolve cell scale and nuclei in the GFP+ cell population, individual image channels are presented for Figure 4B in Supplemental figure 4. Inhibition of Fgf signaling from 3 to 7 dpi resulted in complete failure of lhx1a:EGFP+ cells to aggregate with loss of compact nephrogenic aggregates and the appearance of broadly distributed, single stellate GFP+ cells and disorganized cell clumps (Figure 4C, D). These data indicate that in the absence of Fgf signaling, lhx1a:EGFP+ progenitor cells persist, but fail to migrate or aggregate at sites of regenerative new nephron formation. To test whether Fgf signaling was also required for maintenance of progenitor cell aggregates we inhibited Fgf signaling from 5 to 6 dpi after regenerative aggregates had formed as well as during mesonephric development, where similar lhx1a:EGFP+ cell aggregates precede nephron differentiation (Diep et al., 2011; Diep et al., 2015). Nephrogenic cells form a rosette with a central zo-1 positive apical ring, predicting the site of apical tight junctions in the new tubule lumen which extends in a proximal direction as the new nephron differentiates (Figure 4E, inset). Short term Fgfr inhbition with PD166866 from 5 to 6 dpi induced the appearance of filopodia and lamellopodia which formed first on the most proximal cells which also lose apical zo-1 localization (figure 4F). Ultimately polarity and cell aggregation is lost as cells appear to disperse randomly. In uninjured juvenile zebrafish, characteristic dome-shaped rosettes of lhx1a:EGFP+ nephrogenic cells were numerous and prominent (Figure 4G). Treatment with PD166866 for seven days disrupted pre-existing cell aggregates and promoted the appearance of dispersed single cells and stellate shaped clusters (Figure 4H). Our results suggest that Fgf signaling is required for both cell recruitment and maintenance of cell adhesion and polarity in nephron progenitor cell rosettes.
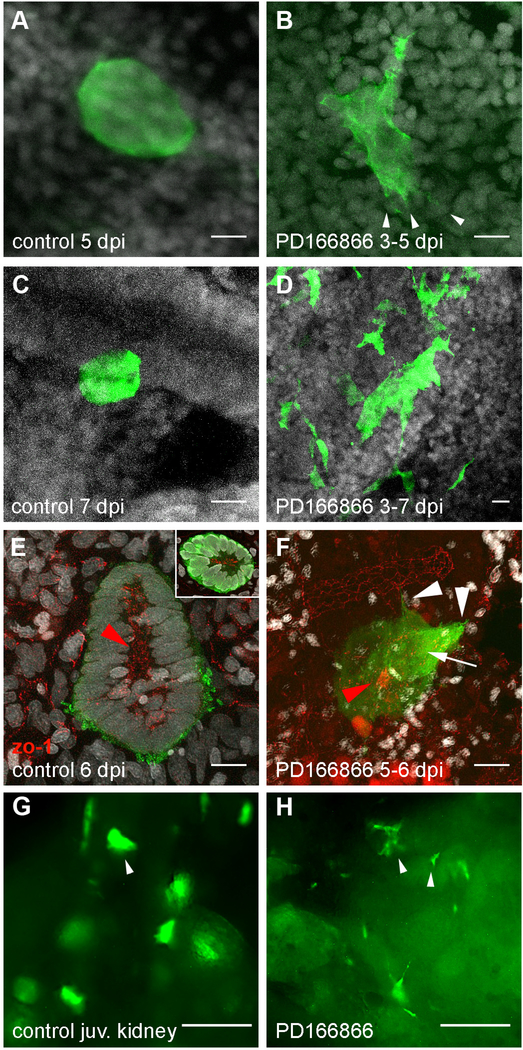
Figure 4. Disruption of nephrogenic cell aggregate formation in Fgf signaling inhibited kidneys.
(A) Adult Tg(lhx1a:EGFP) fish at 5 dpi exhibit condensed, dome-shaped cell aggregates on distal tubules. (B) Treatment of regenerating fish from 3 to 5 days post injury with the Fgfr1 inhibitor PD166866 followed by imaging at 5 dpi showed delayed formation of tight adherent nephrogenic cell domes and the appearance of cell lamellopodia (arrowheads in B). (C-D) Tight, condensed lhx1a:EGFP+ nephrogenic cell aggregates seen in control injured kidneys (C) did not form in fish treated with PD166866 from 3–7 dpi (D); instead lhx1a:EGFP+ cells remained stellate-shaped and disorganized. (E) 6 dpi lhx1a:EGFP+ nephrogenic cell aggregates display a rosette shape with a zo-1 positive apical lumen (inset) extending in the direction of nephron growth (red arrowhead; proximal). (F) 24 hr PD166866 treatment from day 5 to 6 induces filopodia and lamellopodia formation (white arrowheads) and reduction in apical zo-1 localization (white arrow) with a ring of remnant zo-1 only in distal cells (red arrowhead). (G) Juvenile Tg(lhx1a:EGFP) fish at 42 dpf produce nephrogenic aggregates in their developing kidneys identical to injury-induced, dome-shaped aggregates (arrowhead in G). (H) Treatment of juveniles from 35–42 dpf with PD166866 results in the loss of domed aggregates and appearance of single cells and stellate cell clusters (arrowheads in H), indicating a loss of adhesion. Scale bars in Z-projections (A-F) are 10μm and 100μm in (G,H).
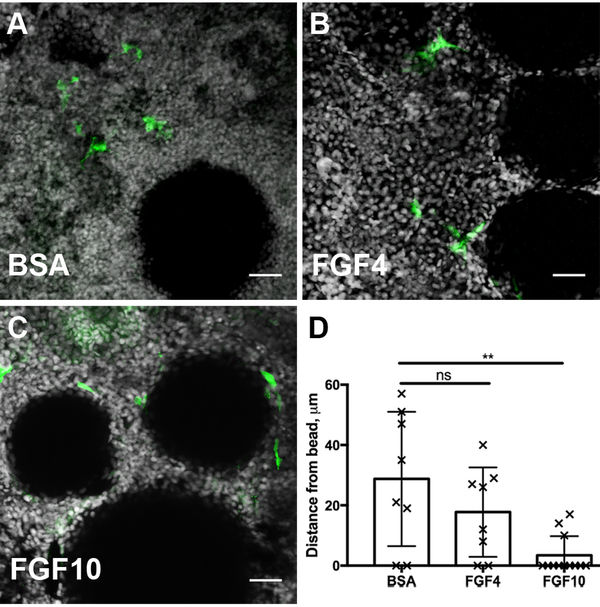
To assess whether Fgf signaling was sufficient to induce kidney progenitor cell recruitment we implanted FGF soaked beads into an uninjured adult lhx1a:EGFP kidney in explant culture. After 24 hours of in vitro culture, lhx1a:EGFP+ progenitor cells were found to preferentially associate with FGF beads compared to BSA bead controls (Figure 5 A–C; D). FGF10, and to a lesser extent FGF4, induced recruitment of cells to the beads with FGF10 causing most cells to spread directly on the bead surface (Figure 5C). Taken together, the data indicate that injury-induced Fgf expression in kidney tubules is sufficient and required for progenitor cell recruitment and rosette formation at sites of new nephron formation in regenerating zebrafish kidneys.
Figure 5. Exogenous FGF beads are sufficient to recruit progenitor cells.
A) Stem cells marked by the transgene lhx1a:eGFP are not attracted to BSA soaked agarose beads after 24 hours of culture. (B) Beads soaked with Fgf4 are more closely surrounded by renal stem cells, indicated by a decrease in cell to bead distance from an average of 28.8μm to 17.75μm. (C) Beads soaked with FGF10 strongly attract renal stem cells which accumulate adjacent to the bead at a mean distance of 3.42μm. (D) Quantification of cell to bead distances after 24 hours of culture demonstrate a chemoattractive role of FGF ligands on the recruitment renal stem cells. 3 biological replicates per condition. Error bars indicate ±SD. Comparisons to BSA beads were made by Kruskal-Wallis test. ns indicates not-significant, starred comparison indicates p=0.008. In Z-projection panels (A-C) scale bar is 25μm.
Discussion
The remarkable capacity of the zebrafish kidney to form new nephrons after injury is mediated by mobilization and differentiation of a resident population of lhx1a:GFP+, adult kidney stem/progenitor cells (Diep et al., 2011; Kamei et al., 2019). While serial transplantation studies provide strong support for lhx1a:GFP+ cells acting as kidney progenitor cells (Diep et al., 2011), the signals that mobilize these cells and drive nephron differentiation were not known. We recently reported that Wnt signaling patterns new nephron aggregates into zones of cell proliferation and tubule invasion (Kamei et al., 2019). Our principle finding here is that acute kidney injury in adult zebrafish initiates expression of multiple FGF ligands and receptors which have an early and essential function in recruiting kidney progenitors to sites of new nephron formation. In addition we find that continued FGFR signaling is required to maintain cell adhesion and polarity of nephron progenitor aggregates, revealing a role for FGF signaling in the earliest stages of mesenchyme to epithelial transformation that drives nephron formation. A similar function for FGF signaling in maintaining polarized epithelial rosettes is observed in the formation of hair cells in the zebrafish lateral line organ (Ma and Raible, 2009). Also, FGF signaling has been shown to mediate apico-basal polarity of the Drosophila mesoderm during gastrulation (Sun and Stathopoulos, 2018). Our findings establish FGF signaling as a key factor in cell polarization and define roles for FGF 4 and 10a as chemotactic factors in the context of kidney regeneration.
During mammalian kidney organogenesis, fibroblast growth factors 1, 2, 7, 8, 9, 10, 11, 12, 20 and receptors FGFR1, FGFR2 and FGFRl1 are expressed in temporally and spatially restricted patterns consistent with discrete cell signaling roles (Barak et al., 2012; Bates, 2011; Brown et al., 2011; Gerber et al., 2009; Grieshammer et al., 2005; Ohuchi et al., 2000; Okazawa et al., 2015; Perantoni et al., 2005; Poladia et al., 2006; Qiao et al., 1999; Trueb et al., 2013). Branching morphogenesis of the ureteric epithelium requires FGF7 and FGF10 for full development of the collecting system (Ohuchi et al., 2000; Qiao et al., 1999). “Cap mesenchyme”, a population mammalian nephron progenitor cells, is maintained by the activity of FGF9 and FGF20 which drive progenitor cell self-renewal at the periphery of the developing kidney (Barak et al., 2012; Brown et al., 2011). Both branching morphogenesis and progenitor self-renewal ultimately define nephron number and kidney organ size, with defects in FGF signaling leading to kidney hypodysplasia (Walker et al., 2016). FGF8 is expressed in the newly forming nephron epithelium and is required for survival and development of tubular epithelia (Grieshammer et al., 2005; Perantoni et al., 2005). FGF receptors acting in the mesenchyme (FGFR½; (Poladia et al., 2006)) and ureteric epithelium (FGFR2; (Zhao et al., 2004)) control multiple steps of kidney development with double mutants exhibiting a severe kidney aplasia phenotype (Poladia et al., 2006). Our results suggest a new role for epithelium-expressed FGFs as chemotactic factors recruiting and/or maintaining kidney mesenchymal cells at sites of new nephron formation. We found that zebrafish fgf4 and fgf10a were expressed in kidney epithelia after injury and were sufficient, when applied ectopically in ligand soaked beads, to recruit lhx1a:GFP+ kidney progenitor cells in uninjured kidneys. This finding is consistent with prior studies (Tang et al., 2017) where we found putative six2a, eya2, osr1+ adult kidney progenitor cells show a roughly 16-fold enriched expression of fgfr1a and fgfr2 over total kidney, suggesting adult kidney progenitor cells in uninjured kidneys are sensitive to Fgf. Although progenitor cell rosettes were not observed in our Fgf bead experiments this may be due to the relatively short, in vitro incubation period we used. The absence of new nephron cell aggregates in Fgfr inhibited zebrafish kidneys further argues that FGF signaling is required for progenitor cell recruitment. While the evidence for Fgf involvement in cell recruitment to tubules is strong, there must be additional factors controlling the size and location of nephron aggregates since progenitor cells are not spread evenly across Fgf-expressing epithelial tubules and instead form spaced cell clusters of uniform size.
Mammalian kidney cap mesenchyme, a cell population orthologous to the zebrafish lhx1a:GFP+ nephron progenitor cells (Diep et al., 2011; Tang et al., 2017), is highly motile and dynamic during nephrogenesis (Combes et al., 2016; Lindstrom et al., 2018). In time lapse studies individual cells are observed to make transient filopodial contacts with the ureteric epithelium and then detach and migrate among other cap mesenchyme and stromal cells (Combes et al., 2016; O’Brien et al., 2018). This behavior suggests that cells destined to generate nephrons maintain a close but dynamic association with the inducing ureteric epithelium by a combination of attractive and repulsive cues (Combes et al., 2016; O’Brien et al., 2018). In mice, deficiency in Robo2, a putative repulsive cue in the ureteric tip, results in an ectopic association of cap mesenchyme with ureteric stalks (Wainwright et al., 2015), implying persistent cell association with the growing collecting system. Mutation in the ureteric bud tip morphogen Wnt11 results in loss of stable attachment of mesenchymal cells to the UB tips and dispersal of cells from the cap mesenchyme niche (O’Brien et al., 2018). Our studies suggest FGFs may also act as chemoattractants during kidney development and regeneration. FGFs have broadly conserved roles in cell migration and adhesion (Bae et al., 2012; Boulet and Capecchi, 2012; Chi et al., 2006; Dalle Nogare and Chitnis, 2017; Hacohen et al., 1998; Li and Muneoka, 1999; Park et al., 1998; Roussigne et al., 2018). Blocking FGF signaling in our experiments allowed lhx1a:GFP+ progenitor cells to disperse away from the distal nephron epithelium while FGF4 or FGF10 soaked beads exerted a strong chemotactic influence on kidney progenitor cells. Taken together, the data suggests that attractive cues like FGFs may maintain progenitor cells in close apposition to an inducing epithelium to promote efficient and localized production of new nephrons. Genetic analysis of this phenomenon in zebrafish will require new conditional fgf mutants as knockdown of fgf4 results in strong left-right asymmetry defects (Yamauchi et al., 2009), fgf10a is required for fin development (Norton et al., 2005) and fgf8 is essential for hindbrain patterning (Reifers et al., 1998).
Candidate mammalian FGF ligands known to stimulate migration and also expressed in the ureteric epithelium include FGF2, and FGF9 (Chi et al., 2006; Joannes et al., 2016; Lin et al., 2009; Song et al., 2016; Wang et al., 2017). Although both ligands are more commonly associated with mesenchymal cell survival and proliferation (Barak et al., 2012; Drummond et al., 1998), FGF ligands can have multiple functions in different cellular contexts. FGF receptor binding activates multiple signal transduction pathways including ERK and PI3K/Akt (Ornitz and Itoh, 2015). ERK activation is associated with cell survival and proliferation while PI3K signals cell survival and cell motility (Francavilla et al., 2013; Ornitz and Itoh, 2015). In Hela cells FGF10 signals cell motility by inducing the rapid phosphorylation of tyrosine 734 on FGFR2b, recruiting PI3K and SH3BP4 and leading to cell migration (Francavilla et al., 2013). Different FGFR splice forms may also lead to differential activation of signaling pathways (Sharma et al., 2015). Finally, expression of different negative feedback inhibitors (sprouty/ERK inhibition vs. Spred/PI3K inhibition) can influence the cellular response to FGF receptor binding (Nutt et al., 2001; Sivak et al., 2005). Future studies should reveal how the cellular context of FGF signaling during kidney development and regeneration promotes cell aggregation at sites of new nephron formation.
Experimental Procedures
Animals
Adult wild type TuAB and Tg(hsp70l:dnfgfr1-EGFP) zebrafish approximately one year of age and adult and juvenile zebrafish carrying the lhx1a:eGFP transgene were used for this study (Lee et al., 2005; Swanhart et al., 2010). Heat shocked animals were injured under standard protocol. After confirmation, they were moved to a rack within the fish facility in which water temperature is raised to 37°C for 3 hours per day. Control fish were maintained under standard maintenance conditions after injury. Experimentally induced acute kidney injury procedures were performed in accordance with an MGH approved IACUC protocol.
Chemicals
Acute kidney injury was induced by intraperitoneal injection of gentamicin (Sigma, Saint Louis, MO) at 80mg/kg (2mg/ml stock in PBS at 10μl per 0.25g body mass) as previously described (Kamei et al., 2015). Sham injury was performed by injecting an equivalent volume of PBS intraperitoneally in place of gentamicin solution. After confirmation of kidney injury by visual identification of cast excretion at 1 day post injury (Kamei et al., 2015), Fgfr1 signaling was inhibited by adding PD166866 (10μM, Millipore Sigma, Burlingame, MA) in DMSO (0.04% final concentration) to fish system water (Panek et al., 1998). Fish water was changed daily, with fresh DMSO or PD166866 added at the same concentration. The FGF inhibition protocol was maintained until animals were sacrificed at 7 days post-injury, except where otherwise noted.
In Situ Hybridization
In situ hybridizations were performed on adult kidney at the time post-injury described. Briefly, Digoxygenin labeled RNA probes for lhx1a and dusp6 were prepared from cDNA clones, while probes for fgf4 and fgf10a were prepared by transcription from a PCR fragment amplified from genomic DNA as described previously (Thisse et al., 2004). Quantitation of lhx1a positive aggregates was performed on 5X images of whole mount tissue on the widest section of the kidney. Positive aggregates were marked and quantified in ImageJ. For histological sections, tissue from in situ hybridizations was embedded in JB4 blocks (Polysciences, Inc., Warrington, PA) and sectioned to a thickness of 10μm. Sections were mounted in Permount (Sigma, Saint Louis, MO) and imaged on a Nikon Eclipse E800 microscope.
Kidney tubule isolation
Adult TuAB trunk kidneys (two per sample) were incubated at room temperature with 0.5mg/ml Collagenase Type A (Sigma, Saint Louis, MO) in HBSS (Walkersville, MD) for 30 minutes on a rocking stage at room temperature. The tissue was vigorously vortexed for 3 minutes to fragment the tissue. The tube was left to stand for 5 minutes to allow the tubules to separate from single cells by gravity. The supernatant was passed through a 40μm filter (Corning, Corning, NY), pelleted and lysed into Qiazol (Qiagen, Venlo, Netherlands) for RNA preparation. The tubular fraction was washed with HBSS and gravity collected again for 5 minutes, then lysed into Qiazol (Qiagen, Venlo, Netherlands) for RNA preparation. Enrichment for hematopoietic cell types in single cell fraction RNA and kidney tubular epithelial cells in tubule fraction RNA was confirmed by qRTPCR for gata1 (single cells) and cdh17 (tubule cells) (Supplemental figure 5).
Immunofluorescence and Microscopy
Tissue was fixed in 4% formaldeyde for 3 hours then stained for indirect immunofluorescence. Primary antibodies used were chick anti-GFP (Abcam, Cambridge, UK) and mouse anti-Dusp6 (Sigma, Saint Louis, MO), and secondary antibodies were anti-chick IgG and anti-mouse IgG linked to Alexafluor 488 and 546 (Life Technologies, Carlsbad, CA). Nuclei were stained with Hoechst 33342 (invitrogen, Carlsbad, CA). Confocal imaging was performed on a Zeiss LSM 510 microscope.
Reverse-Transcriptase PCR and qPCR Assays
For screening expression of FGF related genes, RNA was harvested from three pooled kidney samples from adult TuAB kidneys, either un-injured or at the indicated time post-gentamicin induced AKI. cDNA was synthesized using Quantitect Reverse Transcription kit reagents (Qiagen, Venlo, Netherlands). Primers for initial RTPCR screening are presented in Supplemental Table 1. For qPCR, RNA harvested from individual kidneys was performed at the indicated time after gentamicin injury. RNA was harvested with Qiagen RNeasy columns and cDNA libraries were prepared using SuperScript IV (Invitrogen, Carlsbad, CA). qRTPCR was performed on a StepOnePlus thermal cycler using Sybr Green reagents (Applied Biosystems, Foster City, CA). qRTPCR results are expressed as fold change by ΔΔCT, with analysis described previously (Willems et al., 2008). qRTPCR primers used were as follows: gapdh-forward 5’-cggagcaccaggttgtgtcca-3’, gapdh-reverse 5’-agcaataccagcaccagcgtc-3’, lhx1a-forward 5’-gctgcgagtgcaaatgtaac-3’, lhx1a-reverse 5’-catgcatgtgaagcagttcag-3’, dusp6-forward 5’-tctatctcgagggtggcttca-3’, dusp6-reverse 5’-actcgatgtccgaggagtca-3’, fgf4-forward 5’-ccggcgtacacaacgaaaac-3’, fgf4-reverse 5’-gaactgctcagatccgtaaagc-3’, fgf10a-forward 5’-cgatccgtacagtacactcgaaa-3’, fgf10a-reverse 5’-agcttgcagtcaatgccgaa-3’. Dendrograms of Fgf ligands and receptors were prepared using Phylip 3.695 drawtree program (Felsenstein, 1989).
Progenitor Cell Motility Assay
Kidneys were isolated from un-injured adult Tg(lhx1a:eGFP) fish for explant culture. The tissue was placed atop a polycarbonate filter, on a media equilibrated agarose raft. To deliver growth factors Affi-Gel Blue Gel (Bio-Rad, Hercules, CA) beads were incubated in mouse FGF4 or FGF10 (R&D Systems, Minneapolis, MN) or BSA (Sigma, Saint Louis, MO) at 500ng/ml, then washed in PBS before implantation. Several beads were placed upon the widest part of the kidney by capillary action from a 1μl aspiration of washed beads. Cultures were maintained at 28°C for 24h before formaldehyde fixation and immunostaining. Culture media was prepared following previously described zebrafish explant culture (Cao and Poss, 2016), with amendments for culture without carbon dioxide supplementation. The culture medium was L-15 based (Thermofisher, Waltham, MA), with an addition of HEPES pH7.0 to 10mM.
Statistical Analysis
All quantifications are represented with error bars indicating SD. All data represent three or more biological replicates. Individual comparisons were Mann-Whitney T-tests. Multiple comparisons were made using Kruskal-Wallis tests. All statistical analyses were performed and graphs prepared using Prism7 (GraphPad).
Supplementary Material
Supplemental Table 1. PCR primers used.
Supplemental Figure 1. Dusp6 expression in nephrogenic cell aggregates. (A) Immunostaining with anti-Dusp6 (red) co-localizes with anti-GFP immunostaining of the lhx1a:eGFP transgene (B) at 7 dpi. (C) Merged image of A and B. Dotted line represents outline of adjacent distal tubule. Scale bars are 10 μm. (D) Injury-induced expression of dusp6 is reduced by PD166866 (added to tank water from 1 dpi to 7 dpi) to sham injured levels. qPCR represents N=4 fish per condition. All comparisons not significant by Kruskal-Wallis test.
Supplemental Figure 2. RT-PCR screen of FGF family genes in regenerating kidneys. (A) RT-PCR analysis of FGF ligand and receptor gene expression reveals additional changes in gene expression following injury as well as many Fgf family member mRNAs that remain unchanged after AKI. (B) Dendrogram of the FGF ligands indicating genes that were injury induced (green), repressed (red), or unchanged (yellow). (C) Dendrogram of the FGF receptors indicating genes that were injury induced (green), repressed (red), or unchanged (yellow). Fgf family genes that showed no kidney expression in the RT-PCR screen are not shown.
Supplemental Figure 3. FGF signaling is required for aggregation of cells into nephrogenic condensates. (A) Nephrogenic aggregates are apparent in histological sections as compact basophilic structures adjacent to distal tubules at 7 dpi. Arrows indicate nascent nephrons. (B) FGF inhibition with PD166866 post-injury prevents the formation of nephrogenic aggregates, and no peri-tubular basophilic cellular aggregates can be found in histological sections. Scale bars are 50μm.
Supplemental Figure 4. Channel separated and merged image of figure 4B. To better resolve nuclei and GFP+ cell scale, Figure 4B is represented as separate color channels DAPI, eGFP and the merged image.
Supplemental Figure 5. Characterization of kidney tissue fractions by qRTPCR. (A) qRTPCR analysis of single cell and tubule tissue fraction RNA shows an average 34.9 fold enrichment in gata1 expression in single cells over tubule fractions. (B) qRTPCR analysis revealed an average 5.1 fold enrichment of cdh17 expression in tubule fraction RNA. Each data point indicates a comparison of one replicate’s single cell fraction against its corresponding tubule fraction from the same cell isolation. Statistical comparisons were performed by Mann-Whitney test, ** indicates p=0.0022 in both comparisons.
Highlights Gallegos et al.
Adult zebrafish kidneys regenerate nephrons after injury from a resident kidney stem cell population.
Kidney injury induces expression of multiple FGF ligands and receptors.
FGF signaling is required for kidney progenitor cell aggregation at sites of new nephron formation.
FGF10 and FGF4 function as chemotactic factors to recruit kidney progenitors.
FGF signaling is required to maintain adhesive cell rosettes that give rise to new nephrons.
Acknowledgements
The authors declare no competing interests. The authors thank Dr. Kenneth Poss for providing the Tg(hsp70l:dn-fgfr1) transgenic line. We thank Dr. Neil Hukriede for the Tg(lhx1a:eGFP) transgenic line. This work was supported by NIH grant 5UH3DK107372–04 and grants from the Harvard Stem Cell Institutute to I.A.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bae YK, Trisnadi N, Kadam S, Stathopoulos A, 2012. The role of FGF signaling in guiding coordinate movement of cell groups: guidance cue and cell adhesion regulator? Cell adhesion & migration 6, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschke P, Salomon R, Antignac C, Ornitz DM, Kopan R, 2012. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Developmental cell 22, 1191–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates CM, 2011. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr Nephrol 26, 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet AM, Capecchi MR, 2012. Signaling by FGF4 and FGF8 is required for axial elongation of the mouse embryo. Developmental biology 371, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Adams D, de Caestecker M, Yang X, Friesel R, Oxburgh L, 2011. FGF/EGF signaling regulates the renewal of early nephron progenitors during embryonic development. Development 138, 5099–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Poss KD, 2016. Explant culture of adult zebrafish hearts for epicardial regeneration studies. Nat Protoc 11, 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Itaranta P, Zhang S, Vainio S, 2006. Sprouty2 is involved in male sex organogenesis by controlling fibroblast growth factor 9-induced mesonephric cell migration to the developing testis. Endocrinology 147, 3777–3788. [DOI] [PubMed] [Google Scholar]
- Combes AN, Lefevre JG, Wilson S, Hamilton NA, Little MH, 2016. Cap mesenchyme cell swarming during kidney development is influenced by attraction, repulsion, and adhesion to the ureteric tip. Developmental biology 418, 297–306. [DOI] [PubMed] [Google Scholar]
- Dalle Nogare D, Chitnis AB, 2017. A framework for understanding morphogenesis and migration of the zebrafish posterior Lateral Line primordium. Mech Dev 148, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, Davidson AJ, 2011. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CQ, Peng Z, Ukah TK, Kelly PM, Daigle RV, Davidson AJ, 2015. Development of the zebrafish mesonephros. Genesis 53, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond IA, Mukhopadhyay D, Sukhatme VP, 1998. Expression of fetal kidney growth factors in a kidney tumor line: role of FGF2 in kidney development. Exp Nephrol 6, 522–533. [DOI] [PubMed] [Google Scholar]
- Felsenstein J, 1989. PHYLIP - Phylogeny Inference Package (Version 3.2). Cladistics 5, 164–166. [Google Scholar]
- Francavilla C, Rigbolt KT, Emdal KB, Carraro G, Vernet E, Bekker-Jensen DB, Streicher W, Wikstrom M, Sundstrom M, Bellusci S, Cavallaro U, Blagoev B, Olsen JV, 2013. Functional proteomics defines the molecular switch underlying FGF receptor trafficking and cellular outputs. Mol Cell 51, 707–722. [DOI] [PubMed] [Google Scholar]
- Gerber SD, Steinberg F, Beyeler M, Villiger PM, Trueb B, 2009. The murine Fgfrl1 receptor is essential for the development of the metanephric kidney. Developmental biology 335, 106–119. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR, 2005. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132, 3847–3857. [DOI] [PubMed] [Google Scholar]
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA, 1998. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 92, 253–263. [DOI] [PubMed] [Google Scholar]
- Joannes A, Brayer S, Besnard V, Marchal-Somme J, Jaillet M, Mordant P, Mal H, Borie R, Crestani B, Mailleux AA, 2016. FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and migration and inhibit myofibroblast differentiation of human lung fibroblasts in vitro. American journal of physiology. Lung cellular and molecular physiology 310, L615–629. [DOI] [PubMed] [Google Scholar]
- Kamei CN, Gallegos TF, Liu Y, Hukriede N, Drummond IA, 2019. Wnt signaling mediates new nephron formation during zebrafish kidney regeneration. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei CN, Liu Y, Drummond IA, 2015. Kidney Regeneration in Adult Zebrafish by Gentamicin Induced Injury. J Vis Exp, e51912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD, 2005. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173–5183. [DOI] [PubMed] [Google Scholar]
- Li S, Muneoka K, 1999. Cell migration and chick limb development: chemotactic action of FGF-4 and the AER. Developmental biology 211, 335–347. [DOI] [PubMed] [Google Scholar]
- Lin Y, Chen L, Lin C, Luo Y, Tsai RY, Wang F, 2009. Neuron-derived FGF9 is essential for scaffold formation of Bergmann radial fibers and migration of granule neurons in the cerebellum. Developmental biology 329, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom NO, De Sena Brandine G, Tran T, Ransick A, Suh G, Guo J, Kim AD, Parvez RK, Ruffins SW, Rutledge EA, Thornton ME, Grubbs B, McMahon JA, Smith AD, McMahon AP, 2018. Progressive Recruitment of Mesenchymal Progenitors Reveals a Time-Dependent Process of Cell Fate Acquisition in Mouse and Human Nephrogenesis. Developmental cell 45, 651–660 e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Raible DW, 2009. Signaling pathways regulating zebrafish lateral line development. Curr Biol 19, R381–386. [DOI] [PubMed] [Google Scholar]
- McCampbell KK, Springer KN, Wingert RA, 2015. Atlas of Cellular Dynamics during Zebrafish Adult Kidney Regeneration. Stem Cells Int 2015, 547636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor AE, Golan M, Massasa EE, Lemze D, Weizman T, Shenhav R, Baydatch S, Mizrahi O, Winkler R, Golani O, Stern-Ginossar N, Itzkovitz S, 2017. Global mRNA polarization regulates translation efficiency in the intestinal epithelium. Science 357, 1299–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WH, Ledin J, Grandel H, Neumann CJ, 2005. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development 132, 4963–4973. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, Amaya E, 2001. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev 15, 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LL, Combes AN, Short KM, Lindstrom NO, Whitney PH, Cullen-McEwen LA, Ju A, Abdelhalim A, Michos O, Bertram JF, Smyth IM, Little MH, McMahon AP, 2018. Wnt11 directs nephron progenitor polarity and motile behavior ultimately determining nephron endowment. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N, 2000. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochemical and biophysical research communications 277, 643–649. [DOI] [PubMed] [Google Scholar]
- Okazawa M, Murashima A, Harada M, Nakagata N, Noguchi M, Morimoto M, Kimura T, Ornitz DM, Yamada G, 2015. Region-specific regulation of cell proliferation by FGF receptor signaling during the Wolffian duct development. Developmental biology 400, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N, 2015. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol 4, 215–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek RL, Lu GH, Dahring TK, Batley BL, Connolly C, Hamby JM, Brown KJ, 1998. In vitro biological characterization and antiangiogenic effects of PD 166866, a selective inhibitor of the FGF-1 receptor tyrosine kinase. The Journal of pharmacology and experimental therapeutics 286, 569–577. [PubMed] [Google Scholar]
- Park WY, Miranda B, Lebeche D, Hashimoto G, Cardoso WV, 1998. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Developmental biology 201, 125–134. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M, 2005. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132, 3859–3871. [DOI] [PubMed] [Google Scholar]
- Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM, 2006. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Developmental biology 291, 325–339. [DOI] [PubMed] [Google Scholar]
- Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D, 1999. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126, 547–554. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M, 1998. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrainhindbrain boundary development and somitogenesis. Development 125, 2381–2395. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R, 2001. A fish model of renal regeneration and development. ILAR journal / National Research Council, Institute of Laboratory Animal Resources 42, 285–291. [DOI] [PubMed] [Google Scholar]
- Roussigne M, Wei L, Tsingos E, Kuchling F, Alkobtawi M, Tsalavouta M, Wittbrodt J, Carl M, Blader P, Wilson SW, 2018. Left/right asymmetric collective migration of parapineal cells is mediated by focal FGF signaling activity in leading cells. Proc Natl Acad Sci U S A 115, E9812–E9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Beer K, Iwanov K, Schmohl F, Beckmann PI, Schroder R, 2015. The single fgf receptor gene in the beetle Tribolium castaneum codes for two isoforms that integrate FGF8- and Branchless-dependent signals. Developmental biology 402, 264–275. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Petersen LF, Amaya E, 2005. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Developmental cell 8, 689–701. [DOI] [PubMed] [Google Scholar]
- Song YH, Zhu YT, Ding J, Zhou FY, Xue JX, Jung JH, Li ZJ, Gao WY, 2016. Distribution of fibroblast growth factors and their roles in skin fibroblast cell migration. Mol Med Rep 14, 3336–3342. [DOI] [PubMed] [Google Scholar]
- Sun J, Stathopoulos A, 2018. FGF controls epithelial-mesenchymal transitions during gastrulation by regulating cell division and apicobasal polarity. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanhart LM, Takahashi N, Jackson RL, Gibson GA, Watkins SC, Dawid IB, Hukriede NA, 2010. Characterization of an lhx1a transgenic reporter in zebrafish. The International journal of developmental biology 54, 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Iyer S, Lobbardi R, Moore JC, Chen H, Lareau C, Hebert C, Shaw ML, Neftel C, Suva ML, Ceol CJ, Bernards A, Aryee M, Pinello L, Drummond IA, Langenau DM, 2017. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J Exp Med 214, 2875–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B, Heyer V, Lux A, Alunni V, Degrave A, Seiliez I, Kirchner J, Parkhill JP, Thisse C, 2004. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods in cell biology 77, 505–519. [DOI] [PubMed] [Google Scholar]
- Trueb B, Amann R, Gerber SD, 2013. Role of FGFRL1 and other FGF signaling proteins in early kidney development. Cell Mol Life Sci 70, 2505–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright EN, Wilhelm D, Combes AN, Little MH, Koopman P, 2015. ROBO2 restricts the nephrogenic field and regulates Wolffian duct-nephrogenic cord separation. Developmental biology 404, 88–102. [DOI] [PubMed] [Google Scholar]
- Walker KA, Sims-Lucas S, Bates CM, 2016. Fibroblast growth factor receptor signaling in kidney and lower urinary tract development. Pediatr Nephrol 31, 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lin H, Zhao T, Huang S, Fernig DG, Xu N, Wu F, Zhou M, Jiang C, Tian H, 2017. Expression and purification of an FGF9 fusion protein in E. coli, and the effects of the FGF9 subfamily on human hepatocellular carcinoma cell proliferation and migration. Applied microbiology and biotechnology 101, 7823–7835. [DOI] [PubMed] [Google Scholar]
- Willems E, Leyns L, Vandesompele J, 2008. Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem 379, 127–129. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Miyakawa N, Miyake A, Itoh N, 2009. Fgf4 is required for left-right patterning of visceral organs in zebrafish. Developmental biology 332, 177–185. [DOI] [PubMed] [Google Scholar]
- Yang X, Steinberg F, Zhuang L, Bessey R, Trueb B, 2016. Receptor FGFRL1 does not promote cell proliferation but induces cell adhesion. Int J Mol Med 38, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM, 2004. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Developmental biology 276, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F, 2010. Characterization of mesonephric development and regeneration using transgenic zebrafish. American journal of physiology. Renal physiology 299, F1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. PCR primers used.
Supplemental Figure 1. Dusp6 expression in nephrogenic cell aggregates. (A) Immunostaining with anti-Dusp6 (red) co-localizes with anti-GFP immunostaining of the lhx1a:eGFP transgene (B) at 7 dpi. (C) Merged image of A and B. Dotted line represents outline of adjacent distal tubule. Scale bars are 10 μm. (D) Injury-induced expression of dusp6 is reduced by PD166866 (added to tank water from 1 dpi to 7 dpi) to sham injured levels. qPCR represents N=4 fish per condition. All comparisons not significant by Kruskal-Wallis test.
Supplemental Figure 2. RT-PCR screen of FGF family genes in regenerating kidneys. (A) RT-PCR analysis of FGF ligand and receptor gene expression reveals additional changes in gene expression following injury as well as many Fgf family member mRNAs that remain unchanged after AKI. (B) Dendrogram of the FGF ligands indicating genes that were injury induced (green), repressed (red), or unchanged (yellow). (C) Dendrogram of the FGF receptors indicating genes that were injury induced (green), repressed (red), or unchanged (yellow). Fgf family genes that showed no kidney expression in the RT-PCR screen are not shown.
Supplemental Figure 3. FGF signaling is required for aggregation of cells into nephrogenic condensates. (A) Nephrogenic aggregates are apparent in histological sections as compact basophilic structures adjacent to distal tubules at 7 dpi. Arrows indicate nascent nephrons. (B) FGF inhibition with PD166866 post-injury prevents the formation of nephrogenic aggregates, and no peri-tubular basophilic cellular aggregates can be found in histological sections. Scale bars are 50μm.
Supplemental Figure 4. Channel separated and merged image of figure 4B. To better resolve nuclei and GFP+ cell scale, Figure 4B is represented as separate color channels DAPI, eGFP and the merged image.
Supplemental Figure 5. Characterization of kidney tissue fractions by qRTPCR. (A) qRTPCR analysis of single cell and tubule tissue fraction RNA shows an average 34.9 fold enrichment in gata1 expression in single cells over tubule fractions. (B) qRTPCR analysis revealed an average 5.1 fold enrichment of cdh17 expression in tubule fraction RNA. Each data point indicates a comparison of one replicate’s single cell fraction against its corresponding tubule fraction from the same cell isolation. Statistical comparisons were performed by Mann-Whitney test, ** indicates p=0.0022 in both comparisons.