Abstract
Implantable neurostimulation devices provide a direct therapeutic link to the nervous system and can be considered brain-computer interfaces (BCI). Under this definition, BCI are not simply science fiction, they are part of existing neurosurgical practice. Clinical BCI are standard of care for historically difficult to treat neurological disorders. These systems target the central and peripheral nervous system and include Vagus Nerve Stimulation, Responsive Neurostimulation, and Deep Brain Stimulation. Recent advances in clinical BCI have focused on creating “closed-loop” systems. These systems rely on biomarker feedback and promise individualized therapy with optimal stimulation delivery and minimal side effects. Success of clinical BCI has paralleled research efforts to create BCI that restore upper extremity motor and sensory function to patients. Efforts to develop closed loop motor/sensory BCI is linked to the successes of today’s clinical BCI.
Introduction
Reconsidering Brain-Computer Interfaces
Brain-Computer Interfaces, or BCI, may be a popular topic in science fiction, but there are implantable devices available today that operate within this exact paradigm. These devices allow us to read from (record) and write to (stimulate) the nervous system and have become invaluable tools in treating a wide range of neurological diseases. They target central and peripheral structures, including the vagus nerve[1–5], and the cortical[6–9] and subcortical[10] structures of the brain. BCI are well established treatment options for movement disorders[11,12], pain[13,14], and epilepsy[5,8,10]. Research with the first few generations of implantable devices has led to insights into the relationship between neural recordings and clinical states - opening the door to new and promising applications including memory disorders[15,16], neuropsychiatric conditions[17,18], stroke rehabilitation[19], and chronic pain[20].
The most advanced clinical BCI operate in “closed-loop” fashion, i.e. the device employs internal read-response feedback mechanisms. Table 1. Ideally, neuromodulation devices would sense the patient’s physiology and stimulate only when there is a therapeutic need, thus minimizing side effects while maintaining adequate levels of treatment. To accomplish this, closed-loop BCI rely on “biomarkers” that can be algorithmically identified and reliably indicate the need for therapeutic treatment. For example, in cardiac implantable defibrillators, the biomarker that triggers defibrillation is a shockable cardiac rhythm; in the NeuroPace RNS system the biomarker is neural synchronization or increased neural activity[7]. In Parkinson’s disease, a robust biomarker has yet to be identified, but is under active investigation[21,22]. Additionally, these closed-loop systems could yield significant power savings, reducing battery-replacement surgeries, and thus lowering risks of surgical infection, hardware damage, and exposure to anesthesia[23]. Table 2. Such systems express a true interface between the brain and computer and have had a profound impact on clinical approaches to traditionally difficult disorders of the nervous system.
Table 1.
Summary of available and investigational clinical BCI systems.
| Treatment | Modality | Approval Status | Indication |
|---|---|---|---|
| Vagus Nerve Stimulation (VNS) | |||
| Vagus Nerve Stimulation | Open-loop | 1997: FDA approval | 1997: Epilepsy 2005: Depression |
| AspireSR™ VNS with Cardiac Based Detection Algorithm |
Closed-loop | 2015: FDA approval | 2015: Epilepsy |
| Deep Brain Stimulation (DBS) | |||
| Deep Brain Stimulation | Open-loop | 1997: FDA approval | 1997: Tremor 2002: Parkinson’s Disease 2003: Dystonia (HDE) 2009: OCD (HDE) 2018: Epilepsy |
| Activa® PC+S DBS with sensing and closed-loop capability |
Closed-loop | 2013: Investigational device | |
| Activa® RC+S Rechargeable closed-loop DBS |
Closed-loop | 2019: Clinical trials anticipated | |
| Responsive Neurostimulation (RNS) | |||
| Neuropace Responsive NeuroStimulator |
Closed-loop | 2013: FDA approval | 2013: Epilepsy |
Table 2.
Reported energy savings for experimental closed-loop systems
| Study | Device | Energy Savings | Control System |
|---|---|---|---|
| Swann et al. | DBS Activa® PC+S Patient 1 |
38% energy saved compared to open loop | Nexus D3 (external) |
| 45% energy saved compared to open loop | Nexus E (internal) | ||
| DBS Activa® PC+S Patient 2 |
39% energy saved compared to open loop | Nexus E (internal) | |
| Little et al. | DBS | Adaptive: 132 μW Continuous: 270 μW 51% less energy compared to continuous |
External |
| Molina et al. | RNS (Tourette syndrome) | Open loop duty cycle: 0.8 years battery Closed loop: 2.45 years battery 206% longer battery life compared to open loop duty cycle |
RNS300M (internal) |
This review summarizes the state-of-the-art in clinically-relevant, implantable BCI, and identifies trends in the design and use of these devices. Clinical BCI research is in a period of substantial growth, as improvements across the first generations of implantable devices have led to advances in the understanding of neurological disorders, opportunities for new applications, and improved treatment mechanisms for existing applications. This growth is fueled by parallel efforts in rehabilitation-oriented BCI, that enable physically disabled persons to operate robotic prostheses or other assistive devices. Clinical and rehabilitation BCI operate under the same basic principles (sensors, algorithms, and actuation), use very similar hardware and software, and exhibit a similar trend toward increasingly closed-loop operation. As discussed in this review, these systems and their respective development are fundamentally intertwined. Taking a broad perspective approach to the field of BCI research, this review will provide the context for understanding the most promising developments and pressing challenges in BCI research.
Vagus Nerve Stimulation
Vagus nerve stimulation (VNS) for the adjunctive treatment of medically refractory epilepsy received FDA approval in 1997[4]. The first-generation VNS Therapy® device (from LivaNova, London, UK; previously Cyberonics, Houston, Texas) delivered electrical stimulation to the left vagus nerve in chronic, intermittent, on-and-off periods[2]. Patients could also use a magnetic actuator to trigger additional stimulation during obvious seizure activity or temporarily cease stimulation to quell potential side effects such as hoarseness[3]. Nearly half of all patients self-reported benefit from manual magnetic actuation, with 28% noting decreased seizure intensity and duration[24].
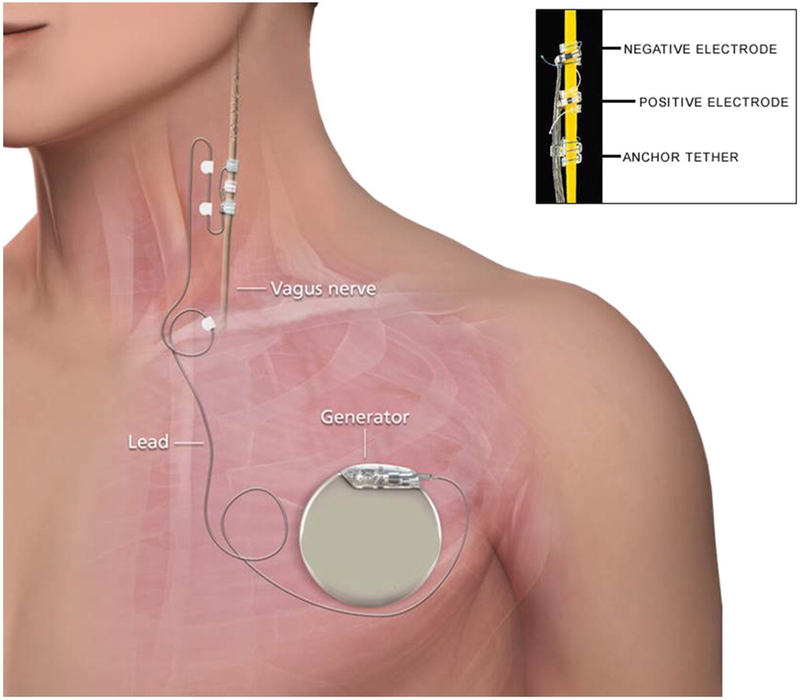
The most recent advance in VNS therapy has been the development of a closed-loop device using a cardiac biomarker. The presence of sinus tachycardia has been associated with epileptic events in up to 82% epilepsy patients[25]. A cardiac-based seizure detection algorithm (CBDA) in the latest VNS device, AspireSR®[24], can detect ictal tachycardia from a ECG vector between VNS leads and a stimulation generator implanted in the chest[26], Figure 1. This method of responsive stimulation offers an additional ≥50% seizure reduction to approximately 70% of patients who previously had open-loop devices[27].
Figure 1.
Schematic of vagus nerve stimulation device placement. For cardiac based closed loop, an ECG vector is read from the generator to the negative electrode. From 26; with permission.
VNS has also been applied to other neurological disorders. After mood improvements were observed in epilepsy patients being treated with VNS, the therapy received FDA approval in 2005 as a depression treatment[5,28]. VNS is also being studied as a potential way to accelerate post stroke rehabilitation by stimulating neuroplasticity[5,29].
Responsive Neurostimulation
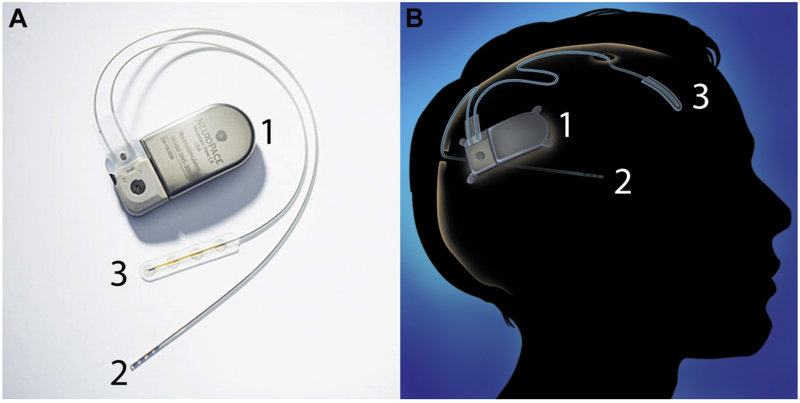
The RNS System from NeuroPace is a true closed-loop device for the treatment of medically refractory epilepsy[6]. Figure 2. Responsive Neurostimulation (RNS) operates by recording brain activity to “listen” for epileptiform activity, and subsequently stimulating at the foci to disrupt the seizure. In 1999, Lesser et al. demonstrated that cortical stimulation could interrupt epileptic activity[30]. The most recent closed-loop RNS device received FDA approval in November 2013[8]. It has gained traction as a viable treatment option for cases where surgical resection is not advised due to multiple foci or localization of foci to eloquent cortex[7].
Figure 2.
(A) Neuropace RNS system with one surface and one depth lead. (B) Schematic of RNS implantation. The RNS device is implanted in the skull and connected to depth or surface electrodes that can be used to sense and stimulate. Each lead has 4 electrode contacts. From 7; with permission.
As the first closed-loop neuromodulation device, there has been substantial interest in RNS for applications beyond epilepsy. Molina et al. used dual RNS systems to stimulate the centromedian-parafascicular thalamus for treatment of Tourette Syndrome. The FDA-approved RNS-300M was configured to provide closed-loop stimulation based on the detection of a patient specific biomarker. This proof of concept study showed significant clinical benefit compared to the open-loop stimulation. Furthermore, due to more efficient use of energy, projected battery life in the closed-loop system was 2.45 years compared to 0.8 years for open loop duty cycle stimulation[31].
The NeuroPace RNS System was the first example of a closed-loop clinical BCI. The successful debut of this device has arguably spurred the neurostimulation market towards more closed-loop devices.
Deep Brain Stimulation
Deep brain stimulation (DBS) has been available clinically since 1997 when the Medtronic Activa™ Tremor Control System was approved for the treatment of tremor[32]. This device delivers stimulation to targeted areas of the brain in an open-loop manner, and stimulation parameters are optimized in a trial-and-error method. DBS has proven effective for treating a variety of motor symptoms of Parkinson’s Disease (PD) and has been used safely in more than 100,000 patients worldwide to treat PD and other movement disorders[33,34].
Progress has focused on expanding indications for open-loop DBS beyond tremor. PD was approved as an indication in 2002[35], dystonia in 2003[36], obsessive-compulsive disorder in 2009[37], and epilepsy in 2018[10,38]. Additional clinical trials and research programs have evaluated DBS for treatment resistant depression[39–41].
As effective as DBS is, side effects include visual changes, facial twitching, dysphonia, and unintended motor movements due to current spreading around the tightly-spaced anatomical stimulation targets. Because DBS systems are open-loop (always on) patients frequently receive stimulation when treatment is not required. One advantage of closed-loop is selective stimulation; ideally, the DBS system would read markers of a heightened disease state and apply stimulation during times of greatest clinical need, minimizing side effects.
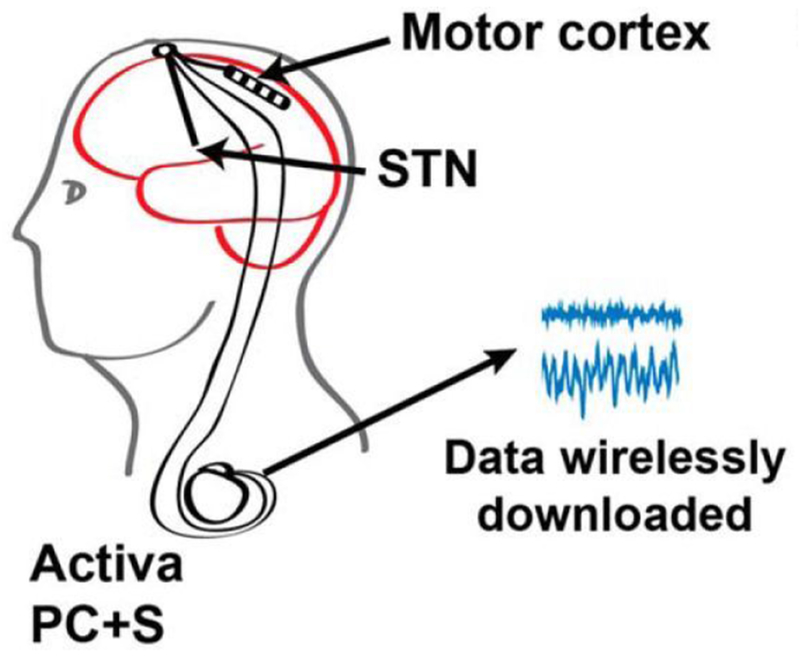
Such adaptive, closed-loop stimulation for PD are in active research and development[23,42,43]. Little et al. used a threshold on beta-band power from the subthalamic nucleus (STN) to trigger stimulation and found significant power savings and better movement scores with less stimulation[43]. This method also reduced stimulation-induced speech side effects[23]. Building on these results, Medtronic subsequently created the Activa® PC+S system. This device can record local field potentials (LFPs) and respond with stimulation[44,45]. The design of this investigational device underscores one of the challenges of a general-use closed-loop DBS system: there is no clear consensus on what the best biomarker is for each indication. This system provides a highly flexible system to design and test solutions. Swann et al. used gamma oscillation in the motor cortex as a control signal and demonstrated 38%-45% energy savings and no worsening of symptoms compared to open-loop stimulation[21,46]. Figure 3.
Figure 3.
Schematic of Activa® PC+S closed-loop system. Gamma oscillation recorded from the motor cortex was used as a control signal for closed-loop stimulation in the STN.From 46; with permission.
The next generation Medtronic device is the Activa® RC+S, which has a rechargeable battery[47]. With investigational devices allowing physicians to customize closed-loop systems for a variety of indications, clinically useful closed-loop DBS will likely soon emerge as a viable treatment tool.
Motor BCI
Unlike the clinical BCI discussed so far, which aim to control symptoms of neurological conditions, motor BCI are focused on producing physical or virtual movement. Motor BCI have the potential to restore independence to patients who have lost function as the result of a limb loss, spinal cord injury, stoke, or amyotrophic lateral sclerosis[48]. These systems are an important parallel to clinical BCI systems, because advances in rehabilitation BCI are often directly applicable to clinical BCI.
Capturing the complex movements of the upper extremity necessitates signal with high spatiotemporal resolution. While less invasive recording modalities including electroencephalography and electrocorticography have been used in research BCI systems[49–51], the 4 mm x 4 mm 96 channel Blackrock Microsystems (Salt Lake City, Utah) microelectrode array (MEA) has been the recording modality of choice for human motor BCI work[52]. This array was used for the first human trial of motor BCI, led by the BrainGate group in 2004. This initial BCI system was implanted in primary motor cortex (M1) of a patient with a spinal cord injury. The signals recorded were used to control a ‘neural cursor’ that was used to perform a number of tasks including controlling a robotic limb[53]. Since this initial trial, MEAs have been used in both M1 and posterior parietal cortex (PPC) to control motor BCI capable of increasingly complex tasks[54,55].
Carrying out complex tasks requires capturing the appropriate signal and using it to control an effector. Effectors can be virtual, physical, or even autologous. For those with severe neurological injury BCI optimized virtual keyboards restore the ability to communicate. Milekovic et al. demonstrated stable communication BCI for up to four and a half months for patients with ALS and locked-in syndrome using recordings from M1[56].
Physical effectors for upper extremity restoration include the LUKE arm from DEKA Integrated Solutions Corporation (Manchester, New Hampshire) and the newer modular prosthetic limb (MPL) from the Johns Hopkins University Applied Physics Laboratory[48]. Both of these limbs have been used as part of BCI systems using recordings from M1. The LUKE arm was used in the BrainGate2 trial and used M1 recordings to accomplish three-dimensional reach and grasp[54]. The MPL was incorporated into a motor BCI by Collinger et al that achieved 7 degrees of freedom[57]. The MPL was designed with 17 degrees of freedom and sensors that can detect force, vibration, temperature, and contact, providing opportunity for future motor BCI to accomplish increasingly complex tasks[58].
Control of an exoskeleton is another effector option for patients. One such system is the CLINATEC BCI platform, which uses a 4-limb exoskeleton[59]. This system was designed to use recordings from WIMAGINE— bilateral epidural wireless ECoG implants. This system features a 64 channel array that is completely integrated into a fully implantable wireless device[60]. Preclinical data has demonstrated control of the exoskeleton arm using WIMAGINE implants in non-human primates[59].
For certain neurological injuries, restoration of motor function to a patient’s own limb can be achieved through the use of functional electrical stimulation (FES). Much like motor BCI, motor intent is decoded from implanted MEAs. This signal is then used to deliver electrical stimulation to the native limb, producing movement. FES has been incorporated into motor BCI systems using both invasive and noninvasive electrodes, and is has been shown to restore reaching and grasping to the paralyzed limb[61].
Effector choices make motor BCI systems adaptable to the patient’s specific condition and allow for optimal restoration of function. For example, a robotic limb might be the best effector for brushing your teeth, but using it to type out an email would be more cumbersome than using a virtual keyboard.
Sensory BCI: Next steps for closed-loop BCI
Sensory feedback is a critical component of how we interact with the world and perform vital motor functions. Closed-loop motor/sensory BCI aim to provide patients with the most intuiative experience possible. Unlike clinical BCI systems discussed so far, which read neural signals to provide feedback in a closed-loop system, sensory BCI rely on delivering neural signals in the form of electrical impulses to primary somatosensory cortex. Figure 4.
Figure 4.
Diagram of a closed-loop motor/sensory BCI. Signals from M1 are used to control a robotic limb with sensors that are used to provide sensory feedback to S1. The feedback is used to direct further movement of the limb.From 65; with permission.
Non-human primate work has shown that closing the loop with sensory stimulation improves performance in motor BCI[62,63]. However, this work provides little insight into the location and quality of the sensation, which will be a critical part of a closed-loop sensory/motor BCI. Work in human subjects has begun to elucidate the best methods for providing useful sensation in closed-loop upper extremity BCI.
Blackrock MEAs are the recording modality of choice in motor BCI, and have been evaluated for sensory BCI in the upper extremity. Flesher et al[64] evaluated the organization and quality of sensations delivered to the upper extremity using two chronically implanted 32-channel MEAs. Salas et al[65] also evaluated upper extremity sensation using two 48-channel MEAs. Providing sensory feedback for a motor BCI effector like a robotic limb would require access to the entire somatopy of the upper extremity. The MEAs in these studies provided useful sensations, but not complete coverage. Lee at al[66] evaluated the utility of 64-channel mini-ECoG grids for sensory BCI. Like the MEA studies, they elicited reliable and reproducible percepts, but also found that the larger surface area of the mini-ECoG grid could potentially provide more complete somatosensation to the hand.
A complete motor/sensory BCI holds promise to restore upper extremity function in a more naturalistic and intuitive way than motor BCI alone. However, for these systems to become a reality, further work needs to be done to understand the best way to provide relevant sensation. Incorporating this early work into motor BCI systems is the first step towards the BCI of tomorrow.
Special Considerations and Barriers
Although the prospect of intelligent closed-loop motor/sensory BCI is promising, there are many clinical, technological, and ethical considerations. Complex systems like RNS, DBS, and newer SCS are significantly more difficult to program than an open-loop system, often requiring specialized centers. This can limit availability to patients and slow widespread adoption. Fully implantable systems with sufficient computing power, battery longevity, and signal stability are not yet feasible.
The MEAs currently used in motor BCI research present several barriers to long lasting systems. While penetrating electrodes provide the highest quality signal for BCI, the penetrating nature of these devices causes an inflammatory response that degrades the signal over time [67]. While degradation may begin as early as 6-12 months after implant, useful signals, including both LFPs and action potentials, may still be recorded for several years [68]. Removal of MEAs requires a craniotomy and can be complicated by the extent of gliosis and encapsulation. Tackling the issue of longevity is essential for these systems to become a viable treatment for chronic neurological diseases.
Ongoing electrode research aims to find the ideal biologic, mechanical, and material approach for neural implants. Elastic hydrogel electrodes have the potential to record and stimulate while producing less inflammation, owing to their mechanical similarity to tissue[69]. Other electrodes adapt to become less rigid after implantation, creating less chronic inflammation[70]. Biologic approaches aim to control the inflammatory response with systemic or local agents including dexamethasone, neural adhesion molecule L1, anti-oxidants, and astrocyte derived extracellular matrix[71].
Conclusion
Since the introduction of the earliest devices designed to constantly deliver electrical stimulation to disrupt essential tremor, there have been substantial advances in the hardware and software underlying BCI devices, making science fiction a reality. New knowledge gleaned from academic research supports the development of more principled approaches to therapy, such as providing electrical stimulation only when needed. The key insight in the most successful devices has been the identification of biomarkers. By adapting the therapy to the disorder, side effects are reduced, benefits improve, and devices last much longer between battery-replacement surgeries.
BCI have become an integral part of the standard of care for neurological disorders which have been historically challenging to treat. With these initial successes, it is likely that funding, research, and product development will continue at increasing rates. Clinicians, scientists, engineers, and patients all play integral roles in developing and using BCI. Interdisciplinary teams which take advantage of expertise from each of these contributors will find the most success in improving today’s implantable device to build tomorrow’s BCI.
Highlights.
Clinical BCI are standard practice for historically difficult to treat conditions
Development of closed-loop systems requires the discovery of reliable biomarkers
Future BCI will include closed-loop devices for expanded neurological indications
Future motor BCI will incorporate sensory feedback, creating a closed-loop system
The development of motor BCI is linked to the success of clinical BCI
Acknowledgments
Funding:
We wish to acknowledge the generous support of National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (KL2TR001854) and the Neurosurgery Research and Education Foundation (NREF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Uthman BM, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reid SA, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology 1993;43:1338–45. [DOI] [PubMed] [Google Scholar]
- [2].Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia 1994;35:616–26. [DOI] [PubMed] [Google Scholar]
- [3].Tatum WO, Helmers SL, Tatum WO IV, Helmers SL. Vagus nerve stimulation and magnet use: Optimizing benefits. Epilepsy Behav 2009;15:299–302. doi: 10.1016/j.yebeh.2009.04.002. [DOI] [PubMed] [Google Scholar]
- [4].Ogbonnaya S, Kaliaperumal C. Vagal nerve stimulator: Evolving trends. J Nat Sci Biol Med 2013;4:8–13. doi: 10.4103/0976-9668.107254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 2018;11:203–13. doi: 10.2147/JIR.S163248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Morrell MJ, RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- [7].Lee B, Zubair MN, Marquez YD, Lee DM, Kalayjian LA, Heck CN, et al. A Single-Center Experience with the NeuroPace RNS System: A Review of Techniques and Potential Problems. World Neurosurg 2015;84:719–26. doi: 10.1016/j.wneu.2015.04.050. [DOI] [PubMed] [Google Scholar]
- [8].Sun FT, Morrell MJ. The RNS System: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev Med Devices 2014;11:563–72. doi: 10.1586/17434440.2014.947274. [DOI] [PubMed] [Google Scholar]
- [9].Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 2014;55:432–41. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015;84:1017–25. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lyons KE, Pahwa R. Deep brain stimulation and essential tremor. J Clin Neurophysiol n.d.;21:2–5. [DOI] [PubMed] [Google Scholar]
- [12].Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA J Am Med Assoc 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132:179–88. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- [14].Deer T, Slavin KV, Amirdelfan K, North RB, Burton AW, Yearwood TL, et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation 2018;21:56–66. doi: 10.1111/ner.12698. [DOI] [PubMed] [Google Scholar]
- [15].Laxton AW, Lipsman N, Lozano AM. Deep brain stimulation for cognitive disorders. Handb Clin Neurol 2013;116:307–11. doi: 10.1016/B978-0-444-53497-2.00025-5. [DOI] [PubMed] [Google Scholar]
- [16].Ezzyat Y, Wanda PA, Levy DF, Kadel A, Aka A, Pedisich I, et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Commun 2018;9:365. doi: 10.1038/s41467-017-02753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Almeida L, Martinez-Ramirez D, Rossi PJ, Peng Z, Gunduz A, Okun MS. Chasing tics in the human brain: Development of open, scheduled and closed loop responsive approaches to deep brain stimulation for tourette syndrome. J Clin Neurol 2015;11:122–31. doi: 10.3988/jcn.2015.11.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hariz M, Blomstedt P, Zrinzo L. Future of brain stimulation: new targets, new indications, new technology. Mov Disord 2013;28:1784–92. doi: 10.1002/mds.25665. [DOI] [PubMed] [Google Scholar]
- [19].Sharififar S, Shuster JJ, Bishop MD. Adding electrical stimulation during standard rehabilitation after stroke to improve motor function. A systematic review and meta-analysis. Ann Phys Rehabil Med 2018. doi: 10.1016/j.rehab.2018.06.005. [DOI] [PubMed] [Google Scholar]
- [20].Shirvalkar P, Veuthey TL, Dawes HE, Chang EF. Closed-Loop Deep Brain Stimulation for Refractory Chronic Pain. Front Comput Neurosci 2018;12:18. doi: 10.3389/fncom.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng 2018;15:046006. doi: 10.1088/1741-2552/aabc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ravina B, Tanner C, Dieuliis D, Eberly S, Flagg E, Galpern WR, et al. A longitudinal program for biomarker development in Parkinson’s disease: a feasibility study. Mov Disord 2009;24:2081–90. doi: 10.1002/mds.22690. [DOI] [PubMed] [Google Scholar]
- [23].Little S, Tripoliti E, Beudel M, Pogosyan A, Cagnan H, Herz D, et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry 2016;87:1388–9. doi: 10.1136/jnnp-2016-313518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fisher RS, Afra P, Macken M, Minecan DN, Bagic A, Benbadis SR, et al. Automatic Vagus Nerve Stimulation Triggered by Ictal Tachycardia: Clinical Outcomes and Device Performance-The U.S. E-37 Trial. Neuromodulation Technol Neural Interface 2016;19:188–95. doi: 10.1111/ner.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eggleston KS, Olin BD, Fisher RS. Ictal tachycardia: the head-heart connection. Seizure 2014;23:496–505. doi: 10.1016/j.seizure.2014.02.012. [DOI] [PubMed] [Google Scholar]
- [26].Verrier RL, Nearing BD, Olin B, Boon P, Schachter SC. Baseline elevation and reduction in cardiac electrical instability assessed by quantitative T-wave alternans in patients with drug-resistant epilepsy treated with vagus nerve stimulation in the AspireSR E-36 trial. Epilepsy Behav 2016;62:85–9. doi: 10.1016/j.yebeh.2016.06.016. [DOI] [PubMed] [Google Scholar]
- [27].Hamilton P, Soryal I, Dhahri P, Wimalachandra W, Leat A, Hughes D, et al. Clinical outcomes of VNS therapy with AspireSR® (including cardiac-based seizure detection) at a large complex epilepsy and surgery centre. Seizure 2018;58:120–6. doi: 10.1016/j.seizure.2018.03.022. [DOI] [PubMed] [Google Scholar]
- [28].Desbeaumes Jodoin V, Richer F, Miron J-P, Fournier-Gosselin M-P, Lespérance P. Long-term Sustained Cognitive Benefits of Vagus Nerve Stimulation in Refractory Depression. J ECT 2018. doi: 10.1097/YCT.0000000000000502. [DOI] [PubMed] [Google Scholar]
- [29].Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, et al. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 2018;49:710–7. doi: 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lesser RP, Kim SH, Beyderman L, Miglioretti DL, Webber WR, Bare M, et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology 1999;53:2073–81. [DOI] [PubMed] [Google Scholar]
- [31].Molina R, Okun MS, Shute JB, Opri E, Rossi PJ, Martinez-Ramirez D, et al. Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J Neurosurg 2017:1–7. doi: 10.3171/2017.6.JNS17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].FDA. Medtronic Activa Tremor Control System P960009. 1997. [Google Scholar]
- [33].Houston B, Blumenfeld Z, Quinn E, Bronte-Stewart H, Chizeck H. Long-term detection of Parkinsonian tremor activity from subthalamic nucleus local field potentials. Conf Proc . Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2015;2015:3427–31. doi: 10.1109/EMBC.2015.7319129. [DOI] [PubMed] [Google Scholar]
- [34].Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 2013;70:163–71. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- [35].FDA. Medtronic Activa® Parkinson’s Control Therapy P960009/S7. 2002. [Google Scholar]
- [36].FDA. Medtronic Activa® Dystonia Therapy - H020007. 2003. [Google Scholar]
- [37].FDA. Reclaim Deep Brain Stimulation for Obsessive Compulsive Disorder (OCD) Therapy. 2009. [Google Scholar]
- [38].FDA. Medtronic DBS Therapy For Epilepsy. 2018. [Google Scholar]
- [39].Clair A-H, Haynes W, Mallet L. Recent advances in deep brain stimulation in psychiatric disorders. F1000Research 2018;7. doi: 10.12688/f1000research.14187.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biol Psychiatry 2015;78:240–8. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- [41].Rao VR, Sellers KK, Wallace DL, Lee MB, Bijanzadeh M, Sani OG, et al. Direct Electrical Stimulation of Lateral Orbitofrontal Cortex Acutely Improves Mood in Individuals with Symptoms of Depression. Curr Biol 2018;28:3893–3902. e4. doi: 10.1016/j.cub.2018.10.026. [DOI] [PubMed] [Google Scholar]
- [42].Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, et al. Closed-Loop Deep Brain Stimulation Is Superior in Ameliorating Parkinsonism. Neuron 2011;72:370–84. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- [43].Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013;74:449–57. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Malekmohammadi M, Herron J, Velisar A, Blumenfeld Z, Trager MH, Chizeck HJ, et al. Kinematic Adaptive Deep Brain Stimulation for Resting Tremor in Parkinson’s Disease. Mov Disord 2016;31:426–8. doi: 10.1002/mds.26482. [DOI] [PubMed] [Google Scholar]
- [45].Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, et al. Design and Validation of a Fully Implantable, Chronic, Closed-Loop Neuromodulation Device With Concurrent Sensing and Stimulation. IEEE Trans Neural Syst Rehabil Eng 2012;20:410–21. doi: 10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- [46].Swann NC, de Hemptinne C, Miocinovic S, Qasim S, Wang SS, Ziman N, et al. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J Neurosci 2016;36:6445–58. doi: 10.1523/JNEUROSCI.1128-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ramirez-Zamora A, Giordano JJ, Gunduz A, Brown P, Sanchez JC, Foote KD, et al. Evolving applications, technological challenges and future opportunities in neuromodulation: Proceedings of the fifth annual deep brain stimulation think tank. Front Neurosci 2018;11:734. doi: 10.3389/fnins.2017.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee B, Attenello FJ, Liu CY, McLoughlin MP, Apuzzo MLJ. Recapitulating flesh with silicon and steel: Advancements in upper extremity robotic prosthetics. World Neurosurg 2014;81:730–41. doi: 10.1016/j.wneu.2014.03.012. [DOI] [PubMed] [Google Scholar]
- [49].Hinterberger T, Schmidt S, Neumann N, Mellinger J, Blankertz B, Curio G, et al. Brain-Computer Communication and Slow Cortical Potentials. IEEE Trans Biomed Eng 2004;51:1011–8. doi: 10.1109/TBME.2004.827067. [DOI] [PubMed] [Google Scholar]
- [50].Buttfield A, Ferrez PW, Millán J del R. Towards a robust BCI: error potentials and online learning. IEEE Trans Neural Syst Rehabil Eng 2006;14:164–8. doi: 10.1109/TNSRE.2006.875555. [DOI] [PubMed] [Google Scholar]
- [51].Pandarinath C, Nuyujukian P, Blabe CH, Sorice BL, Saab J, Willett FR, et al. High performance communication by people with paralysis using an intracortical brain-computer interface. Elife 2017;6. doi: 10.7554/eLife.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee B, Liu CY, Apuzzo MLJ. A primer on brain-machine interfaces, concepts, and technology: A key element in the future of functional neurorestoration. World Neurosurg 2013;79:457–71. doi: 10.1016/j.wneu.2013.01.078. [DOI] [PubMed] [Google Scholar]
- [53].Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006;442:164. [DOI] [PubMed] [Google Scholar]
- [54].Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012;485:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Aflalo T, Kellis S, Klaes C, Lee B, Shi Y, Pejsa K, et al. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 2015;348:906–10. doi: 10.1126/science.aaa5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Milekovic T, Sarma AA, Bacher D, Simeral JD, Saab J, Pandarinath C, et al. Stable long-term BCI-enabled communication in ALS and locked-in syndrome using LFP signals. J Neurophysiol 2018;120:343–60. doi: 10.1152/jn.00493.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 2013;381:557–64. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Armiger RS, Tenore FV, Katyal KD, Johannes MS, Makhlin A, Natter ML, et al. Enabling closed-loop control of the Modular Prosthetic Limb through haptic feedback. Johns Hopkins APL Tech Dig (Applied Phys Lab 2013;31:345–53. [Google Scholar]
- [59].Eliseyev A, Mestais C, Charvet G, Sauter F, Abroug N, Arizumi N, et al. CLINATEC® BCI platform based on the ECoG-recording implant WIMAGINE® and the innovative signal-processing: preclinical results. Conf Proc . Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2014;2014:1222–5. doi: 10.1109/EMBC.2014.6943817. [DOI] [PubMed] [Google Scholar]
- [60].Mestais CS, Charvet G, Sauter-Starace F, Foerster M, Ratel D, Benabid AL. WIMAGINE: wireless 64-channel ECoG recording implant for long term clinical applications. IEEE Trans Neural Syst Rehabil Eng 2015;23:10–21. doi: 10.1109/TNSRE.2014.2333541. [DOI] [PubMed] [Google Scholar]
- [61].Friedenberg DA, Schwemmer MA, Landgraf AJ, Annetta NV, Bockbrader MA, Bouton CE, et al. Neuroprosthetic-enabled control of graded arm muscle contraction in a paralyzed human. Sci Rep 2017;7:8386. doi: 10.1038/s41598-017-08120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].O’Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, et al. Active tactile exploration using a brain-machine-brain interface. Nature 2011;479:228–31. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Klaes C, Shi Y, Kellis S, Minxha J, Revechkis B, Andersen RA. A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback. J Neural Eng 2014;11:056024. doi: 10.1088/1741-2560/11/5/056024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-kabara EC, et al. Intracortical microstimulation of human somatosensory cortex Intracortical microstimulation of human somatosensory cortex. Sci Transl Med 2016:1–11. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- [65].Armenta Salas M, Bashford L, Kellis S, Jafari M, Jo H, Kramer D, et al. Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. Elife 2018;7:1–11. doi: 10.7554/eLife.32904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee B, Kramer D, Salas MA, Kellis S, Brown D, Dobreva T, et al. Engineering Artificial Somatosensation Through Cortical Stimulation in Humans 2018;12:1–11. doi: 10.3389/fnsys.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, et al. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE Trans Neural Syst Rehabil Eng 2003;11:186–8. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- [68].Ryu SI, Shenoy KV. Human cortical prostheses: lost in translation? Neurosurg Focus 2009;27:E5. doi: 10.3171/2009.4.FOCUS0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Liu Y, Liu J, Chen S, Lei T, Kim Y, Niu S, et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat Biomed Eng 2019;3:58–68. doi: 10.1038/s41551-018-0335-6. [DOI] [PubMed] [Google Scholar]
- [70].Nguyen JK, Park DJ, Skousen JL, Hess-Dunning AE, Tyler DJ, Rowan SJ, et al. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J Neural Eng 2014;11:056014. doi: 10.1088/1741-2560/11/5/056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Campbell A, Wu C. Chronically Implanted Intracranial Electrodes: Tissue Reaction and Electrical Changes. Micromachines 2018;9. doi: 10.3390/mi9090430. [DOI] [PMC free article] [PubMed] [Google Scholar]