Abstract
Schistosoma mansoni is a long-lived intravascular trematode parasite that can infect humans causing the chronic debilitating disease, schistosomiasis. We hypothesize that the action of host-interactive proteins found at the schistosome surface allows the worms to maintain a safe, anti-thrombotic and anti-inflammatory environment around them in the bloodstream. One such protein is the ~60kDa alkaline phosphatase SmAP which is known to be expressed in the outer tegument of all intravascular life stages. We demonstrate in this work that the parasites (schistosomula as well as adult males and females) can hydrolyze polyphosphate (polyP) - an anionic, linear polymer of inorganic phosphates that is produced and released by immune cells as well as by activated platelets and that induce proinflammatory and prothrombotic pathways. Purified recombinant SmAP can likewise cleave polyP and with a Km of 6.9 ±1mM. Finally, parasites whose SmAP gene has been suppressed by RNAi are significantly impaired in their ability to hydrolyze polyP. SmAP-mediated cleavage of polyP may contribute to the armamentarium of schistosomes that promotes their survival in the hostile intravascular habitat. This is the first report of any pathogen cleaving this bioactive metabolite.
Graphical Abstract
Introduction
Schistosoma mansoni is one of the three important schistosomes of humans that causes the chronic debilitating disease, schistosomiasis. According to the World Health Organization (WHO), over 200 million people are infected with schistosomes around the world and more than 800 million live at risk of infection [1]. Humans can become infected following direct contact with fresh water containing the free-living larval stage (the cercaria). After penetrating host skin, the cercaria transforms into the juvenile, intra-mammalian form called the schistosomulum. Schistosomula migrate through the vasculature to the portal vein, where they mature into adult males and females. The paired worms migrate to the mesenteric venules, where they lay eggs that may be shed in stool.
Adult worms can live in the vasculature of their hosts for many years, yet they appear not to provoke protective immune responses or blood clot formation around them in vivo [2]. It has been suggested that host-interactive proteins found in the parasite’s tegument (such as Schistosoma mansoni alkaline phosphatase, SmAP) are key to the worm’s ability to block damaging immunity and to impede thrombus formation [3–7]. SmAP is a glycoprotein that requires Mg2+ for activity and has an optimal pH of 9 [8]. SmAP cleaves AMP to generate adenosine - an anti-inflammatory metabolite and an inhibitor of platelet activation [9, 10]. In addition, recombinant SmAP hydrolyzes sphingosine-1-phosphate (S1P), a lipid signaling molecule that can orchestrate immune responses and facilitate platelet aggregation [11–14]. Platelets contain high concentrations of S1P that can be secreted during coagulation [12]. Platelets additionally contain polyphosphate (polyP) - an anionic, linear polymer of inorganic phosphates that can be released following platelet activation [15]. PolyP plays a variety of prothrombotic roles: it enhances the binding of platelets to von Willebrand factor [16], triggers activation of factor XII [17], amplifies factor XI activation by thrombin [18], accelerates factor V activation [19], inactivates tissue factor pathway inhibitor [20] and inhibits fibrinolysis [21]. PolyP can also be released by mast cells [22]. Additionally, polyP-activated factor XII triggers the kallikrein-mediated kininogen pathway, producing high levels of the inflammatory mediator bradykinin, increasing vascular permeability and the inflammatory response [23].
In this work, we set out to determine whether intravascular schistosomes can cleave polyP. Acid and alkaline phosphatases have been shown capable of continuously hydrolyzed polyP via its terminal groups, producing free phosphate ions (Pi) and shorter chained polyphosphates [24]. Since S. mansoni express the alkaline phosphatase SmAP at their host-interactive surface [8, 25], we hypothesized that this enzyme on living worms might be capable of similarly hydrolyzing polyP thereby potentially blunting the pro-coagulation and pro-inflammation impact of this bioactive metabolite.
Material and Methods
Parasites and mice
Schistosoma mansoni cercariae were obtained from ~40-day infected Biomphalaria glabrata snails following exposure to light and schistosomula were prepared as described [26]. Adult parasites were harvested by perfusion from Swiss Webster mice that had been percutaneously infected with ~125 cercariae 7 weeks earlier, as described [27]. All parasites were cultured in complete DMEM/F12 medium supplemented with 10% heat-inactivated fetal bovine serum, 200 U/ml penicillin and 200 µg/ml streptomycin, 0.2 µM Triiodo-L-thyronine, 1 µM serotonin and 8 µg/ml human insulin and were maintained at 37°C, in an atmosphere of 5% CO2 [28]. All protocols involving animals were approved by the Institutional Animal Care and Use Committees (IACUC) of Tufts University.
Hydrolysis of polyP
To measure polyP hydrolysis, living parasites (groups of ~1000 schistosomula, or individual adult males or females) or recombinant SmAP (0.5 µg, produced as described [6]) were incubated at 37 °C in 200µl of 2mM P70 polyP (BK Giulini GmbH, Germany), 20 mM Tris-HCL (pH 9), 135 mM NaCl, 5 mM KCl, 10 mM glucose, 2mM CaCl2, 10mM MgCl2. Aliquots were recovered at different time points thereafter, and any Pi generated was measured using a Phosphate Colorimetric Assay Kit (Biovision Inc, CA, USA) following the manufacturer’s instructions and with a Synergy HT spectrophotometer (Bio-Tek Instruments, Winooski, VT, USA). Km values for rSmAP were determined in the above assay buffer containing 0.5µg rSmAP and different concentrations of polyP (0–10 mM). Km values were calculated using computerized nonlinear regression analysis of the data fitted to the Michaelis-Menten equation using Graphpad Prism v.5.
Treatment of parasites with siRNAs.
Adult male worms were treated either with either a synthetic siRNA targeting SmAP (SmAPsiRNA 1: 5’-AAGAAATCAGCAGATGAGAGATTTAAT-3’) or with a control siRNA that targeted no sequence in the schistosome genome (Control: 5’-CTTCCTCTCTTTCTCTCCCTTGTGA-3’) or, as a further control, with no siRNA. Delivery of siRNAs to the parasites was performed by electroporation, using 3µM of each siRNA as described previously, in a protocol that yields robust suppression of SmAP gene expression [8]. Seven days after treatment, the ability of all worms to hydrolyze polyP was measured, as described above.
Statistical analysis
All experiments were repeated at least 3 times with similar results. Data, presented as mean ±SD, have been analyzed using a Shapiro test and have been determined to be normally distributed. All assay results are shown after subtraction of the minimal values seen in control (no treatment) samples. Means are compared by One-way ANOVA with Tukey post-hoc testing or Two-way ANOVA with Bonferroni post-hoc testing using GraphPad Prism, v. 5; (GraphPad Software, Inc, San Diego, CA, USA). A probability value of less than 0.05 was considered significant.
Results
Intravascular schistosomes cleave polyP
The ability of live intravascular schistosomes to hydrolyze polyP and release Pi was assessed using a colorimetric phosphate assay. Incubating each of the life cycle stages tested (i.e. groups of ~1000 schistosomula or individual adult female or male worms) in 2mM polyP led to increased Pi release with time as shown in figure 1. Adult males generate statistically significantly more Pi compared to their smaller female cohorts (p < 0.05 – 0.001).
Figure 1.
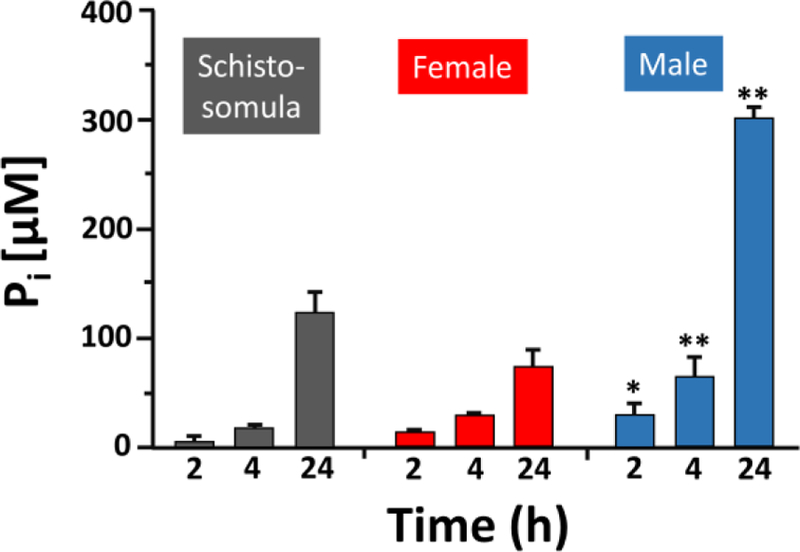
Intravascular S. mansoni cleave polyP. Pi level (µM, mean ± SD) detected 2, 4 and 24 hours (h) after incubating groups of ~1000 living schistosomula (grey bars), individual adult female (red bars) or adult male (blue bars) worms in 2 mM polyP. At least 10 separate male or female parasites were used per assay. Individual males generate statistically significantly more Pi compared to individual females at all comparable timepoints (Two-way ANOVA, * p < 0.05, ** p < 0.001).
Hydrolysis of polyP by S Data. mansoni alkaline phosphatase, SmAP.
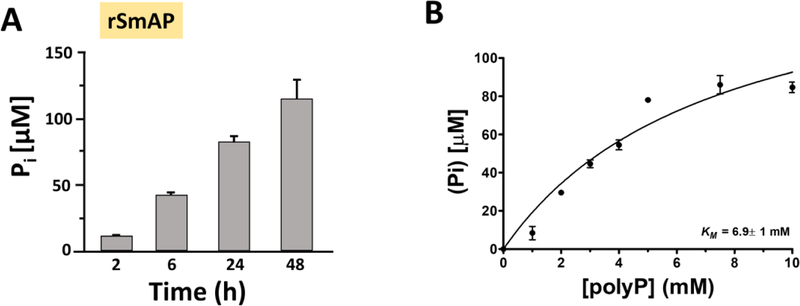
The ability of recombinant SmAP to degrade polyP and release Pi was assessed using the colorimetric assay noted above. Figure 2A shows the significant increase in free Pi detected over time in the presence of rSmAP. Figure 2B is a Michaelis-Menten curve depicting the kinetics of polyP hydrolysis by rSmAP. The Km value is 6.9 ±1 mM. and the Vmax = 167.4 ± 24 µM/h.
Figure 2.
Recombinant S. mansoni alkaline phosphatase SmAP hydrolyzes polyP. A) Pi level (µM, mean ± SD) detected at various time points (as indicated) after incubating 0.5µg rSmAP in 2 mM polyP. Mean values at all time points differ significantly from each other (One-Way ANOVA, p < 0.001) (B) Michaelis-Menten curve depicting the kinetics of polyP hydrolysis by rSmAP. The Km = 6.9 ±1mM and Vmax = 167.4 ±24 µM/h. Data are representative of three separate experiments.
Figure 3 shows that parasites whose SmAP gene were suppressed following administration of siRNA targeting this gene (light grey bar, figure 3) are significantly impaired in their ability to cleave polyP and release Pi compared to control parasites that have been treated with an irrelevant (Control) siRNA or with no (None) siRNA (dark grey bars, figure 3); p<0.05, one-way ANOVA.
Figure 3.
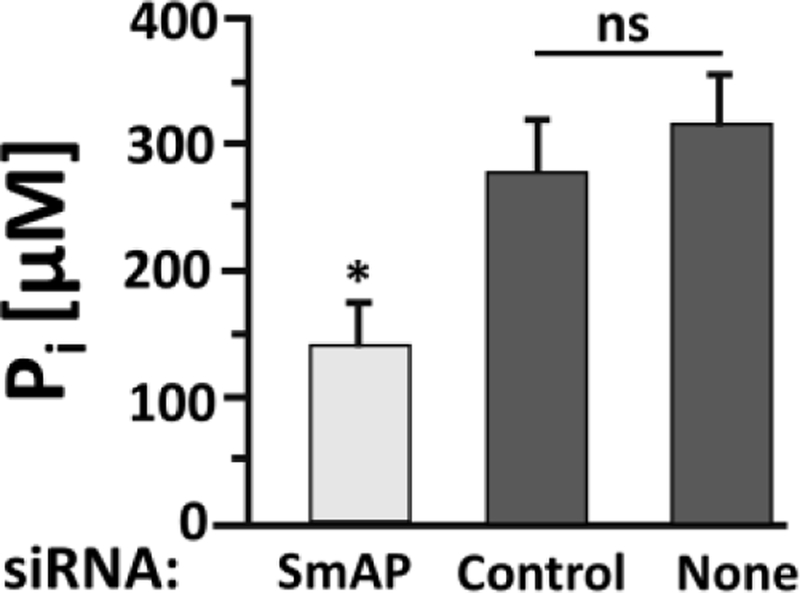
SmAP-suppressed parasites are impaired in their ability to cleave polyP. Adult males were treated with siRNA targeting SmAP or with control siRNA (Control) or with no siRNA (None), as indicated. Seven days later the ability of all worms to cleave polyP was measured. The Pi level (µM, mean ± SD) detected at the 24-hour time point is shown. The SmAP suppressed group generated significantly less Pi compared to either of the control groups, p <0.05 (one-way ANOVA, F=31.07, Df=11). There is no significant (ns) difference between the two control mean values.
Discussion
In this work, we show that living schistosomes can cleave exogenous polyP. This is the case for all life stages examined: schistosomula as well as adult male and female worms. Given the array of prothrombotic [16–21] and proinflammatory [22, 23, 29] effects of polyP, the worm’s ability to diminish the level of this metabolite in their immediate environment might help blunt blood clot formation and damaging immunity around them and so promote their survival.
Some of the capacity of polyP to promote thrombus formation revolves around its ability to enhance the kinetics of coagulation; thrombin and factor XI binding to immobilized polyP causes an ~ 3000-fold increase in the rate of activation of factor XI by thrombin [18] and polyP enhances the rate of factor V activation to Va by factor XIa [19]. Because of these effects, added polyP can correct prolonged clotting times in whole blood samples treated with heparin or in plasma samples from patients with hemophilia A or B [30]. Furthermore, it has been reported that clots formed in the presence of polyP are more resistant to fibrinolysis; polyP alters the structure of fibrin clots, increasing fiber thickness and strength and making fibrin more difficult for fibrinolytic enzymes to digest [31]. Beyond coagulation, FXII activation by polyP initiates several protease cascades in plasma, e.g. involving kallikrein-kinin and this potently triggers bradykinin generation and greatly increases vascular permeability [32]. Additionally, exposure to polyP induces NF-κB activation and leukocyte adhesion in endothelial cells [33].
Phosphatase enzymes have been shown capable of hydrolyzing polyP to generate free Pi [24]. Since schistosomes express a tegumental alkaline phosphatase – the glycoprotein SmAP [8, 25], we tested the hypothesis that this host-exposed parasite enzyme was responsible for the worm’s ability to cleave polyP. This idea is supported by the finding that parasites whose SmAP gene has been suppressed using RNAi have a significantly diminished ability to hydrolyze polyP compared to controls. Furthermore, as shown here, purified rSmAP, produced in a eukaryotic expression system, can itself cleave polyP.
This work extends the range of substrates hydrolyzed by SmAP. The enzyme has been previously shown capable of cleaving sphingosine-1-phosphate (S1P) [6]. Since this bioactive metabolite, like polyP, exerts pro-inflammatory and pro-thrombotic effects [11–14] SmAP-mediated hydrolysis of S1P likely contributes to the ability of the worms to survive in the vasculature unmolested by damaging host hemostatic and immunological effectors. SmAP has also been shown capable of dephosphorylating the nucleoside monophosphates AMP, CMP, GMP and TMP to generate adenosine, cytosine, guanine and thymine, respectively [6]. Such dephosphorylation may facilitate the easy uptake of these metabolites into the worms as food – an important function since schistosomes cannot synthesize purines de novo [34]. Adenosine generated from SmAP action could additionally exert its potent anti-inflammatory effects on a wide host of immune cells (reviewed in [35]).
In addition to its expression at the host-parasite surface, SmAP is also expressed in the parasite’s internal tissues [8, 25]. As at the surface, the protein presumably serves to dephosphorylate metabolites internally also and this may facilitate their movement between tissues. Strong staining in the female vitellaria [25] suggests that the enzyme may have particular importance for female reproduction and egg development.
The Km of rSmAP for polyP is 6.9 mM and this is more than ten-fold higher than its Km for AMP, CMP, GMP or TMP (all ~ 0.6 mM) [6]. Even after prolonged incubation (≥ 24 h), neither whole, living schistosomes nor rSmAP hydrolyze all of the available polyP. The polyP used here has a mean size akin to that of platelet-derived polyP (~ 60–100 residues) and whether the relatively sluggish cleavage of some phosphates from these polymers by schistosomes and rSmAP would be enough to incapacitate polyP function in vivo in a timely manner remains to be established. This is the first report documenting the ability of any pathogen to cleave extracellular polyP.
Highlights.
Schistosoma mansoni is a long-lived intravascular trematode parasite.
Intravascular life stages (schistosomula, adult males & females) can cleave polyphosphate (polyP).
PolyP is an anionic polymer of inorganic phosphates that is proinflammatory & prothrombotic.
Alkaline phosphatase SmAP is a host interactive enzyme that can hydrolyze polyP.
SmAP-suppressed worms are impaired in this ability.
Acknowledgements
This work was supported by grant AI-056273 from the NIH-NIAID. Infected snails were provided by the Biomedical Research Institute via the NIAID schistosomiasis resource center under NIH-NIAID Contract No. HHSN272201000005I. We thank Professor James H. Morrissey and Julie Collins, University of Illinois, Urbana, IL for the kind gift of polyphosphate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gryseels B, Polman K, Clerinx J, Kestens L, Human schistosomiasis, Lancet 368(9541) (2006) 1106–18. [DOI] [PubMed] [Google Scholar]
- [2].Keating JH, Wilson RA, Skelly PJ, No overt cellular inflammation around intravascular schistosomes in vivo, The Journal of parasitology 92(6) (2006) 1365–9. [DOI] [PubMed] [Google Scholar]
- [3].Wu YP, Lenting PJ, Tielens AG, de Groot PG, van Hellemond JJ, Differential platelet adhesion to distinct life-cycle stages of the parasitic helminth Schistosoma mansoni, J Thromb Haemost 5(10) (2007) 2146–8. [DOI] [PubMed] [Google Scholar]
- [4].Bhardwaj R, Skelly PJ, Purinergic signaling and immune modulation at the schistosome surface?, Trends Parasitol 25(6) (2009) 256–60. [DOI] [PubMed] [Google Scholar]
- [5].Da’dara AA, Bhardwaj R, Ali YB, Skelly PJ, Schistosome tegumental ecto-apyrase (SmATPDase1) degrades exogenous pro-inflammatory and pro-thrombotic nucleotides, PeerJ 2 (2014) e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Elzoheiry M, Da’dara AA, Bhardwaj R, Wang Q, Azab MS, El-Kholy EI, El-Beshbishi SN, Skelly PJ, Intravascular Schistosoma mansoni Cleave the Host Immune and Hemostatic Signaling Molecule Sphingosine-1-Phosphate via Tegumental Alkaline Phosphatase, Front Immunol 9 (2018) 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elzoheiry M, Da’dara AA, deLaforcade AM, El-Beshbishi SN, Skelly PJ, The Essential Ectoenzyme SmNPP5 from the Human Intravascular Parasite Schistosoma mansoni is an ADPase and a Potent Inhibitor of Platelet Aggregation, Thromb Haemost (2018). [DOI] [PubMed]
- [8].Bhardwaj R, Skelly PJ, Characterization of schistosome tegumental alkaline phosphatase (SmAP), PLoS Negl Trop Dis 5(4) (2011) e1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Johnston-Cox HA, Ravid K, Adenosine and blood platelets, Purinergic Signal 7(3) (2011) 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fuentes E, Pereira J, Mezzano D, Alarcon M, Caballero J, Palomo I, Inhibition of platelet activation and thrombus formation by adenosine and inosine: studies on their relative contribution and molecular modeling, PLoS One 9(11) (2014) e112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cyster JG, Schwab SR, Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs, Annu Rev Immunol 30 (2012) 69–94. [DOI] [PubMed] [Google Scholar]
- [12].Obinata H, Hla T, Sphingosine 1-phosphate in coagulation and inflammation, Semin Immunopathol 34(1) (2012) 73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mahajan-Thakur S, Bohm A, Jedlitschky G, Schror K, Rauch BH, Sphingosine-1-Phosphate and Its Receptors: A Mutual Link between Blood Coagulation and Inflammation, Mediators Inflamm 2015 (2015) 831059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Urtz N, Gaertner F, von Bruehl ML, Chandraratne S, Rahimi F, Zhang L, Orban M, Barocke V, Beil J, Schubert I, Lorenz M, Legate KR, Huwiler A, Pfeilschifter JM, Beerli C, Ledieu D, Persohn E, Billich A, Baumruker T, y Schnitzler M. Mederos, Massberg S, Sphingosine 1-Phosphate Produced by Sphingosine Kinase 2 Intrinsically Controls Platelet Aggregation In Vitro and In Vivo, Circ Res 117(4) (2015) 376–87. [DOI] [PubMed] [Google Scholar]
- [15].Ruiz FA, Lea CR, Oldfield E, Docampo R, Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes, J Biol Chem 279(43) (2004) 44250–7. [DOI] [PubMed] [Google Scholar]
- [16].Montilla M, Hernandez-Ruiz L, Garcia-Cozar FJ, Alvarez-Laderas I, Rodriguez-Martorell J, Ruiz FA, Polyphosphate binds to human von Willebrand factor in vivo and modulates its interaction with glycoprotein Ib, J Thromb Haemost 10(11) (2012) 2315–23. [DOI] [PubMed] [Google Scholar]
- [17].Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH, Polyphosphate modulates blood coagulation and fibrinolysis, Proc Natl Acad Sci U S A 103(4) (2006) 903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choi SH, Smith SA, Morrissey JH, Polyphosphate is a cofactor for the activation of factor XI by thrombin, Blood 118(26) (2011) 6963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi SH, Smith SA, Morrissey JH, Polyphosphate accelerates factor V activation by factor XIa, Thromb Haemost 113(3) (2015) 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Puy C, Tucker EI, Ivanov IS, Gailani D, Smith SA, Morrissey JH, Gruber A, McCarty OJ, Platelet-Derived Short-Chain Polyphosphates Enhance the Inactivation of Tissue Factor Pathway Inhibitor by Activated Coagulation Factor XI, PLoS One 11(10) (2016) e0165172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mutch NJ, Engel R, de Willige S. Uitte, Philippou H, Ariens RA, Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin, Blood 115(19) (2010) 3980–8. [DOI] [PubMed] [Google Scholar]
- [22].Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R, Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes, J Biol Chem 287(34) (2012) 28435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T, Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo, Cell 139(6) (2009) 1143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huang R, Wan B, Hultz M, Diaz JM, Tang Y, Phosphatase-Mediated Hydrolysis of Linear Polyphosphates, Environ Sci Technol 52(3) (2018) 1183–1190. [DOI] [PubMed] [Google Scholar]
- [25].Araujo-Montoya BO, Rofatto HK, Tararam CA, Farias LP, Oliveira KC, Verjovski-Almeida S, Wilson RA, Leite LC, Schistosoma mansoni: molecular characterization of Alkaline Phosphatase and expression patterns across life cycle stages, Exp Parasitol 129(3) (2011) 284–91. [DOI] [PubMed] [Google Scholar]
- [26].Ramalho-Pinto FJ, Gazzinelli G, Howells RE, Mota-Santos TA, Figueiredo EA, Pellegrino J, Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro, Exp Parasitol 36(3) (1974) 360–72. [DOI] [PubMed] [Google Scholar]
- [27].Tucker MS, Karunaratne LB, Lewis FA, Freitas TC, Liang YS, Schistosomiasis, Current protocols in immunology / edited by Coligan John E. … [et al. ] 103 (2013) Unit 19 1. [DOI] [PubMed] [Google Scholar]
- [28].Basch PF, Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing, J Parasitol 67(2) (1981) 179–85. [PubMed] [Google Scholar]
- [29].Morrissey JH, Smith SA, Polyphosphate as modulator of hemostasis, thrombosis, and inflammation, J Thromb Haemost 13 Suppl 1 (2015) S92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith SA, Morrissey JH, Polyphosphate as a general procoagulant agent, J Thromb Haemost 6(10) (2008) 1750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smith SA, Morrissey JH, Polyphosphate enhances fibrin clot structure, Blood 112(7) (2008) 2810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caen J, Wu Q, Hageman factor, platelets and polyphosphates: early history and recent connection, J Thromb Haemost 8(8) (2010) 1670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bae JS, Lee W, Rezaie AR, Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models, J Thromb Haemost 10(6) (2012) 1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Levy MG, Read CP, Purine and pyrimidine transport in Schistosoma mansoni, J Parasitol 61(4) (1975) 627–32. [PubMed] [Google Scholar]
- [35].Cekic C, Linden J, Purinergic regulation of the immune system, Nat Rev Immunol 16(3) (2016) 177–92. [DOI] [PubMed] [Google Scholar]