Abstract
The prevalence and cost of disorders affecting the musculoskeletal system are predicted to rise significantly in the coming years due to the aging global population and the increase of associated risk factors. Despite being the second largest cause of disability, the clinical options for therapeutic intervention remain limited. The clinical translation of cell-based therapies for the treatment of musculoskeletal disorders faces many challenges including maintenance of cell survival in the harsh in vivo environment and the lack of control over regulating cell phenotype upon implantation. In order to address these challenges, the development of bio-instructive materials to modulate cell behavior has taken center stage as a strategy to increase the therapeutic potential of various cell populations. However, the determination of the necessary cues for a specific application and how these signals should be presented from a biomaterial remains elusive. This review highlights recent biochemical and physical strategies used to engineer bio-instructive materials for the repair of musculoskeletal tissues. There is a particular emphasis on emerging efforts such as the engineering of immunomodulatory and antibacterial materials, as well as the incorporation of these strategies into biofabrication and organ-on-a-chip approaches.
Keywords: Musculoskeletal, stem cell differentiation, electroconductive, biomineralization, tissue engineering
Graphical Abstract
1. Introduction
Musculoskeletal injuries and diseases are among the most costly and prevalent conditions worldwide. It is estimated that one in two adult Americans live with a musculoskeletal condition, comparable to the combined prevalence of cardiovascular and chronic respiratory diseases[1]. Musculoskeletal conditions affected more than 126 million adults in 2012 in the United States alone[1], the occurrence of which will only increase as the population continues to age. Musculoskeletal disorders include many of the most disabling and costly healthcare conditions: back and neck pain, fractures, rheumatoid arthritis and osteoarthritis[2]. Disorders affecting the musculoskeletal system can be caused by trauma (e.g., fractures and sports injuries), genetic conditions (e.g., muscular dystrophy), or by autoimmune and degenerative diseases (e.g., rheumatoid arthritis and osteoarthritis). The pathologies of these disorders are often interconnected, thereby complicating the treatment options and patient recovery[3].
The capacity of the musculoskeletal system to self-repair varies highly among tissues. Whereas long bones and skeletal muscle can heal small injuries, the regeneration of large defects or injuries to articular cartilage, tendon, and ligament remains clinically challenging. Tissue engineering and regenerative medicine (TERM) approaches are under investigation to provide durable and stable repair without the limitations associated with tissue grafts. Cell-based therapies, particularly those leveraging the therapeutic potential of stem and progenitor cells, are under widespread investigation. Mesenchymal stem/stromal cells (MSCs) from bone marrow and other compartments are primary candidate populations for use in cell-based therapies due to their potential to differentiate toward many relevant cell types including osteoblasts, chondrocytes, and adipocytes. Despite their multipotential in vitro, limited evidence exists for MSCs directly forming tissues upon implantation in vivo[4]. MSCs are thought to contribute to tissue formation and regeneration through their potent secretome that acts upon host cells[4]. The lack of FDA-approved stem cell-based therapies for musculoskeletal disorders highlights the difficulties faced by TERM approaches to be translated from bench to bedside. Many limitations remain including effective cell isolation and in vitro expansion, time consuming in vitro maturation of the transplant, maintenance of cell viability upon transplantation to the harsh in vivo microenvironment, control of cell phenotype upon implantation, and donor variability that contributes to variations in the size and quality of neotissues.
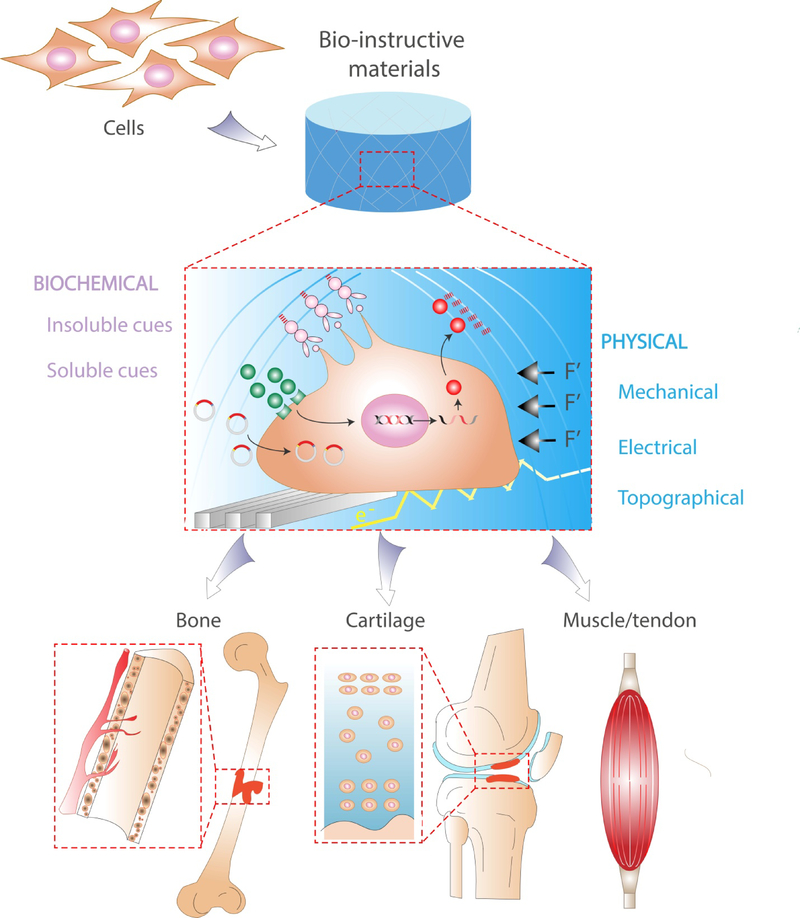
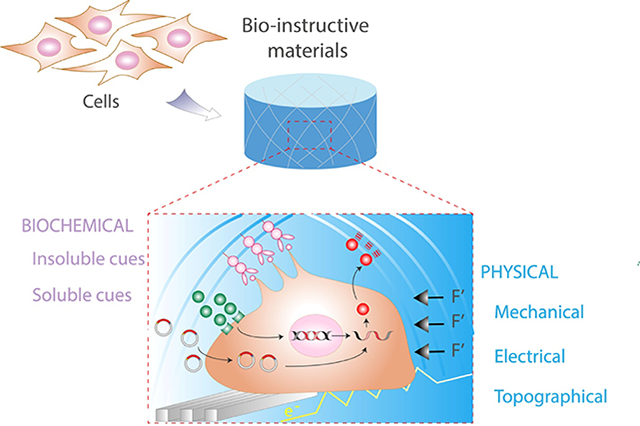
Biomaterials have tremendous potential to address these challenges through the development and use of bio-instructive scaffolds. Compared to early generation materials that were inert in order to limit the inflammatory and immune response, the field has evolved towards engineering scaffolds which elicit specific cellular functions[5,6]. Cells are exquisitely sensitive to mechanical, biological, chemical, and physical signals within their immediate microenvironment (Figure 1). These cues instruct cell adhesion and shape, migration, intracellular signaling pathways, and ultimately, gene expression. However, a primary challenge remains: What is the best strategy to direct cell function and tissue regeneration, and how should one present these cues in biomaterials? This review aims to highlight the biochemical and physical strategies for the engineering of bio-instructive materials and their application for the repair of clinically challenging musculoskeletal tissues.
Figure 1.
Bio-instructive materials present physical and biochemical signals to associated cells at the meso-, micro-, and nanoscale.
2. Strategies for bio-instructive materials
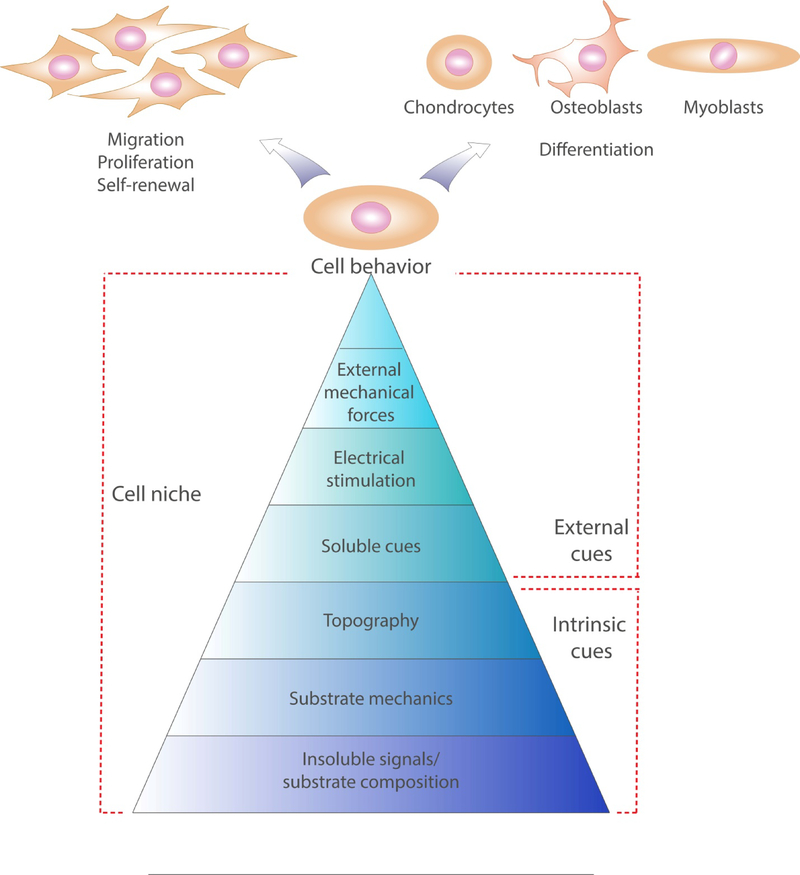
Cells in their native physiological niche are exposed to a complex array of physical and biochemical stimuli (Figure 2). Physical cues are comprised of the mechanical stimuli transmitted to the cells due to their response to the stiffness of underlying adhesive sites (i.e., substrate), an applied mechanical load (i.e., compression or shear stresses), as well as the topography and possibly electroconductivity of the local substrate that influences cellular organization and physiological function. Biochemical stimuli are the combination of soluble signals and insoluble components of the local ECM that guide cell interaction with their substrate and allow for the efficient transmission of previously defined physical cues. Multiple strategies have been used to incorporate these cues into biomaterials to instruct cell function during in vitro culture and in vivo transplantation. In the following sections of this review, we have summarized established and innovative approaches to engineer biomaterials capable of transmitting physical and biochemical signals for musculoskeletal applications.
Figure 2.
The cell niche is determined by intrinsic and external cues of physical (substrate mechanics, topography, external force and electric stimulation) and biochemical (substrate composition and soluble molecules) origin. This complex milieu determines cell behavior and differentiation.
2.1. Physical
2.1.1. Mechanical cues
Cells constantly generate and are exposed to forces, which may be derived from applied load, hydrostatic forces, or fluid shear stress[7]. In solid tissues such as those of the musculoskeletal system, cells are embedded in a dense polymer network known as the extracellular matrix (ECM), which may be exposed to various external loading regimes that induce tissue deformation or strains. Upon exposure to these mechanical stresses, the adaptive ECM evolves to necessary stiffness and elasticity. The cell cytoskeleton is connected to the ECM through integrin adhesion receptors that engage with specific ECM ligands[8], and the interplay between intracellular machinery and the ECM affects all aspects of cell function[7,9]. When the mechanical properties of the local ECM are disrupted due to trauma or disease, the behavior and function of associated cells are also altered. Cells in culture also sense changes in their mechanical microenvironment[7,8]. Thus, the response of cells to mechanical cues has emerged as an important focus to improve our understanding of cell behavior in vivo and assist in the development of strategies to instruct cell function using engineered materials.
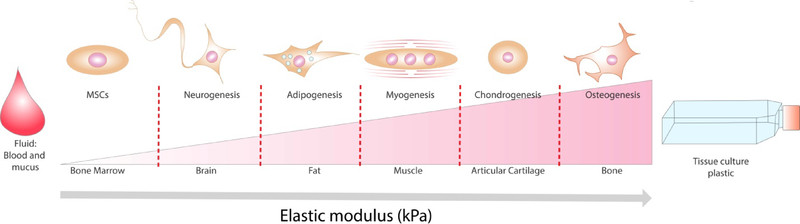
Substrate mechanics is a significant physical cue to guide cell fate, making it an essential design parameter for creating and deploying instructive biomaterials (Figure 3). First reported by Pelham and Wang, fibroblast migration has been shown to be dependent on the physical properties of the underlying matrix[10]. Engler et al. subsequently demonstrated that human MSCs cultured on two-dimensional (2D), tunable polyacrylamide substrates could be differentiated toward neurogenic, myogenic, and osteoblastic lineages as a function of gel elastic modulus ranging from 0.1 to 40 kPa[9]. These results confirmed that material elasticity mimicking those of native tissues (brain, muscle and bone) was an important contributor for inducing MSCs towards a specific lineage. MSCs exhibited similar responsiveness to substrate elastic modulus when entrapped within three-dimensional (3D) microenvironments[11]. Subsequent studies revealed the importance of substrate stiffness on cell attachment, spreading, cytoskeletal tension, and nuclear conformation. Cells in soft substrates exhibit reductions in spreading and attachment, rounded morphology, and a more diffuse actin cytoskeleton, whereas stiffer substrates favor cell attachment through increased focal adhesions, and subsequently, higher cytoskeletal tension, stress fiber formation, and nuclear deformation[12,13].
Figure 3.
Substrate stiffness is an essential design parameter for the incorporation of physical cues into biomaterials to guide stem cell fate.
Material mechanics can regulate stem cell fate, but this physical characteristic can also influence cell proliferation, self-renewal, and senescence[14,15]. Muscle-derived stem cells cultured on polyethylene glycol (PEG) hydrogels mimicking the elastic modulus of muscle (12 kPa) exhibited self-renewal in vitro and contributed extensively to muscle regeneration in vivo[15]. However, these cells lost their differentiation potential and had limited regenerative potential when cultured on tissue culture plastic (~106 kPa). Although soft substrates are effective for stem cell maintenance and to delay senescence[14,15], human MSCs cultured on 0.25 kPa substrates, mimicking the elasticity of bone marrow and adipose tissue, exhibited a halt in cell cycle progression[16]. This quiescent state reverted when the MSCs were transferred to more rigid substrates (7.5 kPa). Similar stiffness-based reprogramming of MSC differentiation was reported when culturing MSCs on soft (0.5 kPa) or stiff (40 kPa) hydrogels, followed by transfer to gels of the opposite stiffness[17]. When transferred, MSCs first cultured on softer gels switched from a neurogenic phenotype to an osteogenic lineage. In contrast, MSCs cultured initially on stiffer substrates retained their osteogenic phenotype after a switch to softer gels[17]. These findings confirm that cells have an inherent response to the mechanical properties of their environment.
Many strategies have emerged to engineer bio-instructive materials with varied mechanical properties to capitalize on the effect of substrate stiffness and elasticity. The most common approaches focus on tuning the mechanical properties of polymeric scaffolds and hydrogels by varying the crosslinker concentration[14,18], crosslinking time[19], polymer concentration[18] and polymer molecular weight[20]. Non-degradable polymeric networks used for tissue engineering applications such as PEG exhibit almost purely elastic behavior[21]. Although variations in initial elastic modulus can be produced with these materials and used to guide cell behavior, most tissues are viscoelastic, and thus, the mechanical stresses of the ECM relax over time upon exposure to load. Alginate gels can be formulated to possess elastic or viscoelastic behavior using covalent or ionic crosslinking strategies, respectively[21]. Purely elastic substrates store elastic energy in the material and possess time-independent storage or elastic moduli, presenting constant resistance to applied forces over time. Viscoelastic materials exhibit stress relaxation, or a decrease in the storage or elastic modulus over time when a constant strain is applied. Unlike covalently crosslinked gels, ionically crosslinked alginate gels undergo stress relaxation due to diffusion of calcium ions, and the stress relaxation time of these gels can be tuned by crosslinker and composition[22]. MSCs entrapped in viscoelastic gels with faster stress relaxation times exhibited enhanced cell adhesion, spreading, proliferation, and osteogenic differentiation[21]. Other musculoskeletal cell populations are also responsive to time-dependent properties of materials. For example, chondrocytes exhibited increased matrix production in viscoelastic gels but increased markers of cartilage degeneration when entrapped in elastic gels[23]. Murine myoblasts proliferated faster and spread more on hydrogels that exhibited stress relaxation[24]. When implanted in a rat critical-sized calvarial defect, fast relaxing gels facilitated increased host cell migration into the defect and more bone formation compared to slow-relaxing gels[25]. Within matrices exhibiting stress relaxation, the resistance to cellular traction forces relaxes over time due to remodeling of the matrix, dispersing the energy that cell-generated forces transmitted to the material. These findings collectively highlight the ability of a substrate to either store or dissipate cellular forces as an important mechanical property in the design of biomaterial systems to instruct cells for tissue formation.
2.1.2. Topographical/morphological cues
The native ECM is a fibrous matrix in the nanometer range (fibers of 10–100 nm in diameter) presenting a complex mixture of pores, ridges and fibers organized into a 3D anisotropic architecture that provides topographical signals to cells at the microscale (10–100 μm)[26,27]. The ECM architecture at the nanoscale is not static, but instead undergoes constant remodeling in response to tissue maturation, external stimuli, damage and repair[28]. ECM organization is tissue-specific and can vary locally throughout the tissue. For example, cortical bone is a dense matrix formed by repeating osteon units in which the collagen fibers are organized periodically in concentric layers around a vascularized central canal. However, trabecular bone is a more porous and interconnected substrate with a disorganized collagen network[29]. Similarly, tendons possess highly variable topography as a function of distance from the bone, with gradients in anisotropy from the enthesis down the length of tissue. Tissue anisotropy can also be found in articular cartilage where collagen fibers are aligned parallel to the articular surface in the superficial zone and perpendicular to the subchondral bone in the deep zone[30].
A number of strategies are under examination to reproduce the nano- and microtopographies of ECM within native tissues and to investigate the capacity of substrate topography to regulate cell migration, proliferation, and differentiation. Lithographic methods including nanoimprint[31], capillary force lithography[32], photolithography[33] and electron beam lithography[34] have been used to fabricate 2D polymeric substrates with topographic features. Nanogratings and arrays of nanoposts and nanopits are the most widely tested topographic geometries created with these techniques[35]. Nanograting topography can promote cellular alignment and elongation in the grating direction. Human MSCs seeded on polydimethylsiloxane (PDMS) matrices possessing a nanograting pattern of 250 nm line width developed aligned stress fibers that upregulated neurogenic and myogenic differentiation pathways[36]. In contrast, cells on nanoposts and nanopits exhibit reduced cell spreading[35], and these nanotopographies guide stem cell differentiation via the density of the patterned features[37]. MSCs seeded on polyurethane hydrogels patterned with variable nanopost (diameter = 700 nm) densities differentiated toward the osteogenic pathway at low densities whereas low post-to-post distances favored the adipogenic phenotype. MSCs on substrates with low nanopost densities exhibited more cell spreading, F-actin expression, stress fiber formation, and cytoskeletal stiffness[37]. This is in good agreement with earlier studies wherein MSC differentiation was regulated by controlling cell shape and thus cytoskeletal tension[38].
While 2D nano- and microtopography represents an effective strategy to investigate cell response as a function of geometry, this approach fails to recapitulate the complexity of the 3D microenvironment. To address this challenge, fiber anisotropic architecture in 3D polymeric scaffolds has been controlled through numerous biofabrication techniques such as ionotropic gelation[39], magnetic fields[40], directional freezing[41,42], 3D bioprinting[43], and electrospinning[44,45], the latter of which is the most broadly used. Electrospinning uses biodegradable polymers to produce scaffolds with fiber diameters in the nano- and microscale, mimicking the ECM structure[45,46]. For example, MSCs seeded on aligned polycaprolactone (PCL) electrospun fiber scaffolds were oriented parallel to the fibers, while cells spread in all directions on randomly-aligned fiber scaffolds[44]. When connective tissue growth factor (CTGF) and transforming growth factor beta 3 (TGF-β3) were added to the culture media, MSCs in aligned scaffolds developed either a ligamentous or chondrogenic phenotype, while osteogenesis was observed in MSCs on randomly aligned scaffolds[44].
In addition to fiber alignment, porosity is an important factor for vascular invasion, cell viability, migration, proliferation, and differentiation in 3D fibrous scaffolds. Small pore sizes may limit host cell infiltration and migration or contribute to rapid death of transplanted cells in the interior of the scaffold due to poor oxygen and nutrient diffusion[47]. In contrast, larger pore sizes (>300 μm) facilitate increased vascular invasion, surface area, and cell attachment[47–49]. Although porosity and pore size enable tissue ingrowth, the frequency and size of these voids compromise scaffold structural integrity, resulting in lower mechanical properties. To decouple the effect of pore size and substrate mechanics on osteogenesis and chondrogenesis of MSCs, Matsiko et al. explored stem cell response to collagen-HyA scaffolds with the same mechanical properties but different pore sizes[50]. This study showed that MSC differentiation was dependent on pore diameter, with chondrogenesis observed in scaffolds with pore sizes of 300 μm compared to a more fibroblastic phenotype at 94 and 130 μm[50]. Pore size and fiber architecture are relatively easy to control in fibrous scaffolds using methods such as temperature modulation during the freeze drying process[51,52]. However, it is more challenging to generate hydrogels with macroporous architecture due to their inherent nanometer-sized pores, which limits cell migration and proliferation[50–53]. Pore-forming hydrogels have been investigated through the addition of a rapidly-lost sacrificial material to a bulk hydrogel with slower degradation kinetics[54,55]. Different combinations of bulk-sacrificial materials such as high molecular weight alginate-oxidized alginate[56], alginate-pluronics[54] and alginate-methylcellulose[55] are promising candidates for use in musculoskeletal regeneration.
2.1.3. Electrical cues
Endogenous electric fields provide signals to control cell function during embryonic development, tissue maintenance, and repair[57]. In native tissues, voltage gradients occur across the cell membrane (membrane potential) due to cellular regulation of intracellular ion concentrations through membrane ion pumps and ion channels[57]. To maintain this potential, cells transport ions against concentration and/or electrochemical gradients. The membrane potential, typically ranging between 50–100 mV[58], not only influences cell function at the single cell level but also at the tissue and organ level to enable muscle function[58], nerve signaling[58], embryonic development[59] and tissue regeneration[60]. In light of its role in tissue development and repair, electrical stimulation has been used clinically for the treatment of diabetic ulcers[61], chronic musculoskeletal disorders[62], bone fractures[63] and to enhance nerve regeneration[64].
Electrical stimulation is a promising strategy to direct stem cell fate. This phenomenon, recently reviewed by Ross et al.[65], is not exclusive for stem cell differentiation into myocytes[66], cardiomyocytes[67] and neurons[68]. MSC differentiation can also be directed towards the osteogenic[69,70] and chondrogenic[71] lineages using electric stimulation. To leverage the potential of electrical stimulation in musculoskeletal TE, hybrid materials have been fabricated through the combination of electroconductive materials with biocompatible scaffolding materials (Table 1). Electroconductive materials used in these hybrids such as metal nanoparticles (iron oxide[72], zinc oxide[73] and gold[74]), graphene[66], and electroconductive polymers such as polyaniline[75] and polypyrrole[69] have been widely investigated for musculoskeletal tissue engineering. However, the application of these materials for TE approaches is limited by slow degradation, possible cytotoxicity, and inflammation[76]. The deployment of bio-instructive materials possessing both bioelectric and topographical cues represents a promising approach to regenerate highly anisotropic tissues such as skeletal muscle. Myotube formation by myoblasts seeded on electrospun PCL nanofiber scaffolds functionalized with polyaniline was dependent on nanofiber alignment and polyaniline concentration, confirming the synergistic effects of electrical and topographical cues[77]. Electroconductive materials have also been explored for their antibacterial activity. Biohybrid implants formed of electroconductive collagen-pristine graphene enhanced the alignment and differentiation of embryonic stem cell-derived cardiomyocytes while inhibiting the attachment of S. aureus[78].
Table 1.
Electroconductive 3D hybrid scaffolds for musculoskeletal tissue engineering
| Electroconductive scaffold | Applied voltage | Cell type | TE application | Reference |
|---|---|---|---|---|
| Polyaniline-PCL | None | Myoblasts | Skeletal muscle TE | [77] |
| Graphene- poly(citric acid-octanediolpolyethylene glycol) | None | Myoblasts | Skeletal muscle TE | [83] |
| Zinc oxide coated- polydimethylsiloxane | 120 mV/mm | Umbilical cord bloodderived MSCs | Skeletal muscle TE | [73] |
| Graphene oxide-PCL | None | Umbilical cord bloodderived MSCs | Skeletal muscle TE | [66] |
| Chitosan/polypyrrolealginate | None | MG-63 osteosarcoma cell line | Bone TE | [84] |
| Polyaniline- | None | MG-63 osteosarcoma | Bone TE | [85] |
| PCL/alginate/gelatin/HyA | cell line | |||
| Polypyrrole-Gelatinhydroxyapatite | None | Osteoblasts | Bone TE | [86] |
| Polypyrrole- polylactide | 200 mV | Adipose derived MSCs | Bone TE | [87] |
| Polyaniline-polylactide | None | Bone marrow-derived MSCs | Bone TE | [88] |
How these materials instruct cell behavior and tissue regeneration is poorly understood, particularly when no external voltage is applied. In living systems, local electrical fields are a consequence of out-of-equilibrium ion concentrations across membranes or interfaces that generate a voltage potential across the membrane[79]. Adherence of cardiomyocytes to an electroconductive substrate is of critical importance for electrical cell-to-cell coupling[80]. This is because the cleft, representing the interface between a cell and material surface, is filled with physiological solution. Tight adhesion is needed to generate a high resistance between this cleft and the ground (also called high seal resistance)[79]. Other common biophysical parameters such as substrate charge density, wettability, and protein adsorption may influence material conductivity when an electrical current is applied[81,82].
2.1.4. Physical cues for immunomodulation and macrophage polarization
Biomaterials possess intrinsic properties that can activate the immune system, and these biomaterial scaffolds must be able to overcome immunological rejection and promote proper healing after implantation. Upon implantation of a biomaterial, neutrophils and other innate immune cells infiltrate into the implant site and recruit macrophages through the secretion of different chemokines and cytokines[89]. Macrophages are a highly plastic cell type that undergo polarization from a pro-inflammatory phenotype (M1) to a more anti-inflammatory, pro-regenerative phenotype (M2) or related subtypes [89]. Whereas an early inflammatory M1 response is required to initiate tissue regeneration after an injury, a shift to the M2 phenotype is also required to re-establish tissue homeostasis and avoid the destructive effects of chronic inflammation.
Macrophage phenotype may be regulated by the physical properties of the implant such as matrix stiffness, porosity, and topography. When implanted in vivo, softer RGD-modified PEG hydrogels (130 kPa) impaired M1 macrophage polarization and the foreign body reaction compared to stiffer gels (840 kPa), suggesting that softer materials may be better for tissue regeneration[90]. Importantly, even the softer gels were significantly stiffer than many hydrogels used for tissue engineering, necessitating other studies in hydrogels possessing more commonly observed mechanical properties. In contrast, macrophage polarization was not directly correlated to stiffness when manipulating collagen scaffolds, yet the crosslinker used to modulate scaffold stiffness induced significant differences in macrophage phenotype[91]. These data highlight the importance of scaffold fabrication method and suggest the interplay between protein adhesion, substrate stiffness, and macrophage polarization. Surface roughness, particle size, and shape also have an important role in macrophage polarization[92–94] and have been manipulated in orthopedic implants to promote M2 polarization and enhance implant integration. Additionally, macrophage phenotype can be influenced by the porosity of 3D scaffolds. M1 macrophages were more prevalent within polyhydroxyethylmethacrylate (pHEMA) scaffolds of small pore sizes (34 μm) compared to larger diameter pores (160 μm)[95], resulting in improved neovascularization within the implant. This study stopped short of characterizing implant integration, yet these findings have important implications on the design of biomaterials to modulate the polarity of invading macrophages.
Charge may be another important factor to influence the immune response. Zwitterionic materials, characterized by balanced pairs of cationic and anionic groups and a net charge equal to zero, possess nonfouling properties that resist nonspecific protein adsorption and avoid macrophage recognition and foreign body reaction[96,97]. Ultra-low fouling poly(carboxybetaine methacrylate) (PCBMA) hydrogels exhibit reduced fibrous capsule formation, recruit fewer pro-inflammatory M1 macrophages, and enhance vascularization when implanted in vivo[96]. More recently, the interplay between substrate stiffness and the immunomodulatory characteristics of zwitterionic hydrogels was established, with stiff (165 kPa) hydrogels eliciting increased immune cell adhesion and foreign body reaction[98].
2.2. Biochemical
The mechanical integrity of tissues is dependent upon the ECM, and this dynamic structure also has an active role in mediating cell attachment and growth factor presentation. Specialized cell surface receptors mediate the interplay between the cell and the biochemical signals present in the ECM, which may be soluble or insoluble ligands. The specific biochemical reaction between a ligand and its receptor elicits a signaling cascade which may ultimately result in a range of cellular actions. Soluble factors such as cytokines, growth factors, RNA, and hormones readily diffuse through the cell microenvironment and regulate cell-cell signaling, cell differentiation, migration, and proliferation[99,100]. Insoluble factors, forming part of the polymeric ECM network, dictate cell adhesion, shape, migration, and response to mechanical stimuli. Furthermore, insoluble components directly interact with soluble factors to shield them from degradation and control their bioactivity and cell presentation[11,101]. To leverage the potential of these biological cues, numerous strategies have been investigated to engineer materials for delivery of soluble factors and to incorporate insoluble ligands for directing cell function and tissue regeneration.
2.2.1. Delivery of soluble signals
2.2.1.1. Protein and nucleic acid delivery
In musculoskeletal TE, the administration of soluble cues including Bone Morphogenetic Protein 2 (BMP-2) and other members of the Transforming Growth Factor β (TGF-β) superfamily to instruct cell fate, drive stem cell differentiation, and speed repair is an area of particular importance. To achieve the desired effect, soluble growth factors and cytokines can be easily incorporated into the biomaterial through physical entrapment, non-specific adsorption[102–104] and covalent coupling[100,105]. A more physiological alternative is to retain exogenous and cell-produced cytokines through the incorporation of ECM components or specific ECM domains[106]. Negatively-charged glycosaminoglycans (GAGs) and proteoglycans can bind cationic cytokines and growth factors, causing oligomerization and increased local concentrations. The incorporation of heparin to an alginate hydrogel enabled sustained release of encapsulated fibroblast growth factor 2 (FGF-2) over one month compared to the one day burst release observed using unmodified hydrogels[107]. However, the use of natural glycoproteins to engineer growth factor-sequestering scaffolds is limited by their high costs and large batch-to-batch variability[108,109]. Therefore, chemical modification of polymers such as poly(lactide-co-glycolide)[110], cellulose[108,111], chitosan[108] and alginate[109,112] with sulfate groups to recapitulate the binding affinities of sulfated glycoproteins has been explored for bone[108,112] and cartilage[111] repair.
As an alternative to recombinant proteins, gene delivery has been proposed as a promising strategy for the cell-mediated production of regulatory factors[100,113,114]. Since the first attempts of nucleic acid incorporation into collagen sponges[115], various strategies including viruses containing the gene of interest (viral gene delivery) or polymer- or nanoparticle-plasmid DNA complexes (non-viral gene delivery) have been explored to maximize gene transfer and cell-mediated protein expression[116,117]. However, safety concerns associated with viral vectors[100,118], as well as effects on cell viability and stem cell differentiation of commonly used non-viral gene delivery vectors[119,120], continue to hamper their translation into the clinic. To overcome these challenges, the delivery of RNA may be a safer and more effective alternative[121]. Different types of RNA such as messenger RNA (mRNA)[122], microRNA (miRNA)[123], and small interference RNA (siRNA)[124] have been incorporated into biomaterials to enhance the repair of muscle[125], bone[123,124] and cartilage[122].
2.2.1.2. Delivery of immunomodulatory cytokines
As described above, it is necessary to modulate the macrophage response to a biomaterial to overcome immunological rejection and promote effective healing. Molecules secreted by M1 macrophages such as TNF-α, IL-1, and IL-6 may accelerate bone fracture repair, matrix mineralization, and the recruitment of MSCs and osteoprogenitor cells[126,127]. However, sustained presentation of higher concentrations of these cytokines inhibit cartilage formation in the early phases of bone induction and are responsible for cartilage damage in disorders such as osteoarthritis[128]. Local cytokine presentation from biomaterials is under investigation to influence macrophage polarization and enhance tissue repair. For example, the sequential delivery of IL-4 and IFN- γ from decellularized bone scaffolds promoted sequential M1 and M2 polarization and higher vascularization when implanted in vivo[129]. More recently, local M2 macrophage polarization was enhanced by adsorption of IL-4 and IL-13 to collagen scaffolds implanted in an osteotomy gap, resulting in improved endochondral ossification and bone regeneration in vivo[130].
2.2.2. Incorporation of insoluble signals
2.2.2.1. Peptide modification to control cell behavior
Cells in their microenvironment can interact with distinct protein moieties present in the ECM through the binding of cell surface receptors such as integrins, cell surface proteoglycans, and the hyaluronan receptor CD44[131]. Due to ligand-receptor specificity, cell adhesion to different ECM proteins triggers different responses that modulate cell function[5,132]. Initially, decellularized animal-derived ECM was explored to recapitulate this native complexity[26]. However, naturally derived ECMs have several limitations for their clinical translation including batch-to-batch variability and immunogenic potential[26,133]. To overcome these issues, various scaffolds and hydrogels have been functionalized with ECM mimics that emulate the native ECM, enabling increased control over material design and properties.
Cell adhesion peptides (CAPs) represent the largest class of ECM mimics to regulate cell function in engineered materials. These short amino acid sequences are the minimal moiety needed for binding to the specific cell receptor responsible for cell attachment[132]. Among CAPs, the arginine-glycine-aspartic acid (RGD) tripeptide sequence[133,134] is arguably the most commonly used cell adhesion motif. In fact, 89% of CAP studies published from 1970–2018 used the RGD sequence[132]. However, depending on the type of tissue, the ECM contains different ratios of multiple cell attachment domains[132]. Thus, more than one peptide motif is necessary to reproduce the native ECM complexity. Many other sequences of native[135–143] or synthetic[144,145] origin (Table 2) have been identified to drive stem cell differentiation towards the osteogenic, myogenic, and chondrogenic pathways. Domains isolated from growth factors such as BMP-2[146,147], VEGF[148], and parathyroid hormone[149] are of special interest to instruct cell behavior and differentiation when incorporated to biomaterials. For example, peptides derived from BMP-2 and parathyroid hormone domains have been explored to enhance osteogenesis[146,147], and chondrogenesis[149] of MSCs, while other motifs such as the QK peptide can promote vascularization[148].
Table 2.
Peptide sequences for material functionalization and improved musculoskeletal regeneration.
| Origin | Sequence name | Cellular effect | References | |
|---|---|---|---|---|
| ECM | Collagen type I | GFOGER | Chondrogenesis/osteogenesis | [135] |
| Collagen type III | CTC | Osteogenesis | [138] | |
| Decorin | KLER | Chondrogenesis | [139] | |
| Fibronectin | RGD | Osteogenesis, chondrogenesis/myogenesis | [133,134] | |
| Laminin | YIGSR | Myogenesis, angiogenesis | [136,137] | |
| Laminin | IKVAV | Adipogenesis/osteogenesis | [137] | |
| N-cadherin | HAVDI | Chondrogenesis | [140] | |
| Bone specific ECM | Bone sialoprotein | CB | Osteogenesis | [141] |
| Bone sialoprotein, | KSRS | Osteogenesis | [142] | |
| fibronectin, osteopontin | ||||
| Osteopontin | CBM | Osteogenesis | [143] | |
| Growth factor | BMP-2 | KIPKASSVPTELSAISTLYL | Osteogenesis, Chondrogenesis | [146,150] |
| BMP-4 | HBD | Osteogenesis | [151] | |
| BMP-7 | BFP-1 | Osteogenesis | [152] | |
| Parathyroid hormone | PTH 1–34 | Osteogenesis | [149] | |
| VEGF receptor | QK | Angiogenesis | [148] | |
| Synthetic | RADA | Chondrogenesis/osteogenesis/ligament | [144,145] | |
| KLD | Osteogenesis/chondrogenesis | [140] | ||
| RRETAWA | Osteogenesis/adipogenesis | [137] | ||
Cells sense the mechanical properties of the matrix only through adhesion to the surrounding microenvironment. The binding between the specific cell receptors and the ligands present in the ECM enables linking of the actin cytoskeleton within the cell through a focal adhesion complex [153]. The development of tension and the maturation of focal adhesions trigger the signaling cascades that control cell spreading, migration, and differentiation[153]. The ability of cells to sense the mechanical properties of their environment and their responses depend on both ligand identity and density[153]. For example, MSCs seeded on peptide-functionalized hydrogels with constant stiffness and ligand density exhibited increased osteogenesis when seeded on laminin-derived IKVAV-functionalized gels, yet cells seeded on laminin-derived YIGSR- or synthetic RRETTAWA-functionalized gels followed the adipogenic route[137]. Despite sharing the same matricellular protein of origin, IKVAV and YISGR induced unique effects on cell differentiation due to different integrins engaging specifically with each peptide[137]. Ligand density is another determinant for cell function. MSCs entrapped in alginate gels that were either non-functionalized or functionalized with a high density of RGD peptides, providing too few or too many adhesion sites, respectively, exhibited impaired migration that translated to increased osteogenesis and bone formation, whereas alginate hydrogels with low RGD density induced more migration but reduced bone formation[154]. The interaction of MSCs with RGD ligands is also mediated by dynamic material properties such as stress relaxation, with faster relaxing RGD-modified alginate gels facilitating increased integrin receptor binding, RGD ligand clustering, and enhanced osteogenesis[21]. The effect of ligand density on cell differentiation is likely due to cytoskeletal tension regulated by the number of bonds between the substrate and the cell[153].
2.2.2.2. Antimicrobial peptides
Infections due to opportunistic microorganisms are one of the most important causes of musculoskeletal repair failure and prosthesis revision, with Staphylococcus aureus (S. aureus) identified as one of the most common microbes. It is estimated that out of the 1 million knee prostheses and 6 million fracture fixation devices implanted per year, 2% and 5%, respectively, fail due to infections[155,156]. Infection-related failure of orthopedic implants results in an economic burden of $2 billion annually. Numerous approaches have been examined to avoid microbial infections in scaffolds for tissue engineering after implantation including the incorporation of antibiotics[157,158] and nanoparticles made of gold[159] and silver[160]. However, increasing bacterial drug resistance and rapid burst release upon implantation[156] limit the efficacy of these approaches. Alternatively, the incorporation of electroconductive materials[78] and their functionalization with antibacterial peptides may provide sustained antibacterial activity and enhanced tissue regeneration. Antimicrobial peptides (AMPs) contain cationic amino acids that bind to negatively charged bacterial membranes, leading to increased membrane permeabilization, and ultimately, cell lysis. In light of their bioactivity, both natural and synthetic materials have been functionalized with AMPs[161,162]. For example, the synthetic peptide Tet213 was incorporated within gelatin methacryloyl (GelMA)-tropoelastin hydrogels, decreasing colonization by S. aureus and Escherichia coli (E. coli) to levels comparable to zinc oxide incorporation within the same hydrogel[162]. Other peptides exhibit synergistic potential with antibacterial properties by simultaneously enhancing musculoskeletal regeneration. The self-assembling peptide KLD modified with variable numbers of cationic arginine residues at its N-terminus possessed increased antibacterial potential against E. coli, while also inducing greater expression of osteogenic genes in vitro and increased callus formation in vivo[163].
2.2.2.3. Enzymatic degradation sequences
The ECM is a dynamic structure that undergoes constant remodeling due to cell-mediated enzymatic degradation and subsequent new ECM synthesis. ECM turnover is critical for maintenance of its function, and disruption of the balance between regenerative and degenerative processes is often associated with a loss of functionality or disease[164]. Degradation kinetics must be considered in light of natural turnover rates, as the goal of many TERM approaches is to provide a temporary structure that can support function and is subsequently replaced by native ECM over time[164]. Many materials are designed to degrade hydrolytically, and the material degradation rate is tuned by changing the fraction of bonds susceptible to hydrolysis in a copolymer. However, natural ECM is degraded enzymatically through the action of matrix metalloproteinases (MMPs) and serine proteinases.
Proteolytically sensitive peptide sequences provide an opportunity to leverage nature’s own mechanisms for ECM remodeling. PEG hydrogels were initially crosslinked with MMP-cleavable oligopeptides, based on the cleavage sites present in collagen type I, which increased cell invasion and healing of a rat critical sized calvarial bone defect[165]. After these foundational studies, novel protease substrates from different origins that can degrade ECM faster or more specifically have been investigated. The degradability of 17 different natural and synthetic sequences specific for MMP-1 and MMP-2 activity were screened by incorporation into PEG hydrogels[166]. When exposed to specific enzymes produced by particular cell types, these hydrogels underwent faster substrate remodeling, cell spreading, and invasion in vitro. PEG has been used successfully as a blank slate to interrogate these processes due to the capacity to incorporate adhesivity and probe specific cell physiological processes. For example, MMP-7, a chondrogenesis-specific enzyme, was incorporated into PEG hydrogels to enable differentiation-mediated hydrogel degradation and increased MSC chondrogenesis[167].
2.2.2.4. Inorganic materials: Calcium phosphates to promote biomineralization
Bone is a highly mineralized tissue composed of up to 65% calcium phosphate (CaP). Thus, many studies focus on the use of CaP-based composites for bone TE scaffolds. The mineral phase in natural bone is a calcium-deficient, nanocrystalline, carbonate-substituted hydroxyapatite (HA). This phase is found in both mineralizing collagen matrix and localized between collagen fibers in a highly organized manner[168]. CaP itself is known to have osteoinductive and osteoconductive properties and has been used in synthetic bone grafts since the 1970s. Many forms of CaP-based bone graft systems are in clinical use and under further development[169]. However, the structure of highly organized and hierarchical mineralized native tissue such as bone or dentin has not yet been replicated in the laboratory. There are few examples in the literature in which synthetic organic/inorganic composites containing ceramics have an organization and properties approaching that of biological material or tissue[170,171]. Therefore, bio-instructive approaches involving mineralized 3D scaffolds or surfaces on which a tissue is formed by an in vivo like process are attractive[172–174].
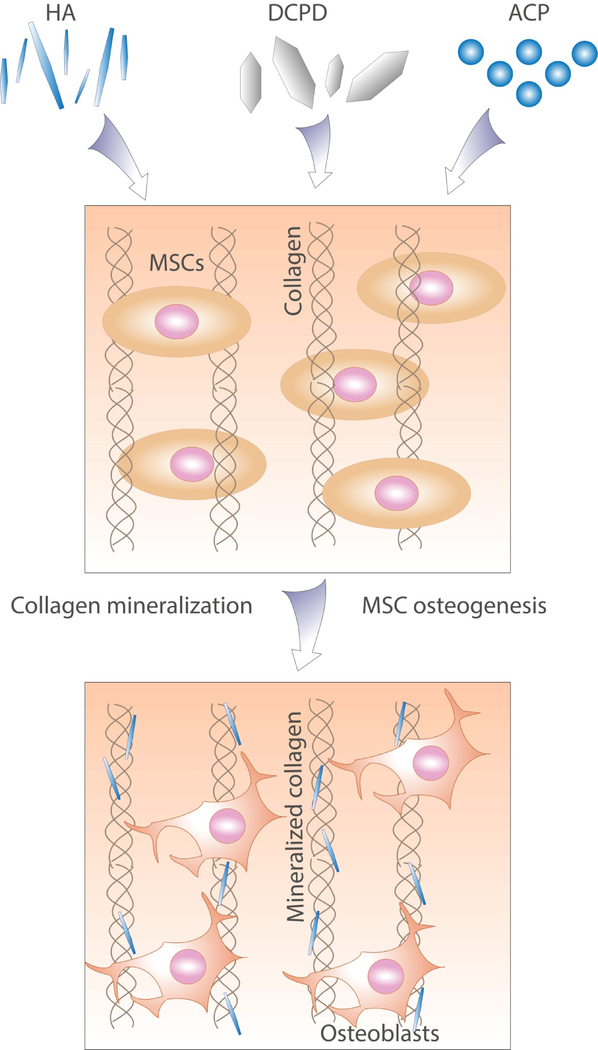
CaP composites can be instructive by providing a source of ions for deposition of new bone[175], surface morphology and chemistry recognized by bone cells[174], or improved mechanical properties[176] (Figure 4). Instructive composite scaffolds based on proteins, biopolymers, or synthetic polymers can be mineralized using a range of strategies including ALP-induced precipitation[177] or precipitation from chemical precursors[173,178]. Polymeric substrata have been coated with carbonated apatite by immersion in simulated body fluid supersaturated with respect to CaP[174], and coated substrates have been used as stand-alone scaffolds[179] or as part of composite materials[180,181] to guide MSC osteogenic differentiation in vitro and in vivo. Polymer-induced liquid precursor (PILP)-driven mineralization has been also widely explored. When applied to densified collagen films with various degrees of chemical crosslinking, this method resulted in homogeneous intra- and extrafibrillar mineralization and composites with mechanical properties approaching those of native bone[176]. Recently, a self‐structuring in vitro model of bone formation was created using a fibrin gel containing rat periosteal cells and cast between bio-instructive β‐TCP/brushite anchors[175]. The β‐TCP/brushite particles were incorporated as a local source of calcium and phosphate for the ossification of developing tissues, while fibrin was chosen to mimic the microenvironment of the initial callus formed during fracture healing[175]. Cell culture was continued for up to 1 year, resulting in a cell-synthesized osseous matrix resembling the composition and structure of native mature bone[175].
Figure 4.
Incorporation of calcium phosphate phases such as HA, dicalcium phosphate dihydrate (DCPD) or amorphous calcium phosphate (ACP) into biomaterials can provide a surface recognized by cells and biomacromolecules or act as a source of ions for the mineralization of incorporated or cell-produced ECM components. Both processes contribute to instructing stem cell phenotype towards de novo bone formation.
3. Future directions
Throughout this review, we have summarized current and emergent strategies to incorporate physical and biochemical cues within biomaterials to modulate cell behavior and tissue formation. This allows for the compilation of the general and tissue specific characteristics needed for musculoskeletal repair (Figure 5). However, most highlighted strategies focus on a single aspect of cell biology, yet more integrative approaches presenting multimodal stimuli are needed to effectively address regenerative processes. For example, the combination of antimicrobial and osteogenic factors loaded in a 4-arm PEG-maleimide (PEG-4MAL) hydrogel simultaneously reduced S. aureus infection and regenerated murine critical-sized segmental bone defects[182].
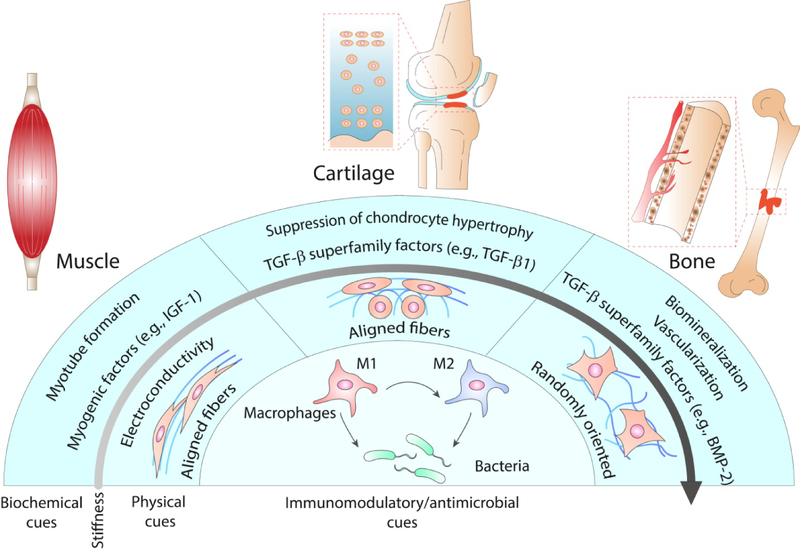
Figure 5. Summary of physical and biochemical cues to enhance regeneration of specific musculoskeletal tissues.
While immunomodulatory and antimicrobial cues are common for the design of bio-instructive materials for musculoskeletal tissues, the necessary biochemical and physical cues are tissue specific. Softer and aligned substrates are preferred for muscle and cartilage, but bone requires stiff and randomly oriented materials to effectively guide cells toward the osteogenic lineage. Muscle regeneration is enhanced by the presentation of myogenic factors (e.g., IGF-1, SDF1α), cartilage by the presentation of members of the TGF-β superfamily (e.g., TGF-β1, TGF-β3) and the suppression of chondrocyte hypertrophy, and bone by other members of the TGF-β superfamily (e.g., BMP-2, BMP-4), angiogenic factors and the promotion of tissue biomineralization.
Moreover, individual musculoskeletal tissues are not autonomous entities that work and regenerate independently from one another. Native tissues are dynamically interconnected, and this connection guarantees physiological function and regulates repair processes after injury. For example, open bone fractures affecting the surrounding skin and muscle exhibit impaired bone healing and delayed union in comparison to closed fractures[183]. Furthermore, the repair of the subchondral bone underlying articular cartilage is necessary for the successful regeneration of osteochondral and superficial cartilage defects[184]. In order to understand how to effectively regenerate musculoskeletal tissues, it is necessary to know what material properties are preferred for each specific organ and also how tissues interact with each other in a more integrated approach. The application of bio-instructive materials to technologies such as organ-on-a-chip and 3D bioprinting offer the possibility of recapitulating this complexity and advancing tissue engineering strategies towards clinical translation.
3.1. Bio-instructive materials for in vitro modelling of organ development and function
There is an urgent need for the development of physiologically relevant 3D in vitro models as an alternative to conventional preclinical in vivo models for basic science and drug screening. Such models have been primarily driven by needs in cancer research due to poor correlation between culturing tumor cells on 2D surfaces and their behavior in vivo[185,186]. Organ-on-a-chip strategies are based on the engineering of biomimetic microsystems that represent the native physiology and functions of living organs through the convergence of tissue engineering and microfluidics. The use of organ-on-a-chip approaches in musculoskeletal regeneration that capture elements such as the complex cell population, mechanical loading, signaling gradients, and fluid flow are needed.
Polydimethylsiloxane (PDMS) is the most commonly used material for the engineering of organs-on-a-chip due to its biocompatibility, gas permeability, transparency, low cost, and ease of fabrication[187]. However, the use of PDMS in musculoskeletal applications is limited by its hydrophobicity, non-specific protein adsorption, low stiffness and high deformation, challenges in maintaining a hypoxic microenvironment, and leaching of uncrosslinked polymer chains[188]. In order to resolve these issues, PDMS surfaces can be coated with matricellular proteins such as fibronectin and collagen type I[189]. However, 2D models fail to reproduce the complexity of musculoskeletal tissues. To address this challenge, 3D scaffolds and hydrogels are being combined with PDMS chips to produce microfluidic in vitro models of bone[190], bone marrow[191], cartilage[192,193] osteochondral unit[194,195] and skeletal muscle[196]. Various biophysical properties of biomaterials can be modified to recapitulate the tissue niche including topography[192] or bioactivity[190,191,194]. Beyond engineering a single tissue, there is an emerging effort to recapitulate interfacial tissues. For example, an organ-on-a-chip in vitro model of the osteochondral unit was developed to study the pathology of osteoarthritis through the spatial differentiation of MSCs in bioactive GelMA scaffolds locally exposed to either osteogenic or chondrogenic media[194]. This strategy effectively reproduced local gene expression and ECM gradients found in native articular cartilage.
3.2. Bio-instructive materials for biofabrication
Additive manufacturing and biofabrication approaches has the potential to revolutionize the tissue engineering field due to control over implant architecture, the precision for cell seeding, and the promise of generating functional, patient-specific organs. The development of bioinks, defined as “a formulation of cells suitable for processing by an automated biofabrication technology that may also contain biologically active components and biomaterials”[197], has taken central stage. However, the development of materials that provide necessary fidelity for high printing resolution and promote cell viability, proliferation, and differentiation remains challenging. Stiff hydrogels containing high polymer concentrations/or crosslink densities are best suited for biofabrication, yet these may not be optimal to support the cell functions required in tissue engineering[198]. Therefore, the engineering of bio-instructive materials for biofabrication applications have the added requirement to meet the physicochemical demands of the additive manufacturing process.
Alginate[199], GelMA[200], agarose[200], collagen[201], PEG[202], HyA[203] and others have been utilized as hydrogel-based bioinks in biofabrication for musculoskeletal tissue engineering. Various biochemical and physical approaches previously reviewed in this article have been used to instill cell-instructive potential in these materials. The regulation of bioink stiffness to modulate stem cell differentiation at different locations within the bioprinted tissue is an effective strategy to locally direct stem cell fate[204]. Control over bioink micro- and nanotopography has also been explored through the engineering of composite materials for the introduction of nanofibers into the bulk bioink hydrogel[205] or the fabrication of pore-forming bioinks[54,55]. To achieve fine control of the topography of printed tissues, 3D inkjet-based cell printing has been combined with electrospinning to manufacture layered nanofibrous scaffolds[206]. Similar to bulk hydrogels, bioinks have been functionalized with cell adhesive peptides[207] or chemical groups[208], decellularized ECM[209], and bioactive moieties such as growth factors[200] or genes encoding for these molecules[210].
Poor mechanical strength of most hydrogels is a major limitation for their use as bioinks in load-bearing applications. Hybrid co-printing has emerged as a promising strategy to provide mechanical stability and biological functionality to 3D printed tissues using a multi-head deposition system (MHDS) of mechanically stable thermoplastic polymers and cell compatible bioinks. The co-deposition of these hydrogels along with polymeric materials such as PCL[211] or PLGA[212] provides the opportunity to fabricate mechanically reinforced constructs with compressive equilibrium moduli in the range of trabecular bone or articular cartilage. This approach enables the decoupling of mechanical and biological properties of 3D printed constructs, allowing for a wider range of bioinks not limited by their mechanical properties. For example, mechanically reinforced 3D-printed vertebral bodies were manufactured with the MHDS approach to co-print PCL and RGD-modified alginate gels[211]. The resulting materials possessed mechanical properties approaching those of trabecular bone. The same co-printing approach was used to prepare constructs with alginate bioinks functionalized through the incorporation of plasmids encoding for TGF-β3 and BMP-2, resulting in increased osteogenesis of MSCs in vitro and in vivo[210].
The use of 3D bioprinting for musculoskeletal tissue engineering provides an important advantage over existing bulk materials, namely the superior spatial control over the deposition of cells and materials that enables the engineering of complex tissue interfaces. The local patterning of physical and biochemical cues by constructing material gradients in bioink mechanical properties[213], growth factors[214], nucleic acids[215], and cell types[216] has been investigated to recapitulate the bone-cartilage interface of the osteochondral unit[215] and the bone-ligament interface between others[214,216].
4. Conclusion
Bio-instructive materials can control cell behavior and influence differentiation towards specific functions and phenotypes. To date, this has primarily been demonstrated via in vitro experiments using model biological systems and often in homotypic cell cultures. As summarized in this review, approaches to enhance biomaterial effects on cell response are inspired by intrinsic and external factors to which cells are exposed in their native niches. These factors were summarized into physical and biochemical cues that can be incorporated into biomaterials using different strategies. However, the translation of these strategies to clinical use remains a major challenge. Most of these strategies are based on the identification of single components of the complex niche milieu which can be recapitulated in vitro and decoupled from the intrinsic properties of the biomaterial. Although a single component may be insufficient to modulate host and transplanted cell response in vivo, the combination of different cues, each of them aiming to control a specific aspect of cell biology, may be a more relevant strategy to successfully guide the repair of musculoskeletal tissues. The engineering of multifactorial bio-instructive materials, together with the spatial and temporal control of these cues and the production of mechanically compliant patient-specific implants, may facilitate the clinical translation of these therapies.
Statement of Significance.
Disorders affecting the musculoskeletal system affect individuals across the lifespan and have a profound effect on mobility and quality of life. While small defects in many tissues can heal successfully, larger defects are often unable to heal or instead heal with inferior quality fibrous tissue and require clinical intervention. Cell-based therapies are a promising option for clinical translation, yet challenges related to maintaining cell survival and instructing cell phenotype upon implantation have limited the success of this approach. Bio-instructive materials provide an exciting opportunity to modulate cell behavior and enhance the efficacy of cell-based approaches for musculoskeletal repair. However, the identification of critical instructive cues and how to present these stimuli is a focus of intense investigation. This review highlights recent biochemical and physical strategies used to engineer bio-instructive materials for the repair of musculoskeletal tissues, while also considering exciting progress in the engineering of immunomodulatory and antibacterial materials.
5. Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 DE025899, R01 DE025475, R01 AI129302). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the decision to publish, or preparation of the manuscript. TGF was supported by an American Heart Association Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yelin E, Weinstein S, King T, The burden of musculoskeletal diseases in the United States, Semin. Arthritis Rheum 46 (2016) 259–260. [DOI] [PubMed] [Google Scholar]
- [2].Briggs AM, Woolf AD, Dreinhöfer K, Homb N, Hoy DG, Kopansky D-, Reducing the global burden of musculoskeletal conditions, Bull. World Health Organ 96 (2018) 366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Atala A, Lanza R, Thomson JA, Nerem RM, Principles of regenerative medicine, 2008.
- [4].Caplan AI, Mesenchymal stem cells: time to change the name!, Stem Cells Transl. Med 6 (2017) 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Custódio CA, Reis RL, Mano JF, Engineering biomolecular microenvironments for cell instructive biomaterials, Adv. Healthc. Mater 3 (2014) 797–810. [DOI] [PubMed] [Google Scholar]
- [6].Navarro M, Michiardi A, Castaño O, Planell JA, Biomaterials in orthopaedics, J. R. Soc. Interface 5 (2008) 1137–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Discher DE, Mooney DJ, Zandstra PW, Growth Factors, Matrices, and forces combine and control stem cells, Science (80-. ). 324 (2009) 1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fletcher DA, Mullins RD, Cell mechanics and the cytoskeleton, Nature. 463 (2010) 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell. 126 (2006) 677–89. [DOI] [PubMed] [Google Scholar]
- [10].Pelham RJ, l Wang Y, Cell locomotion and focal adhesions are regulated by substrate flexibility, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 13661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate, Nat. Mater 9 (2010) 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gupta M, Sarangi BR, Deschamps J, Nematbakhsh Y, Callan-Jones A, Margadant F, Mège R-M, Lim CT, Voituriez R, Ladoux B, Adaptive rheology and ordering of cell cytoskeleton govern matrix rigidity sensing, Nat. Commun 6 (2015) 7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zemel A, Rehfeldt F, Brown AEX, Discher DE, Safran SA, Optimal matrix rigidity for stress-fibre polarization in stem cells, Nat. Phys 6 (2010) 468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Her GJ, Wu H-C, Chen M-H, Chen M-Y, Chang S-C, Wang T-W, Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages, Acta Biomater. 9 (2013) 5170–5180. [DOI] [PubMed] [Google Scholar]
- [15].Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM, Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture, Science. 329 (2010) 1078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Winer JP, Janmey PA, McCormick ME, Funaki M, Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli, Tissue Eng. Part A 15 (2009) 147–154. [DOI] [PubMed] [Google Scholar]
- [17].Lee J, Abdeen AA, Kilian KA, Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment, Sci. Rep 4 (2015) 5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mao AS, Shin J-W, Mooney DJ, Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation, Biomaterials. 98 (2016) 184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Young JL, Engler AJ, Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro, Biomaterials. 32 (2011) 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hung BP, Harvestine JN, Saiz AM, Gonzalez-Fernandez T, Sahar DE, Weiss ML, Leach JK, Defining hydrogel properties to instruct lineage- and cell-specific mesenchymal differentiation, Biomaterials. 189 (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H, Lippens E, Duda GN, Mooney DJ, Hydrogels with tunable stress relaxation regulate stem cell fate and activity, Nat. Mater 15 (2016) 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao X, Huebsch N, Mooney DJ, Suo Z, Stress-relaxation behavior in gels with ionic and covalent crosslinks, J. Appl. Phys 107 (2010) 63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee H, Gu L, Mooney DJ, Levenston ME, Chaudhuri O, Mechanical confinement regulates cartilage matrix formation by chondrocytes, Nat. Mater 16 (2017) 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bauer A, Gu L, Kwee B, Li WA, Dellacherie M, Celiz AD, Mooney DJ, Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts, Acta Biomater. 62 (2017) 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Darnell M, Young S, Gu L, Shah N, Lippens E, Weaver J, Duda G, Mooney D, Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo, Adv. Healthc. Mater 6 (2017) 1601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wade RJ, Burdick JA, Engineering ECM signals into biomaterials, Mater. Today 15 (2012) 454–459. [Google Scholar]
- [27].Li D, Zhou J, Chowdhury F, Cheng J, Wang N, Wang F, Role of mechanical factors in fate decisions of stem cells, Regen. Med 6 (2011) 229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Daley WP, Peters SB, Larsen M, Extracellular matrix dynamics in development and regenerative medicine, J. Cell Sci 121 (2008) 255–264. [DOI] [PubMed] [Google Scholar]
- [29].Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y, Nanomaterials and bone regeneration, Bone Res. 3 (2015) 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gannon AR, Nagel T, Bell AP, Avery NC, Kelly DJ, Postnatal changes to the mechanical properties of articular cartilage are driven by the evolution of Its collagen network, Eur. Cell. Mater 29 (2015) 105–123. [DOI] [PubMed] [Google Scholar]
- [31].Chalut KJ, Kulangara K, Giacomelli MG, Wax A, Leong KW, Deformation of stem cell nuclei by nanotopographical cues, Soft Matter. 6 (2010) 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Suh K-Y, Park MC, Kim P, Capillary force lithography: A versatile tool for structured biomaterials interface towards cell and tissue engineering, Adv. Funct. Mater 19 (2009) 2699–2712. [Google Scholar]
- [33].Miao S, Cui H, Nowicki M, Lee S, Almeida J, Zhou X, Zhu W, Yao X, Masood F, Plesniak MW, Mohiuddin M, Zhang LG, Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation, Biofabrication. 10 (2018) 035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Biggs MJP, Fernandez M, Thomas D, Cooper R, Palma M, Liao J, Fazio T, Dahlberg C, Wheadon H, Pallipurath A, Pandit A, Kysar J, Wind SJ, The functional response of mesenchymal stem cells to electron-beam patterned elastomeric surfaces presenting micrometer to nanoscale heterogeneous rigidity, Adv. Mater 29 (2017) 1702119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bettinger CJ, Langer R, Borenstein JT, Engineering substrate topography at the micro- and nanoscale to control cell function, Angew. Chemie Int. Ed 48 (2009) 5406–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Teo BKK, Wong ST, Lim CK, Kung TYS, Yap CH, Ramagopal Y, Romer LH, Yim EKF, Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase, ACS Nano. 7 (2013) 4785–4798. [DOI] [PubMed] [Google Scholar]
- [37].Ahn EH, Kim Y, Kshitiz, An SS, Afzal J, Lee S, Kwak M, Suh K-Y, Kim D-H, Levchenko A, Spatial control of adult stem cell fate using nanotopographic cues., Biomaterials. 35 (2014) 2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS, Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment, Dev. Cell 6 (2004) 483–495. [DOI] [PubMed] [Google Scholar]
- [39].Yamamoto M, James D, Li H, Butler J, Rafii S, Rabbany S, Generation of stable co-cultures of vascular cells in a honeycomb alginate scaffold, Tissue Eng. Part A 16 (2010) 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guo C, Kaufman LJ, Flow and magnetic field induced collagen alignment, Biomaterials. 28 (2007) 1105–1114. [DOI] [PubMed] [Google Scholar]
- [41].Almeida HV, Sathy BN, Dudurych I, Buckley CT, O’Brien FJ, Kelly DJ, Anisotropic shape-memory alginate scaffolds functionalized with either type I or type II collagen for cartilage tissue engineering, Tissue Eng. Part A 23 (2017) 55–68. [DOI] [PubMed] [Google Scholar]
- [42].Arora A, Kothari A, Katti DS, Pore orientation mediated control of mechanical behavior of scaffolds and its application in cartilage-mimetic scaffold design, J. Mech. Behav. Biomed. Mater 51 (2015) 169–183. [DOI] [PubMed] [Google Scholar]
- [43].Costantini M, Testa S, Mozetic P, Barbetta A, Fuoco C, Fornetti E, Tamiro F, Bernardini S, Jaroszewicz J, Święszkowski W, Trombetta M, Castagnoli L, Seliktar D, Garstecki P, Cesareni G, Cannata S, Rainer A, Gargioli C, Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo, Biomaterials. 131 (2017) 98–110. [DOI] [PubMed] [Google Scholar]
- [44].Olvera D, Sathy BN, Carroll SF, Kelly DJ, Modulating microfibrillar alignment and growth factor stimulation to regulate mesenchymal stem cell differentiation, Acta Biomater. 64 (2017) 148–160. [DOI] [PubMed] [Google Scholar]
- [45].Ingavle GC, Leach JK, Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering., Tissue Eng. Part B. Rev 20 (2014) 277–93. [DOI] [PubMed] [Google Scholar]
- [46].Pauly HM, Sathy BN, Olvera D, McCarthy HO, Kelly DJ, Popat KC, Dunne NJ, Haut Donahue TL, Hierarchically structured electrospun scaffolds with chemically conjugated growth factor for ligament tissue engineering, Tissue Eng. Part A 23 (2017) 823–836. [DOI] [PubMed] [Google Scholar]
- [47].Murphy CM, Haugh MG, O’Brien FJ, The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering, Biomaterials. 31 (2010) 461–466. [DOI] [PubMed] [Google Scholar]
- [48].O’Brien FJ, Harley BA, V Yannas I, Gibson LJ, The effect of pore size on cell adhesion in collagen-GAG scaffolds, Biomaterials. 26 (2005) 433–41. [DOI] [PubMed] [Google Scholar]
- [49].Vissers CAB, Harvestine JN, Leach JK, Pore size regulates mesenchymal stem cell response to Bioglass-loaded composite scaffolds, J. Mater. Chem. B 3 (2015) 8650–8658. [DOI] [PubMed] [Google Scholar]
- [50].Matsiko A, Gleeson JP, O’Brien FJ, Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition, Tissue Eng. Part A 00 (2014) 1–12. [DOI] [PubMed] [Google Scholar]
- [51].Haugh MG, Murphy CM, O’Brien FJ, Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes, Tissue Eng. Part C. Methods 16 (2010) 887–94. [DOI] [PubMed] [Google Scholar]
- [52].Cunniffe GM, Díaz-Payno PJ, Sheehy EJ, Critchley SE, Almeida HV, Pitacco P, Carroll SF, Mahon OR, Dunne A, Levingstone TJ, Moran CJ, Brady RT, O’Brien FJ, Brama PAJ, Kelly DJ, Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues, Biomaterials. 188 (2019) 63–73. [DOI] [PubMed] [Google Scholar]
- [53].Krebs MD, Sutter KA, Lin ASP, Guldberg RE, Alsberg E, Injectable poly(lactic-co-glycolic) acid scaffolds with in situ pore formation for tissue engineering, Acta Biomater. 5 (2009) 2847–2859. [DOI] [PubMed] [Google Scholar]
- [54].Armstrong JPK, Burke M, Carter BM, Davis SA, Perriman AW, 3D bioprinting using a templated porous bioink, Adv. Healthc. Mater 5 (2016) 1724–1730. [DOI] [PubMed] [Google Scholar]
- [55].Schütz K, Placht A-M, Paul B, Brüggemeier S, Gelinsky M, Lode A, Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: towards biofabrication of tissue engineering constructs with clinically relevant dimensions, J. Tissue Eng. Regen. Med 4 (2015) 1574–1587. [DOI] [PubMed] [Google Scholar]
- [56].Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ, Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation, Nat. Mater 14 (2015) 1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Balint R, Cassidy NJ, Cartmell SH, Electrical stimulation: A novel tool for tissue engineering, Tissue Eng. Part B Rev 19 (2013) 48–57. [DOI] [PubMed] [Google Scholar]
- [58].Hodgkin AL, The ionic basis of electrical activity in nerve and muscle, Biol. Rev 26 (1951) 339–409. [Google Scholar]
- [59].Shi R, Borgens RB, Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern, Dev. Dyn 202 (1995) 101–114. [DOI] [PubMed] [Google Scholar]
- [60].Illingworth CM, Barker AT, Measurement of electrical currents emerging during the regeneration of amputated finger tips in children, Clin. Phys. Physiol. Meas 1 (1980) 87–89. [Google Scholar]
- [61].Peters EJ, Lavery LA, Armstrong DG, Fleischli JG, Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial, Arch. Phys. Med. Rehabil 82 (2001) 721–5. [DOI] [PubMed] [Google Scholar]
- [62].Johnson M, Martinson M, Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta-analysis of randomized controlled trials, Pain. 130 (2007) 157–165. [DOI] [PubMed] [Google Scholar]
- [63].Goldstein C, Sprague S, Petrisor BA, Electrical stimulation for fracture healing: current evidence, J. Orthop. Trauma 24 (2010) S62–S65. [DOI] [PubMed] [Google Scholar]
- [64].Chan KM, Curran MWT, Gordon T, The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice, J. Physiol 594 (2016) 3553–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ, Harrison BS, The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation, Stem Cell Res. 15 (2015) 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chaudhuri B, Bhadra D, Moroni L, Pramanik K, Myoblast differentiation of human mesenchymal stem cells on graphene oxide and electrospun graphene oxide–polymer composite fibrous meshes: importance of graphene oxide conductivity and dielectric constant on their biocompatibility, Biofabrication. 7 (2015) 015009. [DOI] [PubMed] [Google Scholar]
- [67].Mooney E, Mackle JN, Blond DJ-P, O’Cearbhaill E, Shaw G, Blau WJ, Barry FP, Barron V, Murphy JM, The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs, Biomaterials. 33 (2012) 6132–6139. [DOI] [PubMed] [Google Scholar]
- [68].Ryan AJ, Lackington WA, Hibbitts AJ, Matheson A, Alekseeva T, Stejskalova A, Roche P, O’Brien FJ, A physicochemically optimized and neuroconductive biphasic nerve guidance conduit for peripheral nerve repair, Adv. Healthc. Mater 6 (2017). [DOI] [PubMed] [Google Scholar]
- [69].Zhang J, Li M, Kang E-T, Neoh KG, Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels, Acta Biomater. 32 (2016) 46–56. [DOI] [PubMed] [Google Scholar]
- [70].Sun L-Y, Hsieh D-K, Lin P-C, Chiu H-T, Chiou T-W, Pulsed electromagnetic fields accelerate proliferation and osteogenic gene expression in human bone marrow mesenchymal stem cells during osteogenic differentiation, Bioelectromagnetics. 31 (2010) 209–19. [DOI] [PubMed] [Google Scholar]
- [71].Mayer-Wagner S, Passberger A, Sievers B, Aigner J, Summer B, Schiergens TS, Jansson V, Müller PE, Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells, Bioelectromagnetics. 32 (2011) 283–290. [DOI] [PubMed] [Google Scholar]
- [72].Bonfrate V, Manno D, Serra A, Salvatore L, Sannino A, Buccolieri A, Serra T, Giancane G, Enhanced electrical conductivity of collagen films through long-range aligned iron oxide nanoparticles, J. Colloid Interface Sci 501 (2017) 185–191. [DOI] [PubMed] [Google Scholar]
- [73].Yoon J-K, Misra M, Yu SJ, Kim HY, Bhang SH, Song SY, Lee J-R, Ryu S, Choo YW, Jeong G-J, Kwon SP, Im SG, Il Lee T, Kim B-S, Thermosensitive, stretchable, and piezoelectric substrate for generation of myogenic cell sheet fragments from human mesenchymal stem cells for skeletal muscle regeneration, Adv. Funct. Mater 27 (2017) 1703853. [Google Scholar]
- [74].Baei P, Jalili-Firoozinezhad S, Rajabi-Zeleti S, Tafazzoli-Shadpour M, Baharvand H, Aghdami N, Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering, Mater. Sci. Eng. C 63 (2016) 131–141. [DOI] [PubMed] [Google Scholar]
- [75].Jun I, Jeong S, Shin H, The stimulation of myoblast differentiation by electrically conductive sub-micron fibers, Biomaterials. 30 (2009) 2038–2047. [DOI] [PubMed] [Google Scholar]
- [76].Zhang Y, Ali SF, Dervishi E, Xu Y, Li Z, Casciano D, Biris AS, Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells, ACS Nano. 4 (2010) 3181–3186. [DOI] [PubMed] [Google Scholar]
- [77].Ku SH, Lee SH, Park CB, Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation, Biomaterials. 33 (2012) 6098–6104. [DOI] [PubMed] [Google Scholar]
- [78].Ryan AJ, Kearney CJ, Shen N, Khan U, Kelly AG, Probst C, Brauchle E, Biccai S, Garciarena CD, Vega-Mayoral V, Loskill P, Kerrigan SW, Kelly DJ, Schenke-Layland K, Coleman JN, O’Brien FJ, Electroconductive biohybrid collagen/pristine graphene composite biomaterials with enhanced biological activity, Adv. Mater 30 (2018) 1706442. [DOI] [PubMed] [Google Scholar]
- [79].Spira ME, Hai A, Multi-electrode array technologies for neuroscience and cardiology, Nat. Nanotechnol 8 (2013) 83–94. [DOI] [PubMed] [Google Scholar]
- [80].Wu Y, Guo L, Enhancement of intercellular electrical synchronization by conductive materials in cardiac tissue engineering, IEEE Trans. Biomed. Eng 65 (2018) 264–272. [DOI] [PubMed] [Google Scholar]
- [81].Wong JY, Langer R, Ingber DE, Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 3201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Brusatori Michelle A., Yanrong Tie A, Van Tassel PR, Protein adsorption kinetics under an applied electric field: An optical waveguide lightmode spectroscopy study, (2003).
- [83].Du Y, Ge J, Li Y, Ma PX, Lei B, Biomimetic elastomeric, conductive and biodegradable polycitrate-based nanocomposites for guiding myogenic differentiation and skeletal muscle regeneration, Biomaterials. 157 (2018) 40–50. d [DOI] [PubMed] [Google Scholar]
- [84].Sajesh KM, Jayakumar R, Nair SV, Chennazhi KP, Biocompatible conducting chitosan/polypyrrole–alginate composite scaffold for bone tissue engineering, Int. J. Biol. Macromol 62 (2013) 465–471. [DOI] [PubMed] [Google Scholar]
- [85].Khorshidi S, Karkhaneh A, Hydrogel/fiber conductive scaffold for bone tissue engineering, J. Biomed. Mater. Res. Part A 106 (2018) 718–724. [DOI] [PubMed] [Google Scholar]
- [86].Zanjanizadeh Ezazi N, Shahbazi M-A, Shatalin YV, Nadal E, Mäkilä E, Salonen J, Kemell M, Correia A, Hirvonen J, Santos HA, Conductive vancomycin-loaded mesoporous silica polypyrrole-based scaffolds for bone regeneration, Int. J. Pharm 536 (2018) 241–250. [DOI] [PubMed] [Google Scholar]
- [87].Pelto J, Björninen M, Pälli A, Talvitie E, Hyttinen J, Mannerström B, Suuronen Seppanen R, Kellomäki M, Miettinen S, Haimi S, Novel polypyrrole-coated polylactide scaffolds enhance adipose stem cell proliferation and early osteogenic differentiation, Tissue Eng. Part A 19 (2013) 882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chen J, Yu M, Guo B, Ma PX, Yin Z, Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration, J. Colloid Interface Sci 514 (2018) 517–527. [DOI] [PubMed] [Google Scholar]
- [89].Taraballi F, Sushnitha M, Tsao C, Bauza G, Liverani C, Shi A, Tasciotti E, Biomimetic tissue engineering: tuning the immune and inflammatory response to implantable biomaterials, Adv. Healthc. Mater 7 (2018) 1800490. [DOI] [PubMed] [Google Scholar]
- [90].Blakney AK, Swartzlander MD, Bryant SJ, The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels, J. Biomed. Mater. Res. A 100 (2012) 1375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sridharan R, Ryan EJ, Kearney CJ, Kelly DJ, O’Brien FJ, Macrophage polarization in response to collagen scaffold stiffness Is dependent on cross-linking agent used to modulate the stiffness, ACS Biomater. Sci. Eng (2018). [DOI] [PubMed] [Google Scholar]
- [92].Shayan M, Padmanabhan J, Morris AH, Cheung B, Smith R, Schroers J, Kyriakides TR, Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization, Acta Biomater. 75 (2018) 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mahon OR, O’Hanlon S, Cunningham CC, McCarthy GM, Hobbs C, Nicolosi V, Kelly DJ, Dunne A, Orthopaedic implant materials drive M1 macrophage polarization in a spleen tyrosine kinase- and mitogen-activated protein kinase-dependent manner, Acta Biomater. 65 (2018) 426–435. [DOI] [PubMed] [Google Scholar]
- [94].Lebre F, Sridharan R, Sawkins MJ, Kelly DJ, O’Brien FJ, Lavelle EC, The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation, Sci. Rep 7 (2017) 2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD, Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction, Ann. Biomed. Eng 42 (2014) 1508–1516. [DOI] [PubMed] [Google Scholar]
- [96].Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, Ratner BD, Jiang S, Zwitterionic hydrogels implanted in mice resist the foreign-body reaction, Nat. Biotechnol 31 (2013) 553–556. [DOI] [PubMed] [Google Scholar]
- [97].Sinclair A, O’Kelly MB, Bai T, Hung H-C, Jain P, Jiang S, Self-healing zwitterionic microgels as a versatile platform for malleable cell constructs and injectable therapies, Adv. Mater 30 (2018) 1803087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jansen LE, Amer LD, Chen EY-T, Nguyen TV, Saleh LS, Emrick T, Liu WF, Bryant SJ, Peyton SR, Zwitterionic PEG-PC hydrogels modulate the foreign body response in a modulus-dependent manner, Biomacromolecules. 19 (2018) 2880–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lee K, Silva EA, Mooney DJ, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments, J. R. Soc. Interface 8 (2011) 153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gonzalez-Fernandez T, Kelly DJ, O’Brien FJ, Controlled non-viral gene delivery in cartilage and bone repair: Current strategies and future directions, Adv. Ther 1 (2018) 1800038. [Google Scholar]
- [101].Wilgus TA, Growth factor-extracellular matrix interactions regulate wound repair., Adv. Wound Care 1 (2012) 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, Guldberg RE, An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects, Biomaterials. 32 (2011) 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].V Almeida H, Liu Y, Cunniffe GM, Mulhall KJ, Matsiko A, Buckley CT, O’Brien FJ, Kelly DJ, Controlled release of transforming growth factor-β3 from cartilage-extra-cellular-matrix-derived scaffolds to promote chondrogenesis of human-joint-tissue-derived stem cells, Acta Biomater. 10 (2014) 4400–9. [DOI] [PubMed] [Google Scholar]
- [104].Baker HB, Passipieri JA, Siriwardane M, Ellenburg MD, Vadhavkar M, Bergman CR, Saul JM, Tomblyn S, Burnett L, Christ GJ, Cell and growth factor-loaded keratin hydrogels for treatment of volumetric muscle loss in a mouse model, Tissue Eng. Part A 23 (2017) 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tayalia P, Mooney DJ, Controlled growth factor delivery for tissue engineering, Adv. Mater 21 (2009) 3269–85. [DOI] [PubMed] [Google Scholar]
- [106].Briquez PS, Hubbell JA, Martino MM, Extracellular matrix-inspired growth factor delivery systems for skin wound healing, Adv. Wound Care 4 (2015) 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y, Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate, J. Biomed. Mater. Res 56 (2001) 216–221. [DOI] [PubMed] [Google Scholar]
- [108].Peschel D, Zhang K, Fischer S, Groth T, Modulation of osteogenic activity of BMP-2 by cellulose and chitosan derivatives, Acta Biomater. 8 (2012) 183–193. [DOI] [PubMed] [Google Scholar]
- [109].Mhanna R, Becher J, Schnabelrauch M, Reis RL, Pashkuleva I, Sulfated alginate as a mimic of sulfated glycosaminoglycans: binding of growth factors and effect on stem cell behavior, Adv. Biosyst 1 (2017) 1700043. [DOI] [PubMed] [Google Scholar]
- [110].Park JS, Na K, Woo DG, Yang HN, Park K-H, Determination of dual delivery for stem cell differentiation using dexamethasone and TGF-beta3 in/on polymeric microspheres, Biomaterials. 30 (2009) 4796–805. [DOI] [PubMed] [Google Scholar]
- [111].Waghmare NA, Arora A, Bhattacharjee A, Katti DS, Sulfated polysaccharide mediated TGF-β1 presentation in pre-formed injectable scaffolds for cartilage tissue engineering, Carbohydr. Polym 193 (2018) 62–72. [DOI] [PubMed] [Google Scholar]
- [112].Schmidt J, Lee MK, Ko E, Jeong JH, DiPietro LA, Kong H, Alginate sulfates mitigate binding kinetics of proangiogenic growth factors with receptors toward revascularization, Mol. Pharm 13 (2016) 2148–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Evans CH, Huard J, Gene therapy approaches to regenerating the musculoskeletal system, Nat. Rev. Rheumatol 11 (2015) 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Goudenege S, Pisani DF, Wdziekonski B, Di Santo JP, Bagnis C, Dani C, Dechesne CA, Enhancement of myogenic and muscle repair capacities of human adipose-derived stem cells with forced expression of MyoD., Mol. Ther 17 (2009) 1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bonadio J, Smiley E, Patil P, Goldstein S, Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration, Nat. Med 5 (1999) 753–9. [DOI] [PubMed] [Google Scholar]
- [116].Brunger JM, Huynh NPT, Guenther CM, Perez-Pinera P, Moutos FT, Sanchez-Adams J, a Gersbach C, Guilak F, Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage, Proc. Natl. Acad. Sci. U. S. A 111 (2014) E798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gonzalez-Fernandez T, Tierney EG, Cunniffe GM, O’Brien FJ, Kelly DJ, Gene delivery of TGF-β3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering, Tissue Eng. Part A 22 (2016) 776–787. [DOI] [PubMed] [Google Scholar]
- [118].Mi Z, Ghivizzani SC, Lechman E, Glorioso JC, Evans CH, Robbins PD, Adverse effects of adenovirus-mediated gene transfer of human transforming growth factor beta 1 into rabbit knees, Arthritis Res. Ther 5 (2003) R132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gonzalez-Fernandez T, Sathy BN, Hobbs C, Cunniffe GM, McCarthy HO, Dunne NJ, Nicolosi V, O’Brien FJ, Kelly DJ, Mesenchymal stem cell fate following non-viral gene transfection strongly depends on the choice of delivery vector, Acta Biomater. 55 (2017) 226–238. [DOI] [PubMed] [Google Scholar]
- [120].Lv H, Zhang S, Wang B, Cui S, Yan J, Toxicity of cationic lipids and cationic polymers in gene delivery., J. Control. Release 114 (2006) 100–9. [DOI] [PubMed] [Google Scholar]
- [121].Curtin CM, Castaño IM, O’Brien FJ, Scaffold-based microRNA therapies in regenerative medicine and cancer, Adv. Healthc. Mater 7 (2018) 1700695. [DOI] [PubMed] [Google Scholar]
- [122].Aini H, Itaka K, Fujisawa A, Uchida H, Uchida S, Fukushima S, Kataoka K, Saito T, Chung U, Ohba S, Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment, Sci. Rep 6 (2016) 18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Menciá Castanõ I, Curtin CM, Duffy GP, O’Brien FJ, Next generation bone tissue engineering: Non-viral miR-133a inhibition using collagen-nanohydroxyapatite scaffolds rapidly enhances osteogenesis, Sci. Rep 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Nguyen MK, Jeon O, Krebs MD, Schapira D, Alsberg E, Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation, Biomaterials. 35 (2014) 6278–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Cheng CS, Ran L, Bursac N, Kraus WE, Truskey GA, Cell density and joint microRNA-133a and microRNA-696 inhibition enhance differentiation and contractile function of engineered human skeletal muscle tissues, Tissue Eng. Part A 22 (2016) 573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, Hardouin P, Magne D, TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells, Life Sci. 84 (2009) 499–504. [DOI] [PubMed] [Google Scholar]
- [127].Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J, TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 1585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Hashimoto J, Yoshikawa H, Takaoka K, Shimizu N, Masuhara K, Tsuda T, Miyamoto S, Ono K, Inhibitory effects of tumor necrosis factor alpha on fracture healing in rats, Bone. 10 (1989) 453–7. [DOI] [PubMed] [Google Scholar]
- [129].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds, Biomaterials. 37 (2015) 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K, Macrophages in bone fracture healing: Their essential role in endochondral ossification, Bone. 106 (2018) 78–89. [DOI] [PubMed] [Google Scholar]
- [131].Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK, Extracellular matrix structure, Adv. Drug Deliv. Rev 97 (2016) 4–27. [DOI] [PubMed] [Google Scholar]
- [132].Huettner N, Dargaville TR, Forget A, Discovering cell-adhesion peptides in tissue engineering: Beyond RGD, Trends Biotechnol. 36 (2018) 372–383. [DOI] [PubMed] [Google Scholar]
- [133].Cai L, Heilshorn SC, Designing ECM-mimetic materials using protein engineering, Acta Biomater. 10 (2014) 1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Pierschbacher MD, Ruoslahti E, Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule, Nature. 309 (1984) 30–33. [DOI] [PubMed] [Google Scholar]
- [135].Liu SQ, Tian Q, Hedrick JL, Po Hui JH, Rachel Ee PL, Yang YY, Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage, Biomaterials. 31 (2010) 7298–7307. [DOI] [PubMed] [Google Scholar]
- [136].Wang P-Y, Wu T-H, Tsai W-B, Kuo W-H, Wang M-J, Grooved PLGA films incorporated with RGD/YIGSR peptides for potential application on skeletal muscle tissue engineering, Colloids Surf. B Biointerfaces 110 (2013) 88–95. [DOI] [PubMed] [Google Scholar]
- [137].Frith JE, Mills RJ, Hudson JE, Cooper-White JJ, Tailored integrin–extracellular matrix interactions to direct human mesenchymal stem cell differentiation, Stem Cells Dev. 21 (2012) 2442–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Agrawal V, Kelly J, Tottey S, Daly KA, Johnson SA, Siu BF, Reing J, Badylak SF, An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation, Tissue Eng. Part A 17 (2011) 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Salinas CN, Anseth KS, Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells., J. Biomed. Mater. Res. A 90 (2009) 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Li R, Xu J, Wong DSH, Li J, Zhao P, Bian L, Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling, Biomaterials. 145 (2017) 33–43. [DOI] [PubMed] [Google Scholar]
- [141].Choi YJ, Lee JY, Chung C-P, Park YJ, Enhanced osteogenesis by collagen-binding peptide from bone sialoprotein in vitro and in vivo., J. Biomed. Mater. Res. A 101 (2013) 547–54. [DOI] [PubMed] [Google Scholar]
- [142].Sun S, Yu W, Zhang Y, Zhang F, Increased preosteoblast adhesion and osteogenic gene expression on TiO2 nanotubes modified with KRSR, J. Mater. Sci. Mater. Med 24 (2013) 1079–1091. [DOI] [PubMed] [Google Scholar]
- [143].Lee J-Y, Choo J-E, Park H-J, Park J-B, Lee S-C, Jo I, Lee S-J, Chung C-P, Park Y-J, Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo, Biochem. Biophys. Res. Commun 357 (2007) 68–74. [DOI] [PubMed] [Google Scholar]
- [144].Florine EM, Miller RE, Porter RM, Evans CH, Kurz B, Grodzinsky AJ, Effects of dexamethasone on mesenchymal stromal cell chondrogenesis and aggrecanase activity: comparison of agarose and self-assembling peptide scaffolds, Cartilage. 4 (2013) 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chen K, Sahoo S, He P, Ng KS, Toh SL, Goh JCH, A hybrid silk/RADA-based fibrous scaffold with triple hierarchy for ligament regeneration, Tissue Eng. Part A 18 (2012) 1399–409. [DOI] [PubMed] [Google Scholar]
- [146].Renner JN, Kim Y, Liu JC, Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells, Tissue Eng. Part A 18 (2012) 2581–9. [DOI] [PubMed] [Google Scholar]
- [147].Saito A, Suzuki Y, Ogata S-I, Ohtsuki C, Tanihara M, Prolonged ectopic calcification induced by BMP-2-derived synthetic peptide, J. Biomed. Mater. Res. A 70 (2004) 115–21. [DOI] [PubMed] [Google Scholar]
- [148].D’Andrea LD, Iaccarino G, Fattorusso R, Sorriento D, Carannante C, Capasso D, Trimarco B, Pedone C, Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 14215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Jung RE, Cochran DL, Domken O, Seibl R, Jones AA, Buser D, Hammerle CHF, The effect of matrix bound parathyroid hormone on bone regeneration, Clin. Oral Implants Res 18 (2007) 319–25. [DOI] [PubMed] [Google Scholar]
- [150].Saito A, Suzuki Y, ichi Ogata S, Ohtsuki C, Tanihara M, Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope., Biochim. Biophys. Acta 1651 (2003) 60–7. [DOI] [PubMed] [Google Scholar]
- [151].Choi YJ, Lee JY, Park JH, Park JB, Suh JS, Choi YS, Lee SJ, Chung C-P, Park YJ, The identification of a heparin binding domain peptide from bone morphogenetic protein-4 and its role on osteogenesis, Biomaterials. 31 (2010) 7226–7238. [DOI] [PubMed] [Google Scholar]
- [152].Kim HK, Kim JH, Park DS, Park KS, Kang SS, Lee JS, Jeong MH, Yoon TR, Osteogenesis induced by a bone forming peptide from the prodomain region of BMP-7., Biomaterials. 33 (2012) 7057–63. [DOI] [PubMed] [Google Scholar]
- [153].Haugh MG, Heilshorn SC, Integrating concepts of material mechanics, ligand chemistry, dimensionality and degradation to control differentiation of mesenchymal stem cells, Curr. Opin. Solid State Mater. Sci 20 (2016) 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ho SS, Keown AT, Addison B, Leach JK, Cell migration and bone formation from mesenchymal stem cell spheroids in slginate hydrogels are regulated by adhesive ligand density, Biomacromolecules. 18 (2017) 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Darouiche RO, Treatment of infections associated with surgical implants, N. Engl. J. Med 350 (2004) 1422–1429. [DOI] [PubMed] [Google Scholar]
- [156].Johnson CT, Wroe JA, Agarwal R, Martin KE, Guldberg RE, Donlan RM, Westblade LF, García AJ, Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing., Proc. Natl. Acad. Sci. U. S. A 115 (2018) E4960–E4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Shah SR, Smith BT, Tatara AM, Molina ER, Lee EJ, Piepergerdes TC, Uhrig BA, Guldberg RE, Bennett GN, Wenke JC, Mikos AG, Effects of local antibiotic delivery from porous space maintainers on infection clearance and induction of an osteogenic membrane in an infected bone defect, Tissue Eng. Part A 23 (2017) 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Xue J, Niu Y, Gong M, Shi R, Chen D, Zhang L, Lvov Y, Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection, ACS Nano. 9 (2015) 1600–1612. [DOI] [PubMed] [Google Scholar]
- [159].Zhou B, Li Y, Deng H, Hu Y, Li B, Antibacterial multilayer films fabricated by layer-by-layer immobilizing lysozyme and gold nanoparticles on nanofibers, Colloids Surf B Biointerfaces. 116 (2014) 432–438. d [DOI] [PubMed] [Google Scholar]
- [160].Ribeiro M, Ferraz MP, Monteiro FJ, Fernandes MH, Beppu MM, Mantione D, Sardon H, Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration, Nanomedicine Nanotechnology, Biol. Med 13 (2017) 231–239. [DOI] [PubMed] [Google Scholar]
- [161].Ageitos JM, Sánchez-Pérez A, Calo-Mata P, Villa TG, Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria, Biochem. Pharmacol 133 (2017) 117–138. [DOI] [PubMed] [Google Scholar]
- [162].Annabi N, Rana D, Shirzaei Sani E, Portillo-Lara R, Gifford JL, Fares MM, Mithieux SM, Weiss AS, Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing, Biomaterials. 139 (2017) 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Tripathi JK, Pal S, Awasthi B, Kumar A, Tandon A, Mitra K, Chattopadhyay N, Ghosh JK, Variants of self-assembling peptide, KLD-12 that show both rapid fracture healing and antimicrobial properties, Biomaterials. 56 (2015) 92–103. [DOI] [PubMed] [Google Scholar]
- [164].Stock UA, Wiederschain D, Kilroy SM, Shum-Tim D, Khalil PN, Vacanti JP, Mayer JE, Moses MA, Dynamics of extracellular matrix production and turnover in tissue engineered cardiovascular structures, J. Cell. Biochem 81 (2001) 220–228. [DOI] [PubMed] [Google Scholar]
- [165].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA, Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 5413–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Patterson J, Hubbell JA, Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2, Biomaterials. 31 (2010) 7836–7845. [DOI] [PubMed] [Google Scholar]
- [167].Bahney CS, Hsu C-W, Yoo JU, West JL, Johnstone B, A bioresponsive hydrogel tuned to chondrogenesis of human mesenchymal stem cells, FASEB J. 25 (2011) 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Reznikov N, Bilton M, Lari L, Stevens MM, Kröger R, Fractal-like hierarchical organization of bone begins at the nanoscale, Science. 360 (2018) eaao2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Habraken W, Habibovic P, Epple M, Bohner M, Calcium phosphates in biomedical applications: materials for the future?, Mater. Today 19 (2016) 69–87. [Google Scholar]
- [170].Elsharkawy S, Mata A, Hierarchical biomineralization: from nature’s designs to synthetic materials for regenerative medicine and dentistry, Adv. Healthc. Mater 7 (2018) e1800178. [DOI] [PubMed] [Google Scholar]
- [171].Elsharkawy S, Al-Jawad M, Pantano MF, Tejeda-Montes E, Mehta K, Jamal H, Agarwal S, Shuturminska K, Rice A, Tarakina NV, Wilson RM, Bushby AJ, Alonso M, Rodriguez-Cabello JC, Barbieri E, del Río Hernández A, Stevens MM, Pugno NM, Anderson P, Mata A, Protein disorder–order interplay to guide the growth of hierarchical mineralized structures, Nat. Commun 9 (2018) 2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Harding JL, Osmond MJ, Krebs MD, Engineering osteoinductive biomaterials by bioinspired synthesis of apatite coatings on collagen hydrogels with varied pore microarchitectures, Tissue Eng. Part A 23 (2017) 1452–1465. [DOI] [PubMed] [Google Scholar]
- [173].Schweikle M, Bjørnøy SH, van Helvoort ATJ, Haugen HJ, Sikorski P, Tiainen H, Stabilisation of amorphous calcium phosphate in polyethylene glycol hydrogels, Acta Biomater. 90 (2019) 132–145. [DOI] [PubMed] [Google Scholar]
- [174].Choi S, Murphy WL, A screening approach reveals the influence of mineral coating morphology on human mesenchymal stem cell differentiation., Biotechnol. J 8 (2013) 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Iordachescu A, Amin HD, Rankin SM, Williams RL, Yapp C, Bannerman A, Pacureanu A, Addison O, Hulley PA, Grover LM, An In vitro model for the development of mature bone containing an osteocyte network, Adv. Biosyst 2 (2018) 1700156. [Google Scholar]
- [176].Li Y, Thula TT, Jee S, Perkins SL, Aparicio C, Douglas EP, Gower LB, Biomimetic mineralization of woven bone-like nanocomposites: role of collagen cross-links, Biomacromolecules. 13 (2012) 49–59. [DOI] [PubMed] [Google Scholar]
- [177].Douglas TEL, Krawczyk G, Pamula E, Declercq HA, Schaubroeck D, Bucko MM, Balcaen L, Van Der Voort P, Bliznuk V, van den Vreken NMF, Dash M, Detsch R, Boccaccini AR, Vanhaecke F, Cornelissen M, Dubruel P, Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means, J. Tissue Eng. Regen. Med 10 (2016) 938–954. [DOI] [PubMed] [Google Scholar]
- [178].Bjørnøy SH, Bassett DC, Ucar S, Strand BL, Andreassen J-P, Sikorski P, A correlative spatiotemporal microscale study of calcium phosphate formation and transformation within an alginate hydrogel matrix, Acta Biomater. 44 (2016) 254–66. [DOI] [PubMed] [Google Scholar]
- [179].Murphy WL, Mooney DJ, Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata, J. Am. Chem. Soc 124 (2002) 1910–1917. [DOI] [PubMed] [Google Scholar]
- [180].Ingavle GC, Gionet-Gonzales M, Vorwald CE, Bohannon LK, Clark K, Galuppo LD, Leach JK, Injectable mineralized microsphere-loaded composite hydrogels for bone repair in a sheep bone defect model, Biomaterials. 197 (2019) 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Harvestine JN, Sheaff CS, Li C, Haudenschild AK, Gionet-Gonzales MA, Hu JC, Athanasiou KA, Marcu L, Leach JK, Multimodal label-free imaging for detecting maturation of engineered osteogenic grafts, ACS Biomater. Sci. Eng 5 (2019) 1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Johnson CT, Sok MCP, Martin KE, Kalelkar PP, Caplin JD, Botchwey EA, García AJ, Lysostaphin and BMP-2 co-delivery reduces S. aureus infection and regenerates critical-sized segmental bone defects, Sci. Adv 5 (2019) eaaw1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Court-Brown CM, Bugler KE, Clement ND, Duckworth AD, McQueen MM, The epidemiology of open fractures in adults. A 15-year review, Injury. 43 (2012) 891–897. [DOI] [PubMed] [Google Scholar]
- [184].Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E, The subchondral bone in articular cartilage repair: current problems in the surgical management, Knee Surgery, Sport. Traumatol. Arthrosc 18 (2010) 434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Cheema U, Brown RA, Alp B, MacRobert AJ, Spatially defined oxygen gradients and vascular endothelial growth factor expression in an engineered 3D cell model, Cell. Mol. Life Sci 65 (2008) 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Fang X, Sittadjody S, Gyabaah K, Opara EC, Balaji KC, Novel 3D co-culture model for epithelial-stromal cells interaction in prostate cancer, PLoS One. 8 (2013) e75187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Harink B, Le Gac S, Truckenmüller R, van Blitterswijk C, Habibovic P, Regeneration-on-a-chip? The perspectives on use of microfluidics in regenerative medicine, Lab Chip. 13 (2013) 3512. [DOI] [PubMed] [Google Scholar]
- [188].Berthier E, Young EWK, Beebe D, Engineers are from PDMS-land, Biologists are from Polystyrenia, Lab Chip. 12 (2012) 1224. [DOI] [PubMed] [Google Scholar]
- [189].Ferreira MM, Dewi RE, Heilshorn SC, Microfluidic analysis of extracellular matrix-bFGF crosstalk on primary human myoblast chemoproliferation, chemokinesis, and chemotaxis, Integr. Biol. (Camb) 7 (2015) 569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lee J-H, Gu Y, Wang H, Lee WY, Microfluidic 3D bone tissue model for high-throughput evaluation of wound-healing and infection-preventing biomaterials, Biomaterials. 33 (2012) 999–1006. [DOI] [PubMed] [Google Scholar]
- [191].Torisawa Y, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE, Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro, Nat. Methods 11 (2014) 663–669. [DOI] [PubMed] [Google Scholar]
- [192].Zhong W, Zhang W, Wang S, Qin J, Regulation of fibrochondrogenesis of mesenchymal stem cells in an integrated microfluidic platform embedded with biomimetic nanofibrous scaffolds, PLoS One. 8 (2013) e61283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Goldman SM, Barabino GA, Cultivation of agarose-based microfluidic hydrogel promotes the development of large, full-thickness, tissue-engineered articular cartilage constructs, J. Tissue Eng. Regen. Med 11 (2017) 572–581. [DOI] [PubMed] [Google Scholar]
- [194].Lin H, Lozito TP, Alexander PG, Gottardi R, Tuan RS, Stem cell-based microphysiological osteochondral system to model tissue response to Interleukin-1β, Mol. Pharm 11 (2014) 2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Shi X, Zhou J, Zhao Y, Li L, Wu H, Gradient-regulated hydrogel for interface tissue wngineering: Steering simultaneous osteo/chondrogenesis of stem cells on a Chip, Adv. Healthc. Mater 2 (2013) 846–853. [DOI] [PubMed] [Google Scholar]
- [196].Agrawal G, Aung A, Varghese S, Skeletal muscle-on-a-chip: an in vitro model to evaluate tissue formation and injury, Lab Chip. 17 (2017) 3447–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Groll J, Burdick JA, Cho D-W, Derby B, Gelinsky M, Heilshorn SC, Jüngst T, Malda J, Mironov VA, Nakayama K, Ovsianikov A, Sun W, Takeuchi S, Yoo JJ, Woodfield F, A definition of bioinks and their distinction from biomaterial inks, Biofabrication. 11 (2019) 13001. [DOI] [PubMed] [Google Scholar]
- [198].Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, a Dhert WJ, Groll J, Hutmacher DW, 25th anniversary article: Engineering hydrogels for biofabrication, Adv. Mater 25 (2013) 5011–28. [DOI] [PubMed] [Google Scholar]
- [199].Daly AC, Critchley SE, Rensock EM, Kelly DJ, Rencsok EM, Kelly DJ, A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage, Biofabrication. 8 (2016) 1–25. [DOI] [PubMed] [Google Scholar]
- [200].Daly AC, Pitacco P, Nulty J, Cunniffe GM, Kelly DJ, 3D printed microchannel networks to direct vascularisation during endochondral bone repair, Biomaterials. 162 (2018) 34–46. [DOI] [PubMed] [Google Scholar]
- [201].Yang X, Lu Z, Wu H, Li W, Zheng L, Zhao J, Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering, Mater. Sci. Eng. C 83 (2018) 195–201. [DOI] [PubMed] [Google Scholar]
- [202].Gao G, Yonezawa T, Hubbell K, Dai G, Cui X, Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging, Biotechnol. J 10 (2015) 1568–1577. [DOI] [PubMed] [Google Scholar]
- [203].Di Bella C, Duchi S, O’Connell CD, Blanchard R, Augustine C, Yue Z, Thompson F, Richards C, Beirne S, Onofrillo C, Bauquier SH, Ryan SD, Pivonka P, Wallace GG, Choong PF, In situ handheld three-dimensional bioprinting for cartilage regeneration, J. Tissue Eng. Regen. Med 12 (2018) 611–621. [DOI] [PubMed] [Google Scholar]
- [204].Wu L, Magaz A, Wang T, Liu C, Darbyshire A, Loizidou M, Emberton M, Birchall M, Song W, Stiffness memory of indirectly 3D-printed elastomer nanohybrid regulates chondrogenesis and osteogenesis of human mesenchymal stem cells, Biomaterials. 186 (2018) 64–79. [DOI] [PubMed] [Google Scholar]
- [205].Narayanan LK, Huebner P, Fisher MB, Spang JT, Starly B, Shirwaiker RA, 3D-bioprinting of polylactic acid (PLA) nanofiber–alginate hydrogel bioink containing human adipose-derived stem cells, ACS Biomater. Sci. Eng 2 (2016) 1732–1742. [DOI] [PubMed] [Google Scholar]
- [206].Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, Atala A, Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications, Biofabrication. 5 (2013) 015001. [DOI] [PubMed] [Google Scholar]
- [207].Ahn CB, Kim Y, Park SJ, Hwang Y, Lee JW, Development of arginine-glycine-aspartate-immobilized 3D printed poly(propylene fumarate) scaffolds for cartilage tissue engineering, J. Biomater. Sci. Polym. Ed 29 (2018) 917–931. [DOI] [PubMed] [Google Scholar]
- [208].Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M, Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications, Ann. Biomed. Eng 45 (2017) 210–223. [DOI] [PubMed] [Google Scholar]
- [209].Rathan S, Dejob L, Schipani R, Haffner B, Möbius ME, Kelly DJ, Fiber reinforced cartilage ECM functionalized bioinks for functional cartilage Tissue Engineering, Adv. Healthc. Mater (2019) 1801501. [DOI] [PubMed] [Google Scholar]
- [210].Cunniffe GM, Gonzalez-Fernandez T, Daly A, Sathy BN, Jeon O, Alsberg E, Kelly DJ, Three-dimensional bioprinting of polycaprolactone reinforced gene activated bioinks for bone tissue engineering, Tissue Eng. Part A 23 (2017) 891–900. [DOI] [PubMed] [Google Scholar]
- [211].Daly AC, Cunniffe GM, Sathy BN, Jeon O, Alsberg E, Kelly DJ, 3D bioprinting of developmentally inspired templates for whole bone organ engineering, Adv. Healthc. Mater 5 (2016) 2353–2362. [DOI] [PubMed] [Google Scholar]
- [212].Yang SS, Choi WH, Song BR, Jin H, Lee SJ, Lee SH, Lee J, Kim YJ, Park SR, Park S-H, Min B-H, Fabrication of an osteochondral graft with using a solid freeform fabrication system, Tissue Eng. Regen. Med 12 (2015) 239–248. [Google Scholar]
- [213].Freeman FE, Kelly DJ, Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues, Sci. Rep 7 (2017) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Ker EDF, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, Campbell PG, Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment, Biomaterials. 32 (2011) 8097–8107. [DOI] [PubMed] [Google Scholar]
- [215].Gonzalez-Fernandez T, Rathan S, Hobbs C, Pitacco P, Freeman FE, Cunniffe GM, Dunne NJ, McCarthy HO, Nicolosi V, O’Brien FJ, Kelly DJ, Pore-forming bioinks to enable Spatio-temporally defined gene delivery in bioprinted tissues, J. Control. Release (2019). [DOI] [PubMed] [Google Scholar]
- [216].Ede D, Davidoff N, Blitch A, Farhang N, Bowles RD, Microfluidic flow cell array for controlled cell deposition in engineered musculoskeletal tissues, Tissue Eng. Part C Methods 24 (2018) 546–556. [DOI] [PubMed] [Google Scholar]