White adipocytes serve as an energy reservoir to store excessive calories in the form of lipid droplets and protect other tissues or organs from ectopic lipid accumulation. Brown adipocytes express uncoupling protein 1 and are integral to adaptive thermogenesis. Whereas the functions of adipocytes in either white or brown adipose tissues are well documented, our knowledge of bone marrow adipocytes (BMA) remains in its infancy. Bone marrow adipose tissue (BMAT) occupies approximately 50-70% of the bone marrow volume in human adults.1 It is a dynamic tissue and responds to multiple metabolic conditions. For example, BMAT increases with obesity, aging, diabetes, caloric restriction, and irradiation.2 Although the significance of BMAT expansion under these conditions is still largely unknown, BMA interact locally with hematopoietic and bone cells, and contribute to global metabolism through secretion of adiponectin, leptin, stem cell factor (SCF), and other functional factors. For example, A-ZIP/F1 mice, which lack adipose tissues throughout the body, including BMAT, have delayed hematopoietic regeneration in long bones after irradiation.3 Our latest work also observed that depletion of BMA by bariatric surgery is associated with a decrease in bone marrow erythroid cells and anemia.4 The importance of BMA and the derived factors on hematopoiesis is further enhanced by a study in this issue of the Journal, in which Zhang et al.5 demonstrate that BMAT-derived SCF mediates metabolic regulation of hematopoiesis.
Stem cell factor, also known as Kit ligand (Kitl), is a hematopoietic cytokine expressed in fibroblasts and endothelial cells, as well as in BMA.3 Together with its receptor, c-Kit, SCF plays important roles in the maintenance of hematopoietic stem cells (HSC) and hematopoiesis. Blockade of the interaction between c-Kit and SCF with antic-Kit antibody promotes the clearance of HSC, which indicates the importance of Kitl/c-Kit signaling in HSC self-renewal.6 Loss-of-function mutations in c-Kit cause macrocytic anemia, or even embryonic lethality under some severe mutations.7 Inversely, mice with c-Kit gain-of-function mutations developed erythrocytosis compatible with myeloproliferative disorders.8 Analyses of multiple cell populations isolated from bone marrow and adipose tissue have demonstrated that BMA and LepR-positive (+) stromal cells are the primary sources of SCF, which is required for the regeneration of HSC and hematopoiesis after irradiation.3 Zhang et al. report that BMA-derived SCF is important for hematopoietic homeostasis under basal (Figure 1), obese and aging conditions, and in response to β3-adrenergic agonists.5
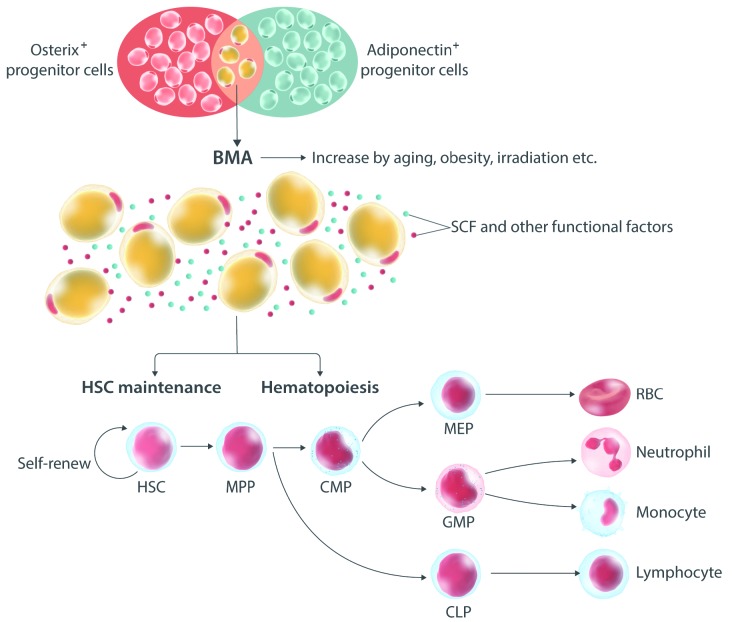
Figure 1.
Bone marrow adipocytes influence the maintenance of hematopoietic stem cell (HSC) and hematopoiesis. Bone marrow cellularity is complex, but is mainly composed of hematopoietic cells and bone marrow adipocytes (BMA), which appear after birth and accumulate with age, obesity and irradiation. BMA originate from osterix-positive (+) progenitor cells and secret adiponectin, stem cell factor (SCF) and other functional factors. In this study, Zhang et al.5 have demonstrated that BMAT-derived SCF plays important roles in HSC maintenance and hematopoietic differentiation under baseline, aging and obese conditions. Deficiency of SCF in BMAT hinders the self-renewal of HSC by influencing the bone marrow microenvironment and hematopoiesis through unknown mechanisms. RBC: red blood cell; MPP: multipotent progenitor; CMP: common myeloid progenitor; MEP: megakaryocyte-erythrocyte progenitor; GMP: granulocyte-monocyte progenitor; CLP: common lymphoid progenitor.
Knockout of SCF in adipocytes with an adiponectin driver does not influence circulating SCF concentrations or phenotypes of the peripheral adipose depots, which is perhaps due to compensatory expression of SCF from other sources, such as endothelial cells, fibroblasts and stromal cells. Interestingly, Zhang et al. observed a significant loss of SCF in the bone marrow supernatant, which indicates that BMAT is a primary source of SCF in bone marrow.5 Deficiency of SCF in BMAT reduces the bone marrow cellularity, hematopoietic stem and progenitor cells (HSPC), common myeloid progenitors (CMP), megakaryocyte-erythrocyte progenitor (MEP) and granulocyte-monocyte progenitors (GMP) under steady-state condition. Consistent with these changes in the progenitor cells of bone marrow, mice deficient for adipocyte SCF develop macrocytic anemia and reduction of neutrophils, monocytes and lymphocytes in circulation. In contrast to results in this study, Zhou et al. reported that the conditional deficiency of SCF in adipocytes driven by adiponectin-Cre/ER had no effect on hematopoiesis under basal conditions.3 Although further investigation is necessary, the discrepancy between these two studies might be due to the time-frame of SCF deletion, tamoxifen injection and/or animal lines. Of note, the deletion of SCF has no effect on the proliferation of HSPC evidenced by colony-forming assays, which suggests that defects in BMAT-derived SCF influences the bone marrow microenvironment rather than the intrinsic function of HSPC.
Since adiponectin-Cre is expressed in both peripheral adipocytes and BMA, it is possible that there might be effects on hematopoiesis that are independent of BMAT. To more specifically study effects of BMA on the bone marrow niche and hematopoiesis, Zhang et al. also deleted the Kitl using osterix promoter, which traces BMA but not the other adipocytes. Again, knockout of Kitl from the osterix-positive (+) cells reduced bone marrow cellularity, hematopoietic progenitor populations and mature blood cells including red blood cells (RBC), neutrophils and monocytes, which is consistent with the phenotypes from mice lacking adipocytic Kitl. Of note, in addition to BMA, osterix+ progenitors also trace to osteoblasts.9,10 Mesenchymal and osteoblast lineage cells are involved in the maintenance and regulation of the supportive microenvironments necessary for quiescence, self-renewal and differentiation of HSC.11,12 However, the SCF from osteoblasts is not required for HSC maintenance in adult bone marrow under steady-state conditions.13 Although the possible effects of SCF derived from osterix+ progenitors on hematopoiesis could not be excluded and the bone phenotypes were not explored in this mouse model, it should be appreciated that authors used both adiponectin- and osterix-driven Cre enzyme to confirm the phenotypes of SCF-deficiency on hematopoiesis. These results strongly point to BMA as an important source of SCF since the common cell type traced by adiponectin and osterix drivers is the BMA; however, development of BMA-specific transgenic mouse tools will be required to truly confirm these observations of BMA and the roles of SCF in the bone marrow niche homeostasis and hematopoiesis.
The authors also investigated whether BMA-derived SCF is required for hematopoietic adaptation to aging or high fat diet (HFD)-induced obesity. Whereas HFD, per se, did not increase the SCF concentrations in bone marrow supernatant, this treatment increased bone marrow cellularity, HSPC, and mature blood cells, including granulocytes, monocytes and lymphocytes, the effects of which were eliminated by SCF deficiency in adipocytes. Aging causes similar increases in the HSPC, especially in the myeloid lineage populations, and most of these effects required adipocyte-derived SCF. Further, these investigators explored a potential role for SCF in mediating effects of a β3-adrenergic receptor agonist. Activation of these receptors induces the lipolysis of white adipocytes, and while although BMAT lipolysis is relatively resistant to β-adrenergic signaling,14 Zhang et al. observed that after administration of a β3-adrenoceptor agonist, CL316, 243, SCF expression was increased in bone marrow without significant changes in the BMA numbers.5 Consistent with the elevated SCF in bone marrow, the numbers of HSPC, including Lin−Sca1+c-Kit+ (LSK) cell, multipotent progenitor (MPP), MEP, GMP and CLP were increased by CL316, 243 injection, the effects of which were compromised by adipocyte-specific deficiency of SCF. Based on the animal models described above, it should be noted that alterations of BMAT, SCF and hematopoiesis were not tightly associated under these conditions, which suggests that hematopoietic metabolism is regulated by factors beyond BMAT and its derived SCF. The global effects of obesity, aging and β3-adrenoceptor activation cannot be excluded from this scenario. In addition, other secreted factors from BMAT may also play significant roles in hematopoiesis under these conditions. Unfortunately, the secretome of BMAT remains largely unexplored.
In summary, Zhang et al.5 have extended our understanding of the roles of BMAT in the bone marrow niche and the interaction between BMA and hematopoietic cells. They thoroughly addressed their hypotheses using a variety of animal models and complete profiling of hematopoietic changes. However, due to the complexity of whole-body metabolism and the lack of BMA-specific transgenic tools, further work will be required to determine whether BMA-derived SCF regulates hematopoiesis directly through Kitl/c-Kit signaling in hematopoietic cells or indirectly by changing the microenvironment of the bone marrow niche.
Acknowledgments
This work was supported by grants from the NIH to OAM (R24 DK092759; R01 DK62876), and from the American Diabetes Association to ZL (1-18-PDF-087).
References
- 1.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, Hardij J, Bagchi DP, Scheller EL, MacDougald OA. Development, regulation, metabolism and function of bone marrow adipose tissues. Bone. 2018;110:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Hardij J, Evers SS, et al. G-CSF partially mediates effects of sleeve gastrectomy on the bone marrow niche. J Clin Invest. 2019;130:2404–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Huang Z, Ong B, Sahu C, Zeng H, Ruan HB. Bone marrow adipose tissue-derived stem cell factor mediates metabolic regulation of hematopoiesis. Haematologica. 2019;104(9):1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nocka K, Majumder S, Chabot B, et al. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice–evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989;3(6):816–826. [DOI] [PubMed] [Google Scholar]
- 8.Bosbach B, Deshpande S, Rossi F, et al. Imatinib resistance and microcytic erythrocytosis in a KitV558Delta;T669I/+ gatekeeper-mutant mouse model of gastrointestinal stromal tumor. Proc Natl Acad Sci U S A. 2012;109(34):E2276–E2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27(11):2344–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29(3):340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. [DOI] [PubMed] [Google Scholar]
- 12.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. [DOI] [PubMed] [Google Scholar]
- 13.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheller EL, Khandaker S, Learman BS, et al. Bone marrow adipocytes resist lipolysis and remodeling in response to beta-adrenergic stimulation. Bone. 2019;118:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]