Abstract
Phenols are attractive starting materials for the preparation of highly substituted cyclohexane rings via dearomative processes. Herein we report an efficient preparation of dearomatized 1-oxaspiro[2.5]octa-4,7-dien-6-ones (para-spiroepoxydienones) via the nucleophilic epoxidation of in situ generated para-quinone methides from 4-(hydroxymethyl)phenols using aqueous H2O2. The developed protocol bypasses the need for stoichiometric bismuth reagents or diazomethane, which are frequently deployed for p-spiroepoxydienone preparation. The p-spiroepoxydienones are further elaborated in numerous downstream complexity-building transformations.
Graphical Abstract

Aromatic feedstock compounds provide an intriguing and economical source for accessing functionalized, complex organic building blocks in a rapid fashion. The latent functionality embedded within these aromatic cores, including phenols, is often unveiled via a wide number of dearomative transformations;1 however, to overcome the aromatic stabilization energy of the benzenoids, stoichiometric quantities of toxic heavy metals (i.e., lead, silver, and bismuth) have frequently been needed to provide the required thermodynamic driving force.2 In addition to their inherent toxicity, the use of these metal reagents frequently entails high costs and results in low product yields, ultimately limiting their applicability in industrial settings; therefore, new dearomative methods involving inexpensive and benign oxidants, such as hydrogen peroxide (H2O2), are desirable for their favorable byproduct profiles and reactivities.
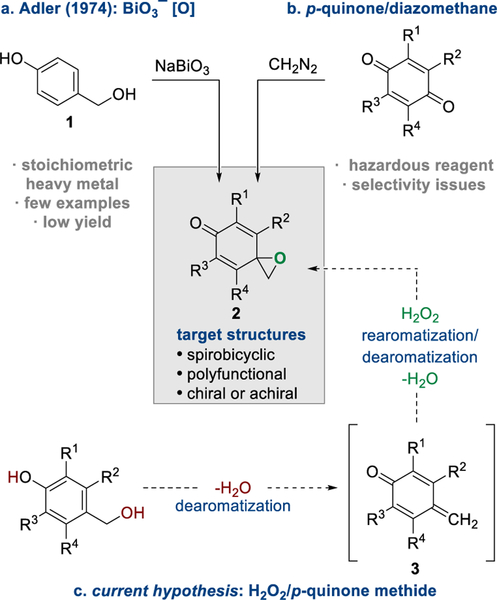
In 1974, Adler described the preparation of dearomatized 1-oxaspiro[2.5]octa-4,7-dien-6-one (p-spiroepoxydienone) 2a via the oxidation of 4-(hydroxymethyl)phenol 1 with sodium bismuthate (NaBiO3) (Scheme 1a).3a While novel, this approach required stoichiometric quantities of NaBiO3 and afforded 2a in poor yield (20–30%).3b Nevertheless, this method for the preparation of p-spiroepoxydienones 2 has been employed in the synthesis of bioactive and complex molecules.4 The low yield obtained with NaBiO3 has resulted in an alternative approach to synthesizing p-spiroepoxydienones. The addition of diazomethane (CH2N2) into highly substituted para-quinone derivatives (Scheme 1b) affords improved yields of the desired product;5 however, the dual quinone carbonyls present regioselectivity complications, and serious hazards associated with CH2N2 prevent its deployment on large scales. NaIO4, an enabling reagent for the analogous oxidation of the ortho-isomer (Adler–Becker oxidation) has not, to the best of our knowledge, been successfully used for the para-isomer, indicating important structural or mechanistic differences.3
Scheme 1.
Methods for Accessing p-Spiroepoxydienones
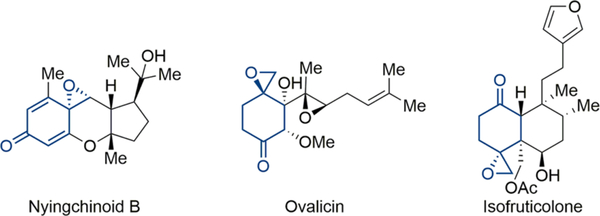
Because of these limitations to the established methods for p-spiroepoxydienone generation as well as the prevalence of naturally occurring bioactive molecules bearing the p-spiroepoxide substructure (Figure 1),6 we were interested in the development of a new approach to these privileged scaffolds.
Figure 1.
Representative bioactive compounds bearing p-spiroepoxide substructure.
The oxidative dearomatization of salicyl alcohol derivatives using H2O2 was recently reported7. The reactions deliver o-spiroepoxydienones via a transient ortho-quinone methide intermediate. Encouraged by the success of this approach, we proposed the reaction design outlined in Scheme 1c that accesses p-spiroepoxydienone 2 from 4-(hydroxymethyl)-phenol 1 via the intermediacy of a para-quinone methide 3. para-Quinone methides 3 (pQMs) are highly reactive dearomatized intermediates that have been extensively explored in recent years due to their ability to form complex building blocks.8 The inherent reactivity exhibited by pQMs is governed by the strong aromatic driving force, which has predicated numerous nucleophilic 1,6-conjugate additions to afford derivatized phenolic products.9 Because of this aromatic driving force, transformations involving pQMs resulting in isolable, dearomatized building blocks for further elaboration are limited.10
Our working hypothesis, outlined in Scheme 1, required a resolution to a procedural challenge in accessing the transient pQMs in situ. Because of the high reactivity of these ephemeral species, a significant majority of methods involving pQM intermediates often rely on the prior synthesis of stable derivatives.11 Because of the ease of preparation and long shelf lives, these stable pQMs have been widely employed;8,9 however, the strict substrate requirements of stable pQMs limit the scope of products available to these transformations (Scheme 2a). In contrast, strategies for the preparation of transient pQMs in situ using basic conditions have been reported.12 These approaches are usually restricted to benzylic aryl substitution, a characteristic that is often incompatible with the stability of p-spiroepoxydienones 2 due to rapid decomposition.13
Scheme 2.
Methods for p-Quinone Methide Generation
Bis(dichloroacetate) esters of phenolic o-benzylic alcohols are appropriately activated for oQM formation in the presence of (−)OOH.7 This cheap and stable activating group exhibited a wide substrate scope, and oQM formation could be easily achieved via base-promoted phenolic deprotection, followed by the expulsion of the benzylic acetate. Considering the known differences in spiroepoxide synthesis from ortho- and para-benzylic alcohol phenols, it was an open question as to whether our previous method could be extended to the para-series. To test this hypothesis, we prepared a variety of 4-(hydroxymethyl)phenols 1 and activated them for projected pQM formation via the single-step preparation of heretofore unknown bis(dichloroacetates) 4 in high yield (>90%) (Scheme 2b).
Using optimized conditions,14 the bis(dichloroacetates) 4 were evaluated as suitable reaction partners in the one-pot pQM formation and subsequent homo-Weitz–Scheffer epoxidation (Scheme 3).15 Bis(dichloroacetate) 4a afforded the achiral p-spiroepoxide 2a in 66% yield, a significant improvement over the NaBiO3-based methodology.3 Scaling up this dearomatization afforded 5.0 g of 2a while maintaining practical yields. Benzylic alkyl substitution (R5) (2b–d) was tolerated, a trend that held true when employing (±)-synephrine as the phenolic precursor (2c).
Scheme 3.
Scope of Oxidative Dearomatization Using Bis(dichloroacetates) of 4-(Hydroxymethyl)phenols
aReactions performed with 1.0 equiv of 4 and 3.0 equiv of H2O2 and KOH in MeCN ([4]0 = 0.05 M). Isolated yields. bFour equiv of KOH was used.
Alkyl substitution (2e–h) provided for the highest yields due to the increased stability of the pQM intermediate.16 Turning our attention to halogenated substrates, we found that similarly good yields could be obtained with chlorine substitution (2i–k); however, bromine and iodine were less tolerated under the optimized reaction conditions (2l,m). Tricyclic spiroepoxides (2n–p) were obtained in high yield.
We identified a few limitations for this reaction. Highly electron-rich substrate 4q was incompatible with the optimized reaction conditions due to the poor electrophilicity of the methide. Additionally, we confirmed the instability of R5 aryl substitution using our reaction conditions.13 Subjecting bis(dichloroacetate) 4r to the reaction conditions resulted in the isolation of hydroquinone and benzaldehyde, byproducts of the rapid decomposition of spiroepoxide 2r.3a,13
1-Oxaspiro[2.5]octa-4,7-dien-6-ones 2 provide an interesting platform for further derivatization into complex organic frameworks. Until now, the reactivity of these compounds has been sparsely evaluated, presumably because of the lack of efficient preparation strategies. Accordingly, we sought to expand the reactivity profile of the dearomatized species (Scheme 4).
Scheme 4.
Chemoselective Transformations of p-Spiroepoxydienonesa
aConditions: acyclopentadiene (3.0 equiv), TFE, 35 °C; bK2CO3 (50 mol %), 30% aq H2O2 (3.0 equiv), acetone, rt; cPh2O, 220 °C; dMeLi (1.1 equiv), THF, −78 °C, then NBS (1.1 equiv), CH2Cl2, rt; eMeMgBr (1.1 equiv), THF, −78 °C; fMeLi (1.0 equiv), THF, −78 °C; gPhNCO (1.1 equiv), DBU (1.0 equiv), CH2Cl2, rt; hNIS (4.0 equiv), MeCN, 50 °C; ipropargyl alcohol (3.0 equiv), Ph2O, 180 °C; jTMSCN (1.5 equiv), KF (10 mol % equiv), MeCN, 0 °C to rt.
Initial attempts at functionalizing p-spiroepoxydienones 2 revealed a unique reactivity as compared with para-quinols17 or 1-oxaspiro[4.5]deca-6,9-dien-8-ones.10b Namely, p-spiroepoxydienone 2 demonstrated a high proclivity toward rearomatization under a variety of reaction conditions, partially ascribable to the highly strained spiroepoxide moiety. Several transformations such as direct epoxidation, 1,4- or 1,2-addition, and hydrogenation resulted in rearomatization to the parent phenolic compound, in agreement with previous literature;4,5 however, the selective functionalization of 2a could instead be achieved by accessing Diels–Alder cycloadduct 53b using 2a and cyclopentadiene, followed by functionalization of the enone and subsequent retro-Diels–Alder cycloaddition. Using this route, bis-epoxide 6 could be prepared as a single diastereomer. Alternatively, pentacycle 7 could be realized via the reaction of 5 with MeLi, followed by bromonium-promoted cyclization using N-bromosuccimide (NBS).18
To evaluate the unconventional electrophilic sites of the molecule, various nucleophiles were tested against p-spiroepoxydienones 2. The reaction of spiroepoxide 2h with MeMgBr resulted in nucleophilic attack on the epoxide oxygen attaching a pendant alkyl group while reestablishing the aromaticity to afford phenol 8. Conversely, upon exposure to MeLi, spiroepoxide 2h was converted to dearomatized dienone 9 via carbonyl addition, followed by a 1,2-alkyl shift to vinylogously open the strained spiroepoxide moiety. Taking advantage of the new hydroxymethyl handle, the reaction of dienone 9 with phenyl isocyanate followed by iodolactamization resulted in spirocarbamate 10. Thermal Diels–Alder cycloaddition between propargyl alcohol and dienone 9 afforded bicyclic diene 11 featuring two primary alcohols. p-Spiroepoxide 2a was highly prone to rearomatization when treated with alkylmetal nucleophiles; however, employing trimethylsilyl cyanide (TMSCN), the 1,2-addition product was isolated as silyl ether 12, preventing the 1,2-cyano shift to restore aromaticity.
In conclusion, we have developed an efficient preparation for p-spiroepoxydienones that proceeds through an in situ generated p-quinone methide. This method bypasses the need for stoichiometric bismuth or hazardous diazomethane by using aqueous H2O2 as the oxidant while allowing superior yield and substrate scope. The unique reactivity these p-spiroepoxydienones exhibit was further explored via a number of complexity-building transformations. This methodology demonstrates the potential for complementary dearomative processes via p-quinone methide intermediates, and our laboratory is currently exploring these possibilities.
Supplementary Material
ACKNOWLEDGMENTS
The project described was supported by Award R35 GM118055 from the National Institute of General Medical Sciences. M.F.M. is an NSF Graduate Research Fellowship. T.C. acknowledges support from the North Carolina School of Science and Mathematics (NCSSM). We thank the UNC Department of Chemistry Mass Spectrometry Core Laboratory, especially Dr. Brandie Ehrmann, for assistance with mass spectrometry analysis on instrumentation acquired through the NSF MRI program under Award CHE-1726291. We thank the UNC Department of Chemistry NMR Core Laboratory, especially Dr. Marc ter Horst, for assistance with NMR analysis on instrumentation acquired through the NSF MRI program under Award CHE-1828183.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.or-glett.9b02372.
1H and 13C NMR spectra of all newly synthesized compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).For recent reviews on dearomatization, see:; (a) Roche SP; Porco JA Dearomatization Strategies in the Synthesis of Complex Natural Products. Angew. Chem., Int. Ed 2011, 50, 4068. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhuo C; Zhang W; You S Catalytic Asymmetric Dearomatization Reactions. Angew. Chem., Int. Ed 2012, 51, 12662. [DOI] [PubMed] [Google Scholar]; (c) Wertjes WC; Southgate EH; Sarlah D Recent Advances in Chemical Dearomatization of Nonactivated Arenes. Chem. Soc. Rev 2018, 47, 7996. [DOI] [PubMed] [Google Scholar]; (d) Pouységu L; Sylla T; Garnier T; Rojas LB; Charris J; Deffieux D; Quideau S Hypervalent Iodine-Mediated Oxygenative Phenol Dearomatization Reactions. Tetrahedron 2010, 66, 5908. [Google Scholar]
- (2).(a) Metlesics W; Wessely F; Budzikiewicz H Dien-Synthesen mit Chinolacetaten. Montash. Chem 1958, 89, 102. [Google Scholar]; (b) Barton SDR; Yadav-Bhatnagar N; Finet J; Khamsi J; Motherwell WB; Stanforth SP The Chemistry of Pentavalent Organobismuth Reagents. Part X. Studies on the Phenylation and Oxidation of Phenols. Tetrahedron 1987, 43, 323. [Google Scholar]; (c) Liao D; Li H; Lei X Efficient Generation of ortho-Quinone Methide: Application to the Biomimetic Syntheses of (±)-Schefflone and Tocopheol Trimers. Org. Lett 2012, 14, 18. [DOI] [PubMed] [Google Scholar]
- (3).(a) Adler E; Holmberg K; Ryrfors L; Szabo-Lin I; Guthenberg C; Mannervik B Periodate Oxidation of Phenols. XIV. Oxidation of p-Hydroxybenzyl Alcohol with Periodate and Bismuthate. Acta Chem. Scand 1974, 28b, 883. [Google Scholar]; (b) Ohkata K; Tamura Y; Shetuni BB; Takagi R; Miyanaga W; Kojima S; Paquette LA Stereoselectivity Control by Oxaspiro Rings During Diels-Alder Cycloadditions to Cross-Conjugated Cyclohexadienones: The Syn Oxygen Phenomenon. J. Am. Chem. Soc 2004, 126, 16783. [DOI] [PubMed] [Google Scholar]
- (4).Salamonczyk GM; Oza VB; Sih CJ A Concise Synthesis of Thyroxine (T4) and 3,5,3′-Triiodo-L-thryonine (T3). Tetrahedron Lett. 1997, 38, 6965. [Google Scholar]
- (5).Eistert B; Bock G Reaktionon von α-Dicarbonylverbindungen und Chinonon mit Diazoalkanen, II. Die Umsetzung Einiger Substituierter p-Benzochinone mit Diazomethan zu p-Epoxymethylcyclohexadienonen. Chem. Ber 1959, 92, 1247. [Google Scholar]
- (6).(a) Huang GH; Hu Z; Lei C; Wang PP; Yang J; Li JY; Li J; Hou AJ Enantiomertic Pairs of Meroterpenoids with Diverse Heterocyclic Systems from Rhododendron nyingchiense. J. Nat. Prod 2018, 81, 1810. [DOI] [PubMed] [Google Scholar]; (b) Corey EJ; Dittami JP Total Synthesis of (±)-Ovalicin. J. Am. Chem. Soc 1985, 107, 256. [Google Scholar]; (c) Coll J; Tandrón, Isolation and Structure Elucidation of Three neo-Clerodane Diterpenes from Teucrium fruticans L. (LABIATAE). Phytochemistry 2005, 66, 2298. [DOI] [PubMed] [Google Scholar]
- (7).McLaughlin MF; Massolo E; Liu S; Johnson JS Enantioselective Phenolic α-Oxidation Using H2O2 via an Unusual Double Dearomatization Mechanism. J. Am. Chem. Soc 2019, 141, 2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For recent reviews on p-QMs, see:; (a) Caruana L; Fochi M; Bernardi L The Emergence of Quinone Methides in Asymmetric Organocatalysis. Molecules 2015, 20, 11733. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Parra A; Tortosa M para-Quinone Methides: A New Player in Asymmetric Catalysis. ChemCatChem 2015, 7, 1524. [Google Scholar]; (c) Li W; Xu X; Zhang P; Li P Recent Advances in the Catalytic Enantioselective Reactions of para-Quinone Methides. Chem. - Asian J 2018, 13, 2350. [DOI] [PubMed] [Google Scholar]
- (9).For a recent example of a 1,6-conjugate addition resulting in rearomatized products, see:; Shirsath SR; Shinde GH; Shaikh AC; Muthukrishnan M Accessing α-Arylated Nitriles via BF3·OEt2 Catalyzed Cyanation of para-Quinone Methides Using tert-Butyl Isocyanide as a Cyanide Source. J. Org. Chem 2018, 83, 12305. [DOI] [PubMed] [Google Scholar]
- (10).For examples of 1,6-conjugate addition to stable pQMs resulting in dearomatized products, see:; (a) Yuan Z; Fang X; Li X; Wu J; Yao H; Lin A 1,6-Conjugated Addition-Mediated [2 + 1] Annulation: Approach to Spiro[2.5]octa-4,7-dien-6-one. J. Org. Chem 2015, 80, 11123. [DOI] [PubMed] [Google Scholar]; (b) Ma C; Huang Y; Zhao Y Stereoselective 1,6-Conjugate Addition/Annulation of para-Quinone Methides with Vinyl Epoxides/Cyclopropanes. ACS Catal. 2016, 6, 6408. [Google Scholar]; (c) Roiser L; Waser M Enantioselective Spirocyclopropanation of para-Quinone Methides Using Ammonium Ylides. Org. Lett 2017, 19, 2338. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Su Y; Zhao Y; Chang B; Zhao X; Zhang R; Liu X; Huang D; Wang K; Huo C; Hu Y [3 + 2] Cycloaddition of para-Quinone Methides with Nitrile Imines: Approach to Spiro-pyrazolinecyclohexadienones. J. Org. Chem 2019, 84, 6719. [DOI] [PubMed] [Google Scholar]
- (11).(a) Richter D; Hampel N; Singer T; Ofial AR; Mayr H Synthesis and Characterization of Novel Quinone Methides: Reference Electrophiles for the Construction of Nucleophilicity Scales. Eur. J. Org. Chem 2009, 2009, 3203. [Google Scholar]; For a single example of a nucleophilic epoxidation of a stable para-quinone methide, see:; (b) Buchanan GL; Jhaveri B Some Studies on Seven-Membered Ring Compounds. J. Org. Chem 1961, 26, 4295. [Google Scholar]
- (12).(a) Fan Y; Zhou L; Li S Catalytic Asymmetric 1,6-Conjugate Addition of in Situ Generated para-Quinone Methides with Tritylthiol. Org. Chem. Front 2018, 5, 1820. [Google Scholar]; (b) Wang J; Pan X; Liu J; Zhao L; Zhi Y; Zhao K; Hu L Diasteroselective Synthesis of Tetrahydroquinolines via [4 + 2] Annulation Between in Situ Generated p-Quinone Methides and Nitroalkenes. Org. Lett 2018, 20, 5995. [DOI] [PubMed] [Google Scholar]
- (13).(a) Weitz E Über einige Anthronabkommlinge. Leibigs Annalen 1919, 418, 29. [Google Scholar]; (b) Nishinaga A; Tomita H; Tarumi Y; Matsuura T Reaction of Superoxo Co(III) Complexes with 2,6-di-t-butyl-p-Benzoquinone Methides. Tetrahedron Lett. 1980, 21, 4849. [Google Scholar]
- (14).See the Supporting Information for the reaction optimization.
- (15).(a) Weitz E; Scheffer A Über die Einwirkung von alkalischem Wasserstoffsuperoxyd auf ungesättigte Verbindungen. Ber. Dtsch. Chem. Ges. B 1921, 54, 2327. [Google Scholar]; (b) Curini M; Epifano F; Marcotullio MC; Rosati O; Guo M; Guan Y; Wenkert E Hydridanone Synthesis: An Incisterol Model. Helv. Chim. Acta 2005, 88, 330. [Google Scholar]
- (16).Weinert EE; Dondi R; Colloredo-Melz S; Frankenfield KN; Mitchell CH; Freccero M; Rokita SE Substituents on Quinone Methides Strongly Modulate Formation and Stability of Their Nucleophilic Adducts. J. Am. Chem. Soc 2006, 128, 11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).For recent examples highlighting the reactivity of para-quinols, see:; (a) Corbett MT; Johnson JS Enantioselective Synthesis of Hindered Cyclic Dialkyl Ethers via Catalytic oxa-Michael/Michael Desymmetrization. Chem. Sci 2013, 4, 2828. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) García-García C; Ortiz-Rojano L;Álvarez S;Álvarez R; Ribagorda M; Carreño MC Friedel-Crafts Alkylation of Indoles with p-Quinols: The Role of Hydrogen Bonding of Water for the Desymmetrization of the Cyclohexadienone System. Org. Lett 2016, 18, 2224. [DOI] [PubMed] [Google Scholar]; (c) Kishi K; Takizawa S; Sasai H Phosphine-Catalyzed Dual Umpolung Domino Michael Reaction: Facile Synthesis of Hydroindole- and Hydro-benzofuran-2-Carboxylates. ACS Catal. 2018, 8, 5228. [Google Scholar]
- (18).For examples where this cyclization strategy has been employed for further elaboration using cyclopentadiene/para-quinone adducts, see:; (a) Honzumi M; Hiroya K; Taniguchi T; Ogasawara K Integrated synthesis of conduritols A–F using a single chiral building block. Chem. Commun 1999, 1985. [Google Scholar]; (b) Donohoe TJ; Johnson PD; Pye RJ; Keenan M Concise and Enantioselective Synthesis of the Aminocyclitol Core of Hygromycin A. Org. Lett 2005, 7, 1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.