Abstract
BACKGROUND & AIMS:
Increasing drug prices lead to payer coverage restrictions, which limit to therapy. We assessed the cost effectiveness of rifaximin in management of patients with irritable bowel syndrome with diarrhea (IBS-D) under common payer coverage restrictions and determined the maximum price at which rifaximin would be cost effective using contemporary cost-effectiveness thresholds.
METHODS:
A decision analytic model was constructed to evaluate quality of life, cost, and cost effectiveness of rifaximin for patients with IBS-D and complete noncoverage (insurer pays none of the drug cost), unrestricted access (insurer pays 100% of the drug cost), and formulary-restricted access (insurer pays 100% of the drug cost after for patients failed by initial therapy). The maximum cost-effective drug price was determined for each level of drug coverage using threshold analysis adjusted for willingness to pay thresholds from $50,000 to $150,000 per quality-adjusted life year (QALY). Analysis was performed from a payer perspective with a 1-year time horizon.
RESULTS:
Unrestricted and formulary-restricted access were more effective than complete non-coverage, resulting in additional 0.03 and 0.05 QALYs gained over noncoverage. However, unrestricted and formulary-restricted coverage were more expensive. At current drug prices, unrestricted or formulary-restricted coverage would cost an additional $171,850 or $1,207,136/QALY gained, compared to complete non-coverage. A 12% to 62% price reduction ($18.46 to $26.34/pill) for formulary-restricted access and 84% to 88% price reduction ($3.53 to $4.71/pill) for unrestricted access would be needed for rifaximin to be a cost-effective treatment strategy. Rifaximin retreatment intervals, response rates, and adverse events were important factors in sensitivity analysis.
CONCLUSION:
Using a decision analytic model, we show that payer coverage for rifaximin for patients with IBS-D exceeds generally accepted cost-effectiveness thresholds at current drug prices. Improved payer coverage could be justified using value-based pricing methods.
Keywords: drug pricing, antibiotic, insurance, drug coverage
INTRODUCTION
Rifaximin is a non-absorbed antibiotic approved by the Food and Drug Administration (FDA) for the treatment of IBS with diarrhea (IBS-D) based on demonstrated efficacy and tolerability in large clinical trials1. The average cost paid by payers for a two-week course of rifaximin for IBS-D is $1,250.76 in the United States, when covered by insurance2.
As the cost of healthcare continues to rise, clinicians are increasingly obliged to discuss and choose high-value treatment options (i.e. care that is both efficacious and cost-effective) and avoid low-value care (i.e. care that may be efficacious but is not cost-effective)3,4. With prescription drug treatments in IBS, cost-effectiveness and value are directly proportional to drug prices. At the right price, a drug can be cost-effective and “high value”. In contrast, if the price is too high, the same drug can be deemed “low value” even with the same level of efficacy evidenced by clinical trial data. From a policy perspective, high drug prices (i.e. perceived “low value” care) can lead commercial payers to indiscriminately limit access to drug therapy through formulary restrictions or complete exclusions from coverage, regardless of whether a drug is shown to be beneficial in clinical trials5–8. This has direct implications for practicing clinicians, as even fully-insured patients may be unable to fill clinically appropriate prescriptions for IBS therapies9. This lack of access can result in poor healthcare outcomes if ultimately there are few available effective treatment options10. To our knowledge, cost-effectiveness methods have not yet been widely utilized in assessing drug pricing or in ascertaining the “value” of drug therapy in IBS and functional bowel disorders.
To evaluate the cost-effectiveness of rifaximin in managing IBS, and to identify potential justifications for barriers to prescription drug coverage, the aim of this study was to develop a model to estimate the cost-effectiveness of rifaximin for IBS-D at its current national average acquisition price ($29.78/pill in October 2016) using three scenarios of prescription coverage: 1) unrestricted access (insurer pays 100% of drug cost), 2) formulary-restricted access (insurer pays 100% of drug cost after patient fails initial therapy), or 3) complete non-coverage. We then used this cost-effectiveness model to determine the maximum price at which the drug would still be cost-effective, which we termed the “value-based price”, of rifaximin under each scenario of prescription drug coverage.
METHODS
A decision-analytic model was constructed to assess quality of life and cost associated with three scenarios of prescription coverage for rifaximin in treating IBS-D compared to standard pharmacotherapy: complete noncoverage (i.e. therapy restricted to tricyclic agents [TCA] only), unrestricted access (i.e. first-line rifaximin therapy followed by second-line TCA therapy for rifaximin treatment failures), and formulary-restricted coverage (i.e. first-line TCA therapy followed by second-line rifaximin therapy for TCA treatment failures).
Our model was designed to evaluate the outcomes and costs associated with single agent chronic pharmacotherapy for IBS-D. The model assumed that patients would receive pharmacotherapy immediately and for as long as the drug was covered by insurance and tolerated. For the purposes of our model, patients would prefer rifaximin therapy if covered by insurance. A TCA was utilized as the standard pharmacotherapy if rifaximin was not covered by insurance or if rifaximin was previously discontinued. Non-pharmacologic treatment options (such as low FODMAP diet or cognitive behavioral therapy) and over-the-counter and adjunct treatment options (such as antidiarrheal agents, antispasmodics, and peppermint oil) were excluded from analysis, as these options are not necessarily mutually exclusive with pharmacotherapy in usual clinical practice11,12. Derivation of cost and quality-adjusted life year data are included in the supplement and Table 1.
Table 1:
Model inputs
| Description | Base-case value |
Min | Max | Distribution | References |
|---|---|---|---|---|---|
| IBS therapy | |||||
| TCA responder rate | 48.9% | 40.0% | 50.0% | Binomial Probability: 66/135 N: 135 |
Drossman, et al. (2003)18 |
| TCA discontinuation rate | 29.6% | 15.0% | 36.4% | Binomial Probability: 40/135 N: 135 |
Drossman, et al. (2003)18 |
| Rifaximin responder rate | 40.7% | 40.0% | 50.0% | Binomial Probability: 254/624 N: 254/624 |
Pimentel, et al. (2011)42 |
| Rifaximin discontinuation rate | 6.6% | 0.0% | 10.0% | Binomial Probability: 41/625 N: 625 |
Pimentel, et al. (2011)42 |
| Rifaximin retreatment interval | 17.3 weeks | 10 weeks | 52 weeks | Lembo, et al. (2016)32
Pimentel, et al. (2011)19 |
|
| QALYs | |||||
| Health utility associated with therapeutic response | 0.78 | 0.77 | 0.83 | Spiegel, et al. (2009)13 | |
| Health utility associated with therapeutic non-response | 0.73 | Spiegel, et al. (2009)13 | |||
| Costs | |||||
| Cost of office-based electrocardiogram | $41.00 | $35.00 | $150.00 | Healthcare Bluebook43 | |
| Cost of amitriptyline 25mg pill | $0.21974 | Medicaid NADAC Database2 | |||
| Cost of rifaximin 550mg pill | $29.78 | Medicaid NADAC Database2 | |||
| Work-days lost per year | 6.0 | 2.4 | 88.4 | Triangular | Drossman, et al. (1993)44
Hahn, et al. (1999)45 Hungin, et al. (2003)46 Hungin, et al. (2005)47 |
| Average daily 2016 US wage | $177.44 | $0.00 | $300.00 | US Bureau of Labor Statistics48 | |
| Cost of admission related to IBS ICD-9 diagnosis in 2014, discounted forward to 2016 at 3% rate | $5,973.93 | HCUPnet49 | |||
| Annual hospitalization rate with poorly controlled IBS-D | 10.0% | 0.0% | 20.0% | Buono, et al. (2016)50 | |
| Cost of office visit for IBS-D (HCPCS 99214) | $108.20 | Center for Medicare and Medicaid Services Physician Fee Schedule51 | |||
| Number of office visits per year associated with poorly controlled IBS-D | 3.5 | 0.0 | 7.0 | Buono, et al. (2016)50 | |
IBS = irritable bowel syndrome; TCA = tricyclic agent; QALY = quality adjusted life year; ICD = International Classification of Diseases; US = United States; IBS-D = irritable bowel syndrome with diarrhea; HCPCS = Healthcare Common Procedure Coding System
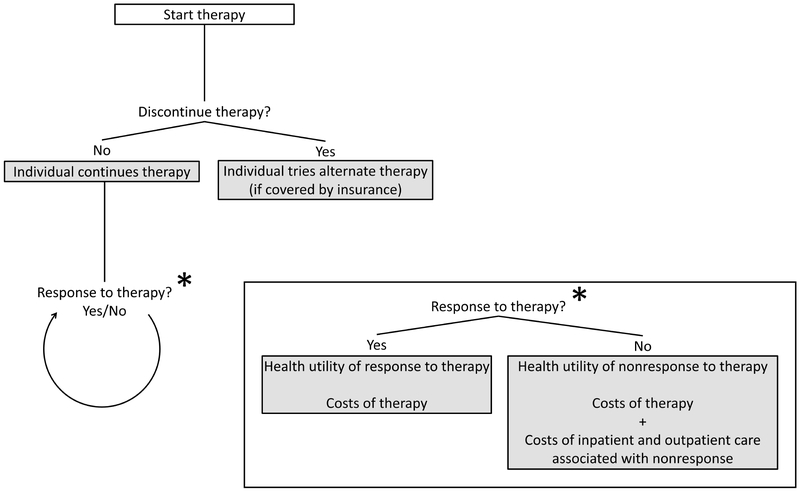
Patient characteristics and outcomes were based on the multi-center PROOF registry which included Rome III IBS patients (79% women, age 43 [SD=15] years)13. Individuals were followed in four-week cycles in the model. Within the first cycle, an individual would determine whether to discontinue therapy (Figure 1), taking into account relative efficacy, tolerability, and other factors14. Intolerable adverse events which were severe enough to cause drug discontinuation were deemed significant and explicitly included in the model. If a treatment was discontinued, the alternative treatment was attempted if covered by insurance. Individuals who discontinued both therapies, or who trialed TCA but could not receive rifaximin due to lack of drug coverage, would receive no further pharmacotherapy and were treated as nonresponders to therapy for remaining cycles. Recognizing the waxing and waning natural history of IBS, response to therapy was determined for each cycle independent of response to therapy in previous cycles15. Gains in health-related quality of life and costs were tallied at the end of each treatment cycle.
Figure 1:
Flowchart of decision-analytic model.
The primary analysis used a one-year time horizon consistent with the time horizon for contemporary coverage decisions and stability of cost estimates over this time period. This was based on a conservative assumption that (1) the payer has an annual budget and (2) the payer prioritizes reducing costs in the annual budget over long-term health gains with longer time horizons (which may not translate into cost savings to the payer). Primary analysis was reported from a payer perspective to better inform coverage decisions. Reference case analysis was conducted from a societal perspective (accounting for work-productivity losses) in the U.S. healthcare system. A discount rate of 3% per annum discount rate was applied to costs and effectiveness outcomes.
IBS therapy
Responder and discontinuation rates for both rifaximin and TCA were derived from systematic reviews of clinical trial data or direct clinical trial data as appropriate14 (Table 1). We defined response using a global endpoint of adequate relief (i.e. “do you feel adequate relief of symptoms?”) which corresponds to health-utility values attributed to clinically meaningful responder and non-responder health states identified in the PROOF study13. Understanding that a recent meta-analysis did not identify significant differences in outcomes among TCAs17, amitriptyline taken 25mg once daily was chosen as the representative TCA due to the low cost of this therapy. Data were extrapolated from a well-conducted multicenter randomized, controlled trial of desipramine for managing IBS18. For TCA, an electrocardiogram was obtained on all individuals prior to initiating therapy due to the risk of prolonged QT. A standard rifaximin treatment course of 550mg three times daily for two weeks was used consistent with FDA-approved labeling. For rifaximin, a four-month retreatment interval was assumed to maintain response which is consistent with clinical trial and observational experience. We assumed no significant loss of response with repeated courses of therapy, consistent with TARGET 3 clinical trial results.
Base-case analysis and sensitivity analysis
Base-case analysis from societal and payer perspectives was performed to determine incremental cost-effectiveness ratios (ICER) for scenarios of unrestricted rifaximin drug coverage or formulary-restricted coverage, relative to a baseline strategy of complete noncoverage rifaximin. The ICER represents the incremental cost to society or to the payer by covering rifaximin, which is incurred in order to gain an additional amount of health, compared to complete non-coverage.
Threshold analysis was conducted from a payer perspective to determine the maximum cost-effective rifaximin pricing at willingness-to-pay levels between $50,000 and $150,000 per QALY. One-way sensitivity analysis was conducted for each variable in the model according to value ranges determined by literature and consensus among authors, with a one-way sensitivity analysis used to evaluate robustness of the model based on variable inputs. Three-way sensitivity analysis was performed according to cost of rifaximin, rifaximin retreatment interval, and TCA responder rate. Analysis was performed using TreeAge Pro 2017 (TreeAge Software Inc, Williamstown, MA).
RESULTS
Base-case analysis
From a payer perspective (Table 2), the total cost of complete non-coverage of rifaximin was $4,355 over one year, compared to $7,608 with unrestricted coverage or $4,783 with formulary-restricted coverage. The QALY gained over one year were again similar among the three coverage strategies (0.747 to 0.752). Formulary-restricted rifaximin coverage was associated with additional $171,850 cost per QALY gained compared to complete non-coverage of rifaximin. Unrestricted rifaximin coverage incurred $1,207,136 additional cost per QALY gained compared to complete non-coverage of rifaximin.
Table 2:
Comparative cost-effectiveness of treatment approaches with and without rifaximin in irritable bowel syndrome with diarrhea (IBS-D)
| Strategy | Total cost ($/yr) |
Total effectiveness (QALY gained/yr) |
Incremental cost ($/yr) |
Incremental effectiveness (QALY/yr) |
ICER ($/QALY gain) |
|---|---|---|---|---|---|
| Societal perspective with one-year time horizon | |||||
| Non-coverage of rifaximin | $4,355 | 0.747 | |||
| Unrestricted rifaximin access | $7,608 | 0.750 | $3,252 | +0.0029 | $1,138,254 (dominated*) |
| Formulary-restricted rifaximin access | $4,783 | 0.752 | $428 | +0.0052 | $82,375 |
| Societal perspective with five-year time horizon | |||||
| Non-coverage of rifaximin | $4,457 | 0.747 | |||
| Unrestricted rifaximin access | $7,529 | 0.750 | $3,072 | +0.0029 | $1,049,586 (dominated*) |
| Formulary-restricted rifaximin access | $4,744 | 0.752 | $287 | +0.0055 | $51,709 |
| Payer perspective with one-year time horizon | |||||
| Non-coverage of rifaximin | $728 | 0.747 | |||
| Unrestricted rifaximin access | $4,177 | 0.750 | $3,449 | +0.0029 | $1,207,136 (dominated*) |
| Formulary-restricted rifaximin access | $1,622 | 0.752 | $894 | +0.0052 | $171,850 |
| Payer perspective with five-year time horizon | |||||
| Non-coverage of rifaximin | $703 | 0.747 | |||
| Unrestricted rifaximin access | $4,110 | 0.750 | $2,485 | +0.0029 | $1,164,033 (dominated*) |
| Formulary-restricted rifaximin access | $1,625 | 0.752 | $921 | +0.0055 | $166,155 |
An unrestricted rifaximin access strategy was dominated (less effective and more expensive) than a formulary-restricted rifaximin strategy at all price points. ICER = incremental cost effectiveness ratio; QALY = quality adjusted life year; TCA = tricyclic antidepressant; IBS-D = irritable bowel syndrome with diarrhea.
From a societal perspective, formulary-restricted rifaximin access was associated with an additional $82,375 cost per QALY gained compared to non-coverage of rifaximin, based on the national average acquisition cost of rifaximin ($29.78/pill). Further results from a societal perspective are reported in the supplement.
Threshold price analysis
The national average reimbursement cost of rifaximin exceeded maximum value-based rifaximin pricing regardless of formulary-restricted or unrestricted coverage. Dependent on evaluated willingness-to-pay ranges from $50,000/QALY to $150,000/QALY, a 12-62% price reduction ($18.46-$26.34/pill) would enable cost-effective formulary-restricted rifaximin coverage compared to complete non- coverage (Table 3). While an 84-88% price reduction ($3.53-$4.71/pill) would enable unrestricted rifaximin coverage to be at least as cost-effective as complete non-coverage of rifaximin, formulary-restricted coverage would remain the most cost-effective strategy.
Table 3:
Maximum price of rifaximin based on contemporary willingness-to-pay levels
| Treatment strategy | Willingness-to-pay threshold | ||
|---|---|---|---|
| $50,000/QALY- gained |
$100,000/QALY- gained |
$150,000/QALY- gained |
|
| One-year time horizon | |||
| Unrestricted rifaximin access | $2.34 per pill $98.28 per treatment |
$3.53 per pill $148.26 per treatment |
$4.71 per pill $197.82 per treatment |
| Formulary-restricted rifaximin access | $10.59 per pill $444.78 per treatment |
$18.46 per pill $775.32 per treatment |
$26.34 per pill $1,106.28 per treatment |
| Five-year time horizon | |||
| Unrestricted rifaximin access | $2.22 per pill $93.14 per treatment |
$3.45 per pill $145.10 per treatment |
$4.69 per pill $197.06 per treatment |
| Formulary-restricted rifaximin access | $11.11 per pill $466.61 per treatment |
$19.15 per pill $804.21 per treatment |
$27.19 per pill $1,141.82 per treatment |
Tests of model robustness
The model appeared robust in one-way sensitivity analysis (Supplemental Figures 1-6) at contemporary willingness-to-pay levels. From a payer perspective and willingness-to-pay of $50,000/QALY-gained, only the assumed rifaximin retreatment interval could change the preferred treatment strategy. At a willingness-to-pay level of $150,000/QALY-gained, the preferred strategy was additionally affected by the assumed rifaximin responder rate as well as health utility gains associated with response to therapy. From a societal perspective, the model was mostly sensitive to number of work-days lost due to illness as well as daily wages of patients (Supplemental Figures 1-3).
Probabilistic sensitivity analysis revealed no overlap in results among simulations for each treatment strategy across the literature-derived range of inputs for each variable in the model from a payer perspective (Supplemental Figure 7).
Sensitivity analyses
Univariate sensitivity analysis was conducted from a payer perspective to determine the effect on net benefit of each strategy based on varying the rifaximin retreatment interval, health utility gains associated with response to therapy, and rifaximin responder rates on of each treatment strategy. A rifaximin retreatment interval shorter than 20 weeks favored a TCA-only strategy excluding rifaximin at a willingness-to-pay of $150,000/QALY-gained, while an interval exceeding one year between rifaximin treatments was required to favor a formulary-restricted rifaximin strategy at a willingness-to-pay level of $50,000/QALY-gained (Supplemental Table 1). Unrestricted rifaximin access was not favored in univariate analysis within literature-derived ranges of variable inputs from societal or payer perspectives. Bivariate sensitivity analysis was conducted from a societal perspective to determine the effects of daily wage and work-days lost due to illness on the preferred strategy (Supplemental Figures 8-10).
We further extended the time horizon to two and five years from a payer perspective, which assumes that payers would consider long-term health gains regardless of the immediate impact on their annual budget. However, longer time horizons were associated with only marginal improvement in cost-effectiveness of rifaximin drug coverage. In contrast to an ICER of $171,850/QALY-gained with a one-year time horizon in primary analysis, the ICER for formulary-restricted rifaximin access was $168,205/QALY-gained with a two-year time horizon and $166,154.76/QALY-gained with a five-year time horizon. Unrestricted rifaximin access had an ICER exceeding $1 million/QALY-gained regardless of time horizon (one, two, or five years).
Multivariate sensitivity analysis to assess effects on threshold pricing
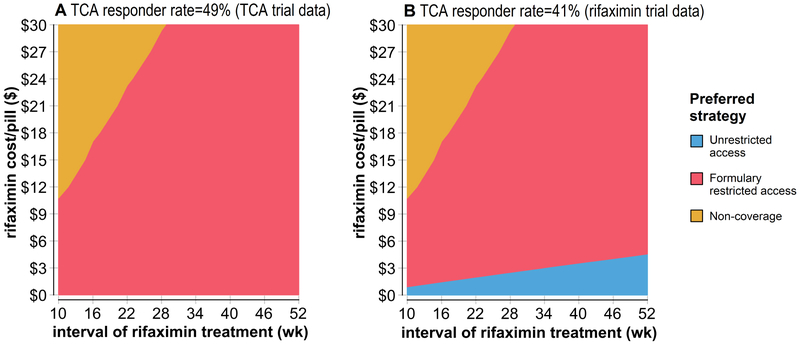
Sensitivity analysis on rifaximin retreatment interval and price of rifaximin were conducted to determine the preferred treatment strategy at each datapoint using a willingness-to-pay of $100,000/QALY-gained (Figure 2a). Unrestricted rifaximin access was the least preferred treatment strategy at any price. For a formulary-restricted strategy to be more cost-effective than rifaximin non-coverage, the maximum potential price of rifaximin had to decrease as the assumed rifaximin retreatment interval decreased. A second sensitivity analysis was conducted assuming a lower TCA responder rate equivalent to the rifaximin responder rate derived from phase III clinical trials (Figure 2b). With this assumption, unrestricted rifaximin could be the preferred strategy albeit at a markedly reduced price. This change did not affect maximum tolerated prices for a formulary-restricted rifaximin coverage approach.
Figure 2:
Sensitivity analysis identifying the optimal level of payer coverage for rifaximin (displayed by color at the corresponding point estimate) based on drug cost (y-axis) and assumed necessary interval of retreatment with rifaximin (x-axis).
DISCUSSION
Prescription coverage for rifaximin improves quality of life and outcomes for patients with IBS. However, prescription drug coverage of rifaximin is not cost-effective using contemporary cost-effectiveness thresholds, based on the national average drug acquisition cost for rifaximin of $29.78/pill. The cost-effectiveness of rifaximin appears sensitive to the rifaximin retreatment interval. Current prices of rifaximin are consistent with an assumption of long periods between retreatment (i.e. a longer and more durable response to a single course of therapy).
In general, prescription drug pricing in the United States is not based on clinical evidence or cost-effectiveness methods21. Instead, drug prices are largely negotiated by commercial payers through pharmacy benefits management (PBM) groups who represent intermediaries for third-party payers5. For a number of reasons, there can be little incentive for PBMs to aggressively negotiate downward on price16,22 with drug sponsors. As a result, drug prices are set at “whatever the market will bear”23. To address high drug costs, payers can impose indiscriminate barriers to prescription drug access (and yet maintain the high drug price) including formulary restriction, high co-pays, requirement for prior authorization, or complete noncoverage5–8. These barriers to access can adversely affect health outcomes by limiting available treatment options to patients and providers24.
Value-based pricing is an alternative method to the current drug pricing which can be defined as “the price of a drug set on the magnitude of its benefit”23. Value-based pricing uses cost-effectiveness analysis, as shown here, to determine drug prices which (1) correlate directly with health outcomes based on clinical evidence and which (2) meet thresholds for high-value care21,25. Leading medical societies including the American Medical Association26 and regulatory agencies including the US Department of Health and Human Services27 have recently voiced support for value-based drug pricing, and value-based pricing has recent been adopted at a state level25. As the delivery of healthcare in gastroenterology moves toward a value-based reimbursement system in general28, it will be equally important for gastroenterologists to consider the value (and price) of drugs prescribed for common indications such as IBS as they do aspects of healthcare such as indications for endoscopic procedures29,30 or costs of endoscopic sedation31.
Our findings were robust in sensitivity analysis. Differences in cost-effectiveness between rifaximin non-coverage and formulary-restricted rifaximin coverage are largely driven by poorer expected outcomes among patients who discontinued TCA therapy in our analysis. Discontinuation is reported to occur in up to one-third of IBS patients for whom TCA are appropriately prescribed18 and is largely attributed to adverse effects including sedation14. Neither price nor responder rate associated with specific TCA agents affect the cost-effectiveness of formulary-restricted rifaximin in sensitivity analysis. In fact, it is possible for formulary-restricted rifaximin to be more cost-effective than a TCA-only strategy even with a zero-cost TCA-based strategy (data not shown).
Similar rates of efficacy reported across all therapies in phase III trials suggest that assuming equivalent efficacy of TCA and rifaximin may be reasonable, for the purposes of sensitivity analysis. In fact, every IBS therapy undergoing rigorous phase III trial evaluation (including alosetron, eluxadoline, and rifaximin for IBS with diarrhea, and tegaserod, lubiprostone, linaclotide, and plecanatide in IBS with constipation) had primary responder rates which did not exceed 60% in phase III data 14,32–34 This observation is independent of the progression of clinical trial endpoints from an assessment of adequate relief to contemporary composite endpoints which incorporate abdominal and motility symptoms. This is in contrast to higher responder rates in smaller and older TCA trials, which can approach 80%17. These differences in efficacy may be related to quality of data collection, population differences, or more pronounced random error in smaller trials35. Comparative efficacy of TCA and rifaximin would ideally be assessed in a head-to-head clinical trial, although such a trial is unlikely to ever be conducted.
The threshold (i.e. maximum tolerated) price of rifaximin is largely dependent on the assumed retreatment interval. In a formulary-restricted rifaximin coverage scenario, the maximum cost-effectiveness price of rifaximin relates to the annual cost-savings achieved by its use in otherwise undertreated patients who already failed TCA. Within this constraint, a shorter rifaximin retreatment interval would require a proportionately lower threshold price. The TARGET 1 and 2 trials evaluated adequate relief of symptoms from a single two-week course of therapy. It is likely that current prices are based on results of these trials and assume a longterm effect from a single course of therapy. While it may be possible to target therapy using serial hydrogen and methane breath testing to achieve cost savings in tertiary care centers36–39, results of published clinical experience as well as TARGET 3 methodology suggest that a shorter retreatment interval between 3 and 4 months may be a reasonable assumption regarding use of this therapy in general clinical practice. These data do not suggest development of antibiotic resistance to repeated therapy, however the effects of repeated courses of rifaximin on gut flora population in IBS remains unknown40. Longitudinal safety data in clinical practice are needed to further assess these risks.
Our cost-effectiveness analysis has a number of strengths. First, the analysis was reported from a societal perspective, comprising a reference case from which further models can be tailored to relevant stakeholders. Second, all inputs and reference ranges were readily available and derived directly from clinical literature or national databases. Third, the model appears robust in sensitivity analysis based on literature-derived ranges of expected values. There are potential limitations which should be considered in interpreting results. First, the reference ranges represent a U.S. national average and should be tailored to the patient population of relevant payers conducting threshold price analysis. Second, we did not include additional therapies which are not generally mutually exclusive with use of rifaximin. These can include alternative antibiotics, adjunct therapies such as on-demand loperamide or hyoscyamine, herbal preparations including peppermint oil, or non-pharmacological treatments such as low FODMAP diet or cognitive behavioral therapy. Third, copays for specialty pharmaceuticals can have a profound effect on medication adherence41 and should be addressed when evaluating effectiveness of rifaximin in high-deductible health plans.
Our study is the first to demonstrate a value-based pricing evaluation of drug therapy for IBS. Our model suggests that value-based pricing for rifaximin could result in rifaximin being a “high-value care” option for IBS-D and would justify better commercial payer coverage. Our results are robust in sensitivity analysis, and most sensitive to the rifaximin retreatment interval. PBMs and industry should strongly consider value-based pricing methods to expand access to rifaximin and other efficacious therapies approved for the treatment of IBS.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
Access to therapy can be limited in clinical practice as rising drug prices lead to payer coverage restrictions. Barriers to payer coverage may be elucidated using value-based pricing methods.
Findings:
Using cost-effectiveness analysis of nationwide data, prescription drug coverage of rifaximin for IBS-D improves health outcomes compared to non-coverage. However, payer coverage for rifaximin for IBS-D requires a lower price to be cost-effective, if prices are linked to health outcomes.
Implications for clinical care:
Improved payer coverage for rifaximin in managing IBS-D could be justified using value-based pricing methods specific to characteristics of covered populations.
Acknowledgments
Grant support: Dr. Shah received funding from the NIH T32 Training Grant in Epidemiology and Health Services (DK062708).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Shah has no disclosures. Dr. Saini has served as a consultant for FMS, Inc. Dr. Chey is a consultant for Ironwood Pharmaceuticals and Allergan.
REFERENCES
- 1.Rifaximin FDA Prescribing Information. Https://Www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2015/021361s012lbledt.Pdf.
- 2.Medicaid National Average Drug Acquisition Cost (NADAC) Database. Https://Data.Medicaid.Gov/.
- 3.Neumann PJ, Cohen JT. Measuring the Value of Prescription Drugs. N Engl J Med 2015;373:2595–2597. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB, Pearson SD. Payer and Policy Maker Steps to Support Value-Based Pricing for Drugs. JAMA 2015;314:2503. [DOI] [PubMed] [Google Scholar]
- 5.Kesselheim AS, Avorn J, Sarpatwari A. The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform. JAMA 2016;316:858. [DOI] [PubMed] [Google Scholar]
- 6.Barua S, Greenwald R, Grebely J, et al. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Ann Intern Med 2015;163:215. [DOI] [PubMed] [Google Scholar]
- 7.Larsen MD, Schou M, Kristiansen AS, et al. The influence of hospital drug formulary policies on the prescribing patterns of proton pump inhibitors in primary care. Eur J Clin Pharmacol 2014;70:859–865. [DOI] [PubMed] [Google Scholar]
- 8.Chen C-C, De AP, Sweet B, et al. Evaluation of Patient Migration Patterns and Related Health Care Costs Within a National Medicare Advantage Prescription Drug Plan After Implementation of an Oxycodone HCl Extended-Release Access Restriction. J Manag Care Spec Pharm 2017;23:902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulman KA, Richman BD. The Evolving Pharmaceutical Benefits Market. JAMA 2018;319:2269. [DOI] [PubMed] [Google Scholar]
- 10.Quigley BM, Sova CC, Brenner DM, et al. (Can’t Get No) Patient Satisfaction: The Predictive Power of Demographic, GI, and Psychological Factors in IBS Patients. J Clin Gastroenterol August 2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the Pharmacological Management of Irritable Bowel Syndrome. Gastroenterology 2014;147:1146–1148. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am J Gastroenterol 2018;113:1–18. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel B, Harris L, Lucak S, et al. Developing Valid and Reliable Health Utilities in Irritable Bowel Syndrome: Results From the IBS PROOF Cohort. Am J Gastroenterol 2009;104:1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah E, Kim S, Chong K, et al. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 2012;125:381–393. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel B, Bolus R, Harris LA, et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther 2009;30:1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabora MC, Turaga N, Schulman KA. Financing and Distribution of Pharmaceuticals in the United States. JAMA 2017;318:21. [DOI] [PubMed] [Google Scholar]
- 17.Ford AC, Lacy BE, Harris LA, et al. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-analysis. Am J Gastroenterol September 2018. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology 2003;125:19–31. [DOI] [PubMed] [Google Scholar]
- 19.Pimentel M, Morales W, Chua K, et al. Effects of rifaximin treatment and retreatment in nonconstipated IBS subjects. Dig Dis Sci 2011;56:2067–2072. [DOI] [PubMed] [Google Scholar]
- 20.Lembo A, Pimentel M, Rao SS, et al. Repeat Treatment With Rifaximin Is Safe and Effective in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2016;151:1113–1121. [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal DM, Goldman DP, Jena AB. Outcomes-Based Pricing as a Tool to Ensure Access to Novel but Expensive Biopharmaceuticals. Ann Intern Med 2017; 166:219. [DOI] [PubMed] [Google Scholar]
- 22.Bai G, Sen AP, Anderson GF. Pharmacy Benefit Managers, Brand-Name Drug Prices, and Patient Cost Sharing. Ann Intern Med 2018;168:436. [DOI] [PubMed] [Google Scholar]
- 23.Kaltenboeck A, Bach PB. Value-Based Pricing for Drugs: Theme and Variations. JAMA 2018;319:2165. [DOI] [PubMed] [Google Scholar]
- 24.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA 2007;298:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang TJ, Kesselheim AS, Sarpatwari A. Value-Based Pricing and State Reform of Prescription Drug Costs. JAMA 2017;318:609. [DOI] [PubMed] [Google Scholar]
- 26.AMA Supports Changing the Fundamentals of Drug Pricing. Https://Www.Ama-Assn.Org/Ama-Supports-Changing-Fundamentals-Drug-Pricing.
- 27.Azar A. Fixing Healthcare: Driving Value Through Smart Purchasing and Policy. https://www.hhs.gov/about/leadership/secretary/speeches/2018-speeches/fixing-healthcare-driving-value-through-smart-purchasing-and-policy.html. May 2018.
- 28.Allen JI. The Road Ahead. Clin Gastroenterol Hepatol 2012;10:692–696. [DOI] [PubMed] [Google Scholar]
- 29.Dorn SD. Academic Gastroenterology Practice in a Value-Based World: One Size No Longer Fits All. Gastroenterology 2017;152:1258–1261. [DOI] [PubMed] [Google Scholar]
- 30.Brill JV, Jain R, Margolis PS, et al. A Bundled Payment Framework for Colonoscopy Performed for Colorectal Cancer Screening or Surveillance. Gastroenterology 2014;146:849–853.e9. [DOI] [PubMed] [Google Scholar]
- 31.Adams MA, Prenovost KM, Dominitz JA, et al. Predictors of Use of Monitored Anesthesia Care for Outpatient Gastrointestinal Endoscopy in a Capitated Payment System. Gastroenterology 2017; 153:1496–1503.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N Engl J Med 2016;374:242–253. [DOI] [PubMed] [Google Scholar]
- 33.Shah ED, Kim HM, Schoenfeld P. Efficacy and Tolerability of Guanylate Cyclase-C Agonists for Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation: A Systematic Review and Meta-Analysis. Am J Gastroenterol 2018;113:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner DM, Fogel R, Dorn SD, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol 2018;113:735–745. [DOI] [PubMed] [Google Scholar]
- 35.Miller LE. Study design considerations for irritable bowel syndrome clinical trials. Ann Gastroenterol 2014;27:338–345. [PMC free article] [PubMed] [Google Scholar]
- 36.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol 2017;112:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Y, Dai N, Fox M. Hydrogen Breath Testing With Measurement of Orocecal Transit for Diagnosis of Small Bowel Bacterial Overgrowth. Clin Gastroenterol Hepatol 2016; 14:1511–1512. [DOI] [PubMed] [Google Scholar]
- 38.Gupta A, Chey WD. Breath Testing for Small Intestinal Bacterial Overgrowth: A Means to Enrich Rifaximin Responders in IBS Patients? Am J Gastroenterol 2016;111:305–306. [DOI] [PubMed] [Google Scholar]
- 39.Bae S, Lee KJ, Kim Y-S, et al. Determination of Rifaximin Treatment Period According to Lactulose Breath Test Values in Nonconstipated Irritable Bowel Syndrome Subjects. J Korean Med Sci 2015;30:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DuPont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther 2014;39:1033–1042. [DOI] [PubMed] [Google Scholar]
- 41.Aitken M, Berndt ER, Cutler D, et al. Has The Era Of Slow Growth For Prescription Drug Spending Ended? Health Aff (Millwood) 2016;35:1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011. ;364:22–32. [DOI] [PubMed] [Google Scholar]
- 43.Healthcare Bluebook. https://healthcarebluebook.com/page_ProcedureDetails.aspx?dataset=md&id=189&g=Electrocardiogram&directsearch=true.
- 44.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569–1580. [DOI] [PubMed] [Google Scholar]
- 45.Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion 1999;60:77–81. [DOI] [PubMed] [Google Scholar]
- 46.Hungin APS, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 2003;17:643–650. [DOI] [PubMed] [Google Scholar]
- 47.Hungin APS, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005;21:1365–1375. [DOI] [PubMed] [Google Scholar]
- 48.Employment, Hours, and Earnings from the Current Employment Statistics survey. US Bureau of Labor Statistics; https://data.bls.gov/timeseries/CES0500000003. [Google Scholar]
- 49.HCUPNet. https://www.ahrq.gov/cpi/about/otherwebsites/hcupnetquery/index.html.
- 50.Buono J, Carson R, Flores N. Healthcare Resource Utilization and Direct Medical Costs Among Patients With Irritable Bowel Syndrome With Diarrhea. 2016 Acad Manag Care Pharm Annu Meet. [Google Scholar]
- 51.Physician Fee Schedule Search. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Cent Medicare Medicaid Serv.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.