1. Introduction
Loss of ovarian function has been associated with increased risk for a variety of neuropsychiatric and neurodegenerative diseases, including anxiety and depressive disorders as well as Alzheimer’s and Parkinson’s disease [1, 2]. Some women experience loss of ovarian function relatively early in adult life, due either to premature ovarian failure or to surgical removal of the ovaries for prevention of cancer or other medical conditions. Studies suggest that these women have significantly increased long-term risk of cognitive impairment and dementia, as well as depressive and anxiety symptoms, compared to women with normal ovarian function [3–7]. Most women, however, undergo a natural menopause due to accelerated loss of ovarian follicles occurring at approximately 51 years of age [8]. Studies suggest that loss of ovarian function due to natural menopause also is associated with increased risks for cognitive impairments and neurodegenerative disease in women [9, 10]. This includes evidence of frontal lobe dysfunction [11], as well as evidence of impaired performance on verbal learning, verbal memory, and working memory tasks [12–14]. The mechanisms responsible for the effects of surgical and natural menopause on cognitive performance are still largely unclear.
It is well known that estrogens can significantly affect neuronal connectivity and function in the brain, with corresponding effects on cognitive performance. Examples include increased neuronal connectivity and synaptic function in areas of the brain critical for learning and memory [8, 15, 16], improved performance on specific cognitive tasks, including spatial learning tasks in rodents [17, 18], and verbal learning and memory tasks in humans [19], and reductions in risk of age-related cognitive decline [6, 9, 10]. Consequently, it is assumed that loss of estrogens contributes significantly to the cognitive impairments that have been associated with surgical and natural menopause. Estrogens exert their effects by binding to specific estrogen receptors (ERs) located throughout the brain. Three receptors have been identified. ERα and ERβ are ligand-dependent transcription factors acting in the nucleus to enhance nuclear transcription [20]. There is evidence that these receptors also can exist in membrane compartments and can participate in rapid effects of estrogens on specific transduction pathways [21]. Recently, a novel membrane bound G protein-coupled receptor, GPR30, was identified which is genetically and structurally unrelated to ERα and ERß, and can initiate estrogen responses at cellular membranes. [22]. All three receptors are expressed in many tissues, and within the brain that are well known to affect neuronal function, plasticity, and cognitive performance [23, 24].
Estrogens also have been shown to significantly affect monoaminergic neurotransmitter (NT) pathways in the brain, which are well known to be critically involved in neuropsychiatric and neurodegenerative diseases. The alteration of NT levels by estrogens and selective ER agonists is complicated and dependent on multiple factors such as brain region, dose, duration of treatment, type of treatment, as well as age and species of the animal. Many studies have been conducted by different laboratories demonstrating effects on specific NT pathways, however data are not always consistent [25–29]. A comprehensive analysis of simultaneous changes that occur both within and across pathways in multiple brain regions has not yet been conducted. Also it is not clear what role the individual estrogen receptors play, or what effects selective estrogen receptor modulators have on these NT pathways.
Recently we conducted an extensive study in rats characterizing monoaminergic endpoints in the brain using two models of menopause, as well as gonadally intact rats collected at proestrus and diestrus stages of the estrous cycle [30]. The two models of menopause included ovariectomy, a model of surgical menopause, and daily injections of 4-vinylcyclohexene diepoxide (VCD), which has been well studied and validated for use as a model of natural menopause in women [31–33]. In contrast to surgical menopause which produces a rapid and complete loss of ovarian hormones, the VCD model produces a gradual loss of ovarian follicles, leading to a corresponding reduction in estrogen production but with continued production of androgens by the remaining ovarian cells [34]. Hence the VCD model more accurately reflects the shift in the ratio of estrogens to androgens that occurs with natural menopause. The study measured multiple endpoints in three different brain regions from each rat at two time points, thus enabling a comprehensive characterization of simultaneous effects on different NT pathways [30]. Results showed significant effects on serotoninergic, dopaminergic, and noradrenergic endpoints that were both model and brain region specific, and that varied by time following loss of ovarian function.
In the current paper we used similar methods to investigate the effects of treating rats with selective ER agonists after undergoing either ovariectomy or VCD-induced follicular atresia. We hypothesize that the different menopausal models lead to differences in the effects of ER agonists on neurotransmitter endpoints in different regions of the brain. This, in turn, may help to explain reports which suggest that the effects of estrogen treatments on cognitive performance differ in women that have undergone transitional vs. surgical menopause. This is the first study to directly and systematically compare the effects of ER agonists on NT pathways in the brain in these two models of menopause.
2. Methods
2.1. Animals:
Female Sprague–Dawley rats (~11 weeks of age) were purchased from Hilltop Laboratories, Inc. All rats were individually-housed and maintained on a 12/12 h light–dark cycle (lights on at 7:00 AM) with free access to food and water. Rats were allowed to acclimate to the housing environment for two weeks prior to use. All experiments were conducted in accordance with the NIH Guide for Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
2.2. Menopausal Models:
Rats were randomly assigned either to the VCD or OVX group. Rats in the VCD group received 4-vinylcyclohexene diepoxide (VCD; Sigma Chemicals; St Louis, MO) at a dose of 80 mg/kg i.p. daily for a period of 30 days as previously described [32]. During this time, rats in the OVX group received daily injections of sesame oil (1ml/kg i.p.). At the end of 30 days, rats that had received sesame oil underwent bilateral ovariectomy, and rats that received VCD received sham surgery. Rats were anesthetized with ketamine (100 mg/kg) and xylazine (7 mg/kg) (0.1 cc/100 g.b.w.). Ovariectomy was performed using a lateral approach. The apical tips of the uterus were ligated, and the ovaries were removed. The peritoneal muscle was closed with 6.0 suture silk, and the overlying skin was closed with metal wound clips. Sham surgery, consisted of skin and muscle incisions and wound closure only. Antibiotic cream was applied on the wound to reduce chance of infection. Rats received Ketofen (3 mg/kg, i.p.) every day for 3 days to reduce post-surgical pain. One week of time was allocated for the rats to recover from surgeries before beginning agonist or vehicle treatment.
2.3. Estrogen receptor agonist treatments:
Rats received continuous administration of E2 (Sigma Chemicals; St Louis, MO), propylpyrazole triol (PPT; Tocris Cookson; Ellisville, MO), diarylpropiolnitrile (DPN; Tocris Cookson; Ellisville, MO), G-1 (1-[4-(6-bromobenzo[1,3] dioxol-5yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c] quinolin-8-yl]-ethanone; Cayman; Ann Arbor, MI), or vehicle (10% DMSO and 20% hydroxypropyl-β-cyclodextrin (HPβCD; Sigma Chemicals; St Louis, MO), beginning one week following ovariectomy or sham surgery. PPT is an ERα-selective agonist that is approximately 410-fold more selective for ERα than ERβ [35, 36]. DPN is an ERβ-selective agonist with 70-fold higher binding affinity and 170-fold higher potency for ERβ than ERα [37]. G-1 is non-steroidal, high-affinity selective GPR30 agonist, with no binding to the classic ERα or ERβ [38]. All drugs were administered by miniosmotic pump (Alzet model 2006; Durect Corp., Inc.) implanted subcutaneously in the dorsal neck region. These pumps have been repeatedly evaluated in our lab (e.g., by filling pumps, placing in normal saline solution at 37 °C and monitoring output for 6 weeks) and deliver a/t a constant rate of 0.15 μl/hr. E2 was administered at a rate of 3 μg/day (0.125 μg/hr). PPT, DPN and G-1 treatments each were administered at a rate of 5 μg/day (0.208 μg/hr). This dose and route of administration was selected based on previous published data from our lab showing that these treatments significantly enhance acquisition of a spatial learning task [39], as well as studies showing that E2 and G-1 significantly enhance potassium-stimulated ACh release in the hippocampus [40]. Treatments continued for either 1 week or 6 weeks. These treatment durations were chosen to evaluate early and late effects of the selective agonists and to evaluate changes in effects as a function of time. Treatment groups are summarized in the Supplemental Table.
2.4. Tissue Collection:
After 1 week or 6 weeks of treatment rats were anesthetized with an overdose of ketamine (3 mg) and xylazine (0.6 mg). Each pump was removed and inspected for any signs of damage or malfunction. The rat was then killed by decapitation. Trunk blood was collected for determination of serum levels of estradiol, testosterone, and androstenedione. Dissections were performed according to plate designations in Paxinos & Watson (1998) [41] and were as follows: hippocampus (HPC; plates 21–41), frontal cortex (FCX; plates 11–21), and striatum (STR; plates 11–21). These regions were selected because of the large body of work showing that each region plays an important role in learning and memory processes as well as important roles in extrapyramidal motor function (e.g., the STR) [42]. In addition, each of these regions have been shown to be influenced by ovariectomy and estrogen treatment [17, 43]. Tissues were stored at −80 °C until processed.
2.5. Brain sample Preparation:
500 μl 10 mM sodium acetate buffer (pH 6.5) was added to 50 mg tissue in a 1.5 ml microfuge tube. Tissues were sonicated at 4 °C until completely dissolved. After sonication, tissues were spun at 14,000 g at 4°C for 10 min. Supernatant was collected and placed at 4 °C. This step was repeated three times and the three supernatants were combined. The cell free supernatants were spun at 15,000g for an additional 40 minutes. A 200 μl aliquot was taken for the determination of protein concentration using the Bradford method [44]. The remaining volume of each supernatant was filtered through a disposable membrane (0.22μm pore size) micropartition device (Millipore Ultrafree-MC) under centrifugation at 14,000g for 30 min at 4 °C to remove any compounds above 10,000 nominal molecular weight limit.
2.6. Hormone assays:
Serum levels of E2, T, and AD were quantified as recently described [30, 45, 46]. For E2, samples were spiked with internal standard 25μl 2,4,16,16,17-d5–17 beta-estradiol (1 ng/ml in methanol) and extracted with 3–4 ml n-Butyl chloride. Samples were then centrifuged, and the organic layer was transferred to salinized culture tubes and dried under a steam of nitrogen. Residues were derivatized with dansyl chloride. Samples were centrifuged, and the supernatant was transferred into glass vials for LC-MS/MS analysis. E2 was eluted using a Waters Acquity UPLC BEH C18, 1.7 μm, 2.1 × 150 mm reversed-phase column, with an acetonitrile: water (0.1% formic acid) gradient.
T and AD levels in serum were quantified using methods similar to the detection of E2. Briefly, samples were spiked with 0.25 ng/ml D3-testosterone or D7-androstenedione as the internal standard and then extracted with n-butyl chloride. After centrifugation and evaporation, the residue was reconstituted in methanol and water (80 μl: 20 μl), centrifuged again and the supernatant transferred into glass vials for UPLC-MS/MS analysis. T and AD were eluted from the same column as E2, with a methanol: water (0.1% formic acid and 2 mM ammonium acetate) gradient from 50 to 85% methanol.
MS detection and quantification of all hormones was achieved in the positive mode. Transitions used for the analysis of estradiol were 506 → 171 for E2, and 511 →171 for the deuterated internal standard. Transitions used for T analysis were 289 →97 for T and 292 →97 for the deuterated T. Transitions used for AD analysis were 287→100 for AD and 294→ 100 for the deuterated AD. Area under the peak was quantified and used to determine absolute levels of E2, T, or AD per ml by comparison with corresponding standards. The limit of detectability for the E2 assay is 2.5 pg/ml. The limit of detectability for the T and AD assays is 25 pg/ml. Intra-assay statistics show relative standard errors below 8.1% and relative standard deviations below 10.4%. Inter-assay statistics show relative standard errors below 5.0% with relative standard deviations below 7.4%.
2.7. Monoamine analysis by HPLC-CMEAS:
Monoamines and metabolites were measured with a modified version of a HPLC-ECD method and as described in our recent paper [30]. The goal was to characterize levels of targets associated with dopaminergic, noradrenergic, and serotoninergic pathways. Targets that were quantified included the amino acids tryptophan (TRP) and tyrosine (TYR); dopamine (DA) and its metabolites, 3–4-dihydroxyphenylalanine (DOPAC) and homovanillic acid (HVA); norepinephrine (NE) and epinephrine (EPI); and serotonin (5-HT) and its metabolite 5-hydroxyindole acetic acid (5-HIAA). These endpoints were selected because these monoaminergic pathways are known to be affected by ovariectomy and estrogen treatment with corresponding effects on cognitive and motor performance[25, 47].
The HPLC methods have recently been described in detail [30]. Briefly, 100 μl of sample was injected into an ESA CoulArray Model 5600 HPLC system, consisting of two Model 582 pumps, one dynamic gradient mixer, two PEEK pulse dampers, a Model 542 refrigerated autosampler injector, a CoulArray organizer module, and a serial array of 16 coulometric electrodes. Mobile Phase A consisted of 1.1% (w/v) of 1-pentane-sulfonic acid (Specrum, Inc.) at pH 3.0. Mobile Phase B consisted of 0.1 M lithium acetate (Sigma-Aldrich, Inc.) in a solvent mixture of methanol (Avantor Performance Materials, Inc.), acetonitrile (Honeywell, Inc.) and isopropanol (Mallinckrodt Baker,Inc.) at the ratio of 80/10/10 (v/v/v) at pH 6.5. Each sample was run on a single column (ESA Meta-250, 5 μm ODS, 250 × 4.6 mm ID) under a 68-minute gradient elution that ranged from 0% to 100% Mobile Phase B and delivered at a fixed flow rate of 0.6 ml/min. The retention time and area of the peaks in tissue homogenates were measured and compared to an external calibrating standard solution containing TYR, TRP, DA, DOPAC, HVA, NE, EPI, 5-HT, and 5-HIAA (Sigma, St. Louis, MO, USA). Concentrations of these substances in the samples were calculated and expressed as ng/mg protein. Turnover ratios (metabolite/monoamine) were calculated as a measure of activity.
2.8. Statistical Analysis:
Results are presented as means ± standard error of the mean (SEM). Statistical analysis was conducted in several stages. An omnibus 3-way ANOVA was performed for each brain region with Model, Time, and Agonist as between factors. These data are reported as main effects of Model, Time, and Agonist as well as 2-way and 3-way interactions. Effects of agonist treatments for a given Model and Time Point were further explored by 1-way ANOVA. Differences between groups were analyzed by Tukey test and also by Dunnet test comparing agonist treatments with corresponding controls. All means, standard errors and 1-way ANOVA results are reported in the supplementary table. Significant effects of agonists targets in specific brain regions and at specific time points also are illustrated in bar graphs. Since our primary goal was to compare effects of treatments in the two models, Cohen’s d was calculated for each comparison (calculated as (M1–M2)/MSE and plotted to illustrate effect size. This provided an efficient means of illustrating effects for the many endpoints and comparisons. Statistical significance was defined a p≤0.05. All p values are corrected for multiple comparisons. All statistics were calculated using JMP v12.
3. Results:
3.1. Serum levels of hormones:
Levels of E2, T, and AD in each of the treatment groups are summarized in Supplemental Table 1. 3-way ANOVAs revealed a significant overall effect for E2 (F[19,72]=8.0, p<0.0001), T F[19,68]=3.3, p<0.001), and AD F[19,70]=4.8, p<0.0001) levels. For E2 levels, significant main effects of Time (F[1,72]=8.1, p<0.01) and Agonist Treatment (F[4,72]=28.4, p<0.0001) were detected, as well as a significant Time × Agonist interaction (F[4,72]=5.7, p<0.001). In OVX rats, levels of E2, as well as AD and T were undetectable or extremely low in rats treated with vehicle, PPT, DPN, or G-1 regardless of Time-Point. As expected, levels of E2 were significantly higher in E2-treated rats compared with all other groups (p<0.05). In addition, levels of E2 detected after 6 weeks of continuous treatment were significantly higher than levels detected after 1 week of treatment. In VCD-treated rats, levels of E2 were significantly higher in E2-treated rats than in all other groups. A 2-way ANOVA of Time and Agonist Treatment on E2 levels in VCD-treated rats revealed a significant main effect of Agonist (F[4, 37]=11.7, p<0.0001), a significant effect of Time (F[1, 37]=5.2, p<0.05) and a significant Time × Agonist interaction (F[4,37]=3.0, p<0.05). Post-hoc analyses revealed that E2 levels were higher in E2-treated rats relative to non-E2-treated rats (irrespective of time-point), and that rats treated with E2 for 6 weeks had significantly higher E2 levels than all other groups (p<0.05).
For AD levels, significant main effects of Model (F[1,70]=45.4, p<0.0001), Time (F[1,70]=6.9, p<0.05), and Agonist Treatment (F[4,70]=3.5, p<0.05) were detected, as well as significant Model × Agonist (F[4,70]=3.1, p<0.05) and Model × Time (F[1,70]=6.0, p<0.05) interactions. Specifically, AD levels were much higher in VCD-treated than in OVX rats, were higher after 1 week than after 6 weeks of treatment, and were substantially lower in E2-treated rats than in all other groups when collapsed across Model and Time Point. Levels of AD in VCD-treated rats were quite variable. There was some indication that E2 treatment produced a decrease and DPN an increase in AD. A 2-way ANOVA of Time and Agonist Treatment on AD levels in VCD-treated rats revealed a significant main effect of Agonist (F[4, 35]=3.4, p<0.05), main effect of Time (F[1, 35]=6.4, p<0.05) but no Time × Agonist interaction (F[4,35]=0.7, p=0.60). Post-hoc analysis revealed that AD levels in DPN-treated rats were significantly higher than in E2-treated rats (irrespective of time-point), although neither group differed significantly from controls. This is likely due to high variability, and to the fact that AD levels in the controls were much lower at 6 weeks than at 1 week. 1-way ANOVA of AD levels in VCD-1W rats show that AD levels were significantly lower in E2-treated rats than in controls or DPN-treated rats. 1-way ANOVA of VCD levels in VCD-6W rats shows that AD levels were significantly higher in DPN-treated rats than in E2-treated rats or controls.
For T levels, a significant main effect of Model (F[1,68]=34.1, p<0.0001) was detected as well as a significant main effect of Agonist (F[4,68]=2.7, p<0.05) interaction. Like AD, levels of T were significantly higher in VCD-treated than in OVX rats. Among the VCD-treated rats, levels also were significantly higher in DPN-treated than in E2-treated rats when collapsed across Time. Like AD, levels of T in VCD-treated rats were quite variable. A 2-way ANOVA of Time and Agonist Treatment on T levels in VCD-treated rats produced a nearly significant effect of Agonist (F[4,33]=2.5, p=0.06), no significant effect of Time (F[1,33]=0.4, p=0.54), and no Time × Agonist interaction (F[4,33]=1.3, p=0.3). 1-way ANOVA of T levels in VCD-6W rats showed that T levels were significantly higher in DPN-treated rats than in controls by Dunnett test. No other significant differences in T-levels were detected, again likely due to the very high variance in these values in the VCD-treated rats.
3.2. Effects of ER Agonists on Monoamines, Monoamine Metabolites, and Amino Acids Levels in OVX-and VCD-treated rats
Detailed analysis consistently revealed significant effects of E2 as well as ER subtype-selective agonists on endpoints associated with noradrenergic, dopaminergic and serotoninergic pathways, in both VCD- and OVX-treated rats (refer to Supplemental Table 2). Effects were model- and region-specific as well as time-dependent. For clarity, results are organized by brain region and by neurochemical pathway.
HPC:
Serotoninergic Pathway:
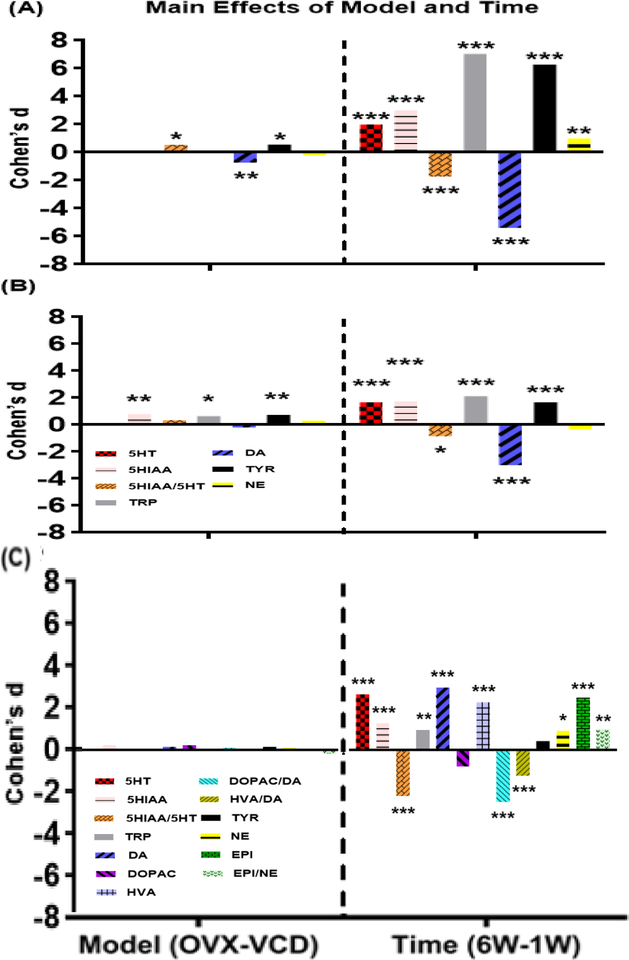
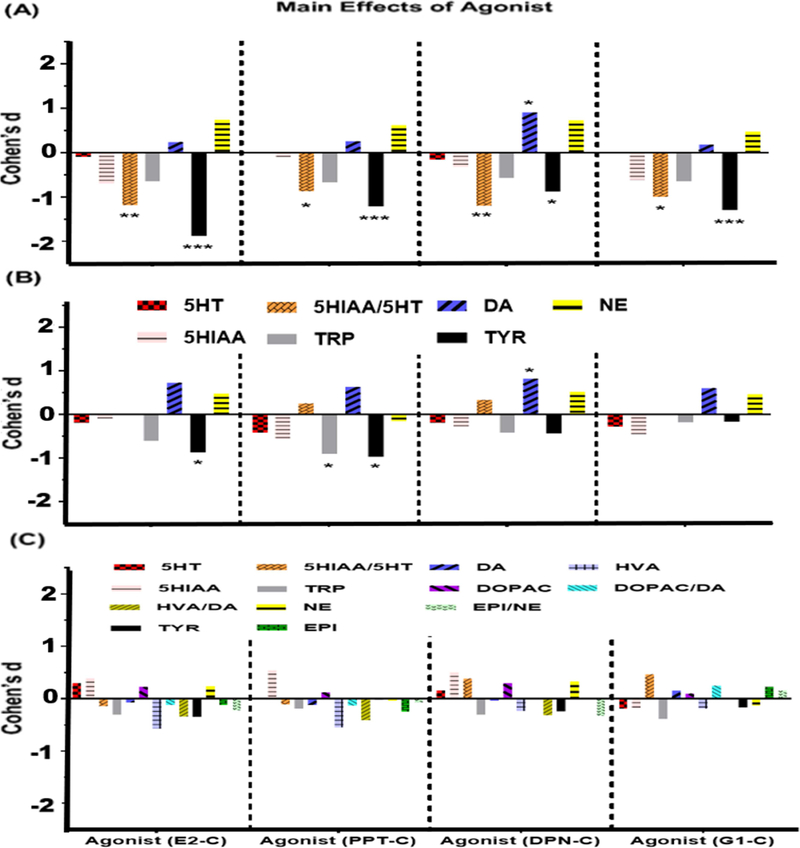
3-way ANOVAs detected significant overall effects on 5-HT (F[19,74]=6.0, p<0.0001), 5-HIAA (F[19,74]=12.1, p<0.0001), the ratio of 5-HIAA/5-HT (F[19,74]=8.1, p<0.0001), and the amino acid TRP (F[19,74]=61.5, p<0.0001). Main effects of Model (F[1,74]=6.8, p<0.05) and Agonist (F[4,74]=4.8, p<0.01) were detected for the ratio of 5-HIAA/5-HT. Specifically, the ratio was higher in OVX than in VCD-treated rats when collapsed across Agonist and Time (Figure 1A), and was greater in controls than in E2, DPN, or G-1 treated rats when collapsed across Model and Time (Figure 2A). A main effect of Time was detected for the ratio of 5-HIAA/5-HT (F[1,74]=73.9, p<0.0001), as well as for 5-HT (F[1,74]=89.8, p<0.0001), 5-HIAA (F[1,74]=207.1, p<0.0001), and TRP (F[1,74]=1144.8, p<0.0001). Specifically, the levels of 5-HT, 5-HIAA, and TRP were significantly lower, and the ratio of 5-HIAA/5-HT was significantly greater, at 1 week than at 6 weeks when collapsed across Model and Agonist Treatment (Figure 1A). A Model × Agonist interaction was detected for the ratio of 5-HIAA/5-HT (F[4,74]=3.0, p<0.05) and for TRP (F[4,74]=3.4, p<0.05). Post-hoc analysis showed that the ratio of 5-HIAA/5-HT in OVX controls was significantly higher than in all E2, DPN, and G-1-treated rats and in VCD-treated rats treated with PPT, when collapsed across Time. Similar analysis showed that levels of TRP were significantly higher in OVX controls than in OVX rats treated with E or PPT, when collapsed across Time. A Model × Time interaction was detected for the ratio of 5-HIAA/5-HT (F[1,74]=7.1, p<0.001). Post-hoc analysis showed that the ratio of 5-HIAA/5-HT was significantly greater in OVX-1W rats than in all other groups when collapsed across Agonist Treatment, followed by VCD-1W rats, and then by both OVX-6W and VCD-6W rats. An interaction between Time × Agonist (F[4,74]=4.2, p<0.005) as well as a full 3-way interaction (F[4,74]=4.1, p<0.005) was detected for the ratio of 5-HIAA/5-HT. Post-hoc analyses showed that the ratio of 5-HIAA/5-HT was greatest in 1W controls compared to all other groups when collapsed across Model. This was followed by PPT-1W and G-1–1W, both of which differed significantly from E2–6W, PPT-6W, and G-1–6W when collapsed across Model.
Figure 1: Main effects of Model and Time on monoamines, metabolites, amino acids and metabolite/monoamine ratios in (A) HPC, (B) FCX and (C) STR.
Bars indicate effect size (Cohen’s d) for Model (OVX-VCD)/MSE and Time (6W-1W)/MSE. For effects of Model, a positive value indicates that levels in OVX were greater than levels in VCD. For Time, a positive value indicates that levels at 6W were greater than levels at 1W. *p<0.05, **p<0.01, ***p<0.001. p-values have been adjusted for multiple comparisons.
Figure 2: Main effects of Agonist on monoamines, metabolites, amino acids and metabolite/monoamine ratios in (A) HPC, (B) FCX and (C) STR.
Bars indicate effect size (Cohen’s d) for comparisons of values in agonist-treated rats vs. controls e.g., (Agonist-Control)/MSE. A positive value indicates that levels in rats treated with the agonist were greater than levels in the controls. Adjusted p values: *p<0.05, **p<0.01, ***p<0.001.
1-way ANOVAs on serotoninergic endpoints in the HPC of OVX rats produced significant overall effects on TRP and the ratio of 5-HIAA/5-HT at 1 week. Post-hoc analyses showed that levels of 5-HT were significantly higher in DPN-treated rats than in controls; levels of 5-HIAA were significantly lower in G-1-treated rats than in controls; levels of TRP were significantly lower in E2-, PPT-, and G-1-treated rats than in controls (Figure 3). The ratios of 5-HIAA/5-HT were significantly lower in E2-, DPN-, and G-1-treated rats than in controls. No significant effects on levels of 5-HT, 5-HIAA, TRP, or the ratio of 5-HIAA/5-HT were detected in OVX rats after 6 weeks of treatment.
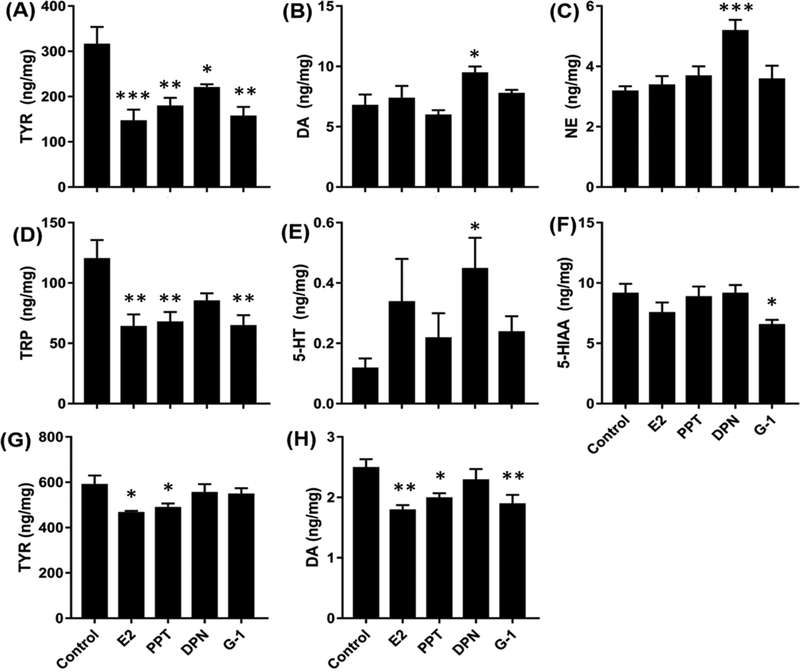
Figure 3: Effects of ER agonists on target levels in the HPC of OVX rats after 1W (A-F) and 6W (G-H) of treatment.
Only those targets showing significant effects are included. Bars indicate Mean ± SEM.*p<0.05, **p<0.01, ***p<0.001, relative to controls by Dunnett’s test.
1-way ANOVAs on serotoninergic endpoints in the HPC of VCD-treated rats showed no significant overall effects after 1 week of agonist treatment. After 6 weeks of treatment a strong trend was observed for the ratio of 5-HIAA/5-HT (F[4,19]=2.7, p=0.06). Slight reductions in E2-and G-1-treated rats relative to controls were detected using Dunnett’s test.
Dopaminergic Pathway:
3-way ANOVAs detected significant overall effects on DA (F[19,74]=40.4, p<0.0001) and TYR (F[19,74]=50.0, p<0.0001). Levels of DOPAC and HVA were below the limit of detection in the HPC. Significant main effects of Model, Agonist Treatment, and Time Point were detected for both DA and TYR (DA: Model F[1,74]=12.5, p<0.001; Agonist F[4,74]=2.7, p<0.05; Time F[1,74]=689.7, p<0.0001; TYR: Model F[1,74]=6.8, p<0.02; Agonist F[4,74]=9.1, p<0.0001; Time F[1,74]=909.9, p<0.0001). Significant interactions also were detected. For DA these included interactions of Model × Time (F[1,74]=12.6, p<0.001), and Agonist Treatment × Time F[4,74]=3.4, p<0.02). Post-hoc analyses revealed that levels of DA were significantly higher in VCD-treated than in OVX rats when collapsed across Agonist and Time (Figure 1A), that levels were significantly higher at 1W than at 6W when collapsed across Model and Agonist (Figure 1A), and that levels of DA were significantly lower in OVX-PPT-treated rats than in VCD-E2, VCD-PPT, VCD-DPN, VCD-G-1 or OVX-DPN-treated rats, when collapsed across time. Analysis also revealed that levels in 1W-DPN treated rats were higher than in 1W-controls, 1WPPT, and 1W-G-1-treated rats, when collapsed across time.
Interaction effects for levels of TYR included Model × Agonist Treatment (F[4,74]=3.5, p<0.02) and a 3-way interaction of Model × Agonist Treatment × Time (F[4,74]=3.3, p<0.02). Post-hoc analysis revealed that levels of TYR were significantly higher in OVX than in VCD-treated rats when collapsed across Agonist Treatment and Time (Figure 1A), were much higher at 6 weeks than at 1 week when collapsed across Model and Agonist Treatment (Figure 1A) and were significantly lower in all treatment groups compared with OVX-controls with the exception of OVX-DPN-treated rats, when collapsed across time.
1-way ANOVAs on levels of DA and TYR in the HPC of OVX rats produced significant overall effects after both 1 week and 6 weeks of treatment. Post-hoc analyses showed that levels of DA were significantly higher in DPN-treated rats than in controls (Figure 3B) after 1 week of treatment, and were significantly lower in E2-, PPT- and G-1-treated rats than in controls after 6 weeks of treatment (Figure 3H). In contrast, levels of TYR were significantly lower in all agonist-treated rats relative to controls after 1 week of treatment (Figure 3A), and remained lower than controls in E2- and PPT-treated rats after 6 weeks of treatment (Figure 3G).
1-way ANOVAs on levels of DA and TYR in the HPC of VCD-treated rats showed no significant overall effects after 1 week of agonist treatment and no effect on DA levels after 6 weeks of treatment. An effect on TYR levels was detected after 6 weeks of treatment at which point levels in E2-treated rats were significantly lower than in PPT-treated rats.
Noradrenergic Pathway:
3-way ANOVA detected a significant overall effect on NE (F[19,74]=3.1, p<0.0005). Levels of EPI were below the limit of detection in the HPC. A significant main effects Time Point was detected (F[1,74]=21.7, p<0.0001) as well as a Model × Time interaction (F[1,74]=12.0, p<0.001). No significant main effects of Model or Agonist Treatment on NE levels were detected. Post-hoc analyses showed that NE levels were significantly higher at 6 weeks than at 1 week when collapsed across Model and Agonist Treatment (Figure 1A), and that NE levels in OVX-1W rats were significantly lower than in VCD-1W rats, which in turn were significantly lower than in OVX-6W rats, when collapsed across Agonist Treatment.
1-way ANOVAs on levels of NE in the HPC of OVX-treated rats produced significant overall effects after 1 week of treatment, but not 6 weeks of treatment. Post-hoc analyses showed that levels of NE were significantly higher in DPN-treated rats than in controls (Figure 3C). No significant effects of treatment were detected in VCD-treated rats when compared at each of the 2 time points.
FCX:
Serotoninergic Pathway:
3-way ANOVAs detected significant overall effects on 5-HT (F[19,72]=3.8, p<0.0001), 5-HIAA (F[19,72]=5.3, p<0.0001) and the amino acid TRP (F[19,72]=7.2, p<0.0001). Main effects of Model were detected for 5-HIAA (F[1,72]=13.1, p<0.001) and TRP (F[1,72]=8.6, p<0.01). Specifically, levels of both 5-HIAA and TRP was higher in OVX than in VCD-treated rats when collapsed across Agonist and Time (Figure 1B). A main effect of Time was detected for ratio of 5-HIAA/5-HT (F[1,72]=16.6, p<0.0001) as well as 5-HT (F[1,72]=61.5, p<0.0001), 5-HIAA (F[1,72]=69.3, p<0.0001), and TRP (F[1,72]=100.6, p<0.0001). Specifically, ratio of 5-HIAA/5-HT as well as the levels of 5-HT, 5-HIAA, and TRP were significantly lower at 1 week than at 6 weeks when collapsed across Model and Agonist Treatment (Figure 1B). No interaction was detected for the endpoints in the serotoninergic pathway.
1-way ANOVAs on serotoninergic endpoints in the FCX of OVX rats produced significant overall effects only on TRP at 1 week. Post-hoc analyses showed that levels of TRP were significantly lower in E2-, PPT-, DPN-, and G-1-treated rats than in controls (Figure 4A). No significant effects on levels of 5-HT, 5-HIAA, TRP, or the ratio of 5-HIAA/5-HT were detected in OVX rats after 6 weeks of treatment.
Figure 4. (A-C) Effects of ER agonists on target levels in FCX and STR of OVX rats.
(A-C) levels of DA, TYR and TRP in the FCX after 1W of treatment. (D-F) levels of DA, 5-HT and 5-HIAA in STR after 1W of treatment. (G) Levels of HVA in STR after 6W of treatment. Only those targets showing significant effects are included. Bars indicate Mean ± SEM.*p<0.05, **p<0.01, relative to control by Dunnett’s test.
1-way ANOVAs on serotoninergic endpoints in the FCX of VCD-treated rats showed no significant overall effects after 1 week or 6 weeks of agonist treatment.
Dopaminergic Pathway:
3-way ANOVAs detected significant overall effects on DA (F[19,72]=13.3, p<0.0001) and TYR (F[19,72]=5.6, p<0.0001). Levels of DOPAC and HVA were below the limit of detection in the FCX. Significant main effects of Model and Time Point were detected for TYR (Model F[1,72]=11.5, p<0.01; Time F[1,72]=62.3, p<0.0001) while significant main effect of Time Point was detected for DA (F[1,72]=210.1, p<0.0001). Main effects of Agonist were detected for TYR (F[4,72]=3.3, p<0.05). Specifically, the levels of TYR were lower in E2- and PPT-treated rats than in controls when collapsed across Model and Time (Figure 2B). Significant interactions were detected for DA, which included interactions of Model × Agonist Treatment (F[4,72]=2.6, p<0.05), Agonist Treatment × Time (F[4,72]=2.8, p<0.05) as well as a full 3-way interaction (F[4,72]=2.5, p<0.05).
Post-hoc analysis revealed that levels of TYR were significantly higher in OVX than in VCD-treated rats when collapsed across Agonist Treatment and Time (Figure 1B); levels of TYR were much higher at 6 weeks than at 1 week while levels of DA were much higher at 1 week than at 6 weeks when collapsed across Model and Agonist Treatment (Figure 1B).
1-way ANOVAs on levels of DA and TYR in the FCX of OVX rats produced significant overall effects after 1 week of treatment. Post-hoc analyses showed that levels of DA were significantly higher in all agonist-treated rats relative to controls after 1 week of treatment. In contrast, levels of TYR were significantly lower in all agonist-treated rats relative to controls after 1 week of treatment (Figure 4B).
1-way ANOVAs on levels of DA and TYR in the FCX of VCD-treated rats showed no significant overall effects after 1 week or 6 weeks of agonist treatment.
Noradrenergic Pathway:
No significant effect on NE was detected through 3-way ANOVA or 1-way ANOVA. Levels of EPI were below the limit of detection in the FCX.
STR:
Serotoninergic Pathway:
3-way ANOVAs detected significant overall effects on 5-HT (F[19,72]=9.4, p<0.0001), 5-HIAA (F[19,72]=3.5, p<0.0001) and ratio of 5-HT/5-HIAA (F[19,72]=6.7, p<0.0001) . Main effects of Time Point were detected for 5-HT (F[1,72]=9.4, p<0.0001), 5-HIAA (F[1,72]=36.4, p<0.0001) , ratio of 5-HT/5-HIAA (F[1,72]=106.3, p<0.0001) and TRP (F[1,72]=20.8, p<0.0001). Specifically, ratio of 5-HIAA/5-HT was significantly lower at 6 weeks than at 1 week while the levels of 5-HT, 5-HIAA, and TRP were significantly higher at 6 weeks than at a week when collapsed across Model and Agonist Treatment (Figure 1C). There was no significant main effect of Agonist for the STR in all three pathways (Figure 2C). The only interaction detected in the serotoninergic pathway was a full 3-way interaction for 5-HIAA (F[4,72] =3.7,p<0.01).
1-way ANOVAs on serotoninergic endpoints in the STR of OVX rats produced significant overall effects only on 5-HT and 5-HIAA at 1 week. Post-hoc analyses showed that level of 5-HT was significantly higher in E2-treated rats than in controls while level of 5-HIAA was significantly higher in DPN-treated rats than in controls (Figures 4E, 4F). No significant effects on levels of 5-HT, 5-HIAA, TRP, or the ratio of 5-HIAA/5-HT were detected in OVX rats after 6 weeks of treatment.
1-way ANOVAs on serotoninergic endpoints in the STR of VCD-treated rats showed significant higher level of 5-HIAA in PPT-treated rats in relative to controls after 6 weeks of agonist treatment (Figure 5C).
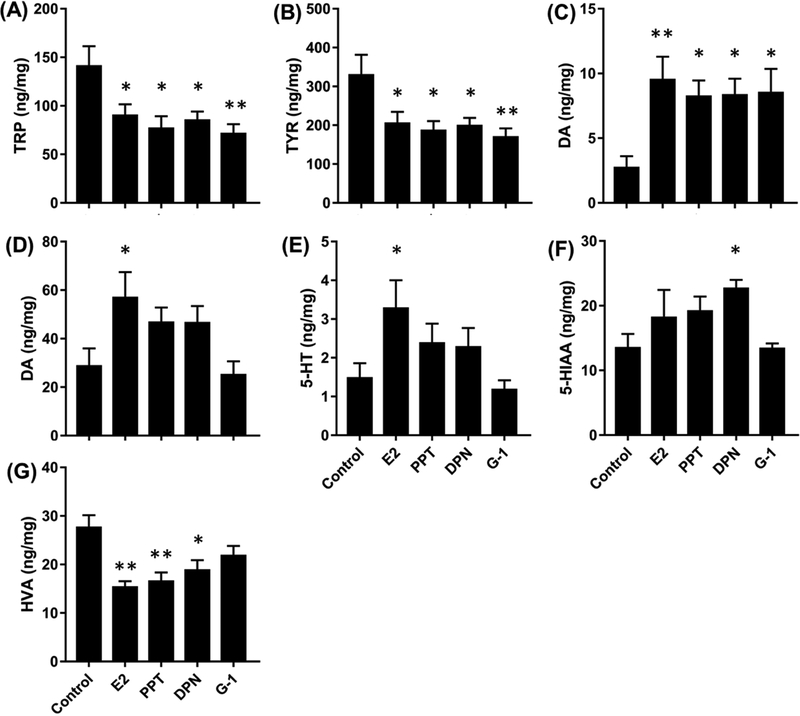
Figure 5. Effects of ER agonists on targets in STR of VCD-treated rats.
(A-B) Levels of HVA and EPI in STR after 1W of treatment. (C) Levels of 5-HIAA in STR after 6W of treatment. Only those targets showing significant effects are included. Bars indicate Mean ± SEM.*p<0.05, **p<0.01, ***p<0.001, relative to control by Dunnett’s test.
Dopaminergic Pathway:
3-way ANOVAs detected significant overall effects on DA (F[19,72]=11.8, p<0.0001) , HVA (F[19,72]=8.7, p<0.0001) and ratio of DOPAC/DA (F[19,72]=8.1, p<0.0001). Main effects of Time Point were detected for all of the endpoints in the dopaminergic Pathway: DA (F[1,72]=199.1, p<0.0001), DOPAC (F[1,72]=13.7, p<0.0001) , HVA (F[1,72]=115, p<0.0001) ratio of DOPAC/DA (F[1,72]=128.1, p<0.0001) and TYR (F[1,72]=4.1, p<0.05). Specifically, ratio of DOPAC/DA and level of DOPAC was significantly lower at 6 weeks than at 1 week while the levels of DA, HVA, and TYR were significantly higher at 6 weeks than at a week when collapsed across Model and Agonist Treatment (Figure 1C). Interaction of Time × Agonist Treatment (F[4,72] =2.7,p<0.05).was detected for DA and interaction of Model × Time(F[1,72]=9,9,p<0.01). , Model × Agonist Treatment (F[4,72]=3.3,p<0.05).and Time × Agonist Treatment (F[4,72]=3.5,p<0.05).were detected for HVA.
1-way ANOVAs on levels of DA and HVA in the STR of OVX rats produced significant overall effects after 1 week of treatment. Post-hoc analyses showed that level of DA was significantly higher in E2-treated rats relative to controls after 1 week of treatment (Figure 4D). Levels of HVA were significantly lower in E2-, PPT-, DPN-treated rats relative to controls after 6 weeks of treatment (Figure 4G)
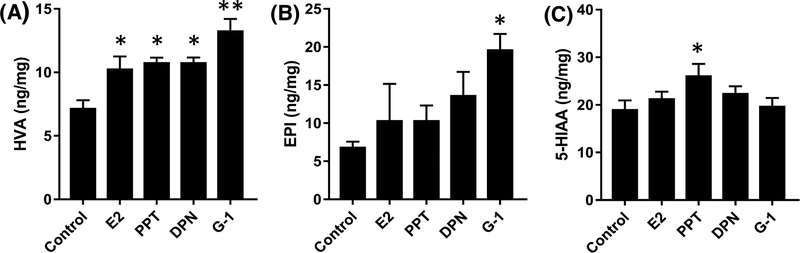
1-way ANOVAs on levels of HVA in the STR of VCD-treated rats showed significant higher levels of HVA in all agonists-treated rats than controls only after 1 week of agonist treatment (Figure 5A).
Noradrenergic Pathway:
3-way ANOVAs detected significant overall effects on NE (F[19,72]=1.9, p<0.05), EPI (F[19,72]=7.8, p<0.0001) and ratio of EPI/NE (F[19,72]=2.0, p<0.05) . Main effects of Time Point were also detected for NE (F[1,72]=19.6, p<0.0001), EPI (F[1,72]=137, p<0.0001) and ratio of EPI/NE (F[1,72]=21.7, p<0.0001) . Specifically, levels of NE, EPI as well as ratio of EPI/NE was significantly higher at 6 weeks than at 1 week when collapsed across Model and Agonist Treatment (Figure 1C). No interaction detected in the noradrenergic pathway in STR.
1-way ANOVAs followed by post-hoc test in the STR of OVX rats revealed significant lower ratio of EPI/NE in PPT-treated rats relative to controls after 1 week of treatment while for the VCD-treated rats, higher level of EPI was detected in G-1-treated rats compared with controls after 1 week of treatment (Figure 5B).
4. Discussion:
4.1. Serum hormone measurements
The goal of the current study was to characterize changes in relevant neurochemical endpoints in the brain associated with surgical and transitional menopause, in response to chronic treatments of estradiol and selective ER agonists. Circulating levels of E2, T and AD are consistent with prior studies [30, 48] and confirm that representation of the two menopausal models was successfully achieved. Results also show that 6 weeks of DPN treatments led to significant elevations in T and a strong trend for elevation of AD in VCD-treated rats. The exact mechanism of this is currently unknown. ERβ is expressed by both thecal and granulosa cells [49]. It may be that activation of ERβ by DPN stimulates thecal cells to increase biosynthesis of androgens, or alternatively inhibits the transformation of androgens to estrogens in the granulosa cells via an effect on aromatase. This needs to be investigated. As expected, rats receiving E2 demonstrated significantly higher plasma E2 levels relative to other groups.
4.2. Main Effects of Model, Time-point and Agonist Treatment
The current data demonstrate significant region-specific effects of Model and Time Point on multiple measures of serotoninergic, dopaminergic and noradrenergic endpoints. In the HPC levels of TYR were higher and levels of DA were lower in OVX vs VCD-treated rats. In the FCX levels of 5-HIAA, TRP and TYR were higher in OVX vs VCD-treated rats. These effects, while statistically significant, were relatively small. Several effects were more striking, when separated by Time-Point. For example, the ratio of 5-HIAA/5-HT in the HPC was much higher in OVX vs VCD-treated rats at 1W, but not at 6W. This could indicate a strong effect of OVX on 5-HT turnover in this region at early time-points. In contrast, levels of DA in the HPC were much lower in OVX vs. VCD-treated rats at 1W, but not at 6W. This corresponds with significantly reduced levels of TYR in the HPC at the 1W time-point, and may indicate a significant reduction in DA activity in this region at early time-points following OVX. In the FCX, levels of 5-HIAA, TRP and TYR all were significantly higher in OVX vs. VCD-treated rats at 6W, but not at 1W. Collectively these findings demonstrate significant differences in the effects of OVX vs. VCD models of menopause on NT endpoints, and that the effects vary depending on brain region and time.
Main effects of ‘Time-Point’ (i.e., collapsed across model and agonist treatment) were much more robust than the main effects of ‘Model’. Effects on several endpoints were consistent across brain regions. For example, levels of 5-HT, 5-HIAA, TRP and the ratio of 5-HIAA/5-HT were higher in each brain region at 6W vs 1W. This suggests significant recovery of endpoints associated with serotonin production and signaling over a period of weeks following OVX or VCD treatment. This is consistent with our prior report showing significant increases in TRP, TYR, 5-HIAA and the ratio of 5-HIAA/5-HT in the HPC at 6W vs 1W following OVX or VCD treatment. This also suggests that these effects reflect increases over time independent of ER agonist treatment. Effects of time-point on other endpoints differed across brain regions as illustrated in Figure 1. Collectively, these findings demonstrate time- and region-specific effects on dopaminergic and noradrenergic endpoints, independent of model and agonist treatment.
In addition to the main effects of Model and Time-Point, the data also show significant main effects of agonist treatments on multiple NT endpoints that differed by brain region. For example, in the HPC, E2, PPT, DPN and G-1 each were associated with decreases in the ratio of 5-HIAA/5-HT and in levels of TYR compared with untreated controls. In contrast PPT, an ERα selective agonist, was associated with an increase in DA whereas DPN and G-1 had no effect. In the FCX E2 was associated with a decrease in TYR, PPT was associated with decreases in TRP and TYR, and DPN was associated with an increase in DA. This suggests that in the FCX activation of ERα is associated with decreases in TRP and TYR whereas activation of ERß is associated with increases in DA. No significant main effects of agonist treatment were detected in the STR. These data indicate that estrogens can influence NT endpoints following loss of ovarian function, that endpoints are differentially affected by activation of specific ER subtypes, and that these effects are brain region-dependent.
4.3. Specific Effects of Agonist Treatment as a Function of Model and Time-Point
This study is the first to analyze levels of TRP and TYR following ER agonist treatments in the same tissues in which multiple monoaminergic endpoints also were measured. Results indicate that the effects of agonist treatments are model- and time-point-dependent. This is clear from the interaction effects detected in the 3-way ANOVAs, and from the data presented in Supplemental Table 2. One general observation is that the majority of ER agonist effects were detected in OVX rats as opposed to VCD-treated rats and were detected after 1W vs 6W of agonist treatment. This suggests that these NT endpoints are particularly sensitive to ER agonist treatments beginning soon following the complete and sudden loss of ovarian function, and that differences between treated rats vs. controls decline over time. In addition, the majority of ER agonist effects were detected in the HPC, suggesting that this region of the brain is particularly sensitive to ER agonist treatments. For clarity, the discussion of specific effects is organized below by brain region.
HPC:
One surprising result was the low levels of TRP (120.6 ± 14.95 ng/mg) and TYR (316.8 ± 37.14 ng/mg) detected in the hippocampus of OVX rats after 1W of agonist treatment. These values are much lower than the levels in normal cycling rats (>230 ng/mg for TRP; >440 ng/mg for TYR) [30]. Previously we reported that levels of TRP and TYR are significantly reduced in the HPC 1W after OVX or VCD treatment and that levels recover by 6W [30]. We predicted that agonist treatment would help to restore the amino acid levels. In contrast, OVX rats treated for 1W with E2 had much lower levels of TRP and TYR than vehicle-treated controls. This was also the case for rats treated with PPT and G-1, and to a lesser extent with DPN, suggesting that activation of any of the three ERs suppresses the amino acid levels even further at this early time point.
Previously we reported that levels of 5HIAA in the HPC and the ratio of 5HT/5HIAA are significantly reduced by more than 50% 1W after OVX or VCD treatment and that levels increase significantly by 6W [30]. As with the effects on TRP and TYR, here we show that rats treated for 1W with E2 also had a significantly lower ratio of 5-HIAA/5-HT than controls, suggesting a reduction in 5-HT turnover. This also was the case for rats treated with DPN and G-1, but not PPT, suggesting that activation of ERß and GPER are sufficient to produce this effect. After 6 weeks of treatment the effects on TRP, 5-HIAA and on the ratio of 5-HIAA/5-HT were no longer evident. The effects on TYR were still evident but were reduced in magnitude, corresponding to the reduction in DA. These findings indicate that negative effects of OVX and VCD treatments on TRP, TYR, and the serotoninergic pathway that are seen at early time points can be exacerbated by ER agonist treatment, but mitigate with time even with continued treatment.
Previously we showed that levels of NE in the HPC were decreased slightly at 1W after OVX and VCD treatment whereas levels of DA and 5HT were unaffected [30]. Here we show that activation of ERβ by DPN treatment at 1W increased levels of all three major neurotransmitters DA, 5-HT and NE simultaneously in HPC, but only in OVX rats and only at the 1W time-point. Furthermore, DPN treatment reinstated the significant reduction in NE levels observed following ovariectomy in our previous study [30]. The differential effects between activation of ERα and ERβ on levels of DA, 5-HT and NE may possibly due to their relative expression in HPC after estrogen deprivation. This, however, would not account for the reductions in TRP and TYR observed in the HPC. Note that none of these effects of agonist treatment were observed in VCD-treated rats despite the fact that levels of TRP and TYR also are significantly reduced at 1W and recover by 6W following treatment with VCD [30]. This demonstrates that the HPC of VCD-treated rats is much less responsive to ER agonist treatment with respect to these endpoints than the HPC of OVX rats.
FCX:
Agonist treatments produced fewer effects on neurochemical endpoints in FCX relative to HPC. Unlike the HPC, our previous study showed that levels of TRP (163.0 ± 23.07 ng/mg) and TYR (296.5 ± 43.84 ng/mg) were not significantly reduced in the FCX of OVX or VCD-treated rats relative to gonadally intact controls [30]. However, as in the HPC, OVX rats treated for 1W with E2 had significantly lower levels of TRP and TYR (35–40%) than vehicle-treated controls. This also was the case for rats treated with PPT, DPN, and G-1. This suggests that, as in the HPC, activation of either ERα, ERß, or GPER, is sufficient to suppress these amino acid levels during the first week after OVX. The reduced levels of TYR may be due to its increased utilization to synthesize DA in the dopaminergic pathway, which may be caused by increased tryptophan hydrolase expression or activity following estrogen receptor activation. Effects were no longer observed after 6 weeks of treatment, demonstrating that, as in the HPC, the effects mitigate with time even with continued treatment. Despite the reduced levels of TYR, no effect on NE levels were detected. Likewise, despite the reductions in TRP, no significant effects on 5-HT, 5-HIAA or the ratio of 5-HIAA/5-HT were detected. Elevated levels of DA were detected in OVX rats treated for 1W with E2, PPT, DPN, and G-1, suggesting either an increase in DA production or a reduction in DA release and degradation at this time point. As in the HPC, the effects of agonist treatments detected at 1W in OVX rats were not detected in VCD-treated rats, suggesting a fundamental difference in the responsiveness of the FCX in these two models.
STR:
Effects of agonist treatments on NT endpoints in the striatum were different in several ways from effects detected in the HPC and FCX. First, no effects of E2 or the other ER agonists on the levels of TRP or TYR were detected. Our previous study [30] showed no effects on OVX or VCD treatments on 5-HT or DA in the striatum relative to gonadally intact controls. Here we show that following OVX, rats treated for 1 week with E2 had significantly higher levels of 5-HT and DA than vehicle-treated controls. This was not observed in rats treated with any of the selective ER agonists. Since E2 will act at all three ERs this suggests that activation of two or more ER receptors is necessary to produce these effects. These results are consistent with either an increase in 5-HT and DA production, or with decreases in 5-HT and DA release and degradation in response to E2.
Treatment with DPN was associated with a significant increase in 5-HIAA relative to vehicle-treated controls, which would be consistent with an increase in 5-HT release. This effect was not observed at 6W despite continuous treatment. In contrast, OVX rats treated for 6W with E2 showed a significant reduction in levels of HVA relative to vehicle-treated controls. Similar effects were observed in rats treated with PPT or DPN but not G-1. This suggests that activation of ERα or ERß, but not GPER, is sufficient to elicit this effect and that the reductions in HVA manifest after longer-term continuous treatment.
In contrast with the reductions in HVA detected in OVX rats after 6 weeks of treatment, significant increases in HVA were detected in VCD-treated rats after 1W of treatment with E2, PPT, DPN, or G-1. This is consistent with an increase in DA turnover, but notably was not accompanied by any increases in DOPAC or the DOPAC/DA or HVA/DA ratios. An increase in the level of EPI also was detected after 1W treatment with G-1, but not with any other agonist. This suggests that activation of GPER may have an effect on the NE pathway associated with increased NE turnover. The fact that a similar effect was not observed in rats treated with E2 suggests that this effect is unique to GPER activation and may be negated by activation of the other ERs. The effects on HVA and EPI levels in VCD-treated rats were not detected after 6W of treatment despite continuous treatment. These data again demonstrate a fundamental difference in the responsiveness to estrogen therapy following transitional vs surgical menopause.
In summary, in contrast with surgical menopause, the VCD model of transitional menopause was associated with far less responsiveness to ER agonists treatment as reflected by fewer effects on NT endpoints.
4.4. Comparisons with existing literature
To our knowledge, this report is the first to provide a comprehensive description of changes in NT endpoints for dopaminergic, serotonergic and noradrenergic pathways in two models of menopause following E2 and selective ER agonist treatments. When combined with our previous report [30], the data provide a comprehensive analysis of the effects of OVX and VCD treatments, with and without ER agonist treatments, on multiple endpoints in comparison with levels in gonadally intact controls. Most prior studies have focused on OVX rats and mice and on the effects of ER agonists on small numbers of NT endpoints. Several of our current findings are consistent with previous results. For example, previous studies found no change in NE and 5-HIAA levels in HPC following 4–6 weeks of chronic E2 treatment in OVX mice [28] and rats [29]. This is consistent with the lack of effect of E2 treatment on these NT endpoints in HPC detected in our study. Inagaki et.al [27] reported that a single s.c. injection of E2 in OVX rats significantly increased levels of DA but had no effect on NE, DOPAC or 5-HIAA in STR. This is consistent with our results in OVX rats treated for 1W with E2. Lubbers et al. [26] reported that 4 days s.c. treatment with E2 but not PPT increased levels of 5-HIAA in the STR of OVX rats. This is consistent with our results suggesting that increased levels of 5-HIAA may be mediated by ERβ activation.
Several inconsistencies with our current findings also have been reported. For example, 4–6 weeks of chronic E2 treatment was reported to decrease levels of 5-HT, but not DA, in HPC of OVX mice [28] and rats [29]. In addition, it has been reported that E2 and PPT treatments increased NE levels [26] while E2 treatment reduced 5-HT levels in the FCX of OVX rats [29]. In another study, 4 days s.c. treatment of E2 was shown not to alter levels of DA and 5-HT in the STR of OVX rats [26]. Jacome et al. [25] reported no effects on monoaminergic endpoints in OVX rat STR 2 days after a single s.c. injection of DPN. Some of these differences with our current results likely are due to differences in dose, timing and duration of treatments.
Note that all of the above studies used OVX rats or mice when studying effects on NT endpoints. Pestana-Olivera et al. [50] recently evaluated the effects of estrogen therapy on serotonin in VCD-treated rats. They reported measurements of 5-HT, as well as mRNA levels of ERβ and progesterone receptor in several brain regions following continuous subcutaneous administration of E2. Specifically, 3W of E2 treatment significantly increased 5-HT levels in dorsal HPC of VCD-treated rats. This differs from our current finding in which no effect on 5-HT level in HPC was reported after 1W or 6W of E2 treatment in VCD-treated rats. Differences in E2 treatment duration (3W vs 1W/6W), circulating E2 levels after E2 supplementation (~40 vs ~70/210 pg/ml), specific brain region analyzed (dorsal HPC vs HPC), VCD dosing regimen (160 mg/kg daily for 15 days vs 80 mg/kg daily for 30 days) could potentially account for the differences in results. This suggests that effects of chronic ER agonist treatment after transitional menopause may vary considerably as a function of dose and time of treatment, as well as how quickly ovarian function is lost. This requires further study.
4.5. Cognitive significance of NT change after ER agonist treatments
Many of the effects on NT endpoints could have important implications for effects on cognitive performance. The HPC is critical to memory consolidation, which is adversely affected by Alzheimer’s disease as well as by normal aging. Studies show that gene expression in the HPC is significantly altered following OVX [51]. E2 as well as specific ER agonists have been shown to affect hippocampal structure and function in several important ways, including effects on spine density in CA1 [52], synaptic formation and plasticity [53], and activation of PI3K/AKT and ERK/MARK neuronal signaling pathways [54].
The FCX also plays an important role in learning and memory, in addition to effects of sensory stimuli on mood and emotion. Effects on mesocortical dopaminergic pathways have been associated with cognitive processes such as short-term memory, anxiety and impulsivity. Previous studies have shown that increasing DA levels in FCX reduces impulsivity in humans [55]. In our current study, DA is the only monoamine affected in the FCX and upregulation of DA levels were reported following E2 and all of the ER agonists treatments. Although 5-HT levels remained unchanged, estrogens also have been shown to affect serotoninergic endpoints through other mechanisms. For example, in the FCX of OVX rats, estrogens can produce rapid receptor-mediated decreases in 5-HT1A receptor function [56]. Chronic exposure to E2 also induces a significant increase in the density of 5-HT2A receptors [57]. Further study is required to provide a better understanding of the potential effects of estrogen replacement on cognitive and emotional functions in menopausal women.
The STR is part of the extrapyramidal motor system. In addition to modulating motor control, it also plays a critical role in motor (i.e., procedural) learning and memory [58]. Studies in rats show that OVX rats have a predisposition for using a response (i.e., procedure) strategy when solving a spatial task whereas E2-treated rats preferentially use an allocentric strategy [59–61]. This is consistent with our data showing effects of E2 on 5-HT, DA and metabolites in the STR of OVX rats, and suggests that E2 treatment can significantly affect extrapyramidal function. However, the finding that effects differed in VCD-treated rats and varied as a function of time and ER agonist indicate that the effects are complex and may not be readily observed in women who undergo transitional menopause. Further study is needed to better understand the potential effects of ER agonists on extrapyramidal function and motor learning in women.
In the current study the HPC was more responsive to ER agonist treatments than other regions. In addition, OVX rats were much more responsive to ER agonist treatments than VCD-treated rats. Similar effects in humans would be consistent with observations that estrogen treatments have less of a positive effect in older women that have undergone transitional menopause than in younger women that have experienced oophorectomy [1]. This may help account for the fewer benefits of estrogen replacement that have been reported in postmenopausal women [62].
Age also is a factor, and previous research suggests a window of opportunity for realizing beneficial effects of estrogen on cognitive performance in women [43, 63]. Note that in such studies it is sometimes difficult to distinguish effects of age from effects of menopause. In female rodents aging results in reproductive senescence which is characterized by a loss of cyclicity and an extended period of estrus. The current study used young adult rats to avoid confounding factors associated with reproductive senescence. The fact that ER agonists had less effect in VCD-treated rats than in OVX rats suggests a fundamental difference between surgical vs transitional menopause in the responsiveness to ER agonist treatment independent of age.
Different estrogen receptors also may contribute to different effects on cognitive performance. Both ERα and ERβ have been implicated in mediating effects on different types of learning and memory [64], as well as on anxiety [65]. In our study, the ERβ agonist DPN simultaneously up-regulated levels of all three major NTs (DA, 5-HT and NE) in HPC of OVX rats following 1W of treatment. Jacome et al. [25] previously reported that ERβ mediates effects of estrogens on recognition memory and that memory enhancements by DPN may occur through alterations in multiple monoaminergic systems primarily in HPC. Walf and Frye [66] showed that administration of an ERβ selective agonist to the HPC decreased anxiety and depressive-like behaviors in OVX rats. If true in humans, this would provide support for selectively targeting ERβ in estrogen therapy to achieve specific beneficial effects while avoiding unfavorable side effects of ERα activation.
5. Conclusions
Collectively, our study is the first to directly and systematically compare the effects of ER agonists on NT pathways in the brain in these two models of menopause. The results of our analysis describe significant changes in local levels of serotoninergic, dopaminergic, and noradrenergic endpoints in association with validated models of surgical and transitional menopause following E2 and selective agonist treatments. Notably, effects were brain-region specific and agonist-dependent. The alternations in neurotransmitter levels following estrogen deprivation are likely relevant to changes in neural function and cognitive performance, which are partially reversed by either estrogen or selective agonists treatments. Furthermore, the transitional menopausal animals seem to be much less sensitive to ER agonist treatments than the OVX model with minimal effects on NT endpoints observed in striatum. This could contribute to the significant differences that have been reported in the effects of estrogen treatments on cognitive performance in older women that have experienced transitional menopause vs. younger women that experience rapid and sudden loss of ovarian function due to oophorectomy [17]. It should be noted that this study also has important limitations. Only young adult rats were used. Effects on neurotransmitter receptor expression and function were not evaluated, nor did we evaluate effects on enzymes involved in neurotransmitter production and degradation. Future studies that include aging as a variable, that evaluate effects on anabolic and catabolic enzymes involved in monoamine regulation, the regional expression and activity of monoamine transporters, neurotransmitter receptors, as well as relevant behavioral tests will further add to our understanding of the mechanisms by which estrogen treatments influence brain physiology and function following surgical and transitional menopause.
Supplementary Material
Highlights.
Used two clinically relevant models of menopause (surgical vs transitional).
Compared effects of estradiol and selective estrogen receptor agonists on neurochemical markers in rat brain.
Detected significant effects on dopaminergic, serotoninergic and noradrenergic endpoints, as well as levels of tyrosine and tryptophan.
Effects were agonist specific, model- and time-dependent, and differed between brain-regions.
Agonist treatments had lesser effects in VCD-treated rats than in OVX rats.
Acknowledgements
The study was supported by the National Institutes of Health (NIH) grant R21 AG043817.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have declared that no conflicts of interest exist.
References:
- 1.Rocca WA, Grossardt BR, and Shuster LT, Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res, 2011. 1379: p. 188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocca WA, Grossardt BR, and Shuster LT, Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol, 2014. 389(1–2): p. 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocca WA, et al. , Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology, 2007. 69(11): p. 1074–83. [DOI] [PubMed] [Google Scholar]
- 4.Rocca WA, et al. , Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause, 2008. 15(6): p. 1050–9. [DOI] [PubMed] [Google Scholar]
- 5.Phung TK, et al. , Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord, 2010. 30(1): p. 43–50. [DOI] [PubMed] [Google Scholar]
- 6.Bove R, et al. , Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology, 2014. 82(3): p. 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan J, et al. , Impact of a premature menopause on cognitive function in later life. BJOG, 2014. 121(13): p. 1729–39. [DOI] [PubMed] [Google Scholar]
- 8.Broekmans FJ, Soules MR, and Fauser BC, Ovarian aging: mechanisms and clinical consequences. Endocr Rev, 2009. 30(5): p. 465–93. [DOI] [PubMed] [Google Scholar]
- 9.Au A, et al. , Estrogens, inflammation and cognition. Front Neuroendocrinol, 2016. 40: p. 87–100. [DOI] [PubMed] [Google Scholar]
- 10.Sliwinski JR, Johnson AK, and Elkins GR, Memory Decline in Peri- and Post-menopausal Women: The Potential of Mind-Body Medicine to Improve Cognitive Performance. Integr Med Insights, 2014. 9: p. 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan PA, et al. , Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology, 2001. 26(6): p. 577–90. [DOI] [PubMed] [Google Scholar]
- 12.Weber MT, Maki PM, and McDermott MP, Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol, 2014. 142: p. 90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki PM and Henderson VW, Cognition and the menopause transition. Menopause, 2016. 23(7): p. 803–5. [DOI] [PubMed] [Google Scholar]
- 14.Pines A, Alzheimer’s disease, menopause and the impact of the estrogenic environment. Climacteric, 2016. 19(5): p. 430–2. [DOI] [PubMed] [Google Scholar]
- 15.Hara Y, et al. , Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol Rev, 2015. 95(3): p. 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frick KM, et al. , Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem, 2015. 22(9): p. 472–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs RB, Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev, 2010. 31(2): p. 224–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel JM and Bohacek J, The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta, 2010. 1800(10): p. 1068–76. [DOI] [PubMed] [Google Scholar]
- 19.Maki PM, Verbal memory and menopause. Maturitas, 2015. 82(3): p. 288–90. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper GG, et al. , Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A, 1996. 93(12): p. 5925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marino M, Galluzzo P, and Ascenzi P, Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics, 2006. 7(8): p. 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prossnitz ER, et al. , The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol, 2008. 109(3–5): p. 350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazell GG, et al. , Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol, 2009. 202(2): p. 223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heldring N, et al. , Estrogen receptors: how do they signal and what are their targets. Physiol Rev, 2007. 87(3): p. 905–31. [DOI] [PubMed] [Google Scholar]
- 25.Jacome LF, et al. , Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem, 2010. 94(4): p. 488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubbers LS, et al. , Estrogen receptor (ER) subtype agonists alter monoamine levels in the female rat brain. J Steroid Biochem Mol Biol, 2010. 122(5): p. 310–7. [DOI] [PubMed] [Google Scholar]
- 27.Inagaki T, Gautreaux C, and Luine V, Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav, 2010. 58(3): p. 415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heikkinen T, et al. , Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav, 2002. 41(1): p. 22–32. [DOI] [PubMed] [Google Scholar]
- 29.Pandaranandaka J, Poonyachoti S, and Kalandakanond-Thongsong S, Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol Behav, 2006. 87(4): p. 828–35. [DOI] [PubMed] [Google Scholar]
- 30.Long T, et al. , Comparison of transitional vs surgical menopause on monoamine and amino acid levels in the rat brain. Mol Cell Endocrinol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyer PB, et al. , Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol, 2001. 29(1): p. 91–9. [DOI] [PubMed] [Google Scholar]
- 32.Muhammad FS, et al. , Effects of 4-vinylcyclohexene diepoxide on peripubertal and adult Sprague-Dawley rats: ovarian, clinical, and pathologic outcomes. Comp Med, 2009. 59(1): p. 46–59. [PMC free article] [PubMed] [Google Scholar]
- 33.Springer LN, et al. , Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol, 1996. 139(2): p. 394–401. [DOI] [PubMed] [Google Scholar]
- 34.Mayer LP, et al. , The follicle-deplete mouse ovary produces androgen. Biol Reprod, 2004. 71(1): p. 130–8. [DOI] [PubMed] [Google Scholar]
- 35.Kraichely DM, et al. , Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-alpha and estrogen receptor-beta: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology, 2000. 141(10): p. 3534–45. [DOI] [PubMed] [Google Scholar]
- 36.Stauffer SR, et al. , Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem, 2000. 43(26): p. 4934–47. [DOI] [PubMed] [Google Scholar]
- 37.Meyers MJ, et al. , Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem, 2001. 44(24): p. 4230–51. [DOI] [PubMed] [Google Scholar]
- 38.Bologa CG, et al. , Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol, 2006. 2(4): p. 207–12. [DOI] [PubMed] [Google Scholar]
- 39.Hammond R, et al. , Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav, 2009. 56(3): p. 309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammond R, Nelson D, and Gibbs RB, GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology, 2011. 36(2): p. 182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paxinos G and Watson C, The Rat Brain in Stereotaxic Coordinates. 1998: Academic Press. [DOI] [PubMed] [Google Scholar]
- 42.Thompson RF and Kim JJ, Memory systems in the brain and localization of a memory. Proc Natl Acad Sci U S A, 1996. 93(24): p. 13438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luine VN, Estradiol and cognitive function: past, present and future. Horm Behav, 2014. 66(4): p. 602–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradford MM, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 1976. 72:p. 248–54. [DOI] [PubMed] [Google Scholar]
- 45.Li J, et al. , A microsomal based method to detect aromatase activity in different brain regions of the rat using ultra performance liquid chromatography-mass spectrometry. J Steroid Biochem Mol Biol, 2016. 163: p. 113–20. [DOI] [PubMed] [Google Scholar]
- 46.Kirshner ZZ and Gibbs RB, Use of the REVERT((R)) total protein stain as a loading control demonstrates significant benefits over the use of housekeeping proteins when analyzing brain homogenates by Western blot: An analysis of samples representing different gonadal hormone states. Mol Cell Endocrinol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benmansour S, et al. , Effects of Long-Term Treatment with Estradiol and Estrogen Receptor Subtype Agonists on Serotonergic Function in Ovariectomized Rats. Neuroendocrinology, 2016. 103(3–4): p. 269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acosta JI, et al. , Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology, 2009. 150(9): p. 4248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drummond AE and Fuller PJ, Ovarian actions of estrogen receptor-beta: an update. Semin Reprod Med, 2012. 30(1): p. 32–8. [DOI] [PubMed] [Google Scholar]
- 50.Pestana-Oliveira N, et al. , Effects of Estrogen Therapy on the Serotonergic System in an Animal Model of Perimenopause Induced by 4-Vinylcyclohexen Diepoxide (VCD). eNeuro, 2018. 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarvari M, et al. , Ovariectomy Alters Gene Expression of the Hippocampal Formation in Middle-Aged Rats. Endocrinology, 2017. 158(1): p. 69–83. [DOI] [PubMed] [Google Scholar]
- 52.Spencer JL, et al. , Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol, 2008. 29(2): p. 219–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolley CS, Effects of estrogen in the CNS. Curr Opin Neurobiol, 1999. 9(3): p. 349–54. [DOI] [PubMed] [Google Scholar]
- 54.Frick KM, Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav, 2015. 74: p. 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kayser AS, et al. , Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci, 2012. 32(27): p. 9402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mize AL, Poisner AM, and Alper RH, Estrogens act in rat hippocampus and frontal cortex to produce rapid, receptor-mediated decreases in serotonin 5-HT(1A) receptor function. Neuroendocrinology, 2001. 73(3): p. 166–74. [DOI] [PubMed] [Google Scholar]
- 57.Cyr M, Bosse R, and Di Paolo T, Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience, 1998. 83(3): p. 829–36. [DOI] [PubMed] [Google Scholar]
- 58.Packard MG and Knowlton BJ, Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci, 2002. 25: p. 563–93. [DOI] [PubMed] [Google Scholar]
- 59.Markowska AL and Savonenko AV, Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci, 2002. 22(24): p. 10985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korol DL and Kolo LL, Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci, 2002. 116(3): p. 411–20. [DOI] [PubMed] [Google Scholar]
- 61.Davis DM, et al. , Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem, 2005. 84(2): p. 132–7. [DOI] [PubMed] [Google Scholar]
- 62.Henderson VW, Action of estrogens in the aging brain: dementia and cognitive aging. Biochim Biophys Acta, 2010. 1800(10): p. 1077–83. [DOI] [PubMed] [Google Scholar]
- 63.Resnick SM and Henderson VW, Hormone therapy and risk of Alzheimer disease: a critical time. JAMA, 2002. 288(17): p. 2170–2. [DOI] [PubMed] [Google Scholar]
- 64.Bean LA, Ianov L, and Foster TC, Estrogen receptors, the hippocampus, and memory. Neuroscientist, 2014. 20(5): p. 534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borrow AP and Handa RJ, Estrogen Receptors Modulation of Anxiety-Like Behavior. Vitam Horm, 2017. 103: p. 27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walf AA and Frye CA, Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav, 2007. 86(2): p. 407–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.