Abstract
Hemorrhagic shock (HS) is a life-threatening condition associated with tissue hypoperfusion, and often leads to injury of multiple organs including the liver. Pregnane X receptor (PXR) is a species-specific xenobiotic receptor that regulates the expression of drug-metabolizing enzymes (DMEs) such as the cytochrome P450 3A (CYP3A). Many clinical drugs, including those often prescribed to trauma patients, are known to activate PXR and induce CYP3A. The goal of this study is to determine whether PXR plays a role in the regulation of DMEs in the setting of HS, and whether activation of PXR is beneficial or detrimental to HS-induced hepatic injury. PXR transgenic, knockout, and humanized mice were subject to HS, and the liver injury was assessed histologically and biochemically. The expression and/or activity of PXR and CYP3A were manipulated genetically or pharmacologically in order to determine their effects on HS-induced liver injury. Our results showed that genetic or pharmacological activation of PXR sensitized wild-type and hPXR/CYP3A4 humanized mice to HS-induced hepatic injury, whereas knockout of PXR protected mice from HS-induced liver injury. Mechanistically, the sensitizing effect of PXR activation was accounted for by PXR-responsive induction of CYP3A and increased oxidative stress in the liver. The sensitizing effect of PXR was attenuated by ablation or pharmacological inhibition of CYP3A, treatment with the antioxidant N-acetylcysteine amide (NACA), or treatment with a PXR antagonist.
Conclusions:
We have uncovered a novel function of PXR in HS-induced hepatic injury. Our results suggest that the unavoidable use of PXR-activating drugs in trauma patients has the potential to exacerbate HS-induced hepatic injury, which can be mitigated by the co-administration of anti-oxidative agents, CYP3A inhibitors, or PXR antagonists.
Keywords: Hemorrhagic shock, Hepatic injury, PXR, CYP3A, ROS
INTRODUCTION
Hemorrhagic shock (HS), a life-threatening condition when the body loses more than 20% of its blood or fluid supply, is the leading cause of death and disability in patients younger than 54 years of age (1). During the acute phase of hemorrhage, in addition to stopping bleeding, fluid resuscitation is a therapeutic priority in the clinic. Although the modern resuscitation techniques developed in the 1960s have led to an improved immediate survival, the emergence of subsequent organ failure remains a major health issue that may lead to late death (2). Among the mechanisms of HS-induced tissue injury, HS has been shown to trigger secondary tissue and organ injury including oxidative stress, which generates free radicals and leads to cellular membrane damage and cell death (3). The liver is an organ sensitive to HS-induced injury (4, 5). In the liver, the cytochrome P450 (CYP) enzymes in the hepatocytes represent an important source of reactive oxygen species (ROS), which may lead to mitochondrial dysfunction and cell necrosis upon HS (6). CYP3A is the most abundant hepatic P450. Although the effect of CYP3A on ROS generation might be substrate dependent, it is believed that CYP3A plays a major role in the overall ability of the human liver to generate ROS (7).
Pregnane X receptor (PXR) is a species specific xenobiotic receptor that regulates the expression of drug-metabolizing enzymes (DEMs) with CYP3A as its primary target gene (8). Seriously injured trauma patients are uniformly prescribed with multiple medications, including common ICU medications, such as fentanyl, protonix, dexamethasone (DEX) (9), benzodiazepines (10), and other medications, such as Tylenol and oxycodone for post-surgery pain management, statins and fibrates for trauma patients with hyperlipidemia, and the antibiotic rifampicin (RIF) for reducing deep surgical site infections (11, 12) or for the treatment of tuberculosis. Many of these drugs are known to activate PXR and induce the expression of CYP3A. For example, DEX and RIF are typical agonists that preferentially activate the mouse and human PXR, respectively (13-15). Besides regulating DMEs, activation of PXR has been shown to sensitize normal and cancerous tissues to oxidative cellular damage, but the mechanism is yet to be defined (16). Although the expression of PXR and DEMs has been reported to be dynamically regulated by HS (17), it is unclear whether and how PXR plays a role in the regulation of DMEs in the setting of HS and if so, whether activation of PXR is beneficial or detrimental to HS-induced hepatic injury.
In this study, we showed that activation of PXR sensitized both the wild-type and hPXR/CYP3A4 humanized mice to HS-induced hepatic injury in a CYP3A dependent manner. The sensitizing effect of PXR on HS-induced hepatic injury can be mitigated by inhibition of CYP3A, or treatment with an antioxidant, or a PXR antagonist.
EXPERIMENTAL PROCEDURES
Animals and hemorrhagic shock
The Pxr−/− (13), VP-PXR transgenic (13), and hPXR/CYP3A4 humanized mice (18) were previously described. VP-PXR/Cyp3a−/− mice were generated by breeding the VP-PXR transgene into the Cyp3a−/− background (19). Eight-week old mice were used for hemorrhagic shock. Under anesthesia, catheters were placed in both femoral arteries. To initiate HS, approximately 0.6 ml of blood were withdrawn using a syringe pump during which the blood pressure was maintained at approximately 25 mmHg. After 2-h of pressure controlled HS, mice were resuscitated using Lactated Ringers solution at a volume of 3x each animal’s shed blood volume given over a 15-min period using a syringe pump. In the sham control mice, the femoral arteries were exposed but not catheterized. Serum and tissue samples were collected 22 hours after resuscitation. The use of mice in this study complied with the relevant federal guidelines and institutional policies.
For histology, immunohistochemistry, and immunofluorescence, real-time PCR and Western blot analysis, serum chemistry, analysis of microsomal metabolism of midazolam and oxycodone, see Supplemental Materials and Methods for details.
Statistical analysis
Results are expressed as mean ± SE. Differences between two individual groups were determined by Student’s t test. Differences between multiple groups were evaluated using one-way analysis of variance followed by post-hoc multiple comparison according to the Student-Newman-Keuls test. Statistical significance was accepted at p < 0.05.
RESULTS
Genetic activation of PXR and Pxr ablation sensitize mice to and protect mice from HS-induced liver injury, respectively
To investigate the role of PXR in hepatic injury following hemorrhagic shock and resuscitation (HS/R), we established a 2-h fixed pressure model of HS/R in which mice were sacrificed 22 h after the resuscitation (20). As expected (4, 5), HS led to hepatic injury, as evidenced by histological necrosis (Supplementary Figure 1A), apoptosis of hepatocytes adjacent to the central vein (CV) (Supplementary Figure 1B) that was consistent with the peri-CV expression of Cyp3a11 (Supplementary Figure 1C), and increased serum level of alanine aminotransferase (ALT) (Supplementary Figure 1D). In addition, qRT-PCR results showed that the expression of Pxr and several of its target genes, including Cyp3a11, Cyp2b10, Cyp2d22 and Cyp2c29, was down-regulated (Supplementary Figure 1E). The down-regulation of Cyp3a11 was verified by Western blotting (Supplementary Figure 1F). The down-regulation of DMEs was also confirmed at the enzymatic level, because compared to the sham group, liver microsomes isolated from the HS group showed a decreased metabolism of sedative midazolam, a substrate of CYP3A (Supplementary Figure 1G) and analgesic oxycodone, a substrate of both CYP3A and CYP2D (Supplementary Figure 1H). Interestingly, we observed an intact expression of Pxr and a modest but significant increase in the expression of Cyp3a11 at the mRNA (Supplementary Figure 1I) and protein (Supplementary Figure 1J) levels in mice that were subject to a 2-h HS but without a resuscitation. These results suggested that the suppression of Pxr and Cyp3a in mice subject to HS/R may have been secondary to the liver injury; whereas the activation of PXR and induction of Cyp3a11 in the HS phase may have played a pathogenic role in the liver injury.
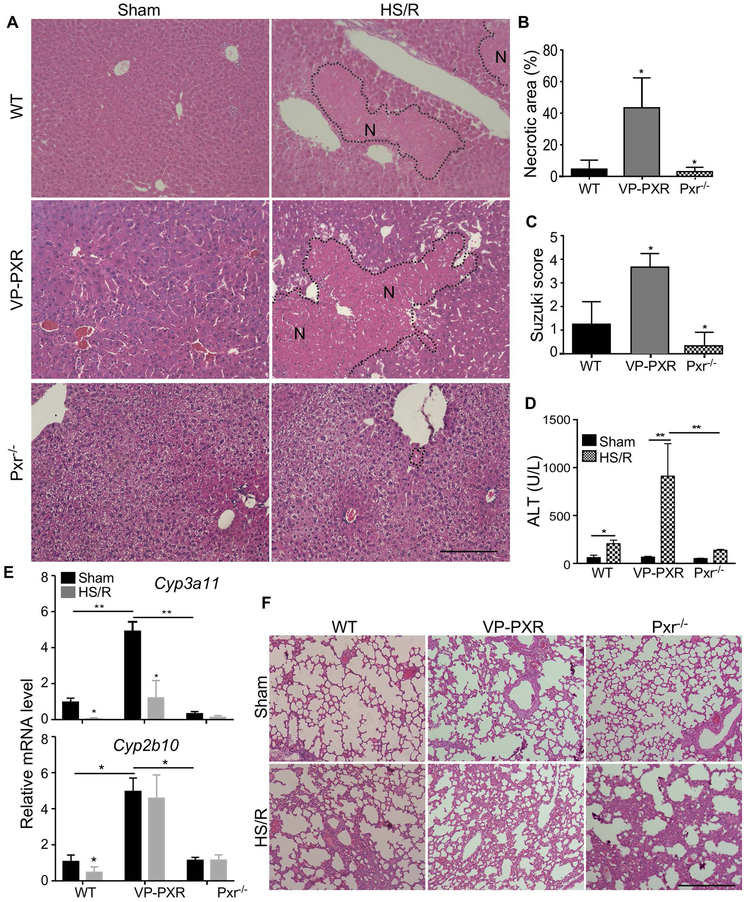
To determine the effect of PXR activation on HS-induced tissue injury, WT, Alb-VP-PXR transgenic, and Pxr−/− mice were subject to HS/R before being analyzed for their injury in the liver and lung, two tissues known to be sensitive to HS (4, 4, 21). The Alb-VP-PXR transgenic mice express the constitutively activated PXR (VP-PXR) under the control of the liver-specific albumin gene promoter (13). Wild type mice subject to HS/R showed hepatic injury, as shown by H&E staining (Figure 1A), quantification of necrotic area (Figure 1B), Suzuki score of the histology based on hydropic degeneration and steatosis (22) (Figure 1C), and the serum ALT level (Figure 1D). Compared to WT mice, VP-PXR transgenic mice and Pxr−/− mice showed heightened and attenuated hepatic injury, respectively (Figure 1A-1D). The expression of both Cyp3a11 and Cyp2b10 was induced in sham VP-PXR transgenic mice as expected, but only the expression Cyp3a11 was suppressed upon HS/R (Figure 1E). Interestingly, the effect of PXR on HS-induced tissue injury was liver-specific, because there were no appreciable differences in HS-induced lung injury, including interstitial edema and infiltration of cells into the interstitial and alveolar spaces, among the three genotypes (Figure 1F). Compared to the liver, the lung had a very low expression of either Pxr or Cyp3a11 (data not shown).
Figure 1. Genetic activation of PXR and Pxr ablation sensitize mice to and protect mice from HS-induced liver injury, respectively.
(A) WT, VP-PXR transgenic, and Pxr−/− mice subject to sham surgery or HS/R were analyzed for liver injury by H&E staining. The “N”s within the dotted circle indicate the necrotic areas. Bar is 100 μm. (B-E) Mice are the same as in (A). Shown are quantification of necrotic areas (B), Suzuki scores of the liver damage (C), and serum levels of ALT (D), and hepatic expression of Cyp3a11 and Cyp2b10 (E). (F) Mice in (A) were analyzed for lung injury by H&E staining. n=4~5 for each group. *, p < 0.05; **, p < 0.01, compared to the WT (B and C), or the comparisons are labeled (D and E).
Pharmacological activation of PXR, but not CAR, sensitizes mice to HS-induced hepatic injury
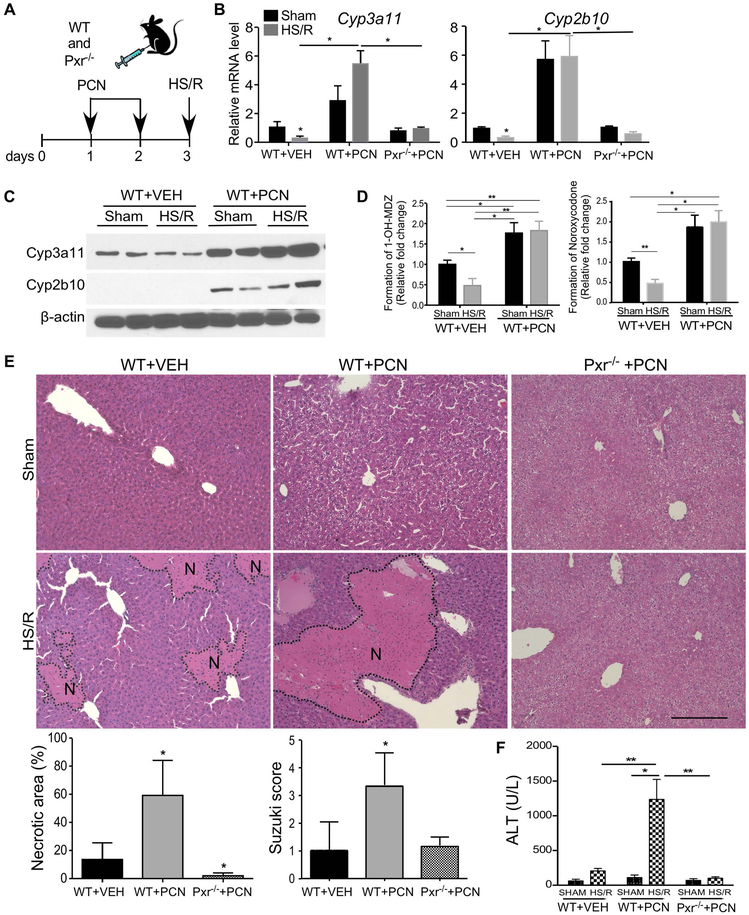
Since many clinical drugs, including those often prescribed to trauma patients, can activate PXR, we investigated whether a pharmacological activation of PXR can also sensitize mice to HS-induced hepatic injury. WT and Pxr−/− mice were pre-treated with PCN (40 mg/kg per day, i.p.) or DEX (20 mg/kg per day, i.p.) for two days before receiving the HS/R surgery. PCN is a prototypical mouse Pxr agonist, whereas DEX is a mouse-preferred Pxr agonist and a widely-prescribed glucocorticoid (13-15). When PCN was used as outlined in Figure 2A, treatment of WT mice with PCN induced the expression of Cyp3a11 and Cyp2b10 at the mRNA (Figure 2B), protein (Figure 2C), and enzymatic (Figure 2D) levels in the sham group. PCN also induced the expression of Cyp3a11 in the HS/R group, which overrode the suppressive effect of HS/R on Cyp3a11 (Figure 2B-2D). As expected, PCN had little effect on the expression of Cyp3a11 in Pxr−/− mice regardless of HS/R (Figure 2B). Consistent with the results from the VP-PXR transgenic mice, treatment with PCN also sensitized mice to HS-induced hepatic injury as shown by H&E staining, quantification of necrotic area, and Suzuki scores (Figure 2E), and the serum ALT level (Figure 2F). The sensitizing effect of PCN was abolished in Pxr−/− mice (Figure 2E and 2F). A similar pattern of Pxr-dependent sensitization was observed in mice pre-treated with DEX (Supplementary Figure 2).
Figure 2. Pharmacological activation of PXR sensitizes mice to HS-induced hepatic injury.
(A) Schematic representation of the PCN pre-treatment model. WT and Pxr−/− mice were intraperitoneally injected with PCN (40 mg/kg per day) for two days before receiving the sham surgery or HS/R. (B and C) The hepatic mRNA (B) and protein (C) expression of Cyp3a11 and Cyp2b10 were measured by qRT-PCR and Western blotting, respectively. (D) The liver microsomal productions of 1’-OH midazolam (left) and noroxycodone (right) were measured by UPLC-MS analysis. (E) Liver histology was analyzed by H&E staining. Bar is 100 μm. Shown below are quantification of necrotic areas and Suzuki scores. (F) Serum levels of ALT. n=4~5 for each group. *, p < 0.05; **, p < 0.01, the comparisons are labeled (B, D and F), or compared to the WT+VEH group (E).
The constitutive androstane receptor (CAR) is a sister xenobiotic receptor of PXR. To determine whether activation of CAR can also sensitize mice to HS-induced hepatic injury, WT mice were pre-treated with TCPOBOP (0.25 mg/kg, i.p.), a potent CAR agonist, for 3 days before the HS/R surgery (Supplementary Figure 3A). As expected, TCPOBOP induced the expression of Cyp2b10, a primary target gene of CAR (Supplementary Figure 3B). However, TCPOBOP had little effect on HS-induced hepatic injury, as shown by histology (Supplementary Figure 3C-3E). These results suggested that the sensitizing effect was PXR specific.
Induction of CYP3A is required for the sensitizing effect of PXR activation on HS-induced hepatic injury
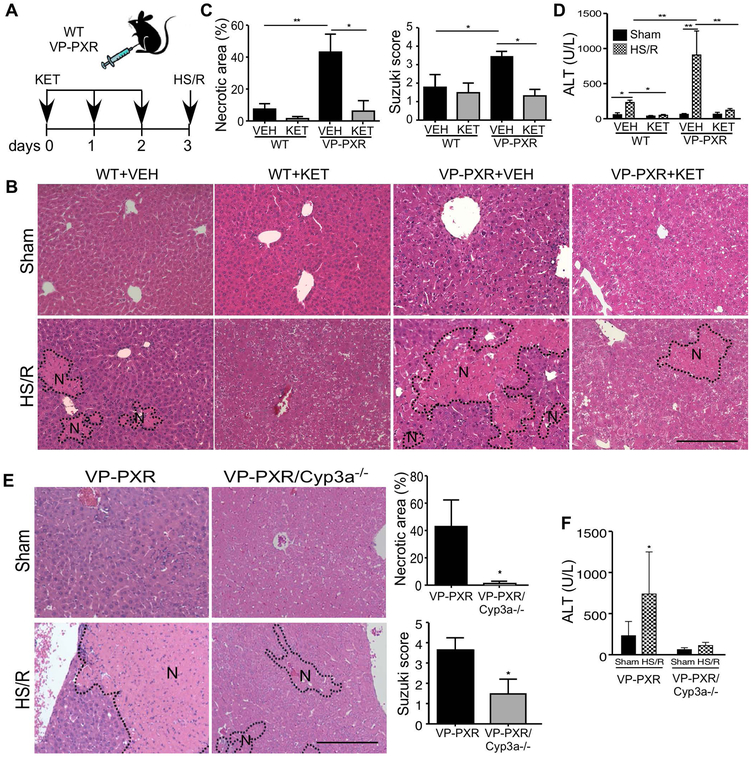
Since CYP3A is the primary target gene of PXR and a major source of ROS production that contributes to liver damage (6, 7), we went on to use CYP3A loss-of-function models to determine whether the expression and regulation of CYP3A were required for the heightened HS-induced hepatic injury in VP-PXR transgenic mice. The inhibition of CYP3A was achieved pharmacologically by treating mice with ketoconazole (KET) (23), or genetically by using the Cyp3a knockout (Cyp3a−/−) mice that bear the deletion of a cluster of mouse Cyp3a genes (19). In the pharmacological model, WT and VP-PXR transgenic mice were pre-treated with vehicle or KET (95 mg/kg per day, p.o.) for three days before receiving the HS/R surgery (Figure 3A). KET attenuated HS-induced hepatic injury in WT mice, and this attenuating effect was more dramatic in VP-PXR transgenic mice, as shown by histology (Figure 3B and 3C) and serum ALT level (Figure 3D).
Figure 3. Induction of CYP3A is required for the sensitizing effect of PXR activation on HS-induced hepatic injury.
(A) Schematic representation of the ketoconazole (KET) pre-treatment model. WT and VP-PXR transgenic mice were treated with KET (20 mg/kg per day) for two days before receiving the sham surgery or HS/R. (B and C) Liver histology was analyzed by H&E staining (B, Bar is 100 μm), and quantification of necrotic areas and Suzuki scores (C). (D) Serum levels of ALT. (E) VP-PXR and VP-PXR/Cyp3a−/− mice subject to the sham surgery or HS/R were analyzed for liver histology by H&E staining. Shown on the right are quantification of necrotic areas and Suzuki scores. (F) Serum levels of ALT. n=4~5 for each group. *, p < 0.05; **, p < 0.01, the comparisons are labeled (C, D, and F), or compared to the VP-PXR group (E).
Since trauma patients often receive treatment after their admissions to ICU, we evaluated the effect of post-HS treatment of KET on PCN-induced and HS/R responsive hepatic injury. Two time points of post-HS KET treatment were chosen: 2 h after the initiation of HS and right before resuscitation when Cyp3a11 was induced (Supplementary Figure 1I), and 12 h after the initiation of HS when the suppression of Cyp3a11 became obvious (Supplementary Figure 4A). The designs of the experiment are outlined in Supplementary Figure 4B. Treatment with KET at the 2-h time point, but not the 12-h time point, was effective to attenuate HS-induced hepatic injury as shown by histology (Supplementary Figure 4C-4E) and the serum ALT level (Supplementary Figure 4F). The loss of protection at the 12-h time point was likely due to the suppression of Cyp3a11. Nevertheless, these results suggested that the effect of post-HS treatment of KET was time-sensitive.
In the genetic model, we generated the VP-PXR/Cyp3a−/− mice by breeding the VP-PXR transgene into the Cyp3a−/− background. The VP-PXR and Cyp3a−/− alleles were verified by PCR genotyping (Supplementary Figure 5A) and the loss of Cyp3a expression was confirmed by qRT-PCR (Supplementary Figure 5B). In the same VP-PXR/Cyp3a−/− mice, the expression of Cyp2b10 was induced (Supplementary Figure 5B), consistent with the notion that Cyp2b10 is a PXR target gene. Compared to their VP-PXR counterparts, VP-PXR/Cyp3a−/− mice showed attenuated liver injury upon HS/R (Figure 3E and 3F). These results suggested that the sensitizing effect of the VP-PXR transgene was Cyp3a11 dependent, but independent of Cyp2b10. Ablation of Cyp3a also attenuated the sensitizing effect of PCN (Supplementary Figure 5C-5F).
Pharmacological activation of hPXR sensitizes the hPXR/hCYP3A4 humanized mice to HS-induced hepatic injury in a CYP3A-dependent manner
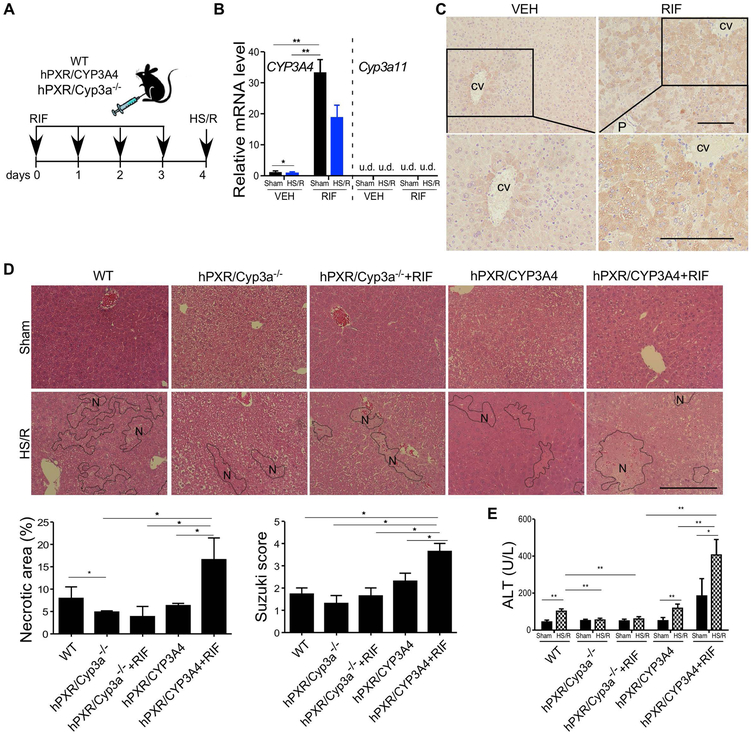
The drug induction of DMEs is known to have species specificity, and original hPXR humanized mice showed that the species origin of the PXR receptor is the determining factor for the species specificity (13). The second-generation of humanized mice were subsequently created by humanizing both hPXR and human CYP3A4. The humanization of CYP3A4 was achieved by introducing the CYP3A4 transgene into the Cyp3a−/− background (18). We used the hPXR/hCYP3A4 humanized mice and the control hPXR/Cyp3a−/− mice to determine whether activation of hPXR by RIF, an antibiotic and potent hPXR-specific agonist, can sensitize mice to HS-induced liver injury and whether this sensitization is CYP3A dependent. The humanized mice were pre-treated with RIF (10 mg/kg per day, i.p.) for 4 days before the HS/R surgery (Figure 4A). Treatment with RIF was efficient in inducing CYP3A4 as expected (Figure 4B), and the massive induction of CYP3A4 caused the loss of zonal expression of CYP3A4 (Figure 4C). The humanized mice showed a basal sensitivity to HS-induced liver injury similar to WT mice, and the liver injury was exacerbated by the treatment of RIF, as shown by histology (Figure 4D) and serum ALT level (Figure 4E). The sensitizing effect of RIF was attenuated in hPXR/Cyp3a−/− mice, suggesting that the sensitizing effect was CYP3A dependent (Figure 4D and 4E).
Figure 4. Pharmacological activation of hPXR sensitizes the hPXR/hCYP3A4 humanized mice to HS-induced hepatic injury in a CYP3A-dependent manner.
(A) Schematic representation of the rifampicin (RIF) pre-treatment model. WT, hPXR/CYP3A4 and hPXR/Cyp3a−/− mice were treated with RIF (10 mg/kg per day) for four days before receiving the sham surgery or HS/R. (B) The hepatic mRNA expression of human CYP3A4 and mouse Cyp3a11 was measured by qRT-PCR. (C) The hepatic expression of CYP3A4 was measured by immunostaining. CV, central vein; P, portal vein. (D) Liver histology was analyzed by H&E staining. Bar is 100 μm. Shown below are quantification of necrotic areas and Suzuki scores. (E) Serum levels of ALT. n=4~5 for each group. *, p < 0.05; **, p < 0.01, the comparisons are labeled.
The sensitizing effect of PXR on HS-induced hepatic injury is accompanied by increased oxidative stress
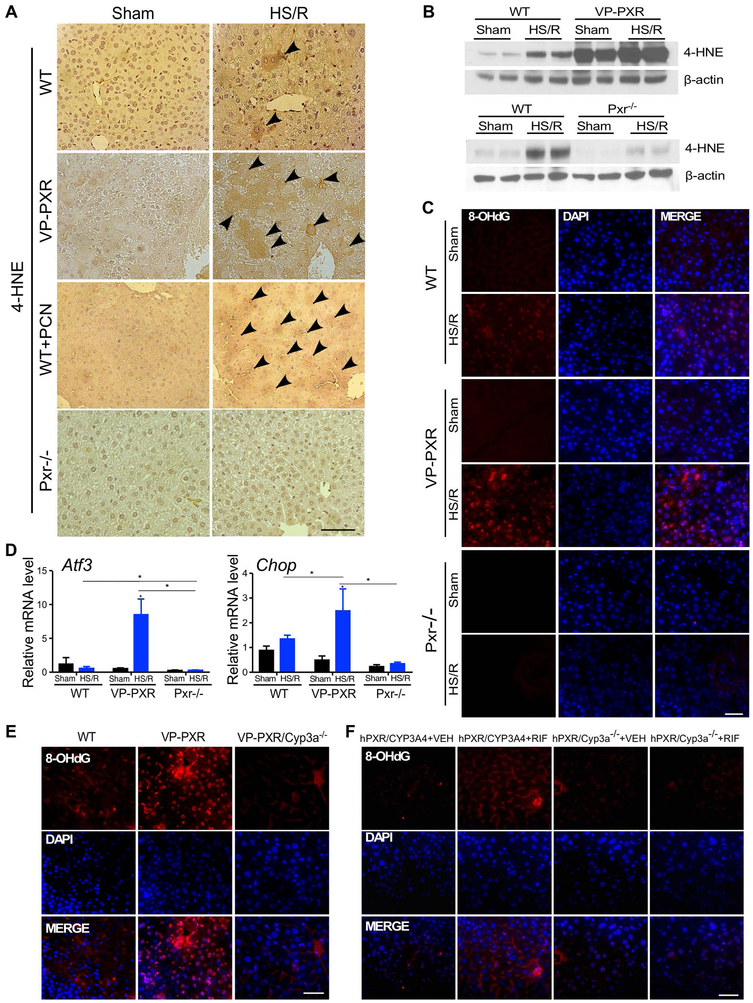
CYP3A-mediated oxidation is an important source of ROSs (6, 7). Activation of PXR has been reported to sensitize mice to oxidative stress, such as those triggered by the oxidative toxicant paraquat (16). Having shown that induction of CYP3A is required for the sensitizing effect of PXR on HS-induced hepatic injury, we went on to determine whether the sensitizing effect of PXR was associated with an increased oxidative stress. Upon HS/R and compared to WT mice, the immunostaining of 4-hydroxynonenal (4-HNE), the α,β-unsaturated hydroxyalkenal produced by lipid peroxidation and an oxidative stress biomarker (24), was increased in VP-PXR transgenic mice and PCN-treated WT mice, but decreased in Pxr−/− mice (Figure 5A). The respective increased and decreased oxidative stress in HS/R-treated VP-PXR and Pxr−/− mice were further verified by Western blot analysis of 4-HNE (Figure 5B) and immunofluorescence of 8-hydroxy-2’-deoxyguanosine (8-OHdG), a biomarker for free radicals induced by oxidative DNA lesions (25) (Figure 5C). Consistent with the notion that Cyp3a enzymes reside in the endoplasmic reticulum, the hepatic expression of activating transcription factor 3 (Atf3) (Figure 5D) and C/EBP homologous protein (Chop) (Figure 5E), two ER stress sensors, was increased in VP-PXR mice upon HS/R. The increased HS-responsive oxidative stress in VP-PXR transgenic mice and RIF-treated hPXR/hCYP3A4 humanized mice was Cyp3a dependent, because these effects were abolished in VP-PXR/Cyp3a−/− mice and hPXR/Cyp3a−/− mice (Figure 5F), respectively, as shown by immunofluorescence of 8-OHdG.
Figure 5. The sensitizing effect of PXR on HS-induced hepatic injury is accompanied by increased oxidative stress.
(A) Immunostaining of 4-HNE in liver tissues from WT, VP-PXR, WT+PCN, and Pxr−/− mice subject to the sham surgery or HS/R. Arrowheads indicate positive staining. (B) The level of 4-HNE was measured by Western blotting. (C) Immunofluorescent staining of 8-OHdG in liver tissue from WT, VP-PXR and Pxr−/− mice. (D) The hepatic expression of Atf3 and Chop was measured by qRT-PCR. (E and F) Immunofluorescent staining for 8-OHdG in liver tissues from WT, VP-PXR and VP-PXR/Cyp3a−/− mice that were subject to HS/R (E), or VEH- or RIF-treated hPXR/CYP3A4 and hPXR/Cyp3a−/− mice that were subject to HS/R (F). Bars are 100 μm. n=4~5 for each group. *, p < 0.05; **, p < 0.01, the comparisons are labeled.
Treatment with the antioxidant NACA attenuates the sensitizing effect of PXR on HS-induced hepatic injury
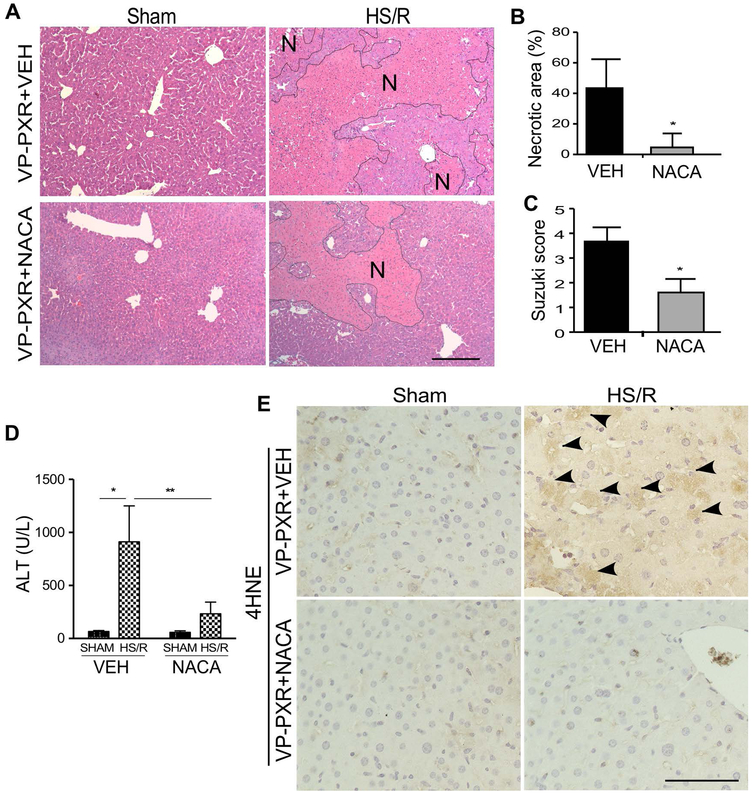
To determine whether treatment with an antioxidant can attenuate the sensitizing effect of the VP-PXR transgene, VP-PXR transgenic mice were pre-treated with the antioxidant N-acetylcystein amide (NACA, 200 mg/kg, i.p.) (26), one hour before the initiation of HS/R. Treatment with NACA attenuated HS-induced hepatic injury, as shown by histology (Figure 6A-6C) and serum ALT level (Figure 6D). The HS-induced increase of 4-HNE immunostaining in the transgenic mice was also attenuated upon the NACA treatment (Figure 6E). Treatment of WT mice with NACA also attenuated the sensitizing effect of PCN and DEX on HS-induced hepatic injury (Supplementary Figure 6). Co-treatment of the VP-PXR transgenic mice with KET and NACA fully abolished the HS-induced hepatic injury (Supplementary Figure 7).
Figure 6. Treatment with the antioxidant NACA attenuates the sensitizing effect of PXR on HS-induced hepatic injury.
(A) Liver histology was analyzed by H&E staining in VEH- or NACA-treated VP-PXR mice that were subject to the sham surgery or HS/R. Bar is 100 μm. (B to D) Mice are the same as in (A). Shown are quantification of necrotic areas (B), Suzuki scores (C), and serum levels of ALT (D) in mice subject to HS/R. (E) Mice are the same as in (A). The level of 4-HNE was measured by immunostaining with the positive staining indicated by arrowheads. Bar is 100 μm. n=4~5 for each group. *, p < 0.05; **, p < 0.01, compared to the VEH group (B and C), or the comparisons are labeled (D).
To evaluate the therapeutic potential of NACA, we conducted the post-HS treatment of NACA 2 h after HS but before the initiation of resuscitation (Supplementary Figure 8A). Post-HS treatment of NACA also attenuated PCN-induced and HS-responsive hepatic injury, as shown by histology (Supplementary Figure 8B-8D) and serum ALT level (Supplementary Figure 8E).
Post-hemorrhagic shock activation of PXR sensitizes mice to HS-induced hepatic injury
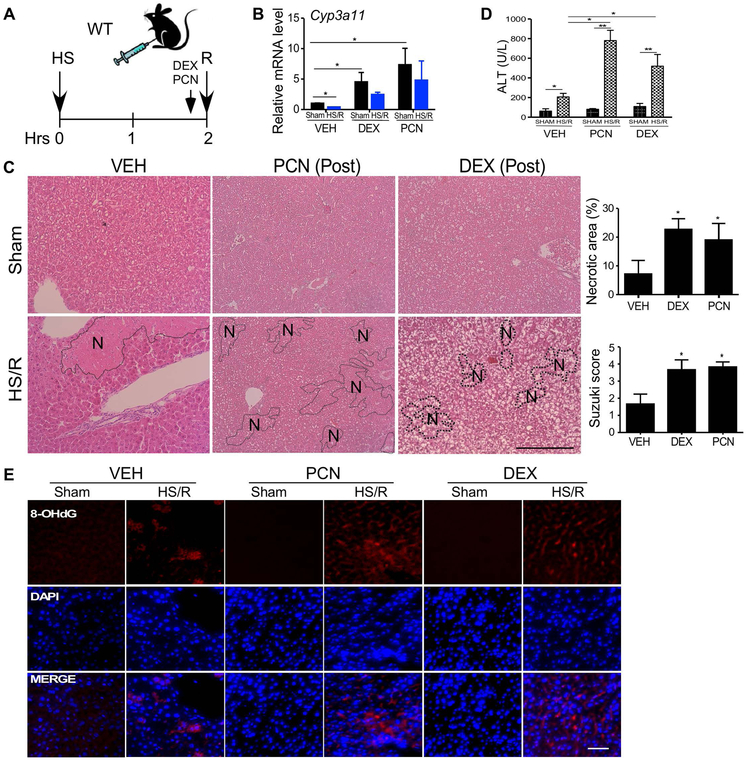
Since many trauma patients receive PXR-activating drugs upon their admissions to ICU, we investigated whether post-HS pharmacological activation of PXR can also sensitize mice to HS-induced hepatic injury. In this experiment, WT mice were subject to the HS surgery first, and right before the resuscitation, mice received a single intraperitoneal injection of PCN (40 mg/kg) or DEX (20 mg/kg) (Figure 7A). The activation of PXR and induction of Cyp3a11 were verified by qRT-PCR (Figure 7B). Post-HS treatment with PCN or DEX also exacerbated HS-induced hepatic injury as shown by histology (Figure 7C) and serum ALT level (Figure 7D). Post-HS treatment with PCN or DEX also increased HS-responsive oxidative stress as shown by immunofluorescence of 8-OHdG (Figure 7E). When the post-HS treatment of PCN was repeated in Cyp3a−/− mice, we found that PCN can no longer sensitize these mice to HS-induced hepatic injury (Supplementary Figure 9), suggesting that the sensitizing effect of post-HS activation of PXR was also Cyp3a dependent.
Figure 7. Post-hemorrhagic shock activation of PXR sensitizes mice to HS-induced hepatic injury.
(A) Schematic representation of the model of post-HS treatment of PCN or DEX. WT mice were subject to a 2-h HS and intraperitoneally injected with PCN (40 mg/kg) or DEX (20 mg/kg) before the resuscitation, and the mice were sacrificed 22 h after. (B) The hepatic mRNA expression of Cyp3a11 was measured by qRT-PCR. (C) Liver histology was analyzed by H&E staining. Shown on the right are quantification of necrotic areas and Suzuki scores. (D) Serum levels of ALT. (E) Immunofluorescent staining for 8-OHdG. Bar is 100 μm. n=4~5 for each group. *, p < 0.05; **, p < 0.01, the comparisons are labeled (B and D), or compared to the VEH group (C).
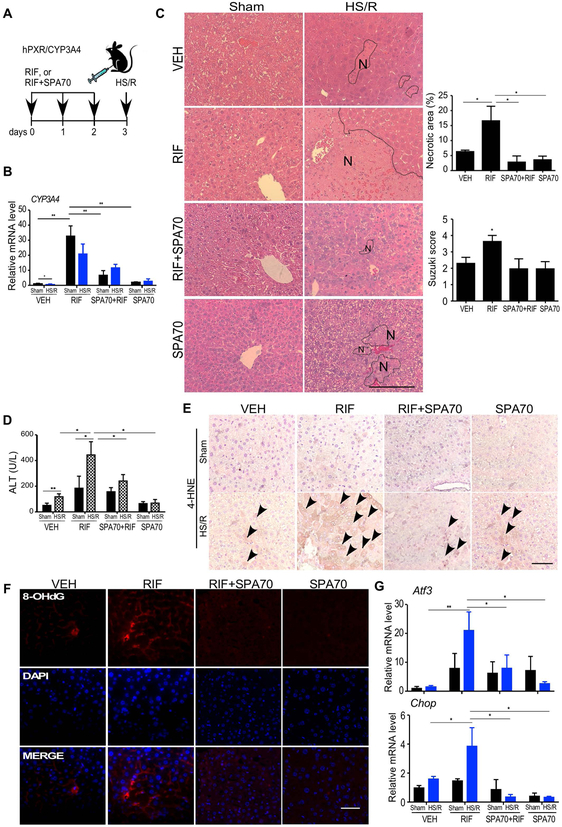
Treatment with the hPXR antagonist SPA70 protects the hPXR/hCYP3A4 humanized mice from HS-induced hepatic injury in a time-sensitive manner
To determine whether a pharmacological inhibition of PXR can protect mice from HS/R-induced hepatic injury, humanized mice treated with RIF (10 mg/kg), in the absence or presence of a recently reported human-specific PXR antagonist SPA70 (150 mg/kg) (27), for 3 days were subject to HS/R (Figure 8A). Treatment with SPA70 efficiently blocked RIF-responsive induction of CYP3A4 as reported (27) (Figure 8B). SPA70 also attenuated HS/R-responsive and RIF-exacerbated hepatic injury as shown by histology (Figure 8C) and the serum ALT level (Figure 8D). In addition, SPA70 attenuated HS/R-induced oxidative stress, as shown by immunostaining of 4-HNE (Figure 8E), immunofluorescence of 8-OHdG (Figure 8F), and measurement of the expression of Atf3 and Chop (Figure 8G).
Figure 8. Treatment with the hPXR antagonist SPA70 protects the hPXR/hCYP3A4 humanized mice from HS-induced hepatic injury.
(A) Schematic representation of the SPA70 pre-treatment model. Mice were intraperitoneally injected with VEH, RIF (10 mg/kg) and SPA70 (150 mg/kg) individually or in combination for 3 days before receiving the sham surgery or HS/R. (B) The hepatic mRNA expression of CYP3A4 was measured by qRT-PCR. (C) Liver histology was analyzed by H&E staining. Shown on the right are quantification of necrotic areas and Suzuki scores. (D) Serum levels of ALT. (E and F) The hepatic levels of 4-HNE (arrowheads indicated the positive staining) (E) and 8-OHdG (F) were measured by immunostaining and immunofluorescence, respectively. (G) The hepatic mRNA expression of Atf3 and Chop was measured by qRT-PCR. Bars are 100 μm. n=4~5 for each group. *, p < 0.05; **, p < 0.01, the comparisons are labeled.
The protective effect of SPA70 was time-sensitive, because when given at 2-h or 12-h after the initiation of HS (Supplementary Figure 10A), SPA70 failed to protect humanized mice from RIF-induced and HS/R-responsive hepatic injury (Supplementary Figure 10B-10E). The lack of post-HS effect of SPA70 was likely due to the insufficient time to inhibit RIF-responsive expression of CYP3A4 (Supplementary Figure 10F). In the same set of experiments, treatment with KET at the 2-h time point, but not the 12-h time point, was effective to attenuate HS-induced hepatic injury (Supplementary Figure 10B-10E), consistent with the results from PCN-treated WT mice (Supplementary Figure 4).
DISCUSSION
Hemorrhagic shock remains a major health concern due to the secondary tissue and organ injuries. A better understanding of the mechanisms by which HS induces tissue injury will help to develop effective therapeutic strategies to reduce the mortality of organ injury, including injury to the liver. Our previous microarray analysis suggested a major suppression in the expression of PXR and its target DMEs (17), based on which we initially speculated that the suppression of PXR might have contributed to HS-induced hepatic injury. To our surprise, we found that Pxr ablation or pharmacological inhibition of PXR in humanized mice by SPA70 protected mice from HS-induced liver injury. In contrast, transgenic or pharmacological activation of PXR sensitized mice to HS-induced liver injury. The pathological role of PXR activation in HS-induced liver injury was consistent with our observation that there was an intact expression of Pxr and elevated expression of Cyp3a11 in mice that received the 2-h HS but without a resuscitation. Our results suggest that HS has a dynamic effect on the expression of Pxr and DMEs, and the HS-responsive suppression of PXR may represent a protective response and/or secondary response to HS-induced liver injury.
Our study is of medical significance. This is because seriously injured trauma patients are uniformly prescribed with multiple drugs, many of which are PXR activators and DME inducers in the liver. The unavoidable use of PXR-activating drugs prior to HS or during the clinical management of trauma may restore drug metabolism, but has the potential to exacerbate HS-induced liver injury. We showed both pre-HS and post-HS activation of PXR sensitized mice to HS-induced liver injury. The pre-treatment with PXR agonists simulates HS patients who happen to take PXR-activating drugs prior to their injury, whereas treatment during resuscitation mimics HS patients who receive PXR-activating medications during their ER visit or ICU stay. The human relevance of this study was also illustrated by our use of the hPXR/hCYP3A4 humanized mice, in which treatment with RIF, an antibiotic to reduce deep surgical site infections (14, 15) or to treat tuberculosis, sensitized these mice to HS-induced liver injury.
The mechanistic insight revealed from this study may provide translational opportunities. We have presented both pharmacological and genetic evidence that the induction of CYP3A is required for the sensitizing effect of PXR on HS-induced hepatic injury. The initial evidence was that treatment with the CYP3A inhibitor KET attenuated the VP-PXR transgenic mice from HS-induced liver injury. In addition to inhibiting Cyp3a enzymes, KET and its derivatives can also function as PXR antagonists (28). Therefore, we cannot exclude the possibility that inhibition of both Cyp3a and PXR may have contributed to the protective effect of KET. To more conclusively define the role of CYP3A, we showed that ablation of the Cyp3a genes abolished the sensitizing phenotype in VP-PXR transgenic mice, PCN- or DEX-treated WT mice, or RIF-treated humanized mice. Pharmacoenhancing by CYP3A inhibition is not uncommon in the clinic. It is interesting to know whether pharmacoenhancers, such as Ritonavir used in the HIV antiretroviral therapy (29), can be used to attenuate the sensitizing effect of PXR activation. The use of antioxidant NACA to relieve the sensitizing effect of PXR was also exciting, because the oral formulation of N-acetylcysteine (NAC) is a drug approved for treating APAP overdose whose mechanism of hepatotoxicity also involves oxidative stress. The use of hPXR antagonist SPA70 to attenuate RIF-induced sensitization in humanized mice provided a proof of concept evidence that a direct use of PXR antagonists may also be used to manage PXR-mediated sensitization of HS-responsive liver injury. Last but not least, it is encouraging that post-HS administration of NACA and KET remained effective in alleviating PXR-induced and HS/R-responsive hepatic injury.
In summary, we have uncovered a previously unrecognized role of PXR in HS-induced liver injury. Cautions need to be applied when PXR-activating drugs are used in trauma patients, or when patients were on PXR-activating drugs prior to their traumatic injuries. Our results also suggest that co-administration of anti-oxidative agents, CYP3A inhibitors, and/or PXR antagonists can be used to mitigate the side effect of liver injury associated with the unavoidable use of PXR-activating drugs in trauma patients.
Supplementary Material
Acknowledgements
We thank Dr. Taosheng Chen (St. Jude Children’s Research Hospital) for the SPA70 compound and Dr. Frank Gonzalez (National Cancer Institute) for the Cyp3a11/CYP3A4 antibody.
Financial Support: This work was supported in part by NIH grants DK117370 and ES023438 to WX.
Abbreviations:
- ALT
alanine aminotransferase
- CAR
constitutive androstane receptor
- CV
central vein
- CYP
cytochrome P450
- DEX
dexamethasone
- HS/R
hemorrhagic shock and resuscitation
- 4-HNE
4-Hydroxynonenal
- KET
ketoconazole
- PCN
pregnenolone 16α-carbonitrile
- RIF
rifampicin
- PXR
pregnane X receptor
- NACA
N-acetylcystein amide
- 8-OHdG
8-hydroxy-2’ -deoxyguanosine
- ROS
reactive oxygen species
REFERENCES
- 1.Krug EG, Sharma GK, Lozano R. The global burden of injuries. Am J Public Health 2000;90:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, O’Keefe GE, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med 2012;40:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toklu HZ, Tumer N: Oxidative Stress, Brain Edema, Blood-Brain Barrier Permeability, and Autonomic Dysfunction from Traumatic Brain Injury In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL), 2015. [Google Scholar]
- 4.Wetzel G, Relja B, Klarner A, Henrich D, Dehne N, Bruhne B, Lehnert M, et al. Myeloid knockout of HIF-1 alpha does not markedly affect hemorrhage/resuscitation-induced inflammation and hepatic injury. Mediators Inflamm 2014;2014:930419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu HP, Hsu JC, Hwang TL, Yen CH, Lau YT. Resveratrol attenuates hepatic injury after trauma-hemorrhage via estrogen receptor-related pathway. Shock 2008;30:324–328. [DOI] [PubMed] [Google Scholar]
- 6.Jaeschke H Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol 2011;26 Suppl 1:173–179. [DOI] [PubMed] [Google Scholar]
- 7.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med 1998;24:1324–1330. [DOI] [PubMed] [Google Scholar]
- 8.Yan J, Xie W. A brief history of the discovery of PXR and CAR as xenobiotic receptors. Acta Pharm Sin B 2016;6:450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zingarelli B, Caputi AP, Di Rosa M. Dexamethasone prevents vascular failure mediated by nitric oxide in hemorrhagic shock. Shock 1994;2:210–215. [DOI] [PubMed] [Google Scholar]
- 10.Abel RM, Reis RL. Intravenous diazepam for sedation following cardiac operations: clinical and hemodynamic assessments. Anesth Analg 1971;50:244–248. [PubMed] [Google Scholar]
- 11.Shiels SM, Tennent DJ, Wenke JC. Topical Rifampin Powder for Orthopaedic Trauma Part I: Rifampin powder reduces recalcitrant infection in a delayed treatment musculoskeletal trauma model. J Orthop Res 2018. [DOI] [PubMed] [Google Scholar]
- 12.Tyas B, Marsh M, Oswald T, Refaie R, Molyneux C, Reed M. Antibiotic resistance profiles of deep surgical site infections in hip hemiarthroplasty; comparing low dose single antibiotic versus high dose dual antibiotic impregnated cement. J Bone Jt Infect 2018;3:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 2000;406:435–439. [DOI] [PubMed] [Google Scholar]
- 14.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998;92:73–82. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg B, Sabbagh W Jr., Juguilon H, Bolado J Jr., van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 1998;12:3195–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong H, Singh SV, Singh SP, Mu Y, Lee JH, Saini SP, Toma D, et al. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol 2006;20:279–290. [DOI] [PubMed] [Google Scholar]
- 17.Edmonds RD, Vodovotz Y, Lagoa C, Dutta-Moscato J, Yang Y, Fink MP, Levy RM, et al. Transcriptomic response of murine liver to severe injury and hemorrhagic shock: a dual-platform microarray analysis. Physiol Genomics 2011;43:1170–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma X, Cheung C, Krausz KW, Shah YM, Wang T, Idle JR, Gonzalez FJ. A double transgenic mouse model expressing human pregnane X receptor and cytochrome P450 3A4. Drug Metab Dispos 2008;36:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Herwaarden AE, Wagenaar E, van der Kruijssen CM, van Waterschoot RA, Smit JW, Song JY, van der Valk MA, et al. Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J Clin Invest 2007;117:3583–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohut LK, Darwiche SS, Brumfield JM, Frank AM, Billiar TR. Fixed volume or fixed pressure: a murine model of hemorrhagic shock. J Vis Exp 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen Z, Fan L, Li Y, Zou Z, Scott MJ, Xiao G, Li S, et al. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: role in posthemorrhagic shock acute lung inflammation. J Immunol 2014;193:4623–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993;55:1265–1272. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JE, Blaschke TF. Ketoconazole inhibits cyclosporine metabolism in vivo in mice. J Pharmacol Exp Ther 1986;236:671–674. [PubMed] [Google Scholar]
- 24.Tjalkens RB, Cook LW, Petersen DR. Formation and export of the glutathione conjugate of 4-hydroxy-2, 3-E-nonenal (4-HNE) in hepatoma cells. Arch Biochem Biophys 1999;361:113–119. [DOI] [PubMed] [Google Scholar]
- 25.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27:120–139. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Hu B, Huang H, Tsung A, Gaikwad NW, Xu M, Jiang M, et al. Estrogen Sulfotransferase Is an Oxidative Stress-responsive Gene That Gender-specifically Affects Liver Ischemia/Reperfusion Injury. J Biol Chem 2015;290:14754–14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin W, Wang YM, Chai SC, Lv L, Zheng J, Wu J, Zhang Q, et al. SPA70 is a potent antagonist of human pregnane X receptor. Nat Commun 2017;8:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Huang H, Li H, Teotico DG, Sinz M, Baker SD, Staudinger J, et al. Activated pregnenolone X-receptor is a target for ketoconazole and its analogs. Clin Cancer Res 2007;13:2488–2495. [DOI] [PubMed] [Google Scholar]
- 29.Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, Sax PE, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA 2018;320:379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.