Abstract
Development of drug addictive behaviors is modulated by both genetic and environmental risk factors. However, the molecular mechanisms remain unknown. To address the role of adolescent stress in the development of drug addiction, we combined a transgenic mouse model in which a putative dominant-negative form of DISC1 under expressional control of the prion protein promoter is used as a genetic risk factor and adolescent social isolation stress as a gene-environmental interaction (GXE). Repeated cocaine exposure induced greater locomotion in the GXE group than in the other groups. In a conditioned place preference (CPP) test, GXE mice exhibited a significant place preference to the cocaine-conditioned area compared with the other groups. In the nucleus accumbens (NAc) of GXE mice, we found increased enzyme activity of phosphodiesterase-4 (PDE4), predominantly located in NAc D2-receptor-expressing neurons, and enhanced effects of the PDE4 inhibitor rolipram, but not the D1 agonist SKF81297, on the phosphorylation of DARPP-32 and GluA1 at PKA sites. Rolipram injection before cocaine exposure completely inhibited cocaine-induced hyperlocomotion and CPP in the GXE group. These results indicate that GXE enhances sensitivity to repeated cocaine exposure via an increase in PDE4 activity in NAc D2-recptor-expressing neurons, leading to the development of cocaine addictive behaviors.
Keywords: addiction, adolescent stress, DISC1, gene-environmental interaction, nucleus accumbens, phosphodiesterase
INTRODUCTION
Development of drug addiction is modulated by both genetic and environmental risk factors (Enoch, 2012; Rovaris et al., 2015). However, the molecular mechanisms of these gene-environment interactions (GXE) in behaviors associated with substance abuse and drug addiction are still largely unknown. Substance use disorders are commonly comorbid with various psychiatric disorders including schizophrenia, and adolescence is a critical period for the onset of substance use disorders as well as that of mood disorders, schizophrenia, and anxiety disorders (Paus et al., 2008).
DISC1 has emerged as an important contributor to molecular pathways underlying the pathophysiology of various psychiatric conditions, most notably schizophrenia, bipolar disorder, and major depression, as well as substance/alcohol abuse (Sawamura et al., 2005; Brandon and Sawa, 2011; Niwa et al., 2016b). DISC1 signaling in the nucleus accumbens (NAc) is important for cocaine self-administration (Gancarz et al., 2016). Accordingly, mice incorporating a putative dominant-negative mutation of DISC1 (DISC1-DN) under the αCaMKII promoter exhibit an elevated acute methamphetamine-induced locomotor response compared with control groups (Hikida et al., 2007; Jaaro-Peled et al., 2013). We have previously reported that social isolation stress imposed during adolescence in DISC1 mutant mice that have a transgenic DISC1-DN under the prion protein promoter (DISC1-DN-Tg-PrP) further increased acute methamphetamine-induced locomotion (Niwa et al., 2013). In the present study, we validated socially isolated DISC1-DN-Tg-PrP mice as a GXE mouse model in the pathophysiology of repeated cocaine abuse.
Phosphodiesterase-4 (PDE4) is an important factor in the DISC1 interactome (Millar et al., 2005; Carlyle et al., 2011; Hikida et al., 2012; Gamo et al., 2013). PDE4 regulates cyclic adenocine monophosphate (cAMP) and many of the actions of dopamine through signal transduction pathways that involve DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa) (Greengard et al., 1999; Svenningsson et al., 2004; Nishi and Snyder, 2010; Heckman et al., 2016). When DARPP-32 is phosphorylated by cAMP-dependent protein kinase (PKA) on Thr34, it is converted into a potent inhibitor of protein phosphatase-1, and thereby controls the phosphorylation state and activity of many downstream physiological effectors, including various neurotransmitter receptors and voltage-gated ion channels. We have reported that pharmacological inhibition of PDE4 with rolipram enhances cAMP/PKA/DARPP-32 signaling in striatal medium spiny neurons as well as prefrontal cortex neurons (Nishi et al., 2008; Kuroiwa et al., 2012). In this study, we investigated whether PDE4 is involved in alterations of cAMP/PKA signaling in GXE mice. Finally, we investigated whether rolipram, a PDE4 inhibitor previously shown to reduce schizophrenia-associated phenotypes in another DISC1 mutant mouse model (Lipina et al., 2012), can ameliorate behaviors associated with cocaine addiction in our mouse model.
MATERIALS AND METHODS
Animals
Male DISC1 dominant-negative transgenic mice under the control of the prion protein promoter (DISC1-DN-Tg-PrP) and their male littermate WT mice were used (Niwa et al., 2013). We reported similar changes in the phenotypes between male and female mice in a previously published study (Niwa et al., 2013), although we acknowledge sex-specific effects of DISC1 and adolescent stress in other animal models (Weiss et al., 2004; Leussis and Andersen, 2008; Pletnikov et al., 2008; Ayhan et al., 2011; Kercmar et al., 2011; Kuroda et al., 2011; Hong et al., 2012; Holley et al., 2013; Abazyan et al., 2014; Nakai et al., 2014). Other labs have also reported no sex-specific findings in a DISC1 animal model (Koike et al., 2006; Clapcote et al., 2007). Mice were group-housed in wire-topped clear plastic cages (21×32×13 cm) or isolated in wire-topped opaque polypropylene cages (12.5×20×11 cm) from 5 weeks of age continuously with free access to food and water (Niwa et al., 2013). All animal handling procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and approved by the animal research committees of Osaka University Institute for Protein Research, Kyoto University Graduate School of Medicine, and Kurume University.
Drug injections and behavioral tests
Cocaine (Shionogi, Osaka, Japan) was dissolved in saline. Cocaine injections and behavioral analyses were started after 8 weeks of age.
Locomotor activity was assessed for a 10-min period with an infrared activity monitor (Coulbourn Instruments, Holliston, MA) immediately after i.p. injection of saline or cocaine (Hikida et al., 2001). Mice received saline once a day and were then habituated in a novel chamber for 3 days. Then, saline or cocaine (10 mg/kg) was injected once a day from day 1 (Acute) to day 5 (Repeated). On day 6, mice that underwent repeated saline treatment received a challenge injection of cocaine (10 mg/kg). In a separate set of experiments, mice also received repeated saline or rolipram (0.1 mg/kg; TOCRIS bioscience, Minneapolis, MN) injections administered 20 mins prior to saline or cocaine injections on Days 1–5.
The Conditioned Place Preference (CPP) test was carried out in a three-chamber apparatus consisting of a small middle chamber that connected the two large side chambers (MED Associates, Fairfax, VT). The two large chambers differed in floor and wall conditions. On day 0, mice were allowed to move freely in the three chambers for 20 min. On days 1–3, mice were confined to one large chamber for 15 min immediately after they had received saline. Four hours later, they received cocaine (10 mg/kg, i.p.) and were confined to the other side chamber for 15 min. Rolipram (0.1 or 0.5 mg/kg) or saline was injected 20 min before the cocaine injection. At day 4, mice were placed in the middle chamber without injection of rolipram or cocaine, and allowed to move freely in the three chambers for 20 min. The time difference was calculated by subtracting the time mice spent in the saline-paired side from the time mice spent in the cocaine-paired side (Hikida et al., 2001).
mRNA isolation, cDNA synthesis and quantitative PCR
NAc, prefrontal cortex (PFC), and ventral tegmental area (VTA) were isolated from 1 mm-thick brain coronal slices by punching (1.5 mm diameter) and cutting out, respectively (Paxinos and Franklin, 2013). Total RNA was isolated using an miRNeasy mini kit (Qiagen, Valencia, CA) and cDNA were synthesized using ReverTra Ace qPCR RT master mix with gDNA remover (TOYOBO, Osaka, Japan) according to the manufacturer’s protocol. Quantitative PCR was conducted using a TaqMan Gene Expression Assay (Applied Biosystems, Waltham, MA) and THUNDERBIRD Probe qPCR Mix (TOYOBO, Osaka, Japan) and the reaction was performed using a StepOnePlus™ Real-Time PCR System (Life Technologies, Calsbad, CA). All data were calculated using the ΔΔCt method normalized with β-actin as the endogenous control gene. The TaqMan gene expression assays used were: Mm01253862_m1 (PDE4B), Mm00456879_m1 (PDE4D), and Mm00607939_s1 (β-actin).
Immunoblotting
Brain tissue was homogenized in RIPA buffer (Santa Cruz Biotechnology, Dallas, TX) containing 2 mM PMSF, 1 mM sodium orthovanadate, and protease inhibitor cocktail. The homogenate was then centrifuged at 25,000g for 5 min at 4 °C and the supernatant was used as whole lysates. Protein concentrations were determined by the Pierce BCA protein assay method. Equal amounts of protein (20 μg) were loaded onto 4–15% polyacrylamide BIS-Tris gels (Bio-Rad), separated by electrophoresis, and then transferred to PVDF membranes (0.45 μm) (Merck-Millipore, Darmstadt, Germany).
Antibodies were generated against PDE4B1 (N-15; 1:200 dilution; Santa Cruz Biotechnology, Dallas, TX), PDE4D (H-69; 1:200; Santa Cruz Biotechnology, Dallas, TX), and β-actin (AC-15; 1:10000; Sigma-Aldrich, St. Louis, MO). Membrane signals were detected using an LAS3000mini image analyzer (GE Healthcare, UK) and quantified using NIH ImageJ software. For each experiment, values obtained from PDEs were normalized by values from β-actin, respectively.
PDE4 activity
PDE4 activity was assayed by PDE-Glo™ Phosphodiesterase Assay (Promega, Madison, WI) according to the manufacturer’s protocol. Briefly, the NAc, PFC, and VTA were homogenized in KHEM buffer ([in mM] 50 KCl, 10 EGTA, 1.92 MgCl2, 1 DTT, and 50 HEPES, pH 7.2) containing protease inhibitor cocktail (nacali tesque, Kyoto, Japan) and PhosSTOP (Roche, Mannheim, Germany), centrifuged, and the supernatant fractions were pre-incubated with 10 μM rolipram or DMSO. cAMP at a final concentration of 2 μM was used as a substrate. PDE-Glo termination buffer containing PDE inhibitor IBMX (Sigma-Aldrich, St. Louis, MO) was added to inhibit most PDEs. After the addition of PDE-Glo detection solution and kinase-Glo reagent, luminescence was measured using a plate-reader. PDE4 activity was designated as the remainder after subtracting the value of PDE activity in the presence of rolipram from that in the presence of DMSO.
Preparation and incubation of brain slices
At 8 weeks old, mice were killed by decapitation. The brains were rapidly removed and placed in ice-cold, oxygenated Krebs-HCO3− buffer [(in mM) 124 NaCl, 4 KCl, 26 NaHCO3, 1.5 CaCl2, 1.25 KH2PO4, 1.5 MgSO4, and 10 D-glucose, pH 7.4]. Coronal slices (350 μm) were prepared using a vibrating blade microtome (Leica Microsystems, Wetzlar, Germany), as described previously (Nishi et al., 2005). Slices of the NAc were dissected from coronal slices in ice-cold Krebs-HCO3− buffer. The slices were preincubated at 30 °C under constant oxygenation with 95% O2/5% CO2 for 60 min. The slices were treated with 10 μM of rolipram for 60 min, or 1 μM of SKF81297 for 10 min (Nishi et al., 2008). After drug treatment, the NAc was frozen on dry ice.
Frozen tissue samples were sonicated in boiling 1% SDS and boiled for an additional 10 min. Protein concentrations were determined by the Pierce BCA protein assay method (Thermo Scientific, Waltham, MA). Equal amounts of protein (40 μg) were loaded onto 4–12% polyacrylamide Bis-Tris gel (Bio-Rad, Hercules, CA), separated by electrophoresis, and then transferred to nitrocellulose membranes (0.2 μm) (Sigma-Aldrich, St. Louis, MO).
The membranes were immunoblotted using phosphorylation-state-specific antibodies raised against phospho-peptides: phospho-Thr34 DARPP-32, a site phosphorylated by PKA (D29E8 and D27A4; 1:4,000 dilution; Cell Signaling Technology, Danvers, MA); phospho-Ser845 GluA1, a site phosphorylated by PKA (p1160–845; 1:250 dilution; PhosphoSolutions, Aurora, CO, USA). Antibodies generated against DARPP-32 (C24–5a; 1:40,000 dilution) and GluA1 (E-6; 1:250 dilution; Santa Cruz Biotechnology, Dallas, TX), which are not phosphorylation-state-specific, were used to determine the total amount of proteins. None of the experimental manipulations used in the present study altered the total levels of specific phosphoproteins.
The membrane was incubated with a goat anti-mouse or rabbit Alexa 680-linked IgG (1:5000 dilution; Life Technologies, Calsbad, CA) or a goat anti mouse or rabbit IRDye800-linked IgG (1:5000 dilution; Rockland Immunochemicals Inc., Limerick, PA). Fluorescence at infrared wavelengths was detected by the Odyssey infrared imaging system (LI-COR Inc., Lincoln, NE) and quantified using Odyssey software.
Statistical and Data Analysis
All data are expressed as the means ± SEM. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) and SPSS 22.0 (IBM Corporation, NY) software. Statistical differences among three groups/factors or more were determined using a two-way or three-way analysis of variance (ANOVA) followed by post hoc tests with Bonferroni correction or where appropriate using student’s t-tests. We checked normality and homogeneity of variance with Mauchly’s test of sphericity. There were no significant differences between all data in Mauchly’s test of sphericity; thus, we evaluated p values without corrections by degrees of freedom. Statistical significance was set at p <0.05.
RESULTS
Enhanced locomotor response to cocaine in GXE mice
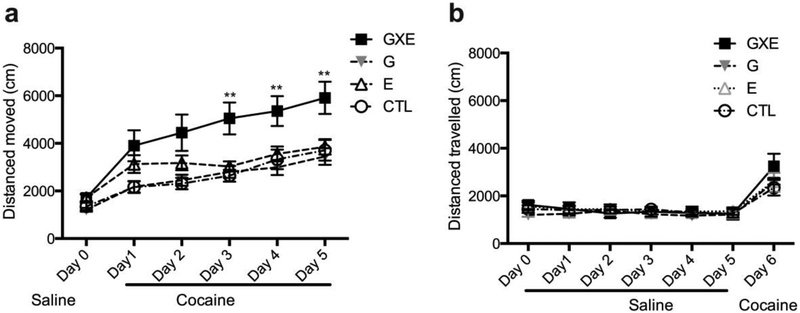
Locomotor sensitization is frequently used as a behavioral paradigm to model neuroplastic changes associated with repeated drug use (Kalivas and Stewart, 1991). We therefore began by exploring cocaine-induced locomotor sensitization in four groups of mice: wild-type mice without isolation (CTL group); wild-type mice with social isolation stress (E group); DISC1-DN-Tg-PrP mice without isolation (G group); and DISC1-DN-Tg-PrP mice with social isolation stress (GXE group). There were no significant differences between the four groups in locomotion after habituation with saline injections for 3 days (Fig 1a, b; Day 0) and after an acute cocaine injection (Fig 1a; Day 1, b; Day 6). Similarly, there were no differences in locomotion between the four groups following daily injections of saline from days 1−5 (Fig 1b; no significant interactions between time and genotype, F6, 96 = 1.67, p = 0.14; between time and environment, F6, 96 = 0.61, p = 0.72; and between time, genotype, and environment; F6, 96 = 1.06, p = 0.39). In contrast, following repeated treatment with cocaine GXE mice demonstrated a significantly augmented locomotor sensitization (Fig 1a; significant interactions between time and genotype, F5, 140 = 2 .82, p = 0.018; between time and environment, F5, 140 = 2.33, p = 0.046; and between time, genotype, and environment; F5, 140 = 3.08, p = 0.011). These data indicate that the GXE mouse model is hypersensitive to the effects of repeated cocaine exposure.
Figure 1.
Cocaine-induced locomotor activity in GXE mice. (a) Locomotor activity in four groups of mice are shown when mice were injected with saline on Day 0 and with cocaine (10 mg/kg, i.p.) on Days 1–5. Repeated cocaine exposure induced a greater increase in locomotor activity in GXE mice. ** p < 0.01, Bonferroni post hoc tests, GXE vs WT, G, or E at the same day. CTL, n = 7; E, n = 6; G, n = 10; GXE, n = 9. (b) Locomotor activity in four groups of mice are shown when mice were injected with saline on Days 0–5, and an acute administration of cocaine (10 mg/kg) on Day 6. No differences between the groups were seen following repeated saline administration (Days 0–5) or during the cocaine challenge (Day 6). n = 5 each.
Increased NAc PDE4 activity in GXE mice.
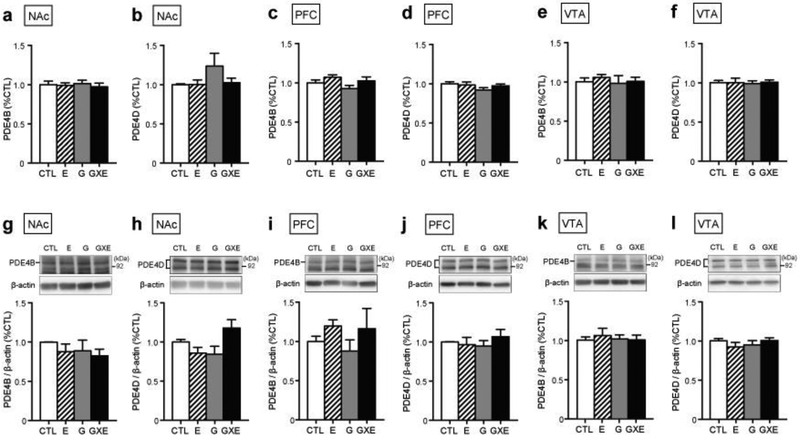
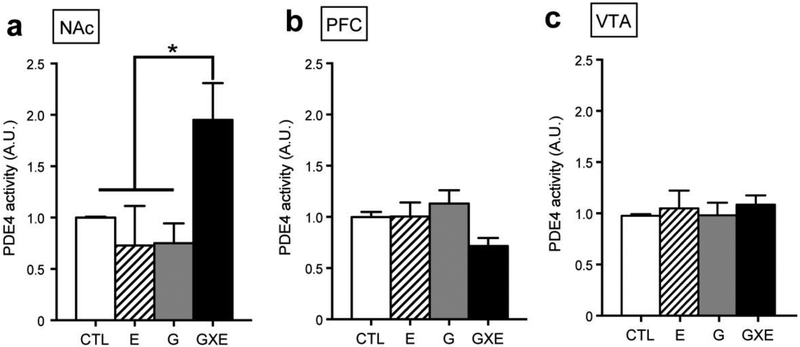
We next evaluated molecular changes in the NAc, PFC, and VTA, which were highlighted as regions of interest for molecular investigation due to their established role in controlling reward and addiction-related behaviors (Di Chiara and Imperato, 1988), as well as evidence of molecular abnormalities in these structures in socially isolated DISC1-DN-Tg-PrP mice (Niwa et al., 2013). PDE4 has four isoforms PDE4A-D, of which PDE4B and PDE4D isoforms interact with the DISC1 protein (Millar et al., 2005; Murdoch et al., 2007). mRNA and protein analyses revealed that expression of PDE4B and PDE4D isoforms were unaltered in the NAc, PFC, and VTA of all experimental groups (Fig 2a–2l). In contrast, PDE4 activity was increased within the NAc (Fig 3a, significant main effect of genotype, F1, 12 = 4.82, p = 0.049, and significant interaction between genotype and environment, F1, 12 = 9.62, p = 0.0092), but not the PFC (Fig 3b) and VTA (Fig 3c), of GXE mice when compared with controls.
Figure 2.
mRNA and protein levels of PDE4 isoforms in the nucleus accumbens (NAc), prefrontal cortex (PFC), and ventral tegmental area (VTA). Expression of PDE4B and PDE4D mRNA (a-f) and protein (g-l) were unaltered in the NAc, PFC, or VTA of GXE, G, and E mice when compared to CTL mice. CTL, n = 5; E, n = 5; G, n = 6; GXE, n = 6.
Figure 3.
Activity levels of PDE4 in the NAc, PFC, and VTA. (a) PDE4 activity was elevated in the NAc of GXE mice. *p < 0.05. n = 4 each. (b, c) There were no significant differences in PDE4 activity in the PFC (b) or VTA (c) of GXE, G, and E mice when compared with CTL mice. CTL, n = 5; E, n = 5; G, n = 4; GXE, n = 6.
Enhanced effects of rolipram on the phosphorylation of DARPP-32 and GluA1 at PKA sites in the NAc of GXE mice.
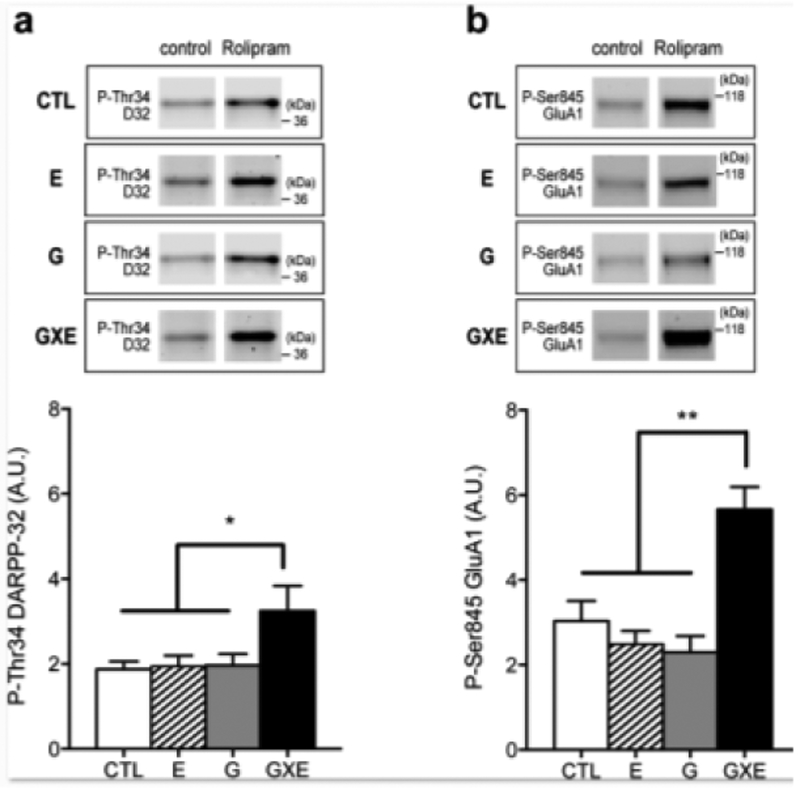
We then investigated the effect of rolipram treatment on intracellular cAMP/PKA signaling cascades downstream of PDE4. Phosphorylation of DARPP-32 and GluA1 in the NAc were chosen as targets due to their involvement in neuroplastic changes associated with cocaine addiction (Shuto et al., 2013). The basal levels of phospho-Thr34 DARPP-32 (Fig 4a, control) and phospho-Ser845 GluA1 (Fig 4b, control) in the NAc were similar among the four groups of mice. In the NAc, treatment of slices with rolipram (10 μM for 60 min) increased the levels of phospho-Thr34 DARPP-32 (Fig 4a, Rolipram) and phospho-Ser845 GluA1 (Fig 4b, Rolipram) in all groups. The increases in DARPP-32 and GluA1 phosphorylation induced by rolipram were significantly larger in GXE mice (Fig 4a, DARPP-32, significant main effects of genotype, F1, 25 = 5.26, p = 0.031, environment, F1, 25 = 5.67, p = 0.0025, and the interaction between genotype and environment, F1, 25 = 4.61, p = 0.042; Fig 4b, GluA1, significant main effects of genotype, F1, 28 = 7.38, p = 0.011, environment, F1, 28 = 9.38, p = 0.0048, and the interaction between genotype and environment, F1, 29 = 19.48, p = 0.0001). Thus, activation of cAMP/PKA signaling and subsequent phosphorylation of DARPP-32 and GluA1 induced by the inhibition of PDE4 with rolipram might be enhanced in the NAc of GXE mice. This analysis with the PDE4 inhibitor suggested the alteration of cAMP/PKA signaling and/or the phosphorylation/dephosphorylation balance of PKA-substrates due to upregulation of PDE4 activity in the NAc under GXE conditions.
Figure 4.
Phosphorylation levels of DARPP-32 and GluA1 in the NAc. Brain slices of the NAc were assessed for phosphorylation levels of DARPP-32 and GluA1 using 10 μM rolipram. Effects of rolipram were measured by phosphorylation levels at Thr34 of DARPP-32 (a) and Ser845 of GluA1 (b), which are PKA sites. (a) Phosphorylation levels at Thr34 of DARPP-32 in the NAc were significantly elevated by treatment with rolipram. The effect of rolipram was significantly greater in the GXE group compared with other groups. * p < 0.05. CTL, n = 8; E, n = 7; G, n = 7; GXE, n = 7. (b) Phosphorylation levels at Ser845 of GluA1 in the NAc were significantly elevated by treatment with rolipram. The effect of rolipram was significantly greater in GXE mice when compared with G, E, and CTL mice. ** p < 0.01. CTL, n = 9; E, n = 8; G, n = 8; GXE, n = 7.
We also examined phosphorylation of DARPP-32 and GluA1 in the NAc after treatment with the D1 agonist SKF81297. In the NAc, treatment of slices with SKF81297 (1 μM for 10 min) increased the levels of phospho-Thr34 DARPP-32 and phospho-Ser845 GluA1 in all groups (Supplementary Fig a&b). However, the increases in DARPP-32 and GluA1 phosphorylation induced by SKF81297 were not significantly changed in GXE mice (Supplementary Fig a, DARPP-32, no significant main effects of genotype, F1, 20 = 0.48, p = 0.50, environment, F1, 20 = 0.42, p = 0.53, and no significant interaction between genotype and environment, F1, 20 = 0.52, p = 0.48; Supplementary Fig b, GluA1, no significant main effects of genotype, F1, 20 = 0.34, p = 0.57, environment, F1, 20 = 0.40, p = 0.54, and no significant interaction between genotype and environment, F1, 20 = 0.04, p = 0.85).
Increased sensitivity to rolipram in cocaine-induced locomotor sensitization and CPP.
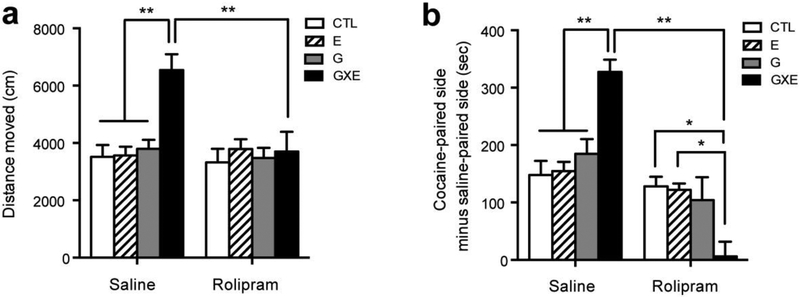
Given the increased PDE4 signaling in GXE mice and the effects this has on intracellular signaling cascades, we questioned whether inhibition of PDE4 activity with rolipram could alter cocaine-induced locomotor sensitization and cocaine-induced CPP in these mice. A low dose (0.1 mg/kg) of rolipram blocked the augmentation of cocaine-induced locomotor sensitization (Fig 5a, significant main effects of genotype, F1, 24 = 6.03, p = 0.022; environment, F1, 24 = 6.62, p = 0.017; and drug, F1, 24 = 7.15, p = 0.013; and significant interaction between genotype, environment, and drug, F2, 23 = 5.51, p = 0.011). Similarly, in a CPP test, GXE mice exhibited a greater preference for a cocaine-paired location when compared to other groups (Fig 5b, Saline). However, when 0.1 mg/kg rolipram was administered alongside cocaine during place conditioning, cocaine-induced CPP was completely inhibited in GXE mice, but not the other groups (Fig 5b; no significant main effects of genotype, F1, 16 = 0.69, p = 0.42; or environment, F1, 16 = 0.29, p = 0.60; but significant main effect of drug, F1, 16 = 93.7, p < 0.001; and significant interaction between genotype, environment, and drug F2, 15 = 11.87, p = 0.001). A higher dose (0.5 mg/kg) of rolipram blocked cocaine-induced CPP in all groups (cocaine-paired side minus saline-paired side: CTL, 28.60 sec ± 38.31; E, 30.84 sec ± 54.89; G, 26.39 sec ± 33.56; GXE, 10.75 sec ± 34.98, n = 5 each), indicating that sensitivity to rolipram is upregulated in GXE mice in response to increased PDE4 activity in the NAc under GXE conditions. Alongside the locomotor sensitization findings, these data suggest that PDE4 activity modulates behaviors related to cocaine reward.
Figure 5.
Effects of rolipram or saline on cocaine-related behaviors (a, b) Rolipram (0.1 mg/kg, i.p.) or saline was administered 20 min before cocaine (10 mg/kg) injections. (a) Repeated cocaine produced a significantly greater sensitization of locomotor activity in GXE mice than CTL, E, and G mice (Saline). Co-administration of rolipram blocked the augmentation of cocaine-induced locomotor sensitization in GXE mice (Rolipram). ** p < 0.01. n = 7 each. (b) In a CPP test, preference for the cocaine-paired side was significantly elevated in GXE mice when compared with CTL, E, and G mice (Saline). Co-administration of rolipram completely blocked cocaine-induced conditioned place preference in GXE, but not CTL, E, or G mice (Rolipram). * p < 0.05, ** p < 0.01. n = 5 each.
DISCUSSION
Previously we have reported that social isolation during adolescence in DISC1-DN-Tg-PrP mice resulted in behavioral and neurochemical alterations (Niwa et al., 2013; Hayashi et al., 2016; Matsumoto et al., 2017). Here for the first time we present evidence that the published model with cocaine exposure demonstrates augmentation of addictive behaviors in DISC1-DN-Tg-PrP mice with social isolation stress (GXE mice). In the current experiments, while all groups demonstrated sensitization of locomotor activity following repeated cocaine administration, this sensitization was significantly more pronounced in GXE mice. GXE mice have epigenetic changes in several genes such as Th, Bdnf, and Fkbp5 (Niwa et al., 2013; Niwa et al., 2016a). Epigenetic mechanisms are known to trigger enduring gene regulation that contribute to cellular alterations influencing the development of addiction (Nestler, 2014).
In this study, we chose to investigate PDE4, an enzyme that regulates cAMP signal transduction and is reported to interact with the DISC1 gene (Millar et al., 2005; Murdoch et al., 2007) as an adolescent stress-dependent regulator of drug addiction. While we found no changes to the total concentrations of PDE4B or PDE4D in the NAc, PFC, or VTA in all groups, GXE mice demonstrated increased PDE4 activity in the NAc. Phosphorylation analyses of DARPP-32 and GluA1 revealed that the increased PDE4 activity in GXE mice resulted in alteration of downstream cAMP/PKA signaling cascades. DISC1 interacts with PDE4 in resting cells and triggers its release in activated cells with high cAMP levels, resulting in activation of PDE4 and inhibition of cAMP signaling (Millar et al., 2005; Murdoch et al., 2007). DISC1-DN dominant-negatively interacts with wild-type DISC1 (Kamiya et al., 2005) and may activate PDE4 in the NAc (Tanaka et al., 2017). The mechanisms underlying stress-induced molecular changes in adolescents remain unclear, but adolescent stress likely potentiates the interaction of DISC1-DN with endogenous DISC1, leading to further activation of PDE4. Such mechanisms may be influenced by pathway-specific epigenetic changes in GXE mice (Niwa et al., 2013; Niwa et al., 2016a).
At a low dose, repeated co-administration of rolipram, a PDE4 inhibitor, was able to completely block the acquisition of cocaine CPP as well as the increase in locomotor sensitization to cocaine in GXE mice, while producing no effect in control groups. Interestingly, repeated intra-VTA co-administration of a larger dose of rolipram in wild-type rats has also been shown to block cocaine-induced CPP (Zhong et al., 2012), and repeated systemic co-administration of high-dose rolipram was able to reduce cocaine-induced locomotor sensitization in wild-type mice (Janes et al., 2009). In agreement, we also observed an inhibitory effect of high-dose rolipram on cocaine-induced CPP in CTL mice. Thus, cocaine sensitization and CPP in GXE mice are highly sensitive to rolipram, presumably due high PDE4 activity.
In NAc projection neurons, PDE4 is predominantly located in dopamine D2-receptor-expressing neurons, rather than in dopamine D1-receptor-expressing neurons (Nishi et al., 2008). Transient neural inhibition of dopamine D2-receptor-expressing neurons in the dorsal striatum facilitates behavioral sensitization (Ferguson et al., 2011). Taken together with our data reveal that the PDE4 inhibitor rolipram, but not the D1 agonist SKF81297, had significant effects on cAMP/PKA signaling in the NAc of GXE mice, increased PDE4 activity may selectively suppress the activity of the dopamine D2-receptor-expressing neurons in GXE mice, resulting in high sensitivity to repeated cocaine treatment. PDE4 is also located at the terminal of dopaminergic neurons (Nishi et al., 2008), and increased PDE4 activity may contribute to high cocaine sensitivity via changes in dopamine release. Taken together, these findings implicate PDE4 activity as a critical determinant of high cocaine sensitivity in GXE mice.
These data indicate that the GXE mouse model may be important in further analyzing epigenetic factors contributing to the development of drug addiction and stress-associated psychiatric disorders. These findings also add to a growing literature demonstrating the role of PDE4 in modulating psychiatric conditions, including schizophrenia (Millar et al., 2005), chronic stress (Plattner et al., 2015), and addiction to drugs of abuse (Janes et al., 2009; Zhong et al., 2012; Lai et al., 2014), and highlight the potential therapeutic benefit of PDE4 inhibitors in the treatment of cocaine addiction and stress-associated disorders.
Supplementary Material
Highlights.
∙ Behavioral sensitization to cocaine was enhanced by GXE.
∙ PDE4 activity was increased in the nucleus accumbens of GXE mice.
Effects of rolipram on the phophrylation of DARPP-32 and GluA1 was enhanced by GXE.
Rolipram inhibited enhanced behavioral sensitization to cocaine in GXE mice.
Acknowledgments
We thank Noriko Otani, Taichi Itou, and Kensuke Kimura for technical support.
Funding and Disclosure
The authors declare that there are no conflicts of interest regarding this article. This work was supported by the Japan Society for the Promotion of Science KAKENHI grants (JP16H06568, JP16K14579 and JP18H02542 to TH; JP16K10198 to MK; JP16H05135 to AN), the Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology program (JPJPR14M6 to MN), Takeda Science Foundation, Smoking Research Foundation, Hyogo Science and Technology Association, Uehara Memorial Foundation of Life Science (TH), Stanley, S-R, RUSK, NARSAD, Maryland Stem Cell Research Fund (AS) and the National Institute of Health (MH-094268 Silvio O. Conte Center, and MH-105660 to AS; DA-040127 to AS and MN). This work was performed, in part, under the International Collaborative Research Program of Institute for Protein Research, Osaka University, ICRa-18–03.
Abbreviations:
- cAMP
cyclic adenocine monophosphate
- CPP
conditioned place preference
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa
- DISC1-DN
dominant-negative mutation of DISC1
- GXE
gene-environment interaction
- NAc
nucleus accumbens
- PDE4
phosphodiesterase-4
- PFC
prefrontal cortex
- PKA
cAMP-dependent protein kinase
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abazyan B, Dziedzic J, Hua K, Abazyan S, Yang C, Mori S, Pletnikov MV, Guilarte TR, 2014. Chronic exposure of mutant DISC1 mice to lead produces sex-dependent abnormalities consistent with schizophrenia and related mental disorders: a gene-environment interaction study. Schizophr. Bull 40, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV, 2011. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol. Psychiatry 16, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A, 2011. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat. Rev. Neurosci 12, 707722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle BC, Mackie S, Christie S, Millar JK, Porteous DJ, 2011. Coordinated action of DISC1, PDE4B and GSK3beta in modulation of cAMP signalling. Mol. Psychiatry 16, 693–694. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC, 2007. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54, 387–402. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A, 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U.S.A 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, 2012. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Curr. Psychiatry Rep 14, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF, 2011. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat. Neurosci 14, 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Duque A, Paspalas CD, Kata A, Fine R, Boven L, Bryan C, Lo T, Anighoro K, Bermudez L, Peng K, Annor A, Raja A, Mansson E, Taylor SR, Patel K, Simen AA, Arnsten AF, 2013. Role of disrupted in schizophrenia 1 (DISC1) in stress-induced prefrontal cognitive dysfunction. Transl. Psychiatry 3, e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz A, Jouroukhin Y, Saito A, Shevelkin A, Mueller LE, Kamiya A, Dietz DM, Pletnikov MV, 2016. DISC1 signaling in cocaine addiction: Towards molecular mechanisms of co-morbidity. Neurosci. Res 105, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC, 1999. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23, 435–447. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Sawa A, Hikida T, 2016. Impaired hippocampal activity at the goal zone on the place preference task in a DISC1 mouse model. Neurosci. Res 106, 70–73. [DOI] [PubMed] [Google Scholar]
- Heckman PR, van Duinen MA, Bollen EP, Nishi A, Wennogle LP, Blokland A, Prickaerts J, 2016. Phosphodiesterase Inhibition and Regulation of Dopaminergic Frontal and Striatal Functioning: Clinical Implications. Int. J. Neuropsychopharmacol 19, pyw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Gamo NJ, Sawa A, 2012. DISC1 as a therapeutic target for mental illnesses. Expert Opin. Ther. Targets 16, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S, 2001. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A 98, 13351–13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andradé M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A, 2007. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc. Natl. Acad. Sci. U.S.A 104, 14501–14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley SM, Wang EA, Cepeda C, Jentsch JD, Ross CA, Pletnikov MV, Levine MS, 2013. Frontal cortical synaptic communication is abnormal in Disc1 genetic mouse models of schizophrenia. Schizophr. Res 146, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Flashner B, Chiu M, ver Hoeve E, Luz S, Bhatnagar S, 2012. Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not in male rats. Physiol. Behav 105, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Niwa M, Foss CA, Murai R, de Los Reyes S, Kamiya A, Mateo Y, O’Donnell P, Cascella NG, Nabeshima T, Guilarte TR, Pomper MG, Sawa A, 2013. Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Hum. Mol. Genet 22, 1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Kantak KM, Cherry JA, 2009. The involvement of type IV phosphodiesterases in cocaine-induced sensitization and subsequent pERK expression in the mouse nucleus accumbens. Psychopharmacol 206, 177–185. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J, 1991. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Brain Res. Rev 16, 223–244. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, Ross CA, Hatten ME, Nakajima K, Sawa A, 2005. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat. Cell Biol 7, 1167–1178. [DOI] [PubMed] [Google Scholar]
- Kercmar J, Budefeld T, Grgurevic N, Tobet SA, Majdic G, 2011. Adolescent social isolation changes social recognition in adult mice. Behav. Brain Res 216, 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA, 2006. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc. Natl. Acad. Sci. U.S.A 103, 3693–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Yamada S, Tanaka M, Iizuka M, Yano H, Mori D, Tsuboi D, Nishioka T, Namba T, Iizuka Y, Kubota S, Nagai T, Ibi D, Wang R, Enomoto A, Isotani-Sakakibara M, Asai N, Kimura K, Kiyonari H, Abe T, Mizoguchi A, Sokabe M, Takahashi M, Yamada K, Kaibuchi K, 2011. Behavioral alterations associated with targeted disruption of exons 2 and 3 of the Disc1 gene in the mouse. Hum. Mol. Genet 20, 4666–4683. [DOI] [PubMed] [Google Scholar]
- Kuroiwa M, Snyder GL, Shuto T, Fukuda A, Yanagawa Y, Benavides DR, Nairn AC, Bibb JA, Greengard P, Nishi A, 2012. Phosphodiesterase 4 inhibition enhances the dopamine D1 receptor/PKA/DARPP-32 signaling cascade in frontal cortex. Psychopharmacol. 219, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Zhu H, Sun A, Zhuang D, Fu D, Chen W, Zhang HT, Zhou W, 2014. The phosphodiesterase-4 inhibitor rolipram attenuates heroin-seeking behavior induced by cues or heroin priming in rats. Int. J. Neuropsychopharmacol 17, 1397–1407. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL, 2008. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse 62, 22–30. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Wang M, Liu F, Roder JC, 2012. Synergistic interactions between PDE4B and GSK-3: DISC1 mutant mice. Neuropharmacol. 62, 1252–1262. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Niwa M, Mouri A, Noda Y, Fukushima T, Ozaki N, Nabeshima T, 2017. Adolescent stress leads to glutamatergic disturbance through dopaminergic abnormalities in the prefrontal cortex of genetically vulnerable mice. Psychopharmacol. 20, 3055–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ, 2005. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310, 1187–1191. [DOI] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, Porteous DJ, Millar JK, Houslay MD, 2007. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J. Neurosci 27, 9513–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T, Nagai T, Wang R, Yamada S, Kuroda K, Kaibuchi K, Yamada K, 2014. Alterations of GABAergic and dopaminergic systems in mutant mice with disruption of exons 2 and 3 of the Disc1 gene. Neurochem. Int 74, 74–83. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, 2014. Epigenetic mechanisms of drug addiction. Neuropharmacol. 76 Pt B, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, 2010. Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: biochemical and behavioral profiles of phosphodiesterase inhibition in dopaminergic neurotransmission. J. Pharmacol. Sci 114, 6–16. [DOI] [PubMed] [Google Scholar]
- Nishi A, Watanabe Y, Higashi H, Tanaka M, Nairn AC, Greengard P, 2005. Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades. Proc. Natl. Acad. Sci. U.S.A 102, 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL, 2008. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J. Neurosci 28, 10460–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Lee RS, Tanaka T, Okada K, Kano S, Sawa A, 2016a. A critical period of vulnerability to adolescent stress: epigenetic mediators in mesocortical dopaminergic neurons. Hum. Mol. Genet 25, 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, Kano S, Ozaki N, Nabeshima T, Sawa A, 2013. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 339, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Cash-Padgett T, Kubo KI, Saito A, Ishii K, Sumitomo A, Taniguchi Y, Ishizuka K, Jaaro-Peled H, Tomoda T, Nakajima K, Sawa A, Kamiya A, 2016b. DISC1 a key molecular lead in psychiatry and neurodevelopment: No-More Disrupted-in-Schizophrenia 1. Mol. Psychiatry 21, 1488–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K, 2013. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, ed 4. Academic Press, London. [Google Scholar]
- Paus T, Toro R, Leonard G, Lerner JV, Lerner RM, Perron M, Pike GB, Richer L, Steinberg L, 2008. Morphological properties of the action-observation cortical network in adolescents with low and high resistance to peer influence. Soc. Neurosci 3, 303–316. [DOI] [PubMed] [Google Scholar]
- Plattner F, Hayashi K, Hernandez A, Benavides DR, Tassin TC, Tan C, Day J, Fina MW, Yuen EY, Yan Z, Goldberg MS, Nairn AC, Greengard P, Nestler EJ, Taussig R, Nishi A, Houslay MD, Bibb JA, 2015. The role of ventral striatal cAMP signaling in stress-induced behaviors. Nat. Neurosci 18, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA, 2008. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol. Psychiatry 13, 173–186, 115. [DOI] [PubMed] [Google Scholar]
- Rovaris DL, Mota NR, Bertuzzi GP, Aroche AP, Callegari-Jacques SM, Guimaraes LS, Pezzi JC, Viola TW, Bau CH, Grassi-Oliveira R, 2015. Corticosteroid receptor genes and childhood neglect influence susceptibility to crack/cocaine addiction and response to detoxification treatment. J. Psychiatric Res 68, 83–90. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Sawamura-Yamamoto T, Ozeki Y, Ross CA, Sawa A, 2005. A form of DISC1 enriched in nucleus: altered subcellular distribution in orbitofrontal cortex in psychosis and substance/alcohol abuse. Proc. Natl. Acad. Sci. U.S.A 102, 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto T, Kuroiwa M, Koga Y, Kawahara Y, Sotogaku N, Toyomasu K, Nishi A, 2013. Acute effects of resveratrol to enhance cocaine-induced dopamine neurotransmission in the striatum. Neurosci. Lett 542, 107–112. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P, 2004. DARPP-32: an integrator of neurotransmission. Ann. Rev. Pharmacol. Toxicol, 44, 269–296. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ishizuka K, Nekooki-Machida Y, Endo R, Takashima N, Sasaki H, Komi Y, Gathercole A, Huston E, Ishii K, Hui KK, Kurosawa M, Kim SH, Nukina N, Takimoto E, Houslay MD, Sawa A, 2017. Aggregation of scaffolding protein DISC1 dysregulates phosphodiesterase 4 in Huntington’s disease. J. Clin. Invest 127, 1438–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J, 2004. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res 152, 279–295. [DOI] [PubMed] [Google Scholar]
- Zhong P, Wang W, Yu F, Nazari M, Liu X, Liu QS, 2012. Phosphodiesterase 4 inhibition impairs cocaine-induced inhibitory synaptic plasticity and conditioned place preference. Neuropsychopharmacology 37, 2377–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.