Abstract
Polypropylene mesh is widely used in urogynecologic surgery, but complications rates (pain and exposure) approach 10%. Emerging evidence implicates the adaptive immune system in regulating the foreign body response to mesh, particularly regulatory T cells (Tregs), which modify macrophage differentiation and down-regulate CD8+ effector T cells. We hypothesize that Tregs protect against a profibrotic response, a likely mechanism of pain complications. Here, thin sections of mesh-tissue complexes removed for the primary complaint of pain (N=14) or exposure (N=15) were labeled for CD8, CD4 (Th), and FoxP3 (Tregs) via immunofluorescence. The same sections were analyzed for localized collagen deposition via a customized semi-quantitative assessment (0.25mm2 grid) after trichrome staining. TGF-β1 concentrations were determined by enzyme-linked immunosorbent assay. Fewer Treg and CD4+ cells were found in fibrotic areas versus non-fibrotic areas (503 and 550/cm2 fewer, respectively, both P <0.001). TGF-β1 was higher in mesh samples compared to autologous control biopsies. TGF-β1 inversely correlated with age, r −0.636 (p = 0.008). No differences were found in T cell subgroups or fibrotic indices between pain and exposure groups. A moderate inverse relationship was found between TGF-β1 and Tregs (r −0.402, P = 0.009). Tregs were present up to 12 years after mesh implantation, challenging the assumption that the adaptive immune response to a foreign body is transient. In conclusion, the inverse relationship between fibrosis and Tregs, and TGF-β1 and Tregs points to a protective role of these cells. Similar immunologic responses in patients with pain and exposure suggest these complications exist along a spectrum.
Keywords: polypropylene mesh, T regulatory cells, fibrosis, prolapse, midurethral sling
Graphical Abstract
1. Introduction
Up to 20% of women will undergo surgery to correct pelvic organ prolapse (POP) or stress urinary incontinence (SUI) before the age of 80 [1]. While the use of polypropylene mesh in these surgeries improves durability and reduces operative time, it also increases the risk of complications which may necessitate removal, most commonly dues to pain at the site of the implanted mesh and/or exposure of the mesh through the vaginal epithelium [2–4]. While a variety of patient and surgical factors could influence the onset of these complications, very little is known about the role of the host immune response in these patient outcomes.
Similar to other types of implants, mesh implantation elicits a prolonged foreign body response with an influx of inflammatory cells and eventual fibrotic encapsulation of the mesh fibers [5, 6]. In some instances, collagen deposition around the mesh fibers may become excessive, leading to a pathologic fibrosis [7, 8], causing the mesh to deform and contract, which may contribute to the complaint of pain [9–11]. Mesh deformation with loading can also cause the mesh pores to collapse, leading to heightened inflammation, increased protease activity and degradation of the surrounding tissues, which may contribute to mesh exposure [12].
Within the fibrotic capsule, a variety of immune cells including macrophages, foreign body giant cells, and lymphocytes have been observed and are the predominant contributors to the foreign body reaction [5, 12, 13]. Among lymphocytes, an increase in T cells, specifically FoxP3+ Treg cells and CD4+ Thelper cells have been associated with complications defined predominately by pain. At the same time, increased CD8+ cytotoxic T cells have been associated with complications defined primarily by exposure [14]. However, the role that these T lymphocytes play in the outcomes of the foreign body reaction to the mesh remains unclear.
Tregs generally function as immune suppressors, preventing over-activation of T effector cells that can lead to a damaging host immune response [15–18]. Tregs also mediate the resolution of inflammation and promote healing, thereby mitigating chronic or prolonged inflammatory responses [19]. Given the prolonged inflammatory response identified in urogynecologic meshes removed for complications, the presence of fibrosis and the association of pathologic tissue fibrosis with persistent inflammation in other conditions [20], we hypothesized that the fibrotic response to urogynecologic meshes would be mediated by Treg cells and the number of Tregs would be inversely related to the amount of fibrosis. Consistent with this hypothesis, we anticipated that complications defined primarily by pain, a response thought to be associated with fibrosis, would be associated with low T regs, increased collagen deposition, and fibrotic encapsulation of the mesh as compared to the complications defined primarily by exposure. We therefore, aimed to measure collagen deposition and Treg cell concentrations in the tissue surrounding mesh fibers in mesh samples removed from women with the complications of pain versus exposure. In addition, the ratio between Treg and CD8+ effector cells [21], was examined as a measure of the overall immune response (tolerogenic/proinflammatory). Finally, because TGF-β1 is an important mediator of fibrosis [22] and indispensable for appropriate function of Treg cells [23, 24], we determined TGF-β1 concentrations and compared the amount of this cytokine in mesh tissue complexed removed for pain vs exposure.
2. Materials and Methods
2.1. Patient selection
This study was approved by the Institutional Review Board at the University of Pittsburgh Medical Center (9/11/2017). Patients who underwent excision of vaginal mesh for the predominant indication of pain versus exposure at Magee Women’s Hospital were offered enrollment in the Magee Mesh Biorepository and underwent informed consent. Samples collected included excised mesh-tissue complexes and an optional biopsy adjacent but not including mesh. Pain was defined as a primary complaint of pain including pain with palpation, movement, or with intercourse, without evidence of exposure. Exposure was defined as a diagnosis of any mesh showing through the epithelium as determined by their surgeon. Exclusion criteria included acute infection as determined by their surgeon, chronic immunosuppressive therapy or diagnosis of an autoimmune disorder. Demographic data including age, BMI, gravity, parity, smoking status, menopausal status, use and type of hormone replacement therapy, and comorbidities as well as variables describing the initial mesh placement, complications and removal were collected at the time of enrollment and mesh removal using a standardized form developed a priori. A randomly selected subset of samples removed for pain (n=14) or exposure (n=15) was analyzed for this study. Tissue obtained from 2 distinct control cohorts was analyzed. For 9 out of 16 samples, a full thickness vaginal biopsy obtained from the same patient at a site in the vagina adjacent to the mesh was available for analysis (autologous control) and for 14 out of 16 samples, a vaginal control biopsy matched for age, BMI and presence/absence of prolapse was available for comparison from women undergoing surgery for prolapse or stress incontinence (matched control). In addition, a total of 30 grids from 6 ungrafted control biopsies from patients with prolapse or stress incontinence were used to determine pathologic collagen content.
2.2. Histologic preparation
Mesh-tissue complexes were placed in a sterile container on ice immediately after s urgical removal. They were then imbedded in O.C.T. compound (Tissue-Tek; Sakura Finetek USA Inc, Torrance, CA), flash frozen in liquid nitrogen, sectioned at 7μm and preserved at −80° C.
2.3. Immunofluorescent labeling
Sections were fixed with acetone and a hydrogen peroxide incubation was used to reduce autofluorescence. Samples were blocked with 2% bovine serum albumin. Primary antibodies to CD4 (CD4 anti-goat 1:50, Santa Cruz) and FoxP3 (FoxP3 anti-mouse 1:150, Abcam) were applied overnight at 4° C followed by secondary antibody incubation (Biotin anti-Goat and HRP anti-mouse at 1:500) and amplification with Alexa Flour 647 streptavidin and Alexa Flour 594 tyramide HRP conjugate. Negative controls were run concurrently with 2% bovine serum albumin omitting the primary antibody. Aqueous mounting medium containing DAPI (Vectashield with DAPI, Vector Laboratories, Burlingame, CA) was applied. Separate slides were triple labeled for CD8+, FoxP3+, and DAPI to assess Treg/cytotoxic T cell ratios. Large immunofluorescent images for each of the whole tissue sections on the slides were taken and analyzed using a Nikon ECLIPSE 90i upright microscope and Nikon NIS Elements Imaging Software (Nikon USA, Melville, NY). Fiber areas were measured. Samples were compared to negative controls to ensure specific labeling and were run in duplicate or triplicate. Two samples were excluded for scant tissue. Immunofluorescent images were taken and analyzed using a Nikon ECLIPSE 90i upright microscope and Nikon NIS Elements Imaging Software (Nikon USA, Melville, NY). CD4+ and FoxP3+ cells per 0.25mm2 were obtained as concentrations and total CD4+ and CD8+ cell counts were obtained per tissue section and normalized by fiber area. Treg cells per 100 CD8+ cells and Treg cells per 100 CD8+ cells per cm2 fiber area were calculated. To correlate the location of CD4+ and FoxP3+ cells to fibrotic response, we developed a customized quantification system, described in detail in 2.5 below.
2.4. Trichrome staining
To evaluate collagen deposition, the coverslips and DAPI mounting medium on the slides that underwent immunofluorescence labeling were gently dislodged and removed by shaking the slides in 1xTBST washing buffer. A standardized Masson’s trichrome staining protocol omitting hematoxylin cell nucleus staining was applied. The samples were reimaged using the Nikon ECLIPSE 90i upright microscope after trichrome staining.
2.5. Fibrosis quantification
A digital grid composed of 0.25 mm2 squares was laid over the trichrome and immunofluorescent images. In each grid, the intensity of collagen fibrosis was quantified using a digital intensity profile. The intensity profile generates a data point for every horizontal pixel in the image; 10 equally spaced profiles were taken along the vertical axis of the grid for representation. The background intensity, determined by histogram with visual confirmation, was subtracted from each data point. Artifacts, such as bubbles induced from the coverslip medium, generally produced a consistent intensity and were excluded.
Intensity in each grid was then averaged and the grids were classified into tertiles of collagen content. By comparing to vaginal control biopsies from patients with prolapse or stress incontinence but without mesh, the intensity profiles in mesh samples were define d as mild, moderate and high (pathologic fibrosis). Grids with high intensity were found to be more than 2.5 standard deviations higher than controls (p<0.001) and was considered pathologic fibrosis. Treg cells and CD4+ cells were counted in each grid giving a concentration of cells/mm2. Treg and CD4+ concentrations in grids with pathologic fibrosis were compared to those with mild or moderate levels of intensity.
2.6. Cytokine Quantification
Sufficient amount of tissue from the mesh-tissue complex was available for cytokine analysis in 16 samples, 9 of which had biopsies taken as a control adjacent to the mesh site. For 14 of these 16 samples, an additional vaginal control biopsy from a patient who did not have mesh was matched based on age (<age 50, 50–70, >70), BMI (by NIH obesity class) and presence or absence of prolapse (≥stage 2) or stress incontinence.
Protein concentrations were determined using a DC protein assay (Bio-Rad,Hercules, CA). TGF-β1 was quantified using a commercially available enzyme-linked immunoabsorbant assay (R&D Systems, Minneapolis, MN). Samples were run in duplicate with 50 μg of protein per sample.
2.7. Statistical analysis
Sample size calculation was based on preliminary data of 7 mesh-tissue complexes with an average Tregs/μm2 of 0.00011 in areas of high fibrosis compared to 0.00027 in areas of low fibrosis. Using the standard deviation of 0.00014 seen in preliminary data, power analysis with a of <0.05 and a power of 80% yielded a desired sample size of 13 samples to detect a difference in T cell populations in high versus low fibrosis areas using a two-tailed t-test. For cytokine analysis it was calculated that 11 mesh samples and 11 control biopsies would be needed to detect the expected 45% difference at 80% power based on prior studies [14]. Treg and CD4+ cell concentrations in pathologic fibrosis versus non-fibrotic grids were compared using mixed effects linear modeling. Treg and CD4+ concentrations, Treg and CD8+ cell counts per tissue section, Treg cells per 100 CD8+ cells, Treg cells per 100 CD8+ cells per cm fiber area and average fibrosis levels were compared using Spearman correlations and between pain and exposure groups and mid-urethral sling and prolapse mesh groups using Mann-U Whitney tests. Simple and multiple linear regression was used to assess for correlations between Treg and CD8+ cells while controlling for duration, mesh type and fiber area.
TGF-β1 levels were compared using Wilcoxon signed ranks tests for comparison between groups, Pearson’s R for correlation between TGFβ–1, Treg concentrations, age, BMI and duration of implantation with multivariate linear regression used to control for variables that had a significant or borderline significant Pearson’s correlation.
Statistical analysis was performed at the 0.05 significance level with STATA software (version 15.1; StataCorp, College Station, Texas) and SPSS (IMB, Armonk, NY). Data is reported as mean ± SD for baseline patient data and TGF-1β concentrations and median (interquartile range) for cell concentrations.
3. Results
3.1. Patient demographics
Women enrolled in the study were middle aged, normal weight and parous (Table 1). Among the pain samples, 10 were mid-urethral slings (MUS) and 4 were prolapse meshes. Of exposure samples, 7 were MUS and 8 were prolapse meshes. There were no differences in age, BMI, parity, tobacco use, rate of diabetes, menopausal status, hormone use, or prior hysterectomy based on removal indication (p>0.05). Mean time of implantation was 37.5 (interquartile range 16–56) months for pain and 58.8 (22–73) months for exposure (p = 0.38).
Table 1.
Demographic information of pain and exposure groups.
| Pain (n=14) | Exposure (n=15) | P value | |
|---|---|---|---|
| Age (y) | 53.5 (42–79) | 56.0 (54–68) | 0.21 |
| BMI (kg/m2) | 28.0 (25.1–31.5) | 30.0 (24.7–32.4) | 0.56 |
| Median parity (range) | 3 (2–5) | 3 (1–5) | 0.89 |
| Midurethral sling | 10 (71.4%) | 7 (46.7%) | 0.18 |
| Prolapse mesh | 4 (28.6%) | 8 (53.3%) | 0.18 |
| Current or smoking history | 7 (53.8%)* | 8 (53.3%) | 0.98 |
| Diabetes | 3 (21.4%) | 1 (6.7%) | 0.33 |
| Postmenopausal | 10 (71.4%) | 13 (86.7%) | 0.39 |
| Hormone therapy use | 7 (50.0%) | 8 (53.3%) | 0.89 |
| Prior hysterectomy | 10 (71.4%) | 9 (60.0%) | 0.70 |
| Duration implantation (mo) | 37.5 (16–56) | 58.5 (22–73) | 0.38 |
Median (interquartile range) is listed for continuous variables, frequencies (%) for categorical variables
smoking information missing on one patient, no difference between groups when data is input as current/former smoker or never smoker.
P values represent Chi2 or Fishers Exact when expected cell value <5.
3.2. CD8+ cells dominated the adaptive immune response
A predominance of CD8+ cells were seen surrounding the mesh fiber in a teardrop shape. Treg cells were seen in close association with CD8+ cells (Figure 2). The overall median number of Tregs per 100 CD8+ cells was 1.85 (IQR 0.61–5.80) with no differences in complication types (exposure vs. pain) and fibrotic intensity (pathologic fibrosis vs. non-fibrotic) (Table 3) (Figure 2). This number was similar for exposure and pain groups as well as non-fibrotic versus fibrosis samples (Table 3). The number of Tregs per 100 CD8+ cells decreased with longer duration of implantation (p = 0.019) but was not correlated with fiber area, average collagen content per patient or sling versus prolapse mesh (all P >0.2).
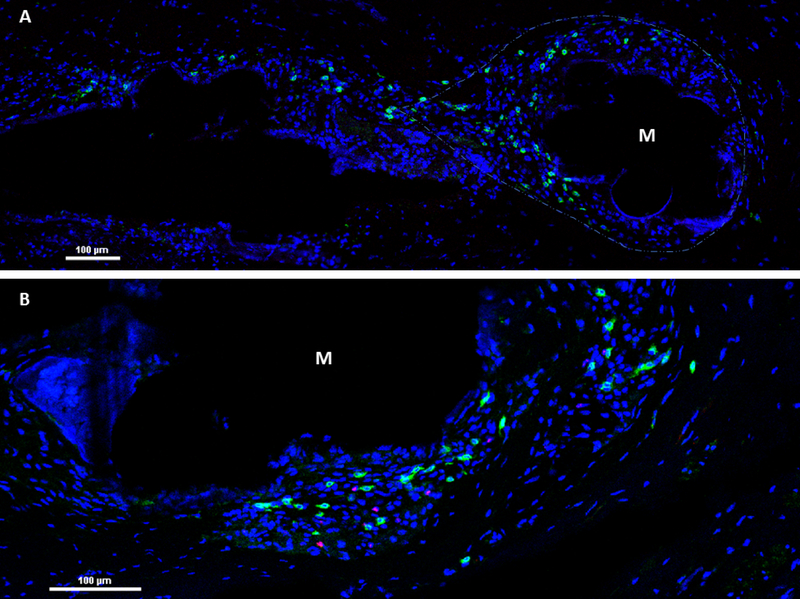
Figure 2. Immunofluorescence of CD8+ and Treg cells surrounding mesh knots.
Proinflammatory response with predominantly CD8+ cells (green) surrounding a mesh knot (M) in a teardrop shape (A). Treg cells (pink) are seen in smaller numbers (B). Nuclei are stained blue.
Table 3.
Median (Interquartile range) total Tregs, CD8+ cells and ratio of Treg per 100 CD8+ cells.
| Tregs/100 CD8+ cells | Total Tregs | Total CD8+ | P value | |
|---|---|---|---|---|
| Exposure | 1.74 (0.60–3.23) | 9 (2–15) | 377 (261–794) | >0.28 |
| Pain | 2.67 (1.05–6.46) | 11 (1–20) | 375 (138–555) | |
| Fibrosis | 1.56 (0.54–7.61) | 10 (2–17) | 377 (231–562) | >0.65 |
| Normal Collagen Content | 1.87 (0.62–5.72) | 9 (1–12) | 258 (116–1654) | |
3.3. Inverse correlation between Tregs and fibrosis
Treg and CD8+ cell populations persisted at similar concentrations for pain and exposure groups with durations ranging from 2 weeks to 12 years after implantation (p=0.5). On mixed effects generalized linear modeling, an inverse correlation between Treg concentration and areas with fibrosis (high intensity collagen profile) was seen with 503 fewer Treg and 550 fewer CD4+ cells per cm2 tissue when compared with non-fibrotic areas (mild/moderate intensity collagen profile; Figure 1, Table 2; P <0.001). Due to the local effect of mesh on the tissue environment, we also examined grids including or immediately adjacent to mesh fibers and saw a strong inverse trend with 716 fewer Treg cells per cm2 (P = 0.053). Sensitivity analysis was performed by excluding an outlier that had a Treg cell concentration >3 standard deviations beyond the mean with similar results.
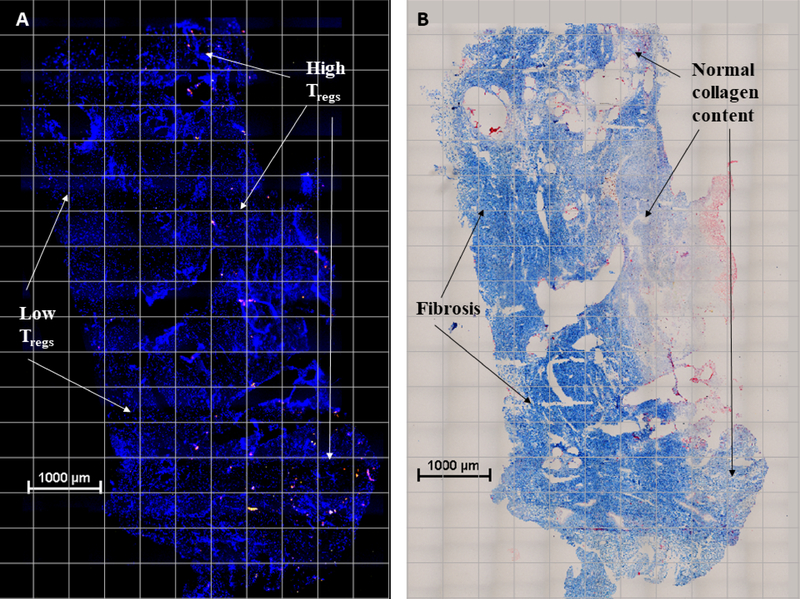
Figure 1. Inverse relationship between Treg cells and fibrosis.
Representative images of Th (CD4+, yellow), Tregs (FoxP3+, red) shown in immunofluorescent micrograph of mesh-tissue explant (A) and trichrome stain of same tissue section with collagen deposition in blue (B) demonstrating low Treg cell concentrations in areas of fibrosis compared to high Treg cell concentrations in areas of normal collagen content.
Table 2.
Treg cell concentration in areas of fibrosis compared to normal collagen content.
| Normal collagen content | Fibrosis | P value | |
|---|---|---|---|
| Treg concentration (cells/cm2) | Referent | −503 | 0.0003 |
| CD4+ concentration (cells/cm2) | Referent | −544 | <0.0001 |
Values represent mixed effects generalized linear modeling, a least biased estimate accounting for multiple areas measured per sample. Raw cell counts are found in Table 3
3.4. Prolapse mesh samples had fewer Treg cells/mm2 compared to sling mesh samples
Prolapse mesh samples had lower Treg cell concentrations than mid-urethral sling mesh samples with 1.05 Treg cells/mm2 (interquartile range [IQR] 0.46 – 6.8) compared to 6.23 (2.2–10.70) Treg cells/mm2, P = 0.024. A similar effect was seen when looking at grids immediately surrounding mesh fibers (1.07 [0.97–9.77] Treg cells/mm2 vs 8.96 [1.50–19.73] Treg cells/mm2, P = 0.041).
3.5. No difference between pain and exposure in Treg concentrations
There were no significant differences between pain and exposure groups in median Treg cell concentration (2.57 Treg/mm2 for exposure with IQR of 1.0–8.3 versus 4.34 [1.59–10.31] pain, P = 0.57). There was also no difference in average fibrosis intensity level between pain and exposure groups (P = 0.83J. When looking only at grids that surrounded fibers, a similar result was seen (2.39 [0.98–13.21] Treg/mm2 for exposure versus 7.88 [1.36–15.22] pain, P = 0.64). These results were the same when controlling for sling vs prolapse mesh.
3.6. TGF-β1 is increased in mesh explants compared to control biopsies obtained from an adjacent site without mesh
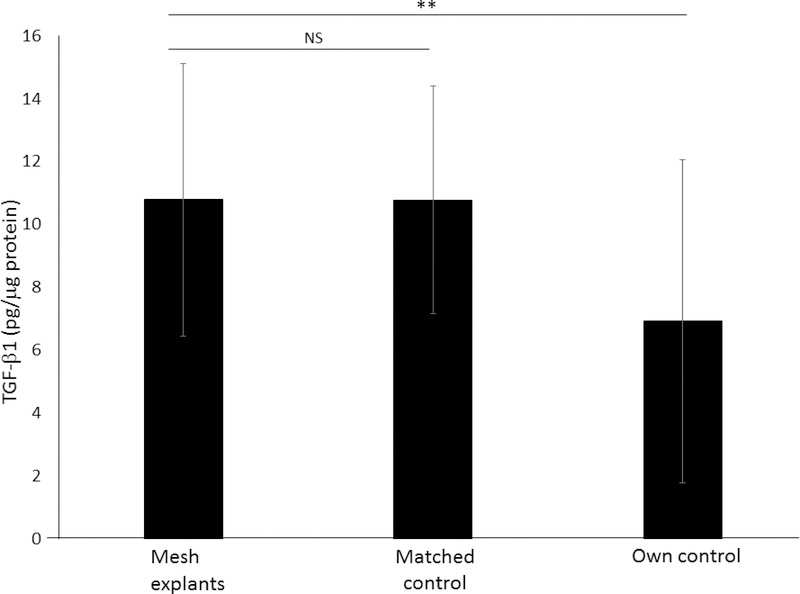
TGF-β1 was significantly higher in mesh samples compared to autologous vaginal control biopsies taken from an adjacent site without mesh at the time of mesh removal (13± 4 vs 7±5pg/μg protein, p = 0.015), but not when compared to a matched control taken from a patient undergoing surgery for pelvic organ prolapse or stress urinary incontinence with no history of prior mesh placement (10±4vs 11±5, P = 0.82, Figure 3).
Figure 3. TGF-β1 (pg/μg protein) in mesh-tissue explants compared to controls.
For controls, biopsies were obtained from 2 control cohorts: 1) matched controls undergoing gynecologic surgery with no history of prior mesh placement and 2) autologous controls in whom tissue was obtained from the vagina at a site adjacent to the mesh. Error bars represent interquartile range. P matched control = 0.82 **P = 0.015.
Age was inversely correlated with TGF-β1, r −0.636 (p = 0.008, Table 4). There was no difference in the amount of TGF-β1 by pain versus exposure or sling versus prolapse mesh (p>0.1). When controlling for duration of implantation, a moderate inverse relationship was seen between TGF-β1 and Treg concentration, r −0.402 (P = 0.009, Table 4).
Table 4.
Effect of age, duration of implantation and Treg concentration on TGF-β1.
| Effect on TGF-β1 | Unadjusted Pearson’s R | P value | Adjusted Coefficient | P value |
|---|---|---|---|---|
| Age | −0.636 | 0.008 | ||
| Duration | −0.492 | 0.053 | −0.073 | 0.005 |
| Treg concentration | −0.402 | 0.122 | −0.050 | 0.009 |
Univariate analysis was performed using Pearson’s R. Multiple linear regression was then run with the covariates duration and Treg concentration, and adjusted coefficients for these two variables and resultant p values are shown.
4. Discussion
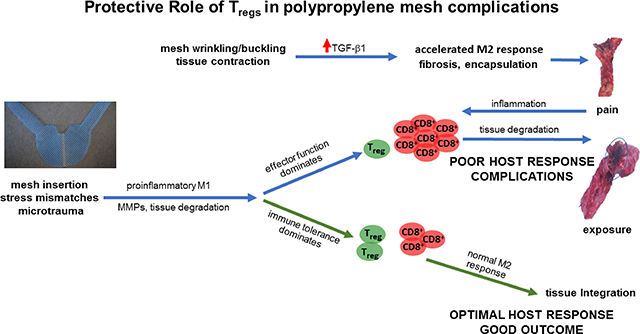
The adaptive immune response has been increasingly recognized to play critical role in the host response to urogynecologic polypropylene meshes. The most important findings in the current study were that 1) T cell subtypes (CD8+, CD4+, FoxP3+) were consistently present in mesh tissue complexes removed from women with complications (pain or exposure) and the Treg/CD8+ ratio was relatively constant with a median of 1 Treg cell per 50 CD8+ cells in all samples indicating ongoing prolonged inflammation; 2) the concentration of Treg cells was inversely co r related with the amount of fibrosis; 3) Lower Treg concentrations were seen in prolapse mesh tissue complexes as compared to mid-urethal slings 4) TGF-β1 was increased in mesh-tissue explants compared to control ungrafted biopsies taken from the same patient and 5) mesh-tissue complexes removed for exposure showed similar immunologic and histologic properties as those removed for pain suggesting they exist on the same pathophysiologic spectrum.
4.1. Consistent Treg/CD8+ ratio
Regardless of mesh fiber area, all samples showed a similar ratio of Treg/CD8+ cells with a decreased ratio over duration of implantation, driven by both a decrease in Tregs and an increase in CD8+ cells over time. A strong proinflammatory cytokine response with predominantly Ml macrophages has also been demonstrated in prior studies examining the impact of polypropylene mesh on the vagina [12, 25]. Although this may represent the normal host response, the chronicity here suggests poorly regulated inflammation in women with complications that does not diminish with time. This response may be in part due to repetitive microinjury associated with movement of a stiff biomaterial against a soft tissue like the vagina (the so-called “brillo pad effect”); however, it is also possible that baseline differences in the inflammatory status of women receiving mesh (implantation environment) impact the way in which the tissue responds. Further studies are needed to determine whether factors which favor a proinflammatory environment (obesity, diabetes, aging, smoking) increase the risk of complications.
4.2. The concentration of Treg cells was inversely correlated with fibrosis
In this study, significantly fewer CD4+ FoxP3+ T cells/cm2 were found in areas of fibrosis compared to non-fibrotic areas. Tregs are generated in response to TGF-β1 and are involved in self-tolerance and immune suppression. Tregs demonstrate the ability to suppress experimentally-induced autoimmune responses and impaired Treg function has been implicated in a variety of inflammatory and fibrosing conditions affecting humans [26–28]. For example, Tregs protect against liver and cardiac fibrosis by reducing influx of macrophages, T cells and myofibroblasts, decreasing cell proliferation and downregulating interstitial cell production of TGF-β1 [29, 30]. However, in pulmonary fibrosis, overproduction of Tregs deficient in immune suppressor activity is thought to contribute to fibrotic deposition by production of IL-4 and IL-13, and initiation of an overly robust TH2 response [31, 32]. Thus, both high and low Treg responses may lead to matrix accumulation and fibrosis, suggesting that future therapies aimed at maintaining T-cell homeostasis may benefit a wide range of conditions, including aberrant responses to foreign bodies such as urogynecologic meshes.
4.3. Lower Treg concentrations in prolapse mesh samples compared to slings
Prolapse samples had lower Tregs concentrations than sling samples, including near mesh fibers. This finding is consistent with clinical observations of higher complication rates with sheets of prolapse mesh as compared to the much smaller sling mesh. This may be because the mesh burden, or the amount of mesh in contact with the host, is increased in prolapse surgeries, which may lead to a stronger pro-inflammatory response. In addition, meshes used for prolapse have been shown to have unstable geometries with tensioning and loading [33], lea ding to both planar (pore collapse) and nonplanar (wrinkling) deformation limiting tissue incorporation into mesh pores and heightening the host immune response[12, 25, 34, 35].
4.4. TGF-β1 depletion
TGF-β1 was increased in mesh-tissue complexes compared to autologous biopsies from adjacent vaginal tissue without mesh (13±4 vs 7±5pg/μg protein, P = 0.015) similar to prior studies [14]. However, comparison of the amount of TGF-β1 in excised mesh tissue complexes and matched control biopsies obtained from gynecologic surgery patients without mesh did not show a similar decrease. This differential finding regarding TGF-β1 levels in autologous control tissue vs a matched control indicates a possible depletion effect in women with mesh complications. In other words, cells producing this profibrotic cytokine are recruited to the site of the mesh in women with mesh complications “depleting” their numbers and hence, TGF-β1, from adjacent sites without mesh.
TGF-β1 is known to induce pro-fibrotic signaling that can transform interstitial cells into myofibroblasts [36, 37]; however, it is also produced by Tregs and may contribute to immunotolerance [21, 38, 39]. In this study, we saw high levels of TGF-β1 in the mesh-tissue complex and an inverse relationship between TGF-β1 and Treg concentration, indicating that here, TGF-β1 likely contributes to fibrosis. These findings also support our conclusions that Tregs are protective against fibrosis.
4.5. Pain and exposure complications may exist on the same spectrum
In the multiple immunologic and histologic studies described here, we saw similar outcomes for complications dominated by mesh exposure and those dominated by pain, with a consistent trend towards slightly fewer Tregs and increased fibrosis in exposure patients. In addition, 3 out of 4 patients with areas of the highest collagen content were exposure patients. This indicates that pain and exposure likely exist on a spectrum of a dysregulated immune response, with exposure representing the end-stage complication.
4.6. Strengths, limitations and future directions
This is a robust, multifaceted study examining the T cell mediated adaptive immune response and its correlation with fibrosis. By using a collagen stain and immunofluorescent labeling of Tregs on the same slide, we were able to define and correlate the T cell and fibrotic responses at a very local level which could not be done using other methods of collagen quantification. These results are consistent with prior studies showing a strong correlation between TGF-β1 and collagen I, collagen III, and collagen III/I ratio in patients with pain [14]. In addition, by simultaneously examining both Treg and CD8+ cell concentrations, we were able to determine the balance between proinflammatory and tolerogenic cells. Finally, by assessing TGF-β1 we were able to provide additional support for our findings.
Limitations include lack of a true control group consisting of mesh removed without complication, as these are rarely removed from patients. Data from well-integrated mesh must come from animal studies. Although animal studies do not allow direct comparison with our present data, in rhesus macaques a stronger pro-inflammatory response has been seen in heavier weight, lower porosity mesh which are more prone to complications when compared with lighter weight, higher porosity mesh types [25]. This suggests that the pro-inflammatory response seen here is part of the pathophysiology of clinical complications. In addition, the use of vaginal control biopsies to define pathologic fibrosis vs. non-fibrosis in prolapse/SUI and to compare TGF-β1 levels provide additional control information.
Finally, although we defined our groups as pain in the absence of exposure or exposure, many of our exposure patients also reported pain albeit, to a lesser degree than patients with predominant pain symptoms. This overlap in clinical phenotypes is not well defined and future studies looking at pain scores and immunologic and histologic outcomes are planned.
4.7. Conclusions
T cell subtypes were present years after mesh implantation, challenging the common notion that the adaptive immune response to a foreign body is transient. The inverse relationship between fibrosis and Tregs as well as TGF-βl and Tregs points to a protective role of these cells. Similar Treg and CD8+ cell concentrations and levels of fibrosis in patients with pain and exposure suggests these complications exist along the same spectrum. Further research into a potential therapeutic role of Tregs is warranted.
Statement of Significance.
The use of polypropylene mesh has been associated with improved outcomes in urogynecologic surgery, but is associated with significant complications, including pain and exposure through the vaginal epithelium. The host immune response features a prolonged inflammatory reaction containing innate immune cells and T lymphocytes clustered in capsules around the mesh fibers. This study uncovers the inverse relationship between T regulatory cells and the extent of fibrosis around the mesh, suggesting an anti-fibrotic effect. In addition, concentrations of T regulatory and T effector cells and levels of fibrosis connect these two most common complications into one mechanistic pathway. These new insights into the immune response to implanted mesh are an important step in understanding the causes of these surgical complications.
Acknowledgments
Funding
This work was supported by the Pennsylvania Department of Health Tobacco Grant [grant number 6220] and the Eunice Kennedy Shriver NICHD NIH R01 HD083383-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amanda M ARTSEN, Magee-Womens Research Institute, Department of Obstetrics and Gynecology and Reproductive Sciences at Magee Womens Hospital, University of Pittsburgh, 204 Craft Avenue, Lab A320, Pittsburgh, PA 15213..
Mr. Matthew RYTEL, Magee-Womens Research Institute, Department of Obstetrics and Gynecology and Reproductive Sciences at Magee Womens Hospital, University of Pittsburgh, 204 Craft Avenue, Lab A320, Pittsburgh, PA 15213.
Rui LIANG, Magee-Womens Research Institute, Department of Obstetrics and Gynecology and Reproductive Sciences at Magee Womens Hospital, University of Pittsburgh, 204 Craft Avenue, Lab A320, Pittsburgh, PA 15213..
Mrs. Gabrielle E. KING, Magee-Womens Research Institute, Department of Obstetrics and Gynecology and Reproductive Sciences at Magee Womens Hospital, University of Pittsburgh, Pittsburgh, PA, USA.
Leslie MEYN, Magee-Womens Research Institute, Department of Obstetrics and Gynecology and Reproductive Sciences at Magee Womens Hospital, University of Pittsburgh, 204 Craft Avenue, Lab A320, Pittsburgh, PA 15213..
Steven D. ABRAMOWITCH, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA; Magee-Womens Research Institute, Department of Obstetrics and Gynecology and Reproductive Sciences at Magee Womens Hospital, University of Pittsburgh, Pittsburgh, PA, USA.
Pamela A. MOALLI, Magee-Womens Research Institute, Department of Obstetrics and Gynecology and Reproductive Sciences at Magee Womens Hospital, University of Pittsburgh; Department of Bioengineering, University of Pittsburgh, 204 Craft Avenue, Lab A320, Pittsburgh, PA 15213..
References
- [1].Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M, Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery, Obstet Gynecol 123(6) (2014) 1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wallace SL, Syan R, Sokol ER, Surgery for Apical Vaginal Prolapse after Hysterectomy: Transvaginal Mesh-Based Repair, Urol Clin North Am 46(1) (2019) 103–111. [DOI] [PubMed] [Google Scholar]
- [3].F. C, Update on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse, In: FDA, editor, July 2011. [Google Scholar]
- [4].Maher C, Feiner B, Baessler K, Schmid C, Surgical management of pelvic organ prolapse in women, Cochrane Database Syst Rev 4 (2013) CD004014. [DOI] [PubMed] [Google Scholar]
- [5].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin Immunol 20(2) (2008) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chung L, Maestas DR, Housseau F, Elisseeff JH, Key players in the immune response to biomaterial scaffolds for regenerative medicine, Adv Drug Deliv Rev 114 (2017) 184–192. [DOI] [PubMed] [Google Scholar]
- [7].Karabulut A, Simavli SA, Abban GM, Akyer Ş, Keskin N, Tan S, §ahin B, Tissue reaction to urogynecologic meshes: effect of steroid soaking in two different mesh models, Int Urogynecol J 27(10) (2016) 1583–9. [DOI] [PubMed] [Google Scholar]
- [8].Klinge U, Dietz U, Fet N, Klosterhalfen B, Characterisation of the cellular infiltrate in the foreign body granuloma of textile meshes with its impact on collagen deposition, Hernia 18(4) (2014) 571–8. [DOI] [PubMed] [Google Scholar]
- [9].Ben Amar M, Wu M, Trejo M, Atlan M, Morpho-elasticity of inflammatory fibrosis: the case of capsular contracture, J R Soc Interface 12(111) (2015) 20150343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klein F OC, Rudolph B, Wüstefeld J, Denecke T, Neuhaus P, Schmidt SC, Formation of a chronic pain syndrome due to mesh shrinkage after laparoscopic intraperitoneal onlay mesh (IPOM), Surg Laparosc Endosc Percutan Tech 22(5) (2012) 288–90. [DOI] [PubMed] [Google Scholar]
- [11].Wassenaar E, Schoenmaeckers E, Raymakers J, van der Palen J, Rakic S, Mesh-fixation method and pain and quality of life after laparoscopic ventral or incisional hernia repair: a randomized trial of three fixation techniques, Surg Endosc 24(6) (2010) 1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nolfi AL, Brown BN, Liang R, Palcsey SL, Bonidie MJ, Abramowitch SD, Moalli PA, Host response to synthetic mesh in women with mesh complications, Am J Obstet Gynecol 215(2) (2016) 206.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klopfleisch R, Jung F, The pathology of the foreign body reaction against biomaterials, J Biomed Mater Res A 105(3) (2017) 927–940. [DOI] [PubMed] [Google Scholar]
- [14].Tennyson L, Rytel M, Palcsey S, Meyn L, Liang R, Moalli P, Characterization of the T Cell Response to Polypropylene Mesh in Women with Complications, Am J Obstet Gynecol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Corthay A, How do regulatory T cells work?, Scand J Immunol 70(4) (2009) 326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lloyd CM, Hawrylowicz CM, Regulatory T cells in asthma, Immunity 31(3) (2009) 438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hawrylowicz CM, O’Garra A, Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma, Nat Rev Immunol 5(4) (2005) 271–83. [DOI] [PubMed] [Google Scholar]
- [18].Sakaguchi S, Setoguchi R, Yagi H, Nomura T, Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease, Curr Top Microbiol Immunol 305 (2006) 51–66. [DOI] [PubMed] [Google Scholar]
- [19].Julier Z, Park AJ, Briquez PS, Martino MM, Promoting tissue regeneration by modulating the immune system, Acta Biomater 53 (2017) 13–28. [DOI] [PubMed] [Google Scholar]
- [20].Wynn TA, Cellular and molecular mechanisms of fibrosis, J Pathol 214(2) (2008) 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wick G, Grundtman C, Mayerl C, Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R, Wolfram D, The immunology of fibrosis, Annu Rev Immunol 31 (2013) 107–35. [DOI] [PubMed] [Google Scholar]
- [22].Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R, The immunology of fibrosis: innate and adaptive responses, Trends Immunol 31(3) (2010) 110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M, Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance, J Exp Med 210(4) (2013) 775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA, Transforming growth factor-beta regulation of immune responses, Annu Rev Immunol 24 (2006) 99–146. [DOI] [PubMed] [Google Scholar]
- [25].Brown BN, Mani D, Nolfi AL, Liang R, Abramowitch SD, Moalli PA, Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque, Am J Obstet Gynecol 213(5) (2015) 668.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wolfram D, Rabensteiner E, Grundtman C, Bock G, Mayerl C, Parson W, Almanzar G, Hasenohrl C, Piza-Katzer H, Wick G, T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis, Plast Reconstr Surg 129(2) (2012) 327e–337e. [DOI] [PubMed] [Google Scholar]
- [27].Hu X, Liu G, Hou Y, Shi J, Zhu L, Jin D, Peng J, Zhao Y, Induction of M2-like macrophages in recipient NOD-scid mice by allogeneic donor CD4(+)CD25(+) regulatory T cells, Cell Mol Immunol 9(6) (2012) 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tan T, Xiang Y, Chang C, Zhou Z, Alteration of regulatory T cells in type 1 diabetes mellitus: a comprehensive review, Clin Rev Allergy Immunol 47(2) (2014) 234–43. [DOI] [PubMed] [Google Scholar]
- [29].Kanellakis P, Dinh TN, Agrotis A, Bobik A, CD4+CD25+Foxp3+ regulatory T cells suppress cardiac fibrosis in the hypertensive heart, J Hypertens 29(9) (2011) 1820–8. [DOI] [PubMed] [Google Scholar]
- [30].Nunoya J, Washburn ML, Kovalev GI, Su L, Regulatory T cells prevent liver fibrosis during HIV type 1 infection in a humanized mouse model, J Infect Dis 209(7) (2014) 1039–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moore MW, Herzog EL, Regulatory T Cells in Idiopathic Pulmonary Fibrosis: Too Much of a Good Thing?, Am J Pathol 186(8) (2016) 1978–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xiong S, Guo R, Yang Z, Xu L, Du L, Li R, Xiao F, Wang Q, Zhu M, Pan X, Treg depletion attenuates irradiation-induced pulmonary fibrosis by reducing fibrocyte accumulation, inducing Th17 response, and shifting IFN-y, IL-12/IL-4, IL-5 balance, Immunobiology 220(11) (2015) 1284–91. [DOI] [PubMed] [Google Scholar]
- [33].Barone WR, Amini R, Maiti S, Moalli PA, Abramowitch SD, The impact of boundary conditions on surface curvature of polypropylene mesh in response to uniaxial loading, J Biomech 48(9) (2015) 1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Feola A, Pal S, Moalli P, Maiti S, Abramowitch S, Varying degrees of nonlinear mechanical behavior arising from geometric differences of urogynecological meshes, J Biomech 47(11) (2014) 2584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liang R, Zong W, Palcsey S, Abramowitch S, Moalli PA, Impact of prolapse meshes on the metabolism of vaginal extracellular matrix in rhesus macaque, Am J Obstet Gynecol 212(2) (2015) 174.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Heinzmann D, Fuß S, Ungern-Sternberg SV, Schreieck J, Gawaz M, Gramlich M, Seizer P, TGFβ Is Specifically Upregulated on Circulating CD14++ CD16+ and CD14+ CD 16++ Monocytes in Patients with Atrial Fibrillation and Severe Atrial Fibrosis, Cell Physiol Biochem 49(1) (2018) 226–234. [DOI] [PubMed] [Google Scholar]
- [37].Seki E, Schnabl B, Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut, J Physiol 590(3) (2012) 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Johnston CJ, Smyth DJ, Dresser DW, Maizels RM, TGF-β in tolerance, development and regulation of immunity, Cell Immunol 299 (2016) 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Esebanmen GE, Langridge WHR, The role of TGF-beta signaling in dendritic cell tolerance, Immunol Res 65(5) (2017) 987–994. [DOI] [PubMed] [Google Scholar]