Abstract
Alcohol consumption is often assessed over weeks to months, but few attempts have been made to characterize rate of alcohol consumption at the level of an individual drinking session. Here, we aimed to compare the rate of alcohol consumption in social drinkers at high risk for alcohol use disorder (AUD) and heavy drinkers. One hundred and sixty social drinkers and 48 heavy drinkers participated in an alcohol self-administration study. Social drinkers were classified as low-risk or high-risk for AUD based on sex, impulsivity, and family history of alcoholism. Participants received a priming dose of intravenous alcohol to assess alcohol-induced craving and completed a 125-minute intravenous alcohol self-administration session to assess rate of achieving a binge-level exposure (blood alcohol concentration ≥ 80mg%). There were no differences between rates of binging in high-risk and heavy drinkers (hazard ratio = 0.87, 95% CI 0.48 to 1.56). Heavy drinkers reported higher levels of craving than high-risk and low-risk drinkers at baseline. However, following a priming dose of alcohol, there were no longer differences in craving between high-risk and heavy drinkers. These results indicate that high-risk social drinkers demonstrate binging behavior that is similar to heavy drinkers, which may be driven by alcohol-induced craving. Prospective studies are needed to elucidate whether these patterns of craving and consumption in high-risk social drinkers are predictive of future AUD.
Keywords: Addictive Behavior, Alcohol Self-Administration, Alcoholism, Binge Drinking, Craving, Risk Factors
INTRODUCTION
Drinking patterns have profound effects on morbidity and mortality (Laramee, Leonard, Buchanan-Hughes et al., 2015). Whereas low to moderate consumption, such as a daily glass of wine, is linked to lower risk of cardiovascular disease and a longer life (Gaziano, Gaziano, Glynn et al., 2000; Ronksley, Brien, Turner et al., 2011; Stampfer, Colditz, Willett et al., 1988), heavier drinking has been associated with hepatic disease (Hart, Morrison, Batty et al., 2010; Savolainen, Liesto, Mannikko et al., 1993) and increased mortality (Smyth, Teo, Rangarajan et al., 2015). Alarmingly, the prevalence of both binge drinking and alcohol use disorder has increased in recent decades (Grant, Chou, Saha et al., 2017). While epidemiological studies have elucidated how broad patterns of drinking such as weekly and annual consumption levels affect health, few attempts have been made to characterize inter-individual differences in alcohol consumption patterns during a single occasion. Recent work has shown that healthy young adults with a greater number of risk factors for alcohol use disorder have markedly higher rates of binging during a two-hour alcohol self-administration session (Gowin, Sloan, Stangl et al., 2017). However, it is not clear how drinking patterns differ between high-risk social drinkers and those who have already developed heavy drinking patterns.
In this study, drinking patterns between high-risk social drinkers and heavy drinkers were examined using a human laboratory paradigm that has several important advantages. We used intravenous alcohol administration, which greatly reduces variability in alcohol exposure compared to oral administration by bypassing absorption and first-pass metabolism and controlling for pharmacokinetic factors that affect alcohol distribution and brain exposure (Ramchandani, Bolane, Li et al., 1999). Intravenous alcohol self-administration uses real-time breathalyzer readings to automatically adjust alcohol infusion rates, enabling precisely controlled, standardized alcohol exposure following each infusion. Intravenous self-administration also eliminates cues such as smell, taste, and appearance, so consumption should be primarily driven by the pharmacodynamic effects of alcohol, such as dopamine release in the nucleus accumbens (Ramchandani, Umhau, Pavon et al., 2011). This paradigm has been used to test the effects of medications on self-administration (Vatsalya, Gowin, Schwandt et al., 2015), to show that males consume more than females (Junger, Gan, Mick et al., 2016; Stangl, Vatsalya, Zametkin et al., 2017), and to examine genetic effects on alcohol consumption and sensitivity (Hendershot, Wardell, McPhee et al., 2017; Sloan, Klepp, Gowin et al., 2018). There is evidence that phenotypes elucidated in the laboratory have important clinical implications. For example, subjective response to alcohol in a laboratory setting prospectively predicted AUD symptom counts throughout six years of follow up (King, McNamara, Hasin et al., 2014). Furthermore, medications that reduce alcohol craving and self-administration in human laboratory studies (O’Malley, Krishnan-Sarin, Farren et al., 2002) have been shown to be effective in clinical trials (Anton, O’Malley, Ciraulo et al., 2006).
In the present study, we recruited the largest sample to date of social and heavy drinkers to participate in an intravenous alcohol self-administration session. We examined both alcohol consumption patterns and self-reported alcohol craving as there has been evidence that risky drinking is driven by higher levels of craving (Fatseas, Serre, Alexandre et al., 2015). High-risk drinkers were determined based on three of the genetic and physiological risk factors for alcohol use disorder listed in DSM-5: male sex (Dawson & Grant, 1998), family history of alcoholism (Dawson & Grant, 1998), and high levels of impulsivity (Caspi, Moffitt, Newman et al., 1996; Gowin, Sloan, Swan et al., 2018). These risk factors have previously been shown to affect alcohol self-administration in social drinkers (Gowin, Sloan, Stangl et al., 2017). During the alcohol self-administration session, we hypothesized that high-risk social drinkers would exhibit greater craving and rates of binging than low-risk social drinkers but lower craving and rates of binging than heavy drinkers.
METHODS AND MATERIALS
Participant Characteristics
One hundred and sixty social drinkers and 48 heavy drinkers were recruited to participate in an alcohol self-administration paradigm as part of three studies. The purpose of the first study (Gowin, Sloan, Stangl et al., 2017; Stangl, Vatsalya, Zametkin et al., 2017) was to examine determinants of intravenous alcohol self-administration in social drinkers. The second and third studies sought to examine the effects of medications on intravenous alcohol self-administration in heavy drinkers (Gowin, Vatsalya, Westman et al., 2016; Vatsalya, Gowin, Schwandt et al., 2015). Participants in all three studies completed a similar intravenous alcohol self-administration session which was used to compare alcohol consumption across groups. For the medication studies, the session occurred prior to receiving the study drug.
Participants initially completed a phone screen and were then asked to complete an in-person clinical screening evaluation and a psychiatric assessment for Axis-I disorders using the Structured Clinical Interview for DSM-IV (SCID-IV) (First, Spitzer, Gibbon et al., 2002) at the National Institutes of Health Clinical Center in Bethesda, MD. To be included, participants were required to have consumed at least five standard drinks on one occasion in their lifetime, where a standard drink was defined as 14 grams of pure ethanol. Participants in the social drinking groups consumed less than 14 drinks per week for females and 21 drinkers per week for males and were excluded if they had met criteria for alcohol dependence at any point in their lifetime. Participants in the heavy drinking group were required to consume an average of ≥ 14 drinks per week for females and ≥ 21 drinks per week for males. Participants in both groups were excluded if they met any of the following criteria: (1) lifetime history of Axis I mood, anxiety or psychotic disorders based on SCID-IV interview; (2) current substance dependence other than alcohol or nicotine; (3) recent or regular use of illicit or non-prescribed psychoactive substances (e.g. positive urine toxicology screen); (4) history of clinically significant alcohol withdrawal (CIinical Institute Withdrawal Assessment for Alcohol Scale Revised (Sullivan, Sykora, Schneiderman et al., 1989) score greater than eight); (5) current or chronic medical conditions requiring inpatient treatment or frequent medical visits; (6) use of medications known to interact with alcohol within 2 weeks prior to the study (including antidepressants, anticonvulsants, opioids, benzodiazepines, medications for diabetes, and medications that affect alcohol metabolism); (7) history of clinically significant withdrawal symptoms (e.g. withdrawal seizures) . Further, females were excluded if they were breastfeeding, pregnant, or intended to become pregnant. All study protocols were approved by the NIH Addictions Institutional Review Board and participants were enrolled after providing written informed consent.
Measures
Recent alcohol consumption was assessed using the 90-day Timeline Followback interview (Sobell & Sobell, 1992), which asks participants to retrospectively assess daily drinking over the previous 90 days using a calendar format to enhance recall. Participants also completed the Alcohol Use Disorders Identification Test (AUDIT) (Saunders, Aasland, Babor et al., 1993). Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker et al., 1991). Since this measure only assigns scores to individuals who smoke, we scored non-smokers as 0 in order to use this measure in continuous analyses.
Sex, impulsivity, and family history of alcoholism were used as markers of risk for alcohol use disorder based on risk factors listed in the DSM-5 and on our previous findings (Gowin, Sloan, Stangl et al., 2017). Family history of alcoholism was assessed via the Family Tree Questionnaire (Vogel-Sprott, Chipperfield & Hart, 1985) and defined as the presence of alcoholism in a biological parent, sibling, or grandparent. Impulsivity was measured using a monetary delay discounting task (Gowin, Sloan, Stangl et al., 2017; Richards, Zhang, Mitchell et al., 1999) in which participants expressed their preference for a larger amount of money to be received after a delay or a smaller, immediately available amount of money. Two versions of the task were used. In the first version of the task, immediate values ranged from $0 to $10.50, and in the second version, values range from $0 to $100. The delay periods ranged from seven to 365 days in the first version, and from seven to 30 days in the second version of the task. The degree of discounting delayed rewards, k, was calculated using a hyperbolic decay function (Mazur, 1987), but as k was not normally distributed, we used the natural log transformed values, ln (k). 18 participants did not complete this task and were therefore excluded from analysis.
Categorizing Social Drinkers as High Versus Low Risk
Social drinkers were divided into high-risk and low-risk social drinkers based on the presence of three AUD risk factors: male sex, family history of alcoholism, and high impulsivity. As we previously found that social drinkers with 2 or more AUD risk factors have higher rates of alcohol consumption, we divided our sample into a low-risk group (0–1 risk factors, N = 92) and a high-risk group (2–3 risk factors, N = 50). High impulsivity was determined by a median split of ln (k). Median splits were done separately for each version of the task as the median value for ln (k) varied by task. Individuals who did not complete the delay discounting task (N = 18) were excluded from this categorization. For a comparison of groups, see Table 1.
Table 1.
Characteristics of Low-Risk and High-Risk Social Drinkers and Heavy Drinkers a
| Characteristic | Low Risk Social Drinkers (n = 92) | High Risk Social Drinkers (n = 50) | Heavy Drinkers (n = 48) |
|---|---|---|---|
| Age, median (IQR), years b | 24.0 (6.0) | 24.0 (6.0) | 35.0 (24.0) |
| Female, No. (%) c | 61 (66.3) | 2 (4.0) | 13 (27.1) |
| Family History Positive, No. (%) d | 7 (7.6) | 16 (32.0) | 18 (37.5) |
| Delay Discounting e | |||
| Task 1, median (IQR) | −5.9 (2.9) | −3.9 (1.9) | −4.5 (3.3) |
| Task 2, median (IQR) | −4.5 (2.2) | −3.5 (1.1) | −3.3 (1.1) |
| Total Drinks (90-day timeline followback), median (IQR) f | 65.0 (70.0) | 67.0 (52.0) | 414.5 (315.0) |
| Binge Drinking Days (90-day Timeline followback), median (IQR) g | 4.0 (9.0) | 3.0 (5.0) | 44.0 (39.0) |
| Nicotine Dependence Severity Score, median (IQR) h | 0.0 (0.0) | 0.0 (0.0) | 0.0 (3.0) |
| Household Income, median bracket, USD | 30000 – 39999 | 30000 – 39999 | 30000 – 39999 |
| Craving score at baseline i | 13.0 (7.0) | 12.0 (11.0) | 18.0 (14.0) |
| Craving score post-alcohol prime j | 15.0 (11.0) | 18.5 (15.0) | 22.0 (18.0) |
| Current DSM-IV Alcohol Abuse Diagnosis, No. (%) k | 3 (3.3) | 1 (2.0) | 7 (14.6) |
| Current DSM-IV Alcohol Dependence Diagnosis, No. (%) l | 0 (0.0) | 0 (0.0) | 16 (33.3) |
Social drinkers at high risk for alcohol use disorder had 2 or more of the following risk factors: male sex, family history of alcoholism, or high impulsivity (as indexed by greater discounting of delayed rewards). Social drinkers at low risk for alcohol use disorder had 0–1 risk factors.
Drinking groups had significantly different distributions of age (H(2) = 24.6, p < 0.01).
There was a significant relationship between drinking group and sex (χ2(2) = 56.9, p < 0.01).
There was a significant relationship between drinking group and family history of alcoholism (χ2(2) = 21.0, p < 0.01).
Drinking groups had significantly different distributions of ln (k) for Task 1 (H(2) = 16.2, p < 0.01) and for Task 2 (H(2) = 18.7, p < 0.01).
Drinking groups had significantly different distributions of total drinks (H(2) = 105.3, p < 0.01).
Drinking groups had significantly different distributions of binge drinking days (H(2) = 90.5, p < 0.01).
Drinking groups had significantly different distributions of nicotine dependence severity scores (H(2) = 39.1, p < 0.01).
Drinking groups had significantly different distributions of craving scores at baseline (H(2) = 11.3, p < 0.01).
Drinking groups had significantly different distributions of craving score post-alcohol prime (H(2) = 13.4, p < 0.01).
There was a significant relationship between drinking group and alcohol abuse diagnosis (χ2(2) = 9.2, p = 0.01).
There was a significant relationship between drinking group and alcohol dependence diagnosis (χ2(2) = 51.7, p < 0.01).
Alcohol Self-Administration Paradigm
Participants were instructed not to drink alcohol in the 48 hours prior to study procedures. Smokers were allowed to smoke one cigarette one hour before the start of the paradigm to reduce nicotine craving and withdrawal effects. Participants arrived around 9:00 AM and provided a baseline breathalyzer reading (Drager Safety Inc., Irving, TX) and a urine drug screen that tested for cannabis, cocaine, amphetamines, opioids, and benzodiazepines. Both tests needed to be negative to proceed with the study session. In addition, females had to provide a negative urine pregnancy test to proceed with the session. CIinical Institute Withdrawal Assessment for Alcohol Scale Revised (CIWA-Ar) scores were measured in all but two of the heavy drinkers prior to the alcohol self-administration session. CIWA-Ar scores ranged from 0 to 5 with a median score of 1 (IQR = 2), indicating that these participants were not experiencing clinically significant withdrawal. After eating a standardized 350 kcal meal, an intravenous catheter was inserted into a vein in the forearm. Intravenous alcohol self-administration was conducted using the Computer Assisted Infusion System (CAIS), which computes the rate of infusion for each individual using a physiologically-based, pharmacokinetic model for alcohol that accounts for sex, age, height and weight (Ramchandani, Bolane, Li et al., 1999).
The intravenous alcohol self-administration session lasted 2.5 hours and consisted of a 25-minute priming phase followed by a 125-minute free access phase. During the priming phase, the software prompted participants to infuse alcohol four times over a 10-minute period. Each infusion raised the estimated arterial blood alcohol concentration (BAC) by approximately 7.5 mg% in 2.5 minutes, resulting in a median BAC of 23.41 mg% (IQR = 5.20) after 10 minutes. During the next 15 minutes, the button remained inactive to allow participants to feel the alcohol’s effects. Then, the free access phase began, and participants had 125 minutes to self-administer alcohol infusions ad libitum. To prevent adverse events, a safety limit was imposed such that estimated BAC could not exceed 100mg% for the participants recruited as social drinkers or 120mg% for the participants recruited as heavy drinkers. Blood alcohol concentration was estimated continuously by the infusion software and a readout was provided at 30-second intervals. Breathalyzer readings were obtained at 15-minute intervals to confirm the software’s estimates and to calibrate the rate of infusion.
Craving at a given timepoint was assessed using the Alcohol Urge Questionnaire (Bohn, Krahn & Staehler, 1995), which consists of eight items rated on a seven-point likert scale. The total score ranges from 8 to 56, with higher scores indicating higher levels of craving. The questionnaire was administered at baseline, prior the priming dose of alcohol, and at minute 20, following the priming dose of alcohol.
Statistical Analysis
Cox proportional hazards models were used to assess whether rate of binging during the session differed across low-risk drinkers, high-risk drinkers, and heavy drinkers while controlling for age and nicotine dependence severity. Binging was defined as achieving an estimated blood alcohol concentration of 80mg%. We also assessed whether drinking groups and risk groups differed in their craving during the priming phase of the session and in their alcohol consumption during first 30 minutes of the free access phase of the session. We analyzed the differences in craving (at baseline and following the priming dose of alcohol) and change in blood alcohol concentration (from minute 0 to minute 30 of the free access phase) between groups using Kruskal-Wallis tests followed by Bonferonni-corrected pairwise comparisons using Mann-Whitney tests.
RESULTS
Rates of Binging in Low-Risk, High-Risk, and Heavy Drinkers
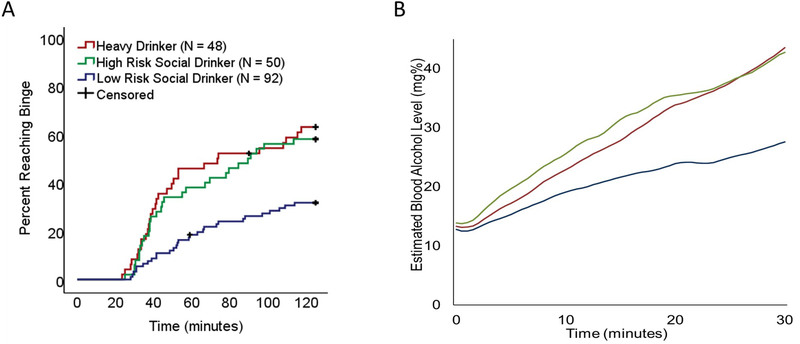
We plotted Kaplan-Meier survival curves to assess the effects of risk group on rate of binging (Figure 1A). There were significant differences between risk groups (log-rank test = 17.34, p < 0.001), with a higher percentage of bingers among heavy drinkers (62.5%) and high-risk drinkers (58.0%) relative to low-risk drinkers (31.5%).
Figure 1. Alcohol Self-Administration Patterns in Low-Risk, High-Risk, and Heavy Drinkers.
(A) Cumulative probability of achieving a binge-level exposure (estimated blood alcohol concentration of 80mg%) during the alcohol self-administration session. Cumulative probability of achieving a binge-level exposure was similar in heavy drinkers and high-risk social drinkers. (B) Total alcohol consumption during the first 30 minutes of the alcohol self-administration session. High-risk social drinkers and heavy drinkers had similar increases in blood alcohol concentration. Lines represent mean blood alcohol concentration for the respective groups.
A Cox proportional hazards model was conducted to assess the effect of drinking group on rate of binging throughout the session while controlling for age and nicotine dependence severity. Relative to low-risk drinkers, both high-risk drinkers (hazard ratio = 2.42, 95% CI 1.44 to 4.06, p = 0.001) and heavy drinkers (hazard ratio = 2.80, 95% CI 1.54 to 5.08, p = 0.001) had significantly higher rates of binging during the session. Rates of binging did not significantly differ between high-risk drinkers and heavy drinkers (hazard ratio = 0.87, 95% CI 0.48 to 1.56, p = 0.630, Table 2).
Table 2.
Cox Proportional Hazards Models Examining Rate of Binging in Low-Risk, High-Risk, and Heavy Drinkers
| Risk Group Analyses | |||
|---|---|---|---|
|
| |||
| Hazard Ratio | 95% CI | ||
| Model 1 | Low Risk Drinkers (reference group) | — | — |
| High Risk Drinkers | 2.42 | 1.44, 4.06 | |
| Heavy Drinkers | 2.80 | 1.54, 5.08 | |
| Age | 0.97 | 0.94, 0.99 | |
| Nicotine Dependence Severity Score | 1.23 | 1.09,1.40 | |
| Model 2 | Low Risk Drinkers | 0.36 | 0.20, 0.65 |
| High Risk Drinkers | 0.87 | 0.48, 1.56 | |
| Heavy Drinkers (reference group) | — | — | |
| Age | 0.97 | 0.94, 0.99 | |
| Nicotine Dependence Severity Score | 1.23 | 1.09, 1.40 | |
| Model 3 | Low Risk Drinkers (reference group) | — | — |
| High Risk Drinkers | 1.96 | 1.15, 3.32 | |
| Heavy Drinkers | 2.25 | 1.22, 4.15 | |
| Age | 0.97 | 0.94, 0.99 | |
| Nicotine Dependence Severity Score | 1.18 | 1.04,1.34 | |
| Craving After Alcohol Prime | 1.05 | 1.02, 1.07 | |
Effect of Risk Group on Alcohol Exposure
Heavy drinkers and high-risk social drinkers had greater increases in blood alcohol concentration across the first 30 minutes of the alcohol self-administration session than low-risk drinkers, with median increases of 28.2 mg%, 23.6 mg%, and of 8.6 mg% respectively. There was a significant effect of risk group on the distribution of change in blood alcohol level (H(2) = 15.15, p < 0.001, Figure 1B). Bonferroni-corrected pairwise comparisons indicated that the distribution of change in blood alcohol concentration was significantly different between high-risk and low-risk drinkers (p < 0.01) and heavy and low-risk drinkers (p < 0.01) drinkers. High-risk drinkers did not significantly differ from heavy drinkers.
Effect of Risk Group on Alcohol-Induced Craving
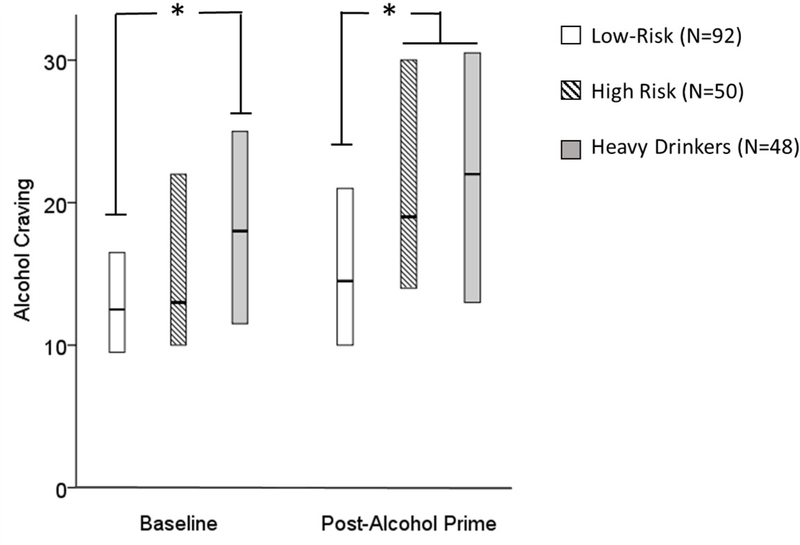
Craving scores differed between groups at baseline (H(2) = 11.32, p = 0.003) and following the priming exposure of alcohol (H(2) = 13.42, p = 0.001) . At baseline, Bonferroni-corrected pairwise comparisons revealed higher craving scores in heavy versus low-risk drinkers (p < 0.01), but no differences in craving scores between high-risk and low-risk drinkers. Following the priming dose of alcohol, craving scores were higher in both high-risk and heavy drinkers than in low-risk drinkers (p < 0.05 for both comparisons, Figure 2), however there was no difference in craving scores between high-risk and heavy drinkers.
Figure 2. Alcohol Craving in Low-Risk, High-Risk, and Heavy Drinkers.
Self-reported alcohol craving was measured using the Alcohol Urge Questionnaire at the beginning of the alcohol self-administration session (baseline) and after the participants received a priming dose of alcohol (post-alcohol prime, at minute 20). High-risk social drinkers reported similar levels of craving to low-risk social drinkers at baseline. After receiving a priming dose of alcohol, they reported similar levels of craving to heavy drinkers. *p < 0.01.
To determine whether craving following the priming dose of alcohol was associated with rate of binging, a Cox proportional hazards model was conducted including post-prime craving score, drinking group, age, and nicotine dependence severity as independent variables. Craving was significantly associated with rate of binging (hazard ratio = 1.05, 95% CI 1.02 to 1.07, p < 0.001, Table 2).
DISCUSSION
Our study shows that social drinkers at high-risk for AUD show similar rates of binging to heavy drinkers during an alcohol self-administration session. Rapid alcohol self-administration in high-risk individuals may be partially explained by inherent differences in their response to alcohol. Despite similar baseline craving between low- and high-risk groups, high-risk drinkers reported craving comparable to heavy drinkers following alcohol exposure, while low-risk drinkers continued to report lower levels of craving. Our findings therefore indicate that high-risk drinkers display psychopharmacological and behavioral responses to alcohol that are similar to heavy drinkers prior to the development of alcohol-related problems.
These variations in psychopharmacological response may be due to inherent differences in reward signaling between high- and low-risk individuals. Both blunted striatal activity during reward anticipation and greater discounting of delayed rewards at age 14 have been shown to predict problematic drug use (Buchel, Peters, Banaschewski et al., 2017). Young adults with a family history of substance use disorder also demonstrate reduced amphetamine-induced dopamine release in the ventral striatum compared to age- and substance-use matched controls (Casey, Benkelfat, Cherkasova et al., 2014). Attenuated reward signaling may alter the rate of drug self-administration, especially given that increasing the rate of brain exposure to a drug appears to change its reinforcing properties and liability for abuse (Henningfield & Keenan, 1993). We did not systematically acquire measures of neural response to reward in our study, but protocols investigating correlations between neural response to reward and alcohol self-administration in low- and high-risk individuals are warranted. Other explanations for differing rates of self-administration could include personality differences between high- and low-risk individuals. For example, high-risk individuals may display higher levels of impulsivity and novelty seeking, which may predispose them to self-administer alcohol at binge-levels. Determining the underlying mechanisms by which sex, family history of alcoholism, and delay discounting impact craving and alcohol self-administration will be an important goal of future studies.
The observed similarity in alcohol self-administration between high-risk and heavy drinkers requires validation in real-world settings. Detailed characterization of drinking patterns in the field has been difficult in the past, but may be facilitated by recent technological advances. For example, ecological momentary assessment has been used to provide temporal and geospatial profiles of drug and alcohol use (Epstein, Tyburski, Craig et al., 2014; Preston, Jobes, Phillips et al., 2016). Such assessments should eventually be coupled with real-time blood alcohol level measurement using wearable biosensors (Leffingwell, Cooney, Murphy et al., 2013). These technologies may prove useful in stratifying clinical risk and providing individually-tailored interventions (Nahum-Shani, Smith, Spring et al., 2016).
This study has several limitations. The study design was cross-sectional, so it remains unknown how alcohol self-administration patterns progress over time and predict addiction liability. Given that the median age of our heavy drinkers was older than that of our social drinkers, it is possible that certain high-risk drinkers would have progressed to heavy drinking status as they reached a comparable age. Furthermore, given that we used sex as a risk-factor and the majority of our high-risk subjects were male, these findings may not be readily extrapolated to females. Future studies should stratify recruitment by sex to assess the effects of these risk factors in females. It is also necessary to assess how other risk factors for AUD, such as comorbid mental illness, influence consumption. Additionally, it remains unclear whether nicotine use affects binging rates in the laboratory. Almost a third of our heavy drinkers were smokers (N = 15), whereas a much smaller proportion of our social drinkers reported nicotine use (N = 2). A study with a balanced proportion of smokers and non-smokers in each drinking group would be better suited to answer this question. Finally, although intravenous alcohol self-administration eliminates much of the pharmacokinetic variability associated with oral self-administration, it also lacks important social and environmental cues associated with real-world drinking.
The results of this study indicate that alcohol craving and consumption patterns are similar in high-risk social drinkers and heavy drinkers during an intravenous alcohol self-administration session. Problematic drinking patterns in young adults may therefore provide valuable prognostic information, but this remains to be determined in prospective studies. Understanding why high-risk drinkers consume alcohol differently than low-risk drinkers and finding ways to prevent the transition to heavy drinking will be of crucial importance in order to reduce the public health burden associated with alcohol use disorder.
Acknowledgements
This study was supported by the National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research (Z1A AA000466 VAR and K99 AA024778 JLG). Development of the CAIS software used for the intravenous alcohol self-administration session was supported by Sean O’Connor, M.D. and Martin Plawecki, M.D., Ph.D., at the Indiana Alcohol Research Center (NIH P60 AA007611). We would like to thank the medical staff who oversaw the safety of these participants, including Nancy Diazgranados, Mary Lee, Ted George, and Dan Hommer. We would like to thank Markus Heilig, Reza Momenan and Melanie Schwandt for operational support for this study and for thoughtful discussion of the results. We thank Dr. Monique Ernst for comments on an early draft of this manuscript. We thank Ruth Pfeiffer for analytic suggestions. We could not have completed this study without the help of the staff on the 5-SW day hospital at the NIH Clinical Center. Lastly, for their help collecting this data, we are grateful to our post-baccalaureate trainees: Megan Cooke, Marion Coe, Molly Zametkin, Kristin Corey, Jonathan Westman, Lauren Blau, Courtney Vaughan, Honoree Brewton, and Allie Schneider.
TRIAL REGISTRATION: clinicaltrials.gov Identifiers: NCT00713492, NCT00695500, NCT02639273
Footnotes
Financial Disclosures
All authors declare no conflicts of interest
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama 295:2003–2017. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research 19:600–606. [DOI] [PubMed] [Google Scholar]
- Buchel C, Peters J, Banaschewski T, Bokde AL, Bromberg U, Conrod PJ, Flor H, Papadopoulos D, Garavan H, Gowland P, Heinz A, Walter H, Ittermann B, Mann K, Martinot JL, Paillere-Martinot ML, Nees F, Paus T, Pausova Z, Poustka L, Rietschel M, Robbins TW, Smolka MN, Gallinat J, Schumann G, Knutson B, consortium I (2017) Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nat Commun 8:14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M (2014) Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol Psychiatry 76:23–30. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA (1996) Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of general psychiatry 53:1033–1039. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF (1998) Family history of alcoholism and gender: their combined effects on DSM-IV alcohol dependence and major depression. Journal of studies on alcohol 59:97–106. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Tyburski M, Craig IM, Phillips KA, Jobes ML, Vahabzadeh M, Mezghanni M, Lin JL, Furr-Holden CDM, Preston KL (2014) Real-time tracking of neighborhood surroundings and mood in urban drug misusers: application of a new method to study behavior in its geographical context. Drug and alcohol dependence 134:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatseas M, Serre F, Alexandre JM, Debrabant R, Auriacombe M, Swendsen J (2015) Craving and substance use among patients with alcohol, tobacco, cannabis or heroin addiction: a comparison of substance- and person-specific cues. Addiction 110:1035–1042. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Gaziano JM, Gaziano TA, Glynn RJ, Sesso HD, Ajani UA, Stampfer MJ, Manson JE, Hennekens CH, Buring JE (2000) Light-to-moderate alcohol consumption and mortality in the Physicians’ Health Study enrollment cohort. Journal of the American College of Cardiology 35:96–105. [DOI] [PubMed] [Google Scholar]
- Gowin J, Sloan ME, Swan JE, Momenan R, Ramchandani VA (2018) The relationship between delay discounting and alcohol dependence in individuals with and without comorbid psychopathology. Psychopharmacology [DOI] [PMC free article] [PubMed]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. The American journal of psychiatry 174:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Vatsalya V, Westman JG, Schwandt ML, Bartlett S, Heilig M, Momenan R, Ramchandani VA (2016) The Effect of Varenicline on the Neural Processing of Fearful Faces and the Subjective Effects of Alcohol in Heavy Drinkers. Alcoholism, clinical and experimental research 40:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA psychiatry 74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G (2010) Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ (Clinical research ed) 340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, McPhee MD, Ramchandani VA (2017) A prospective study of genetic factors, human laboratory phenotypes, and heavy drinking in late adolescence. Addict Biol 22:1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM (1993) Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol 61:743–750. [DOI] [PubMed] [Google Scholar]
- Junger E, Gan G, Mick I, Seipt C, Markovic A, Sommer C, Plawecki MH, O’Connor S, Smolka MN, Zimmermann US (2016) Adolescent Women Induce Lower Blood Alcohol Levels Than Men in a Laboratory Alcohol Self-Administration Experiment. Alcoholism, clinical and experimental research 40:1769–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biological psychiatry 75:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramee P, Leonard S, Buchanan-Hughes A, Warnakula S, Daeppen JB, Rehm J (2015) Risk of All-Cause Mortality in Alcohol-Dependent Individuals: A Systematic Literature Review and Meta-Analysis. EBioMedicine 2:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, Luczak S, Rosen G, Dougherty DM, Barnett NP (2013) Continuous Objective Monitoring of Alcohol Use: Twenty-First Century Measurement Using Transdermal Sensors. Alcoholism-Clinical and Experimental Research 37:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (1987) An adjusting procedure for studying delayed reinforcement. Commons, ML; Mazur, JE; Nevin, JA:55–73.
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, Murphy SA (2016) Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support. Ann Behav Med [DOI] [PMC free article] [PubMed]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ (2002) Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology 160:19–29. [DOI] [PubMed] [Google Scholar]
- Preston KL, Jobes ML, Phillips KA, Epstein DH (2016) Real-time assessment of alcohol drinking and drug use in opioid-dependent polydrug users. Behavioural pharmacology 27:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S (1999) A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcoholism, clinical and experimental research 23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M (2011) A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry 16:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H (1999) Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav 71:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA (2011) Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ (Clinical research ed) 342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M (1993) Development of the alcohol use disorders identification test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption-II. ADDICTION-ABINGDON- 88:791–791. [DOI] [PubMed] [Google Scholar]
- Savolainen VT, Liesto K, Mannikko A, Penttila A, Karhunen PJ (1993) Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcoholism, clinical and experimental research 17:1112–1117. [DOI] [PubMed] [Google Scholar]
- Sloan ME, Klepp TD, Gowin JL, Swan JE, Sun H, Stangl BL, Ramchandani VA (2018) The OPRM1 A118G polymorphism: converging evidence against associations with alcohol sensitivity and consumption. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43:1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth A, Teo KK, Rangarajan S, O’Donnell M, Zhang X, Rana P, Leong DP, Dagenais G, Seron P, Rosengren A, Schutte AE, Lopez-Jaramillo P, Oguz A, Chifamba J, Diaz R, Lear S, Avezum A, Kumar R, Mohan V, Szuba A, Wei L, Yang W, Jian B, McKee M, Yusuf S (2015) Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet 386:1945–1954. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back. In: Measuring alcohol consumption Springer. pp 41–72. [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH (1988) A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. The New England journal of medicine 319:267–273. [DOI] [PubMed] [Google Scholar]
- Stangl BL, Vatsalya V, Zametkin MR, Cooke ME, Plawecki MH, O’Connor S, Ramchandani VA (2017) Exposure-Response Relationships during Free-Access Intravenous Alcohol Self-Administration in Nondependent Drinkers: Influence of Alcohol Expectancies and Impulsivity. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 20:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- Vatsalya V, Gowin JL, Schwandt ML, Momenan R, Coe MA, Cooke ME, Hommer DW, Bartlett S, Heilig M, Ramchandani VA (2015) Effects of Varenicline on Neural Correlates of Alcohol Salience in Heavy Drinkers. Int J Neuropsychopharmacol [DOI] [PMC free article] [PubMed]
- Vogel-Sprott M, Chipperfield B, Hart DM (1985) Family history of problem drinking among young male social drinkers: reliability of the Family Tree Questionnaire. Drug and alcohol dependence 16:251–256. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory. Current topics in behavioral neurosciences 13:315–353. [DOI] [PubMed] [Google Scholar]