Abstract
A key challenge facing immunotherapy is poor infiltration of T cells into tumors, along with suppression of cells reaching these sites. However, macrophages make up a majority of immune cell infiltrates into tumors, creating natural targets for immunotherapies able to direct macrophages away from tumor-supportive functions and toward anti-tumor phenotypes. Recent studies demonstrate that toll-like receptors (TLRs) – pathways that quickly trigger early immune responses – play an important role in polarizing macrophages. Here we present emerging ways in which TLR signaling is being manipulated in macrophages to create new opportunities for cancer immunotherapy. In particular, we discuss approaches to deliver TLR agonists, to leverage biomaterials in these therapies, and to couple TLR-based approaches with other frontline treatments as combination cancer therapies.
Graphical Abstract
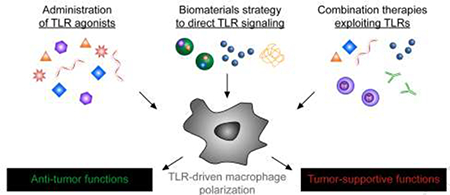
Introduction
Macrophages are key cellular players in the tumor microenvironment, making this population an important target for cancer immunotherapy. In particular, one of the challenges facing new therapies is the poor infiltration of T cells into tumors; these tissues also host an immunosuppressive environment that limits the activity of immune cells that do arrive. Macrophages, however, are a major component of the leukocyte infiltrate to tumor, which create some unique therapeutic opportunities to exploit macrophages relative to other populations such as T cells [1]. Of note, macrophages exhibit plasticity that can result in either favorable or detrimental outcomes with respect to tumor immunotherapy. As with macrophages in other tissue environments, tumor-associated macrophages (TAMs) have historically been segmented into M1 and M2 phenotypes, though this characterization is a simplification of the gradient of functions these cells can exert [2]. Generally speaking, M2-like macrophages support tumor growth and maintenance, whereas M1-like macrophages facilitate inflammation and tumorcidal effects [3–5]. Unfortunately, in most established tumors, M2-like macrophages are the dominant phenotype, fostering tumor growth and evasion of the immune system, as well as metastasis [4,6]. However, the morphology, phenotypes, and functions of macrophages can be altered by exposure to a range of microenvironmental, pharmacological, and biologic cues. As a result, strategies to promote M1-like macrophages and deplete or inhibit M2-like macrophage are being explored as powerful new levers to combat cancer [3–5].
One emerging approach to manipulate macrophage function is to control signaling in innate immune sensing pathways. These sensors, such as inflammasomes, the cGAS–STING pathway, and toll-like receptors (TLRs) are ubiquitous across many cell and tissue types. TLRs, for example, make up a series pathways evolved to detect molecular patterns common in pathogens, but uncommon in humans [7]. These receptors are expressed in distinct cellular locations (e.g., cell surface, endosome) of macrophages, allowing sensing of specific types of pathogens in subcellular locations that facilitate the most-appropriate type of response to a given pathogen. Because agonists (TLRas) for a specific TLR are molecularly defined, TLRs present an attractive target for more precise manipulation of the function of macrophages or other immune cells. This possibility has driven substantial research and some clinical use of a subset of TLR agonists – TLR3, TLR4, TLR7/8 and TLR9, for example – as more selective human adjuvants for vaccines against infectious disease and cancer [8,9]. These efforts have revealed significant insight into how TLR signaling in dendritic cells – which is a professional antigen presenting cell, can be harnessed. However, less work has focused on manipulating TLR signaling in macrophages to enhance immune response, but the unique role and prevalence of macrophages in the tumor microenvironment create exciting potential around this strategy.
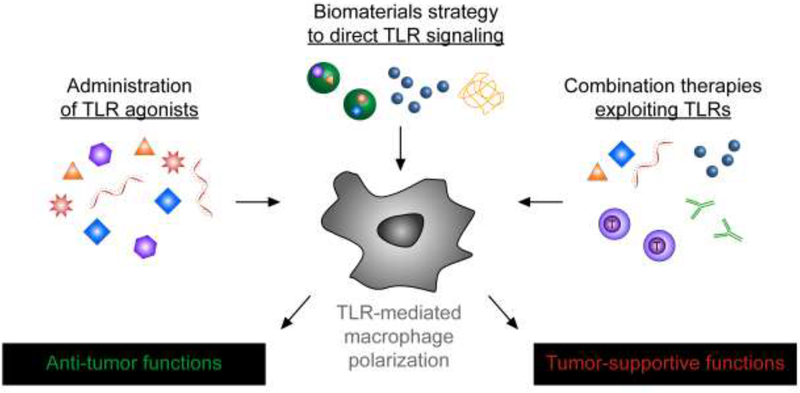
Below we review efforts to direct macrophage function during cancer by manipulating TLR signaling, emphasizing research from the most recent 3 years (Fig. 1). The first section focuses on traditional strategies to deliver TLRa to bias macrophages toward anti-tumor phenotypes, while the subsequent section highlights approaches to harness nanotechnology and biomaterials to control TLR signaling. Lastly, we discuss new reports combining manipulation of TLR signaling in macrophages with other cancer treatments as potential combination therapies.
Figure 1.
Approaches to manipulate toll-like receptor (TLR) signaling in macrophages to enhance cancer immunotherapy. Delivery of free TLR agonists (left) or TLR agonists loaded in biomaterials (center) can polarize macrophages away from tumor-supportive phenotypes and toward antitumor phenotypes. Some biomaterials also provide a second route to bias TLR signaling through intrinsic material properties that directly trigger TLR signaling (center). Combining existing therapies – such as check-point blockade or adoptive T cell therapy – with TLR-mediated polarization of macrophages (right) can create synergistic therapeutic outcomes.
TLR agonists are important cues to induce macrophages with anti-tumor functions
TLRs are key regulators of the innate immune system, functioning in part by triggering release of specific cytokines that define distinct immune cell compartments. In particular, a number of unique macrophage phenotypes can be generated by stimulation with TLRa in tandem with cytokines or other modulatory cues [10]•. One phenotype of interest for anti-tumor response is the “classically-activated” macrophage, inducible using LPS (TLR4a) and IFN-γ. These and related phenotypes produce key proinflammatory cytokines and chemokines to combat suppressive tumor microenvironments, including IL-12, IL-23, tumor necrosis factor (TNF), CC chemokines, and nitric oxide synthase. Agonists for TLR7/8 have recently been used to re-educate TAMs from tumor-supportive phenotypes to the M1-like phenotypes just mentioned [11]••. A key finding from this work is that the efficiency of polarization driven by these TLRa is comparable to strong common induction signals. For example, motolimod (TLR7a), GS9620 (TLR8a) and R848 (resiquimod, dual TLR7/8a) all enriched M1-like phenotypes to levels similar to those achieved with standard LPS and IFN-γ triggers.
Because TLRs recognize molecular patterns, the repertoire of available agonists is rapidly expanding. This is enabled by the evolutionary processes by which the TLR family has evolved to detect a range of classes of pathogen-associate moelcules. For example some TLRs – such as TLR4 – detect bacterial polyscharrides, while others – such as TLR9, detect bacterial DNA, while still others – such as TLR7, detect viral RNA. This diversity creates opportunities to engineer agonists with a range of chemistries and solution structures across the full range of specificity of TLR receptors. As illustration, a new synthetic TLR7a was recently shown to strongly activate macrophages in culture and in mice, enhancing phagocytosis of tumor cells in vitro, and enabling depletion of specific target cells in mice [12]. Taking this idea further, TLRas have been used to repolarize macrophages generally associated with tumor suppressive properties back to M1-like phenotypes. One recent report showed TLR3a skews macrophages in this manner – as indicated by upregulation of activation markers, suppression of co-inhibitory receptors, and release of proinflammatory cytokines – to significantly restrict tumor growth in a tumor-bearing mouse model of colorectal cancer [13]•. Another finding from this work is that IFNα mediated the polarization, identifying this pathway as a target for future therapies centered on macrophage polarization. Likewise, other recent studies have confirmed the ability of additional TLRas, such as TLR1/2 (Pam3CSK4), to skew macrophages toward pro-inflammatory functions [14].
Biomaterials offer synergistic features for manipulating TLR signaling to promote macrophages with anti-tumor functions
Biomaterials include degradable polymer nanoparticles (NPs), liposomes, scaffolds, and other other natural and synthetic materials that offer advanced capabilities for biological applications. For immunotherapy, some of the key features include co-delivery of multiple drugs or ligands, targeting to specific cell populations or tissues, and controlled release or protection of drugs and biologic cargo [15,16]•. Encapsulation and release of immune-active cargos from biomaterials can also reduce systemic side effects. For example, responsive NPs were recently used to target IL-12 to tumor-resident macrophages [17]. This approach promoted M1-like phenotypes without significant systemic toxicity, ultimately improving response during a melanoma xenograft model in mice. From another perspective, biomaterials exhibit tunable mechanical properties that can be used to direct immune cell migration, differentiation, and function [18]. This idea has been gaining traction as a way to control macrophage function in the tumor environment, where properties such as stiffness are variable [19]. TLR signaling in macrophages was recently linked to the mechanical properties of extracellular surfaces these cells encountered. In these studies, responses to TLRas were influenced by the stiffnesses of biomaterial surfaces that macrophages were cultured on [20]. These examples highlight the role of biomaterials both as delivery devices, and as active materials that directly influence the function and differentiation of macrophages. Below we discuss ways in which each of these roles are being exploited to direct TLR-signaling in macrophages.
NPs exhibit intrinsic features that can polarize macrophage function.
Many biomaterials – even some of those already used in the clinic – are now known to exhibit intrinsic physicochemical features that can directly alter innate immune signaling, including TLR activity [21]. While this discovery can complicate rational design because of the immunogenic features of these materials, these interactions also create new opportunities to design carriers that help tune immune function [22]. With respect to macrophages, for example, graphene oxide-based NPs have recently been shown to promote TLR2 signaling and activate macrophages [23]. Recent studies with degradable polymers such as poly(β-amino esters) also demonstrate activation of macrophages. However, these polymers did not activate TLR3, TLR7, or TLR9, or involve NF-κβ, suggesting there may be multiple innate pathways to leverage in directing macrophage phenotype [24,25].
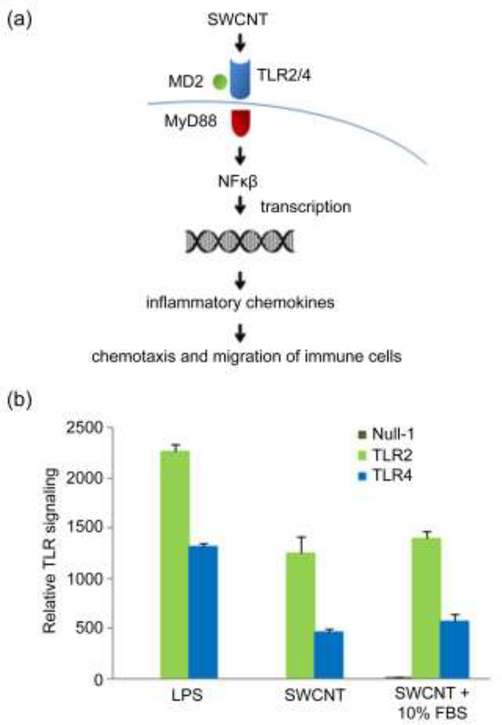
As just alluded to, the mechanisms involving NP-induced macrophage polarization have not been fully elucidated, but a number of materials clearly trigger TLR involvement. Alginate, a natural polysaccharide, promotes macrophage phagocytosis by upregulating TLR4 expression, ultimately stimulating the Akt/NF-κB and p38 MAPK pathways [26]. Thus, the intrinsic immunogenic effects of alginate may result in part from the ability of alginate to trigger increased uptake after smaller amounts are initially internalized to trigger TLR4. Interestingly, other recent studies demonstrate that different subclasses of macrophages preferentially internalized NPs at different rates, lending additional credence to the link between uptake, TLR signaling, and macrophage phenotype [27]•. Using single walled carbon nanotubes (SWCNTs) and a variety of transciptomic, cellular, and computational tools, another research team elucidated a potential mechanism by which these nanomaterials activate primary human monocyte derived macrophages (HMDM) [28]••. In these studies, SWCNTs were found to directly stimulate TLR signaling, resulting in signal transduction through a master regulator of TLR signaling, MyD88. This cascade activated NF-κβ and led to secretion of inflammatory chemokines that drive chemotaxis and recruitment of immune cells (Fig. 2a). For example, using TLR reporter cells, the team demonstrated SWCNTs triggered both TLR2 and TLR4 in both the presence and absence of serum-contianing media (Fig. 2b).
Figure 2.
SWCNT (single walled carbon nanotubes) as materials to trigger TLR signaling in macrophages. (a) Proposed mechanism by which macrophages may sense nanomaterials such as SWCNT. The mechanism was generated based on aggregation of cellular data, trascriptomics studies, and computational models. (b) SWCNT activate TLR2 and TLR4 independently of the presence of serum in the culture medium. Reporter HEK 293 cells co-transfected with human TLR2 (HEK-Blue™ hTLR2) or TLR4 (HEK-Blue™ hTLR4 cells) and an NF-κB/AP-1-secreted embryonic alkaline phosphatase (SEAP) reporter gene were exposed to SWCNT or a positive control (LPS). The cells that only express the SEAP reporter gene without TLR2/4 genes were included as a negative control (null). The relative TLR signaling is proportional to the % increase in SEAP activation. Adapted with permission from [28].
As the illustrations discussed thus far reveal, an emerging theme is that different materials – and most likely, different fundamental properties (e.g., shape, size, chemical functionality) – are able to drive distinct mechanisms by which TLRs and other innate sensing pathways impact macrophage polarization [29]. The size of particles formed from titanium dioxide, for example, preferentially polarizes macrophages toward different phenotypes [30]. Understanding the link between a range of biomaterial properties and macrophage polarization is important if these technologies are to be effectively exploited in the clinic. As one clinically-relevant example focused on cancer, Zanganeh et al. demonstrated that Ferumoxytol - a Food and Drug Administration (FDA) approved iron supplement NPs – inhibits adenocarcinoma and reduces liver metastasis in the absence of any other “treatment” [31]. Interestingly, these effects were associated with an increase in TAMs displaying M1-like functions. Although TLR signaling was not directly assessed in these studies, many of the cytokines and pathways TLRs help direct were altered during this repolarization, suggesting a possible direction for follow-on studies.
NPs offer features to enhance delivery of TLRas.
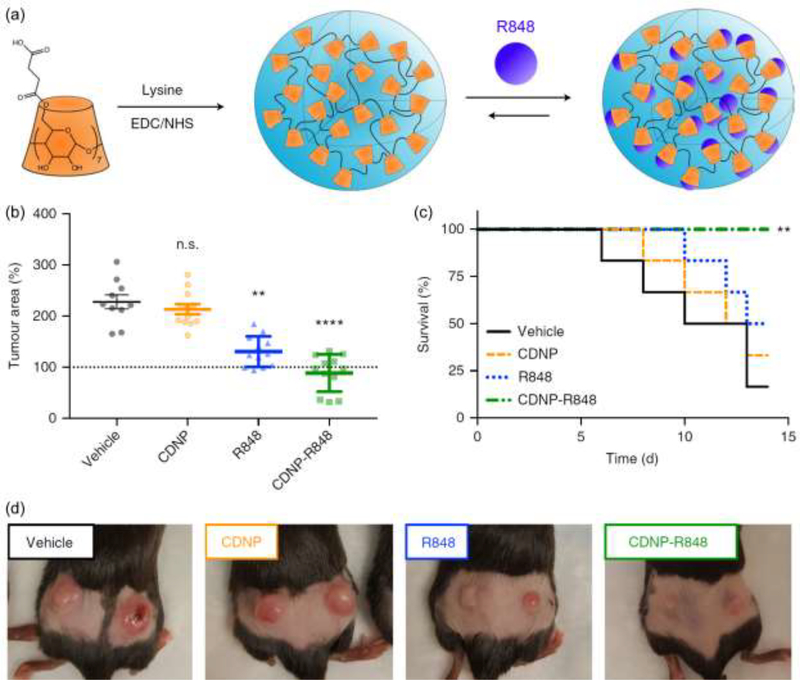
One important way in which NPs are being used to direct the function of immune cells is as carriers to deliver one or more TLRas [32–35]. Incorporating these ligands in NPs can improve targeting, reduce side effects, and increase potency compared to delivery of free TLRas. This idea is now receiving increasing attention specifically to polarize macrophages during cancer immunotherapy [11,36,37]. For example, R848 (TLR7/8) loaded in cyclodextrin NPs (CDNPs-R848) were recently used to target TAMs in several mouse models of cancer, including MC38 colon adenocarcinoma. (Fig. 3a) [11]••. These NPs conferred an M1-like bias to TAMs that was unmatched using systemic delivery of free ligand (R848). Importantly, this treatment controlled established tumors and protected mice against rechallenge (Fig. 3b-d). As with efforts to understand the immunological mechanisms behind the intrinsic immune activity of biomaterials, significant effort is also being exerted to understand how biomaterials enhance the response to TLRas. For example, manipulating the size and hydrophobicity of silica NPs changes the amount of IL-1β – an important innate cytokine – generated in response to TLR3a and TLR4a, ultimately altering the downstream cytokines that are generated [38]. This role of NP size in modulating the response to TLRas has also been observed with other biomaterials, and also specifically in macrophages [39]. Thus using biomaterials as carriers could also help reduce side effects through dose sparing during administration of TLRas with potential toxicity.
Figure 3.
Delivery of TLR7/8a (R848) in cyclodextrin NPs (CDNPs) enhances tumor therapy in the MC38 colon adenocarcinoma mouse model. (a) Scheme to assemble R848 in CDNPs (CDNP-R848). Treatment of mice with CDNP-R848 (b) reduces tumor area, (c) improves survival, and (d) reduces overall tumor burden relative to free R848. Adapted with permission from [11].
Coupling TLR-driven polarization of macrophages with other treatments could create new combination therapies
While breakthrough treatments such as adoptive cell therapy have provided remarkable gains for a subset of patients, these treatments are not yet widely applicable because of the heterogeneity of cancer across tissue type and across patients. This challenge means most patients will require combination therapies. In the last few years, several combination strategies have explored polarization of macrophage function by directing TLR signaling. As one example, antibody-mediated inhibition of the programmed cell death PD-1 receptor – a checkpoint that tumors exploit to suppress immune cells – has developed as an important component of human combination therapies [40]. This checkpoint blockade strategy was recently coupled with intra-tumoral injection of TLR7a and TLR9a in several mouse cancer models [41]. This treatment enhanced efficacy compared to PD-1 checkpoint inhibitors alone or to TLRas alone. Importantly, the therapeutic effects were correlated with activation of TAMs and an increase in the ratio of M1-like:M2-like function. Along similar lines, an important macrophage checkpoint – signal regulatory protein-α (SIRPα) – is a myeloid-specific immune checkpoint that tumor cells engage on macrophages to stop phagocytosis. Several recent studies have demonstrated that blocking SIRPα polarizes macrophages to M1-like functions and promotes anti-tumor activity [42–44]. Coupling this idea with TLRas might create combination therapies that specifically leverage distinct functions of macrophages to combat cancer, including increased phagocytic activity and increased activation.
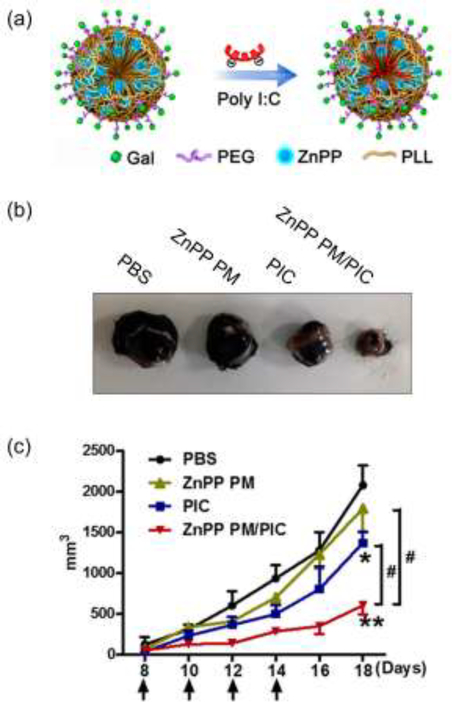
Nanoparticles are also being explored to deliver TLRas as part of combination therapies. For example, the CDNPs-R848 discussed earlier have been combined with anti-PD-1 therapy to improve the success of immunotherapy in mouse models [11]••. Of note, this combination therapy improved outcomes in a tumor model that was resistant to anti-PD-1 therapy alone. Another developing area is coupling TLRas with therapeutic cytokines. A combinatorial in vitro study recently revealed complementary roles for polarization of macrophages using co-administration of TLRas and IFN-γ, for example [45]. Many studies confirm an important role for oxidative processes in inflammation, and one recent study linked oxidant-induced macrophage activation to activation of TLR signaling [46]. Building on these ideas, other recent studies leveraged engineered polymer particles loaded with TLRas (polyI:C) and zinc protoporphyrin IX (ZnPP), a molecule that increases reactive oxygen species (ROS) (Fig. 4a) [47]•. This combination therapy repolarized TAMs to M1-like macrophages in mice, leading to reduced tumor burden in the B16 melanoma tumor-bearing mice model that was not achieved with monotherapies (Fig. 4b,4c). The examples in this section underscore the potential of combination therapies integrating TLR signaling in macrophages, as well as the synergies that can be achieved by integrating nanotechnologies into these approaches.
Figure 4.
Combination therapy using NPs to deliver TLRas and ZnPP in the B16 melanoma tumor-bearing mice model. (a) Scheme for assembling particles integrating polyI:C (PIC) and ZnPP. Treatment with NPs integrating both ZnPP and polyIC (ZnPP PM/PIC) drives the greatest reduction in tumor burden (b) visually and (c) quantitatively. These effects are unmatched by monotherapies of PIC alone, or ZnPP without PIC (ZnPP PM). Adapted with permission from [47].
Conclusions
Compared to T cells underpinning many existing immunotherapies, macrophages comprise a much larger fraction of the immune infiltrate in the tumor microenvironment; this distinction provides new and unique opportunities for exploiting macrophages in immunotherapy. While a gradient of functions and subclasses exist, polarizing TAMs to classically-activated (i.e. M1-like functions) has generally been the focus of cancer therapies, and the discussion above illustrates the multi-dimensional role that TLRas could play in this polarization. Promoting these therapeutic macrophages also reduces the frequency of TAMs with tumor-protective or immune-suppressive properties. Unsurprisingly, then, strategies to promote tumorcidal macrophages by directing TLR signaling are emerging in several strategies. These include delivery of TLRas as both monotherapies and combination therapies. Biomaterials also offer significant potential in this arena, both as carriers of TLRas and because of the intrinsic features these materials exhibit to directly trigger TLR signaling. Despite the potential of TLR signaling as a lever to enhance macrophage function in cancer therapy, there are some important considerations arising. First, TLRs are expressed on a broad range of cells, not just macrophages. Any translatable strategy much assess this risk, or perhaps mitigate it with targeting strategies (e.g., using biomaterials). Second, many TLRs play a dual role in balancing pro-inflammatory and regulatory immune functions [48,49]. As a result, the durability of any induced macrophage polarization must be tunable to ensure long-term disruption of regulatory/tolerogenic pathways are avoided. Dose sparing achieved through biomaterials or synergies of combination therapies might help address this concern [50]. Last, although biomaterials offer exciting potential, the link between the material properties and activation of specific pathways remains unclear. Elucidating these connections may enable more rational design of materials that directly polarize macrophages toward specific functions. Despite the points just outlined, the progress in controlling macrophage function through TLR signaling in the last few years has been significant, foreshadowing therapies that leverage multiple dimensions of the immune system and interdisciplinary technologies to maximize therapeutic response.
Highlights.
Macrophages are unique targets because they naturally infiltrate tumors
Macrophages can exhibit tumor-supportive or anti-tumor phenotypes
TLR signaling plays a significant role in polarizing macrophage phenotype
Biomaterials polarize macrophages with intrinsic properties and by delivery of TLRa
Combining TLR-based therapies with existing approaches creates synergies
ACKNOWLEDGEMENTS
This work was supported in part by the United States Department of Veterans Affairs (Award # 1I01BX003690), the National Institutes of Health (Award # R01EB026896), the Damon Runyon Foundation (Award # DRR3415), the Alliance for Cancer Gene Therapy (Award # 15051543), the Melanoma Research Alliance (Award # 348963), and NSF CAREER Award # 1351688.
Footnotes
DISCLOSURES
C.M.J. is an employee of the Baltimore VA Medical Center. The views reported in this paper do not reflect the views of the Department of Veterans Affairs or the United States Government. C.M.J. has an equity position in Cellth Systems, LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECOMMENDED READINGS
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M et al. : The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med (2015) 21(8):938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M et al. : Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity (2014) 41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P: Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol (2017) 14(7):399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JM, Recht L, Strober S: The promise of targeting macrophages in cancer therapy. Clin Cancer Res (2017) 23(13):3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehne N, Mora J, Namgaladze D, Weigert A, Brune B: Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol (2017) 35(12–19. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen SR, Schmid MC: Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm (2017) 2017(9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawasaki T, Kawai T: Toll-like receptor signaling pathways. Front Immunol (2014) 5(461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson EA, Lore K: Non-human primates as a model for understanding the mechanism of action of toll-like receptor-based vaccine adjuvants. Curr Opin Immunol (2017) 47(1–7. [DOI] [PubMed] [Google Scholar]
- 9.Holldack J: Toll-like receptors as therapeutic targets for cancer. Drug Discov Today (2014) 19(4):379–382. [DOI] [PubMed] [Google Scholar]
- 10.Edwards JP, Zhang X, Frauwirth KA, Mosser DM: Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol (2006) 80(6):1298–1307.The paper provides a side-by-side comparison of physiology and functionality of three distinct populations of activated macrophages using IFN-γ, IL-4, and TLR4 agonists as stimulators.
- 11.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R: Tlr7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng (2018) 2(8):578–588.This paper uses TLR7 (R848)-loaded nanoparticles to polarize macrophages and control tumors in multiple mouse models, driving a synergistic effect with anti-PD-1 therapy, even in an anti-PD-1 resistant tumor model.
- 12.Dahal LN, Gadd A, Edwards AD, Cragg MS, Beers SA: Uc-1v150, a potent tlr7 agonist capable of activating macrophages and potentiating mab-mediated target cell deletion. Scand J Immunol (2018) 87(6):e12666. [DOI] [PubMed] [Google Scholar]
- 13.Vidyarthi A, Khan N, Agnihotri T, Negi S, Das DK, Aqdas M, Chatterjee D, Colegio OR, Tewari MK, Agrewala JN: Tlr-3 stimulation skews m2 macrophages to m1 through ifn-alphabeta signaling and restricts tumor progression. Front Immunol (2018) 9(1650.This paper demonstrates the important role of TLR-3 agonists in skewing M2-like macrophages to M1-like macrophages by regulating IFN-αβ signaling in murine tumor models.
- 14.Davis S, Cirone AM, Menzie J, Russell F, Dorey CK, Shibata Y, Wei J, Nan C: Phagocytosis-mediated m1 activation by chitin but not by chitosan. Am J Physiol Cell Physiol (2018) 315(1):C62–C72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gammon JM, Dold NM, Jewell CM: Improving the clinical impact of biomaterials in cancer immunotherapy. Oncotarget (2016) 7(13):15421–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM: Improving vaccine and immunotherapy design using biomaterials. Trends Immunol (2018) 39(2):135–150.This broad review discusses the intrinsic immunogenicity of biomaterials in relation to their shape, size, and chemistry, as well as recent research using biomaterials to manipulate innate and adaptive immunity in improving vaccines and immunotherapies for infection, cancer, and immune tolerance/autoimmunity.
- 17.Wang Y, Lin YX, Qiao SL, An HW, Ma Y, Qiao ZY, Rajapaksha RP, Wang H: Polymeric nanoparticles promote macrophage reversal from m2 to m1 phenotypes in the tumor microenvironment. Biomaterials (2017) 112(153–163. [DOI] [PubMed] [Google Scholar]
- 18.Gosselin EA, Eppler HB, Bromberg JS, Jewell CM: Designing natural and synthetic immune tissues. Nat Mater (2018) 17(6):484–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springer NL, Fischbach C: Biomaterials approaches to modeling macrophage-extracellular matrix interactions in the tumor microenvironment. Curr Opin Biotechnol (2016) 40(16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber E, Heyward C, Cameron J, Leifer C: Toll-like receptor signaling in macrophages is regulated by extracellular substrate stiffness and rho-associated coiled-coil kinase (rock1/2). Int Immunol (2018) 30(6):267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andorko JI, Jewell CM: Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng Transl Med (2017) 2(2):139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tostanoski LH, Jewell CM: Engineering self-assembled materials to study and direct immune function. Adv Drug Deliv Rev (2017) 114(60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, Kim YS, Lim MY, Kim HY, Kong S, Kang M, Choo YW, Jun JH, Ryu S, Jeong HY, Park J et al. : Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano (2018) 12(2):1959–1977. [DOI] [PubMed] [Google Scholar]
- 24.Andorko JI, Hess KL, Pineault KG, Jewell CM: Intrinsic immunogenicity of rapidly-degradable polymers evolves during degradation. Acta Biomater (2016) 32(24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dold NM, Zeng Q, Zeng X, Jewell CM: A poly(beta-amino ester) activates macrophages independent of nf-kappab signaling. Acta Biomater (2018) 68(168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi D, Zhou R, Cai N, Lai Q, Han Q, Peng Y, Jiang Z, Tang Z, Lu J, Bao W, Xu H et al. : Alginate enhances toll-like receptor 4-mediated phagocytosis by murine raw264.7 macrophages. Int J Biol Macromol (2017) 105(Pt 2):1446–1454. [DOI] [PubMed] [Google Scholar]
- 27.MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WC, McGilvray ID: Phenotype determines nanoparticle uptake by human macrophages from liver and blood. ACS Nano (2017) 11(3):2428–2443.This report confirms the phenotype of macrophages plays an important role in nanoparticle uptake, with M2-like macrophages preferentially internalizing nanoparticles compared with M1-like macrophages.
- 28.Mukherjee SP, Bondarenko O, Kohonen P, Andon FT, Brzicova T, Gessner I, Mathur S, Bottini M, Calligari P, Stella L, Kisin E et al. : Macrophage sensing of single-walled carbon nanotubes via toll-like receptors. Sci Rep (2018) 8(1):1115.This results of this study suggests carbon nanotubes (CNTs) are ‘sensed’ as pathogen by immune cells via TLR2/4 signaling pathway, triggering a cascade that leads to immune cell migration/chemotaxis.
- 29.Silva AL, Peres C, Conniot J, Matos AI, Moura L, Carreira B, Sainz V, Scomparin A, Satchi-Fainaro R, Preat V, Florindo HF: Nanoparticle impact on innate immune cell pattern-recognition receptors and inflammasomes activation. Semin Immunol (2017) 34(3–24. [DOI] [PubMed] [Google Scholar]
- 30.Schoenenberger AD, Schipanski A, Malheiro V, Kucki M, Snedeker JG, Wick P, Maniura-Weber K: Macrophage polarization by titanium dioxide (tio2) particles: Size matters. Acs Biomater Sci Eng (2016) 2(6):908–919. [DOI] [PubMed] [Google Scholar]
- 31.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM et al. : Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol (2016) 11(11):986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR et al. : In vivo characterization of the physicochemical properties of polymer-linked tlr agonists that enhance vaccine immunogenicity. Nat Biotechnol (2015) 33(11):1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tostanoski LH, Chiu YC, Andorko JI, Guo M, Zeng X, Zhang P, Royal W 3rd, Jewell CM: Design of polyelectrolyte multilayers to promote immunological tolerance. ACS Nano (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng Q, Zhang P, Zeng X, Tostanoski LH, Jewell CM: Advanced manufacturing of microdisk vaccines for uniform control of material properties and immune cell function. Biomater Sci (2017) 6(1):115–124. [DOI] [PubMed] [Google Scholar]
- 35.Hess KL, Andorko JI, Tostanoski LH, Jewell CM: Polyplexes assembled from self-peptides and regulatory nucleic acids blunt toll-like receptor signaling to combat autoimmunity. Biomaterials (2017) 118(51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebrahimian M, Hashemi M, Maleki M, Hashemitabar G, Abnous K, Ramezani M, Haghparast A: Co-delivery of dual toll-like receptor agonists and antigen in poly(lactic-co-glycolic) acid/polyethylenimine cationic hybrid nanoparticles promote efficient in vivo immune responses. Front Immunol (2017) 8(1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He XY, Liu BY, Wu JL, Ai SL, Zhuo RX, Cheng SX: A dual macrophage targeting nanovector for delivery of oligodeoxynucleotides to overcome cancer-associated immunosuppression. ACS Appl Mater Interfaces (2017) 9(49):42566–42576. [DOI] [PubMed] [Google Scholar]
- 38.Kakizawa Y, Lee JS, Bell B, Fahmy TM: Precise manipulation of biophysical particle parameters enables control of proinflammatory cytokine production in presence of tlr 3 and 4 ligands. Acta Biomater (2017) 57(136–145. [DOI] [PubMed] [Google Scholar]
- 39.Yue J, Pallares RM, Cole LE, Coughlin EE, Mirkin CA, Lee A, Odom TW: Smaller cpg-conjugated gold nanoconstructs achieve higher targeting specificity of immune activation. ACS Appl Mater Interfaces (2018) 10(26):21920–21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribas A, Wolchok JD: Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Varner JA, Pu M, Messer KS, Guiducci C, Coffman RL et al. : Combination immunotherapy with tlr agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight (2017) 2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, Lietzenmayer M, McKenna KM, Naik TJ, McCarty A, Zheng Y et al. : Anti-sirpalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A (2017) 114(49):E10578–E10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulkarni A, Chandrasekar V, Natarajan SK, Ramesh A, Pandey P, Nirgud J, Bhatnagar H, Ashok D, Ajay AK, Sengupta S: A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat Biomed Eng (2018) 2(8):589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veillette A, Chen J: Sirpalpha-cd47 immune checkpoint blockade in anticancer therapy. Trends Immunol (2018) 39(3):173–184. [DOI] [PubMed] [Google Scholar]
- 45.Muller E, Christopoulos PF, Halder S, Lunde A, Beraki K, Speth M, Oynebraten I, Corthay A: Toll-like receptor ligands and interferon-gamma synergize for induction of antitumor m1 macrophages. Front Immunol (2017) 8(1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Igwe OJ: Exogenous oxidants activate nuclear factor kappa b through toll-like receptor 4 stimulation to maintain inflammatory phenotype in macrophage. Biochem Pharmacol (2018) 147(104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L, He H, Liang R, Yi H, Meng X, Chen Z, Pan H, Ma Y, Cai L: Ros-inducing micelles sensitize tumor-associated macrophages to tlr3 stimulation for potent immunotherapy. Biomacromolecules (2018) 19(6):2146–2155.This study demonstrates ROS-inducing polypeptides can target macrophages in vitro and in vivo, elevating the level of ROS, and lowering STAT3 expression in macrophages. TLR3 agonist-loaded in ROS-inducing micelles efficiently repolarize tumor-associated macrophages to M1-like phenotypes in a ROS-dependent manner, ultimately driving tumor regression in mice.
- 48.Butcher SK, O’Carroll CE, Wells CA, Carmody RJ: Toll-like receptors drive specific patterns of tolerance and training on restimulation of macrophages. Front Immunol (2018) 9(933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dajon M, Iribarren K, Cremer I: Toll-like receptor stimulation in cancer: A pro- and anti-tumor double-edged sword. Immunobiology (2017) 222(1):89–100. [DOI] [PubMed] [Google Scholar]
- 50.Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, Garcia-Flores Y, Baltimore D: An nf-kappab-microrna regulatory network tunes macrophage inflammatory responses. Nat Commun (2017) 8(1):851. [DOI] [PMC free article] [PubMed] [Google Scholar]