Abstract
Recent studies indicate a role for the constitutive androstane receptor (CAR), pregnane X-receptor (PXR), and hepatic xenobiotic detoxifying CYPs in fatty liver disease or obesity. Therefore, we examined whether Cyp3a-null mice show increased obesity and fatty liver disease following 8-weeks of exposure to a 60% high-fat diet (HFD). Surprisingly, HFD-fed Cyp3a-null females fed a HFD gained 50% less weight than wild-type (WT; B6) females fed a HFD. In contrast, Cyp3a-null males gained more weight than WT males, primarily during the first few weeks of HFD-treatment. Cyp3a-null females also recovered faster than WT females from a glucose tolerance test; males showed no difference in glucose tolerance between the groups. Serum concentrations of the anti-obesity hormone, adiponectin are 60% higher and β-hydroxybutyrate levels are nearly 50% lower in Cyp3a-null females than WT females, in agreement with reduced weight gain, faster glucose response, and reduced ketogenesis. In contrast, Cyp3a-null males have higher liver triglyceride concentrations and lipidomic analysis indicates an increase in phosphatidylinositol, phosphatidylserine and sphingomyelin. None of these changes were observed in females. Last, Pxr, Cyp2b, and IL-6 expression increased in Cyp3a-null females following HFD-treatment. Cyp2b and Fatp1 increased, while Pxr, Cpt1a, Srebp1 and Fasn decreased in Cyp3a-null males following a HFD, indicating compensatory biochemical responses in male (and to a lesser extent) female mice fed a HFD. In conclusion, lack of Cyp3a has a positive effect on acclimation to a HFD in females as it improves weight gain, glucose response and ketosis.
Keywords: P450, non-alcoholic fatty liver disease (NAFLD), ketosis, toxicology
1. Introduction
Obesity is a complex disorder that affects nearly 1 out of 3 Americans [1] and is considered an epidemic throughout much of the developed world [2, 3]. For example, the percent of obese individuals is increasing in Latin America (i.e. Panama 35%, Chili 22%), Europe (Greece 23%, Malta 26%), and the Middle East (United Arab Emirates 34%, Saudi Arabia 36%, Egypt 30%) [2]. In turn, the rates of dyslipidemia, hypertension, diabetes, and other metabolic disorders increased dramatically placing a large burden on our healthcare systems [4, 5].
The primary problem is excess food coupled with inactivity. Therefore, healthier food choices and lifestyle changes are common treatment regimens used to control obesity. However, there are multiple internal and external factors that regulate metabolic activity and allocation of dietary lipid and carbohydrate resources [6, 7]. Understanding these forces provides targets for initiating dietary and environmental changes and tackling this disease. Many cytochrome P450s (CYPs) are crucial detoxication enzymes that metabolize drugs [8], environmental chemicals [9], steroids [10] and fatty acids [11], and changes in CYP activity and expression are observed during obesity and diabetic conditions [12–15].
The constitutive androstane receptor (CAR) [16, 17] and pregnane X-receptor (PXR) [18] are two nuclear receptors involved in chemical detoxication, and associated with metabolic diseases although possibly in opposing directions. CAR activation alleviates symptoms of obesity and fatty liver disease and murine PXR activation increases obesity [14, 15, 19], although there are conflicting reports on PXR’s effects on obesity and insulin resistance [20]. These nuclear receptors are key regulators of CYPs such as Cy2b and Cyp3a subfamily members [18, 21, 22]. In a world in which we are exposed to numerous environmental toxicants each and every person may show different CYP activity and in turn show differential metabolism of xeno- and endobiotics, including unsaturated fatty acids because of the different diets and chemicals to which they have been exposed [23, 24]. Therefore, it is possible that chemicals that modulate CYP activity are obesogens or metabolic disruptors [5, 25].
Interestingly, complete loss of hepatic CYP activity in a conditional knockout of P450-oxidoreductase (HRN) caused enlarged livers, and hepatic steatosis, especially after the mice were fed sunflower oil (88% unsaturated fatty acids). In turn, increased hepatic concentrations of unsaturated fatty acids in the HRN mice activated CAR and induced Cyp2b10 and Cyp3a11 providing a putative new role for CAR in the recognition of unsaturated fatty acids and suggests that CYP induction may be a protective mechanism from fatty acid toxicity [19]. Subsequent studies also demonstrated CYP3A induction by fatty acids in human cells [26]. Further, Cyp3a expression and activity is decreased in obese guinea pigs and diabetic humans [27, 28], but increased in diabetic mice [15, 29]. These results suggest a role for Cyp3a in obesity given that Cyp3a expression changes with diet, loss of hepatic CYPs causes fatty liver disease, and the Cyp3a’s are the most abundant hepatic CYPs.
CYP3A4 accounts for approximately 30–40% of total CYP protein in the human liver and is responsible for the metabolism of more than 60% of the drugs on the market [30]. Cyp3a subfamily members produce a number of epoxidated products of linoleic acid and arachidonic acid in human and rodents. CYP3A4 primarily metabolizes linoleic acid to 11-hydroxy-octadecadienoic acid (11-HODE), and the production of 11-HODE is increased 10X by the CYP3A inducer, dexamethasone, in rats [31]. CYP3A4 oxygenates arachidonic acid to a number of metabolites including 13-, 10-, and 7-hydroxyeicosatrienoic acid (13-HETE, 10-HETE, 7-HETE) [31]. The epoxides formed are stable, but also metabolized by epoxide hydrolases to diols [31]. Whether these epoxides have a specific purpose in the liver is unknown; however, inhibition assays suggest that a Cyp3a-mediated arachidonic acid metabolite is in part responsible for relaxation of arterial endothelium [32]. A Cyp3a and Cyp2j produced metabolite of linoleic acid is a key product in the activation of nociception [33], and CYP3A4 metabolizes the arachidonic acid derivative, anandamide, to a high affinity cannabinoid ligand [34–36]. Thus, Cyp3a metabolizes unsaturated fatty acids for specific functions and disruption of this metabolism by xenobiotics or genetic impairments may have consequences that perturb fatty acid metabolism, distribution, and use [37, 38].
Ultimately, we decided to investigate the role of Cyp3a in obesity because Hepatic P450 Reductase-null (HRN) mice with no P450 activity show hepatic steatosis with increased Cyp2b10 and Cyp3a11 expression, perturbations in liver size, lipid homeostasis, and increased liver polyunsaturated fatty acids (PUFAs) [19]. In addition, activation of PXR, the key regulator of Cyp3a members, increases obesity in females but not males [14, 39]. This implicates detoxification CYPs in obesity, and Cyp3a members are the most prominent hepatic CYPs. However, the potential role of Cyp3a on obesity and diet-induced fatty liver disease has not been investigated. We used mice lacking seven of the eight Cyp3a genes on a B6 background to monitor the effects of a high-fat diet (HFD) (diet-induced obesity) on weight gain, glucose and insulin tolerance, fatty liver, and alterations in hepatic gene expression consistent with metabolic disorders.
2. Materials and methods
2.1. Livers from untreated WT and Cyp3a-null mice
Frozen livers from untreated 8–9 week old WT and Cyp3a-null mice were purchased from Taconic (Hudson, NY USA) and shipped on dry ice. These mice lack the Cyp3a11, Cyp3a16, Cyp3a25, Cyp3a41, Cyp3a44, Cyp3a57, and Cyp3a59 genes located within 0.8Mb from each other on chromosome 5. Cyp3a13, which is separate from the rest of the Cyp3a cluster by 7Mb, is still present within this knockout mouse model [40, 41]. The liver tissue was diced into three fractions such that half a liver was frozen for microsome preparation, a quarter of a liver was stored in Tri-Reagent (Sigma, St. Louis, MO USA) for RNA extraction while the remaining liver was frozen immediately and stored at −80°C.
2.2. High-fat Diet Treatment of WT and Cyp3a-null mice
All mice studies were done according to the National Institute of Health guidelines for humane use of research animals and were pre-approved by Clemson University’s Institutional Animal Care and Use committee. Seven - nine week old male and female, WT (C57Bl/6) and Cyp3a-null mice (n=8) were purchased from Taconic and housed for a week for acclimation prior to HFD treatment.
Eight-ten week-old acclimated WT and Cyp3a-null mice were fed pelleted adjusted calorie diet (60% kcal from fat, TD.06414; Harlan, Madison, WI USA) for eight weeks. One male Cyp3a-null mouse was eight weeks old and all other mice were 9–10 weeks old. This mouse also lost weight early in the study and therefore was eliminated from the study. Thus, all remaining mice were 9–10 weeks old at the start of the HFD treatment. Changes in body weight, feed consumption rate, and metabolic tests such as glucose (GTT) and insulin tolerance tests (ITT) were performed. A timeline of procedures is provided (Suppl. File 1). Weight gain was monitored weekly and feed consumption was determined every other day. Fasting plasma glucose levels were determined during week 2, 4 and 6. Glucose and insulin tolerance tests were performed during weeks 4 and 6, respectively. At the end of the study the mice were anesthetized, blood collected by heart puncture, euthanized and liver, kidney, white adipose tissue and testis were excised and weighed. The organs were immediately frozen on dry ice and stored at −80°C or placed in 10% formalin (Fisher, Fairlawn, NJ USA) for further studies. The liver was dissected into four fractions and frozen immediately on dry ice for microsome preparation, RNA isolation, and lipidomics. The portion of the liver used for RNA extraction was stored in Tri-Reagent (Sigma Aldrich). The last portion of the liver was placed in formalin for histopathology.
2.3. Glucose and Insulin tolerance tests
Mice were fasted for 4–5 hr prior to GTT or ITT during weeks four and six of the HFD study, respectively. Fasting blood glucose was determined using an Alphatrak 2 (Chicago, IL USA) blood glucose meter following a tail bleed. Glucose tolerance was determined following an intraperitoneal injection of 1 g/kg of their body weight of D-glucose (Sigma Ultra) with blood glucose readings every 20 minutes for the first hour and every 30 minutes for the second hour. ITT was determined after intraperitoneal injection of 0.75 U/g of their body weight of Novolin N (Nova Nordisk, Bagsvaerd, DK) followed by blood glucose measurements every 30 min for 2 hours.
2.4. cDNA and microsome preparation
RNA was extracted using Tri-Reagent (Sigma Aldrich) according to the manufacturer’s instruction and residual genomic DNA was removed by DNase (Bio-Rad, Hercules, CA USA) treatment. RNA was quantified by determining 260/280 using a spectrophotometer (Molecular devices, Ramsey MN USA). Microsome and cytosol fractions were prepared by homogenization with a dounce homogenizer followed by differential centrifugation as previously described [42]. Protein concentrations were determined with Bradford reagent (Bio-Rad).
2.5. Quantitative real-time PCR (qPCR):
qPCR was used to quantify changes in gene expression. Briefly, cDNA was prepared from 2 μg RNA with 200 units of MMLV reverse transcriptase, 10mM dNTP mixture and 0.5 mg random hexamers (Promega corporation, Madison WI USA). Changes in gene expression were determined using previously published primers for CYPs [43, 44] and key genes related to energy homeostasis and lipid metabolism (Suppl. File 2). Samples were diluted 1:5 and amplified in triplicates using a 96-well plate IQ™ Real-Time PCR detection system (Bio-Rad) with 0.25X RT2 SybrGreen (Qiagen Frederick, MD USA) to quantify gene expression compared to a reference gene as previously described using Muller’s equation to determine changes in gene expression [45]. PCR efficiency was determined based on a standard curve prepared using a sample mixture containing all the cDNA samples diluted at 1:1, 1:5, 1:25, 1:125, 1:625 and 1:3125 dilutions [46].
2.6. Serum concentrations of testosterone, adiponectin and β-hydroxybutyrate
Serum testosterone and β-hydroxybutyrate concentrations were detected by using Enzyme Immunoassay (EIA) and colorimetric kits, respectively, purchased from Cayman Chemical Co (Cayman Chemical, Ann Arbor, MI USA). Serum adiponectin concentrations were determined using an EIA kit from Bertin pharma (Montigny Le Bretonneux, FR).
2.7. Histopathological analysis using H&E and oil red O
A clean slice of liver was made following necropsy and was placed in 10% formalin (Fisher). Hematoxylin and Eosin (H&E) and oil red O staining was performed at Colorado Histoprep (Fort Collins, CO USA).
2.8. Quantification of liver triglycerides and polar lipids
Liver triglyceride concentrations were determined using a colorimetric kit from Cayman Chemical Co. Liver tissue (40–60 mg) was homogenized in a dounce homogenizer with 0.5 ml phosphate buffer mixed with 0.5 ml of a 3:2 mix of hexane:isopropyl alcohol. The homogenate was transferred into a fresh tube and the extraction repeated with 3:2 hexane:isopropyl alcohol. The organic layer was transferred into a fresh tube and the hexane extraction was repeated twice. The combined organic layers were then dried under nitrogen and re-constituted using standard diluent provided in the kit to determine liver triglyceride concentrations according to the manufacturer’s protocol.
Polar lipids were extracted from microsomes (2 mg/ml) by the addition of chloroform (0.1% butylated hydroxytoluene) and methanol in 1:2 parts into a glass tube. The contents of the tube were vortexed and centrifuged at 600 rpm for a minute. The lower layer was transferred into a fresh tube the chloroform extraction was repeated 3X. The combined lower layers were washed with 1M potassium chloride and then with water. Samples were dried under nitrogen and shipped on dry ice to the Kansas Lipidomics Research Center for analysis by electrospray ionization triple quadruple mass spectrometry (Applied Biosystems API 4000) as described previously [47, 48].
2.9. Statistical Analysis
Data are presented as mean ± SEM (n = 7 – 8). Statistical analyses were performed by Student’s t-test using Graphpad Prism version 6. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Changes in the expression of hepatic energy metabolism-related genes in untreated Cyp3a-null mice
Mice lacking all hepatic CYP activity have steatosis [19] and several CYPs are either regulated by transcription factors involved in energy homeostasis or metabolize crucial lipids and bile acids involved in digestion, absorption and utilization of lipids [49]. Therefore, we first investigated whether the liver of 8–9 week-old Cyp3a-null mice show differences in the expression of several energy metabolism-related genes compared to WT mice by qPCR. Most of the genes investigated were not changed. However, carnitine palmitoyl transferase (Cpt1a) is significantly up-regulated by 134X and 18X in Cyp3a-null male and female mice, respectively, compared to their WT counterparts. Fatty acid binding protein-4 (Fabp4) is up-regulated in Cyp3a-null females 13X compared to their WT counterparts (Table 1). Pparα and Srebp1c regulate Cpt1a and Fabp4, respectively, suggesting compensatory changes in liver lipid metabolism in Cyp3a-null mice that help metabolism excess lipids [46, 50, 51].
Table 1:
Compensatory changes in energy and lipid metabolism genes in Cyp3a-null mice.
| FEMALES | MALES | |||
|---|---|---|---|---|
| Genes | WT | Cyp3a-null | WT | Cyp3a-null |
| Acox | 1.00±0.428 | 0.326±0.054 | 1.00±0.520 | 1.318±0.431 |
| Cpt1a | 1.00±0.388 | 18.706±5.562* | 1.00±0.265 | 134.76±60.91* |
| Fabp4 | 1.00±0.215 | 12.923±5.297* | 1.00±0.954 | 2.441±1.697 |
| Fasn | 1.00±0.259 | 1.537±0.258 | 1.00±0.330 | 0.855±0.053 |
| Foxa2 | 1.00±0.348 | 4.808±2.486 | 1.00±0.713 | 6.512±4.854 |
| Pparα | 1.00±0.266 | 1.424±0.374 | 1.00±0.558 | 3.366±1.545 |
| Pparγ | 1.00±0.342 | 2.616±1.791 | 1.00±0.988 | 0.172±0.143 |
| Srebp1 | 1.00±0.256 | 0.908±0.228 | 1.00±0.245 | 1.342±0.272 |
Data represented as relative mean +/− SEM (n = 4). Statistical significance determined by Student’s t-test.
Indicates a p-value ≤ 0.05.
3.2. Weight gain is mitigated in HFD-treated Cyp3a-null female mice in comparison to HFD-treated WT female mice
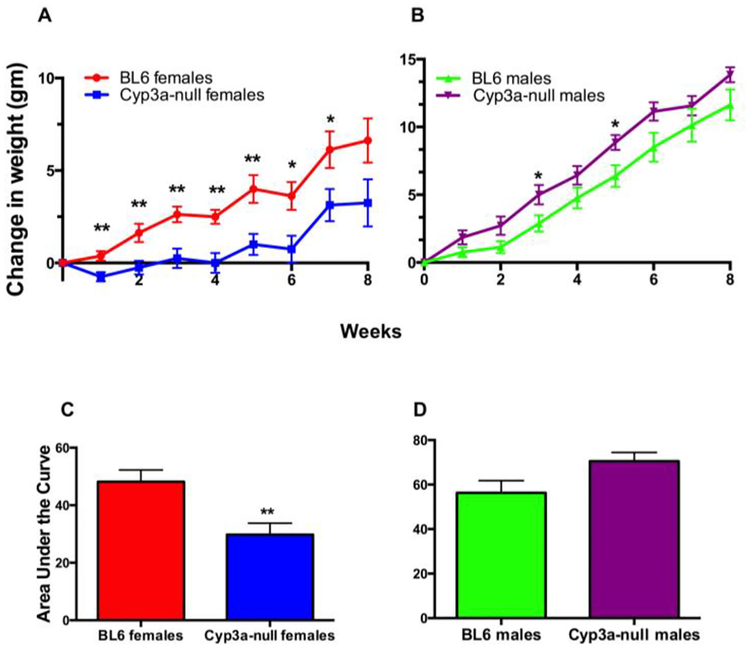
Weight gain was monitored in WT and Cyp3a-null male and female mice fed a HFD over eight-weeks. Female Cyp3a-null mice gained significantly less weight than their WT counterparts as early as week one and by the seventh week Cyp3a-null female mice only gained 3.13g while WT mice gained 6.13g (Fig. 1). In contrast, Cyp3a-null male mice did not show a significant change in weight compared to their WT counterparts over the full course of the study; however, Cyp3a-null male mice gained more weight (p-value = 0.06) initially and weighed more than their WT counterparts over the course of the study (Fig. 1). Differences in weight gain are irrespective of feed consumption as there are no significant differences in feed consumption between the genotypes (Suppl. File 3). Overall, gender plays a role in the Cyp3a-null mice’s response to a HFD.
Fig. 1: Gender is crucial in the response of Cyp3a-null mice to HFD treatment in comparison to WT mice.
Change in body weight of female (A) and male (B) mice during eight-weeks of HFD-treatment. Changes in body weight are also represented as area under the curve to confirm results in females (A) and males (B). Data are presented as mean ± SEM. Statistical significance was determined by Student’s t-tests (n = 7 or 8)(* p<0.05, ** p<0.01).
3.3. Cyp3a-null female mice, but not Cyp3a-null male mice, respond faster to a glucose challenge than their WT counterparts
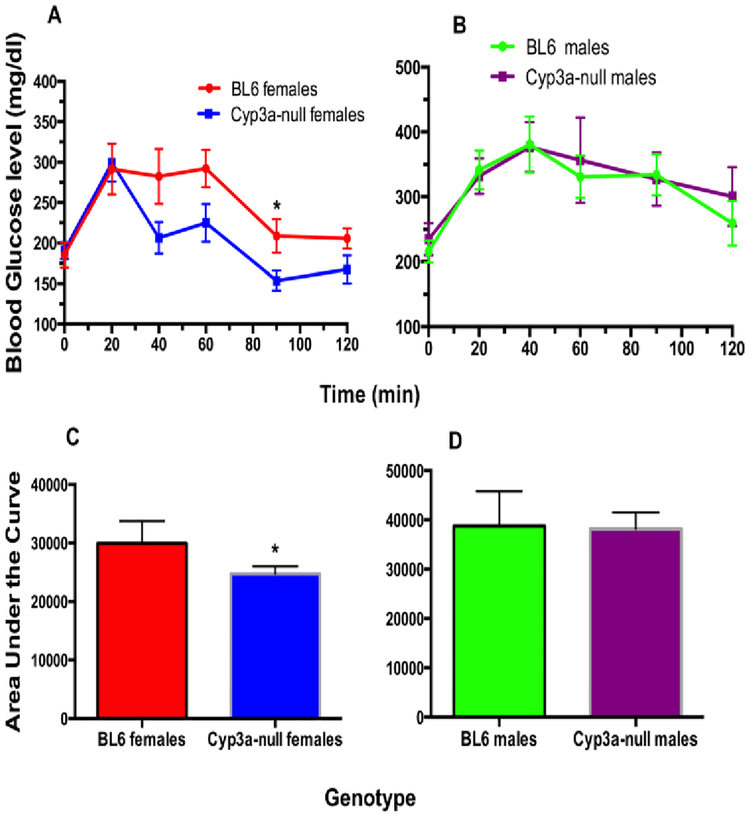
Fasting blood glucose concentrations were similar between the different genotypes after 2, 4, or 6-weeks of HFD-treatment (Suppl. File 4). A GTT was performed on the fourth week of the HFD treatment because of differences in weight gain. Interestingly, Cyp3a-null female mice recover faster than the WT mice at the 40–90 minute intervals following the initial glucose injections (p-value = 0.03 – 0.07). Area under the curve measurements confirmed the rapid recovery in the Cyp3a-null female mice (Fig. 2), and indicates higher lean mass in the Cyp3a-null female mice than their WT counterparts [52]. In contrast, there was no significant difference in glucose tolerance between the male genotypes (Fig. 2).
Fig. 2: Cyp3a-null female mice, but not Cyp3a-null male mice, fed a HFD show increased sensitivity to a glucose challenge in comparison to WT mice.
A GTT was performed as described in the Materials and Methods after 4-weeks of HFD-treatment in female (A) and male (B) mice. GTT results are also represented as area under the curve (AUC) to confirm results for females (C) and males (D). Data are presented as mean blood glucose levels ± SEM. Statistical significance was determined by Student’s t-tests (n = 7–8) (* p<0.05).
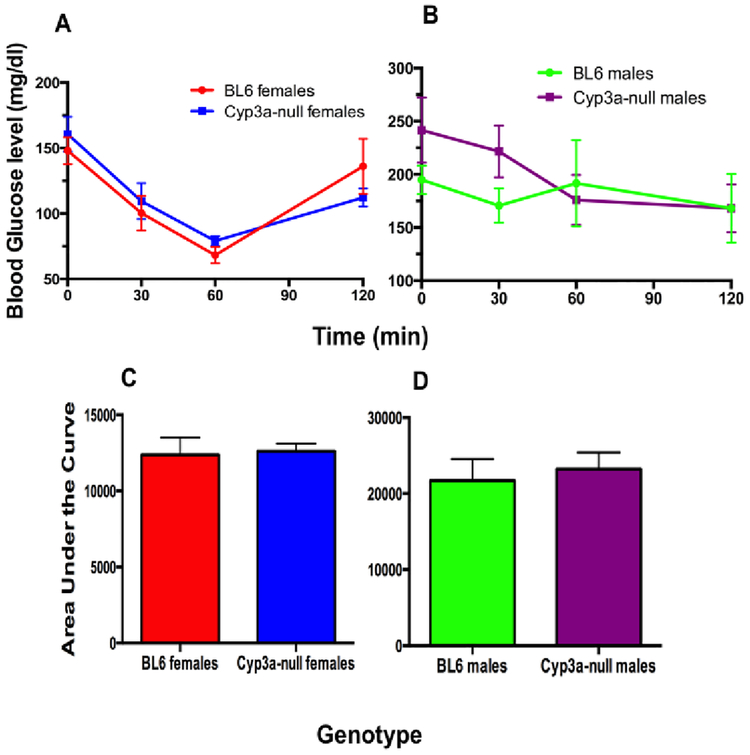
An insulin tolerance test was performed on week 6 to determine insulin sensitivity [53]. There were no significant differences in insulin tolerance between the Cyp3a-null and WT mice in either gender (Fig. 3). We also did not find any differences in fasting insulin levels between the Cyp3a-null and WT mice in either gender (Suppl. File 4). This suggests that the faster glucose recovery observed in the Cyp3a-null female mice is independent of insulin response.
Fig. 3: HFD does not significantly perturb insulin concentrations or action in Cyp3a-null mice.
An ITT was performed as described in the Materials and Methods after 6-weeks of HFD-treatment in female (A) and male (B) mice. ITT results are also represented as area under the curve (AUC) to confirm results for females (C) and males (D), and ITTs are not different between WT and Cyp3a-null mice of either gender. Data are presented as mean ± SEM. Statistical significance was determined by Student’s t-test (n = 7–8).
3.4. Differences in organ weights between WT and Cyp3a-null mice
Liver, kidney, spleen, abdominal and inguinal white adipose tissue (WAT), and testis were excised and weighed (Table 2). WAT weighed 30% more in the WT females compared to their Cyp3a-null counterparts consistent with increased weight gain in WT females; however, increased WAT was not statistically significant (p-value = 0.12). In contrast, the kidneys of Cyp3a-null females are 20% heavier than kidneys from WT females, suggesting compensation for a toxic burden such as ketosis [54]. In addition, testes weights are 29.2% lower in Cyp3a-null males than WT males fed a HFD although the decreased testes weights are primarily due to two males that showed very low testis weights. A drop in testis weights sometimes in only one testis is not uncommon in rodent HFD studies [55]. In contrast, liver weights are 8% higher in Cyp3a-null males than WT males fed a HFD and this result is consistent (Table 2).
Table 2:
Organ weights determined at the termination of study after 8 weeks of HFD treatment.
| Models | Body | Liver | Kidney | WATa | Testis |
|---|---|---|---|---|---|
| WT-F | 23.75±1.03 | 0.91±0.04 | 0.25±0.01 | 1.13±0.20 | |
| Cyp3a-null F | 23.88±1.34 | 0.88±0.04 | 0.30±0.01*** | 0.78±0.23 | |
| WT-M | 35.88±1.22 | 1.39±0.06 | 0.40±0.01 | 3.20±0.36 | 0.24±0.01 |
| Cyp3a-null M | 39.14±0.74* | 1.50±0.54* | 0.42±0.01 | 3.57±0.32 | 0.17±0.02** |
White adipose tissue (WAT)
Data represented as mean +/− SEM (n = 7 or 8). Statistical significance determined by Student’s t-test.
Indicates a p-value ≤ 0.05,
indicates a p-values ≤ 0.01, and
indicates a p-value ≤ 0.001.
3.5. Serum concentrations of testosterone, adiponectin, and β-hydroxybutyrate
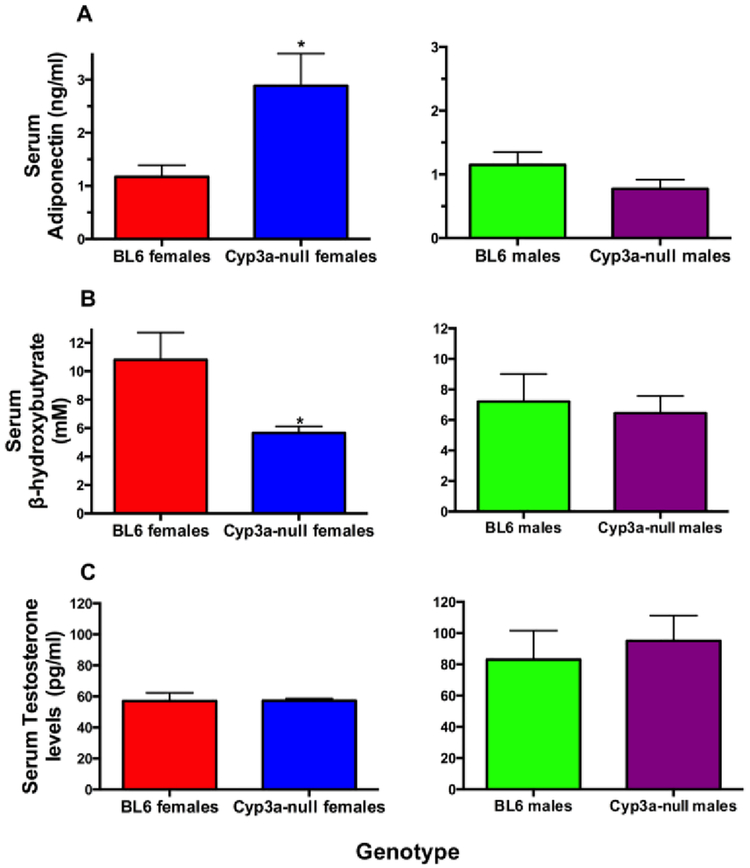
Adiponectin increases insulin sensitivity and is frequently increased in lean compared to obese individuals [56]. The leaner Cyp3a-null females have 1.7X greater serum adiponectin concentrations than WT females further demonstrating that the lower weight gain and adipose levels had physiological/endocrine effects (Fig. 4A). β-hydroxybutyrate (B-OHB) is produced along with acetoacetate from fatty acids as an alternative fuel source during fasting, low carbohydrate diets, or exercise. Because the kidney eliminates B-OHB we hypothesized that the increase in kidney weight in the Cyp3a-null females was due to an increase in B-OHB. Instead, Cyp3a-null female mice show significantly lower B-OHB concentrations (49% lower) than the WT females (Fig. 4B), indicating that the WT mice have significantly more ketosis, potentially due to increased WAT and consistent with the slower response to the glucose challenge. In contrast, we did not observe any significant changes in serum adiponectin and B-OHB levels in male mice. Furthermore, we did not observe a significant change in serum testosterone concentrations in the male mice despite smaller testes (Fig. 4C), probably because the lower testes weights were due to two outlier individuals.
Fig. 4: Serum adiponectin, β-hydroxybutyrate and testosterone in WT and Cyp3a-null mice fed a HFD.
Commercial kits were used to measure (A) adiponectin, (B) β-hydroxybutyrate and (C) testosterone in serum samples as described in the Materials and Methods. Data are presented as mean ± SEM. Statistical significance was determined by Student’s t-tests (n = 7 – 8) (* p<0.05).
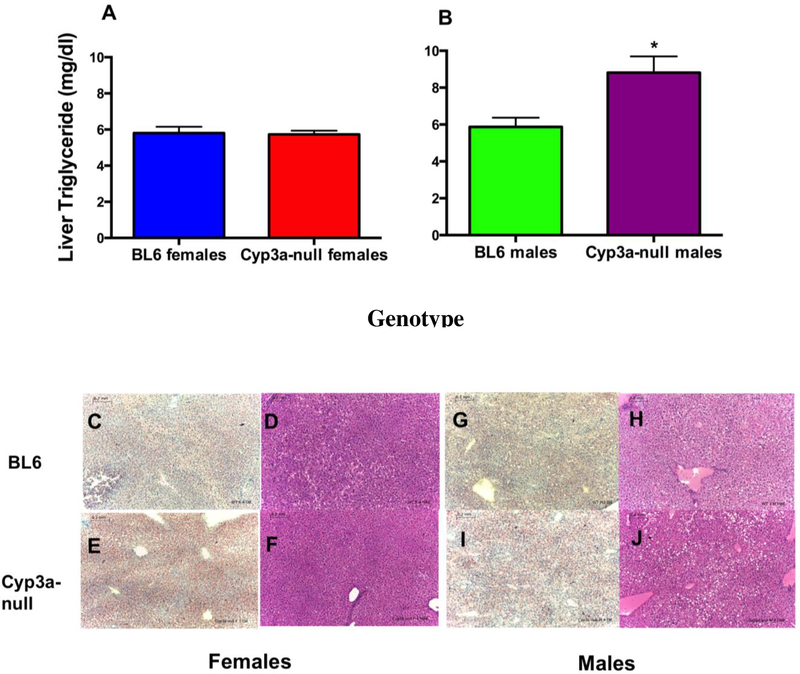
3.6. Liver triglyceride concentrations are higher and accompanied by macrovesicular steatosis in Cyp3a-null male mice
Higher adiponectin and lower WAT coupled with a faster response to a glucose challenge and lower ketones in HFD-treated Cyp3a-null female mice indicate lower liver triglycerides and steatosis, and greater feedback inhibition of β-oxidation in HFD-treated WT female mice [57]. Therefore, liver triglyceride concentrations were measured. Surprisingly, there was no significant difference in liver triglycerides between the WT and Cyp3a-null female mice (Fig. 5A).
Fig. 5: Hepatic lipid levels and histopathological changes observed in WT and Cyp3a-null mice fed a HFD.
Liver triglycerides in female (A) and male (B) mice were determined as described in the Materials and Methods. Data are represented as mean ± SEM. Statistical significance was determined by Student’s t-tests (n = 7 – 8) (* p<0.05). Histopathological changes were evaluated by H&E or Oil red O staining of liver tissues following 8-weeks of HFD treatment in WT and Cyp3a-null mice. WT (C,D) and Cyp3a-null (E,F) female liver slices stained with H&E and Oil red O, respectively. WT (G,H) and Cyp3a-null (I,J) male liver slices stained with H&E and Oil Red O, respectively. Scale 0.2 mm.
In contrast, Cyp3a-null males showed a (1.5X) increase in liver triglycerides compared to WT males (Fig. 5B) that was coupled with a 8% greater liver weight. Oil Red O and H&E staining were used to determine zonation and types of steatosis (microvesicular and macrovesicular) in the liver tissue, which is one of the significant markers for the development of metabolic syndrome [58]. Histopathology results (Fig. 5C–I) indicate mild macrovesicular steatosis without inflammation in the midzonal regions of the liver in male mice while female mice showed diffuse microvesicular steatosis without inflammation in HFD-fed mice. These changes were more visible following Oil red O staining and indicate accumulation of lipid droplets due to HFD-treatment in both the genders although in different ways. However, histopathological analysis did not associate increased steatosis with the absence of Cyp3a genes.
3.7. Phosphatidylserine and phosphatidylinositol are increased in Cyp3a-null male mice
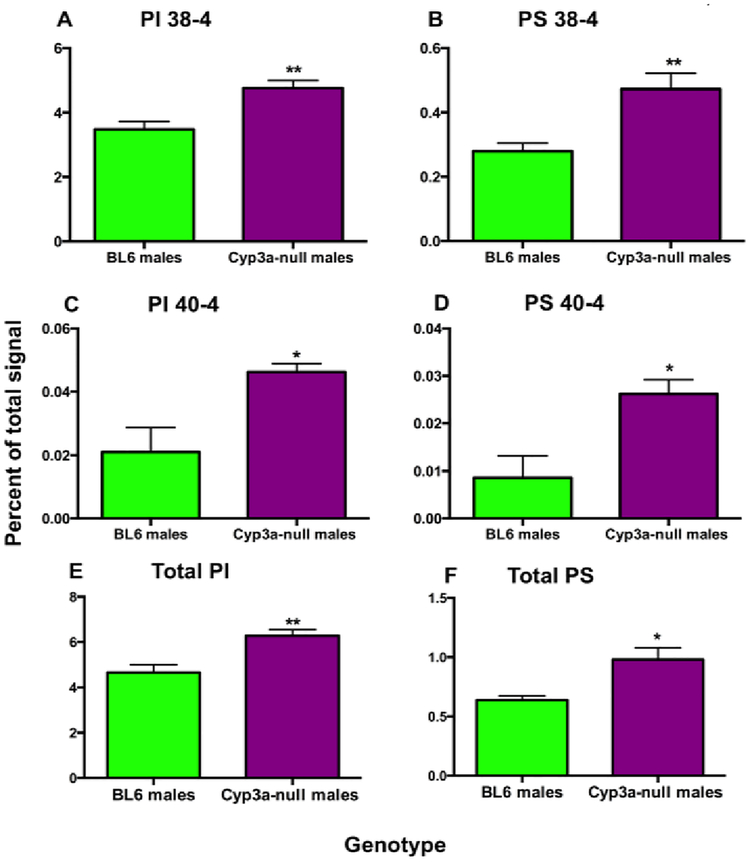
Fatty acids are responsible for the formation of lipid signaling molecules. Lipidomic analysis demonstrated a few significant changes between the lipid profiles of WT and Cyp3a-null males fed a HFD; however no differences were observed between WT and Cyp3a-null females (Table 3). Cyp3a-null males display a two-fold increase in total polar lipids as well as an increase in the concentrations of several specific lipid groups including total phosphatidylserine (PS; 2.65X), phosphatidylinositol (PI; 2.3X), sphingomyelins (SM; 2.7X), phosphatidylglycerol (PG; 2.7X), and phosphatidic acid (PA; 3.44X) in comparison to WT males (Table 3). Principle component analysis (PCA) indicates lipid species are primarily clustered by gender; not genotype with the exception of large-chain PS, some sphingomyelin, and mid-chain phosphatidylcholine (PC) species (Suppl. File 5). When normalizing to relative concentrations to look for changes in composition between WT and Cyp3a-null males, differences are observed only in PS, PI, and SM concentrations by 1.6X, 1.3X and 1.98X respectively (Suppl. File 6). The relative increases in polar lipids is primarily due to higher concentrations of long chain lipid species containing 38:4 and 40:4 in PS and PI, respectively (Fig. 6).
Table 3:
Lipidomics measured after eight weeks of HFD treatment on Cyp3a-null mice.
| FEMALES | MALES | |||
|---|---|---|---|---|
| Lipids | WT | Cyp3a-null | WT | Cyp3a-null |
| Total LysoPC | 0.775±0.240 | 0.466±0.297 | 1.083±0.239 | 1.834±0.424 |
| Total PC | 45.200±10.050 | 36.980±16.570 | 58.750±11.550 | 98.720±13.940 |
| Total SM / DSM | 3.115±0.691 | 2.272±1.217 | 2.855±0.961 | 7.739±1.398* |
| Total ePC | 1.459±0.312 | 1.317±0.509 | 2.082±0.434 | 3.359±0.517 |
| Total LysoPE | 0.217±0.061 | 0.195±0.111 | 0.358±0.087 | 0.631±0.114 |
| Total PE | 8.005±2.107 | 7.912±4.225 | 11.950±2.681 | 19.840±3.300 |
| Total ePE | 0.144±0.042 | 0.156±0.086 | 0.309±0.076 | 0.535±0.093 |
| Total PI | 2.512±0.487 | 2.348±1.220 | 3.907±0.961 | 9.007±1.437* |
| Total PS | 0.510±0.118 | 0.553±0.241 | 0.542±0.138 | 1.434±0.286* |
| Total ePS | 0.003±0.001 | 0.003±0.001 | 0.005±0.002 | 0.014±0.003* |
| Total PA | 0.021±0.003 | 0.026±0.010 | 0.025±0.004 | 0.086±0.020* |
| Total PG | 0.009±0.004 | 0.013±0.007 | 0.034±0.012 | 0.092±0.020* |
| Total lipids | 61.97±14.01 | 52.06±24.45 | 81.89±16.97 | 163.6±7.685** |
Data represented as mean signal per mg of protein +/− SEM (n = 5). Statistical significance determined by Student’s t-test.
Indicates a p-value ≤ 0.05 and
indicates a p-value ≤ 0.01.
Fig. 6: Phosphatidylinositol and phosphatidylserine are increased in Cyp3a-null male mice.
Data are represented as mean ± SEM. Statistical significance was determined by Student’s t-tests (n=5) (* p<0.05, ** p<0.01).
3.8. Changes in the expression of hepatic energy metabolism related genes in HFD-treated Cyp3a-null mice
Following HFD-treatment for 8-weeks, Cpt1a, as well as Fasn, ApoE, Pxr, and Srebp1a are down-regulated by approximately 0.5X in Cyp3a-null male mice compared to their WT counterparts (Table 4). In contrast, IL-6 and Pxr are upregulated about 2.0X in Cyp3a-null females, but not males. Fatp1 is up-regulated 2.1X in males and 2.25X in females; however only the up-regulation in males is significant. Overall, the expression data suggests a decrease in lipid oxidation and synthesis and an increase in transport in the male mice, which may explain the increase in liver triglycerides. These changes in gene expression are consistent with a decrease in AMPK activity. However, we found no significant change in AMPK phosphorylation levels between the WT and Cyp3a-null mice (Suppl. File 7). The lower PXR expression in males may also help explain minor steatosis coupled with no increase in apoptosis or inflammation [59].
Table 4:
Disregulation of energy and lipid metabolism genes in Cyp3a-null mice after eight weeks of HFD treatment.
| Genes | WT | Cyp3a-null | WT | Cyp3a-null |
|---|---|---|---|---|
| Acox | 1.00±0.111 | 0.885±0.145 | 1.00±0.140 | 0.623±0.111 |
| ApoE | 1.00±0.160 | 1.411±0.273 | 1.00±0.156 | 0.437±0.107* |
| Car | 1.00±0.116 | 1.769±0.430 | 1.00±0.198 | 0.564±0.052 |
| Cpt1a | 1.00±0.093 | 1.152±0.196 | 1.00±0.129 | 0.510±0.083** |
| Fasn | 1.00±0.152 | 0.871±0.164 | 1.00±0.184 | 0.450±0.067* |
| Fatp-1 | 1.00±0.312 | 2.259±0.637 | 1.00±0.217 | 2.088±0.416* |
| Foxa2 | 1.00±0.182 | 1.354±0.247 | 1.00±0.202 | 1.052±0.137 |
| IL-6 | 1.00±0.150 | 2.018±0.454* | 1.00±0.222 | 0.640±0.141 |
| Pepck1 | 1.00±0.193 | 1.199±0.327 | 1.00±0.298 | 0.397±0.105 |
| Pparα | 1.00±0.122 | 1.201±0.164 | 1.00±0.211 | 0.970±0.168 |
| Pparγ | 1.00±0.053 | 1.296±0.207 | 1.00±0.327 | 0.878±0.153 |
| Pxr | 1.00±0.158 | 1.919±0.386* | 1.00±0.234 | 0.407±0.075* |
| Srebp1 | 1.00±0.127 | 0.967±0.213 | 1.00±0.145 | 0.567±0.058* |
| Srebp1a | 1.00±0.254 | 1.118±0.346 | 1.00±0.188 | 0.466±0.120* |
| Srebp1c | 1.00±0.127 | 1.355±0.266 | 1.00±0.160 | 0.536±0.131 |
| Cyp2b9 | 1.00±0.137 | 1.495±0.154* | 1.00 ±0.340 | 4.250±3.585 |
| Cyp2b10 | 1.00±0.210 | 1.283±0.176 | 1.00 ±0.202 | 1.819±0.331 |
Data represented as relative mean +/− SEM (n = 7 or 8). Statistical significance determined by Student’s t-test.
Indicates a p-value ≤ 0.05 and
Indicates a p-value ≤ 0.01.
Because recent studies indicated that several Cyps are regulated by a HFD [13, 19], we also investigated the expression of several Cyps (and PXR). With the exception of the knocked out Cyp3a members, there are only a few Cyps that showed changes in gene expression and only Cyp2b protein expression in males (2.8X) was increased significantly; consistent with increases in Cyp2b9, a gene controlled by the metabolic receptors, FoxA2 and CAR, or potentially Cyp2b10, a gene controlled by the nuclear receptors CAR and PXR (Suppl. File 7) [60–63].
4. Discussion
Cyp3a-null female mice gained 50% less weight than WT female mice and Cyp3a-null male mice are slightly heavier than their WT counterparts following treatment with a HFD (diet-induced obesity) (Fig. 1). This indicates gender specific responses to the HFD and demonstrates a role of Cyp3a in the metabolism and utilization of fatty acids. Diverse gender responses might be expected as several Cyp3a members are sexually dimorphic in the liver. For example, Cyp3a41 and Cyp3a44 are both female specific [44, 62, 64] and human CYP3A4 is expressed slightly more in females than males [8, 65].
Cyp3a-null female mice reacted to a glucose challenge more rapidly than WT mice, which is consistent with their reduced weight gain and 30% lower WAT weight. In addition, plasma adiponectin concentrations are higher and serum B-OHB concentrations are lower in Cyp3a-null female mice, which is consistent with lower WAT, faster recovery from glucose, and rapid β-oxidation [66–68]. Adiponectin plays a central role as it increases hepatic glucose and fatty acid utilization, and in turn protects the liver from fatty liver disease [69].
Interestingly, resveratrol protects from alcohol-induced fatty liver disease in part by increasing fatty acid oxidation as observed through greater B-OHB coupled with enhanced adiponectin, and increased expression of Cpt1a as well as other genes associated fatty acid oxidation [70, 71]. Adiponectin also decreases lipid uptake by the liver in part by decreasing CD36 and this could provide a mechanism for compensating for a diet rich in fatty acids [72]. In addition, the Cyp3a inhibitor naringin found in grapefruit and other citrus also reduces serum B-OHB concentrations [73, 74], is associated with reduced adiposity, weight gain in mice and humans, and increased Cpt1a expression through Pparα activation coupled with Srebp-1 inhibition [75, 76]. However, Cpt1a expression was much greater in Cyp3a-null mice than WT mice prior to the HFD, but no significant difference in Cpt1a or other energy-related genes was found after a HFD in female mice. It is also possible that the liver is not the only organ in which Cyp3a plays a role in the metabolism and utilization of fatty acids in HFD-fed female mice as several of the perturbed parameters are regulated by WAT, kidney and skeletal muscle [66, 77, 78].
In contrast to females, Cyp3a-null male mice gained weight relative to WT mice; albeit a relatively small amount. In addition, Cyp3a-null male mice have increased liver lipids including a 1.5X increase in liver triglycerides (Fig. 5B) and 2X increase in total polar lipids (Table 3). Specific lipid groups (PI, PS, SM) and long chain fatty acids that are 38–40 carbons are increased in the livers of Cyp3a-null male mice relative to WT mice (Fig. 6). An increase in long-chain fatty acids is consistent with increased storage and increased metabolism [79], perturbations in the transport of long-chain fatty acids is associated with liver disease [80], and increased SM is associated with obesity [81, 82]. However, while SM is associated with obesity, PI is associated with anti-obesity effects by regulating hepatic lipid metabolism genes [83]. Therefore, most but not all lipid markers are consistent with fatty liver disease and obesity.
Furthermore, down-regulation of Cpt1a, ApoE, Fasn, Pxr, and Srebp-1 genes indicates a down-regulation of lipid metabolism associated genes while Fatp1 is up-regulated and indicates an increase in fatty acid transport genes involved in accumulation [84, 85]. These changes in gene expression are consistent with increased liver lipids [86, 87]. Interestingly, a drop in Srebp1 was also observed in steatotic HRN mice lacking all hepatic CYP activity [88], probably due to the increased retention of unsaturated fatty acids in the liver [89]. Typically, increases in free fatty acids are associated with increases in Srebp1 and Fasn [90] leading to increased Srebp1c activation that causes increased deposition of fat in the liver [91]. This suggests that there are other forces regulating the hepatic increase in triglycerides in Cyp3a-null mice or the down-regulation of Srebp is a compensatory response to the increased liver lipids. Overall, most but not all of these lipid markers are consistent with increased weight, liver triglycerides and polar lipids, and it is likely that decreased Cyp3a-mediated liver metabolism plays a role in the increased liver lipids through changes in metabolism.
In addition to xenobiotics, CYP3A isoforms are involved in the metabolism of steroids, bile acids, and unsaturated fatty acids [30, 49]. CYP3A4 has been shown to metabolize fatty acids such as arachidonic acid to generate epoxyeicosatrienoic acids (EETs) that have anti-inflammatory effects [92]. High-fat diets generate an endogenous fatty acid molecule, anandamide that can be converted to arachidonic acid by fatty acid amide hydrolase that can be further epoxygenated by Cyp3a to EETs. Anandamide and Cyp3a-mediated metabolites [36] bind cannabinoid receptors such as cannabinoid receptor 1 (CB1) in order to alter nociception, cognition, memory and orexigenic effects in the peripheral and central nervous system; however, there were no differences in food consumption between WT and Cyp3a-null mice. For example, CB1 regulates adiponectin activity in obese mice, and Rimonabant, a CB1 inverse agonist causes weight loss, increases Cpt1a, while decreasing Fasn in WT male mice, but not adiponectin-null mice [72]. Cannabinoid receptor 2 (CB2) is associated with immunosuppression and agonists reduce obesity [93]. Given that the fatty acid, anandamide and its Cyp3a metabolites are potent activators of CB1/2, it is possible that the loss of Cyp3a plays a role in perturbing fatty acid metabolism and epoxidation, including the production of anandamide and other fatty acid derivatives, that typically would act as signaling molecules [94, 95], and reduce obesity.
However, this does not explain why males and females react differently except for the possibility that the gender specific Cyp3a members in mice differentially metabolize steroids, arachidonic acid, anandamide, and other fatty acids. Murine 6β-hydroxylation of testosterone is significant higher in females than males demonstrating the higher Cyp3a activity in females than males [46]. Of course this difference in 6β-hydroxylation is lost in Cyp3a-null mice, indicating the differences between males and females for obesity and NAFLD is not directly caused by Cyp3a metabolism of steroids, but may be due to the metabolic differences that lead to sexually dimorphic differences in Cyp3a expression. It is possible that there are distinct differences in Cyp3a-mediated fatty acid metabolism in males compared to females as females express different Cyp3a isoforms, primarily Cyp3a41 and Cyp3a44. However, the substrate profiles for these murine isoforms are not known and that includes there metabolism of anandamide, arachidonic acid, and other PUFAs.
Gender differences in PXR regulation and feedback within Cyp3a-null mice may also play a role in the sexually dimorphism of weight gain. For example, mPXR is associated with increased weight gain and hPXR with protection from weight gain in male mice with increased Cpt1a and PPARα levels [14]. In contrast, hPXR activation/presence causes greater weight gain than mPXR in female mice [39]. Because PXR positively regulates Cyp3a, the PXR studies are consistent with our in which loss of Cyp3a increased obesity in males and protected females. Therefore, while we don’t know the exact mechanism of the sexually dimorphic weight gain in Cyp3a-null mice, it appears that differential PXR/Cyp3a activation and subsequent regulation of gene expression plays a role.
In summary, the loss of seven Cyp3a genes lead to sexual dimorphic changes during the eight weeks of high fat diet treatment with Cyp3a-null female mice showing a healthier acclimation to a high fat diet through decreased weight gain, higher adiponectin, lower B-OHB levels, and a better response to glucose; while the Cyp3a-null males succumb to increased fatty liver including slightly increased weight, higher hepatic polar lipids and triglycerides, and greater liver weight. Overall, the changes in HFD-induced health parameters in females are consistent with other studies showing that compounds that inhibit Cyp3a or perturb Cyp3a-mediated fatty acid metabolism protect from obesity [74, 93], and the increases in weight in males are moderate. Interestingly, PXR activation and subsequent CYP3A induction is associated with weight gain in females [39]. Ultimately, inhibition of CYP activity by environmental compounds or drugs may alter hepatic and non-hepatic lipid levels with different effects in males than females in which males may present increased steatosis and females show a mitigation of the effects of a HFD.
Supplementary Material
Suppl. File 1: Timeline of the procedures performed during the eight weeks of High-Fat Diet (HFD) treatment.
Suppl. File 2: Primer sequences used to determine changes in gene expression by qPCR.
Suppl. File 3: Feed consumption rate of female (A) and male (B) WT and Cyp3a-null mice. Feed consumed was measured every other day during the entire eight-week period. Data are presented as mean ± SEM per week. There are no differences in feed consumption between genotypes. Statistical significance was determined by Student’s t-tests (n = 7 – 8).
Suppl. File 4: Fasting blood glucose and insulin levels. Blood glucose concentrations were determined during weeks 2,4 and 6 (A-C) as described in Materials and Methods. Blood insulin concentrations were determined using ELISA (D) kit purchased from EMD Millipore Corporation (EMB Millipore Corporation, Billerica MA USA). Mice were fasted 4–5hr during weeks 2,4 and 6 and fasting plasma glucose levels determined each time by tail bleed and serum fasting insulin determined using blood collected by tail bleed during week 6. Data are presented as mean blood plasma ± SEM. Statistical significance was determined by Student’s t-tests (n = 7 – 8).
Suppl. File 5: Principal component analysis (PCA) indicates gender differences in hepatic lipid species. Hepatic lipid species are primarily influenced by gender not the lack of Cyp3a with the exception of a few SM, PS and PI species.
Suppl. File 6: Relative change in polar lipid concentrations after eight-weeks of HFD treatment on Cyp3a-null and WT mice.
Suppl. File 7: Compensatory changes in hepatic CYP gene expression after eight weeks of HFD treatment in Cyp3a-null mice. Immunoblots were performed and quantified as described previously with β -actin as the reference protein [62]. β-actin was used as the reference protein (Sigma Aldrich, St.Louis MO).
Highlights:
Cyp3a-null female mice are resistant to the effects of a high-fat diet
Cyp3a-null females show improved metabolic responses, including glucose tolerance
In contrast, Cyp3a-null male mice show increased liver triglycerides
Gene expression changes indicate decreased lipid metabolism and increased uptake
Acknowledgements
This work was supported by NIEHS grant R15ES017321
Abbreviations:
- Acox
Acyl-CoA oxidase
- ApoE
Apolipoprotein E
- Cpt1a
Carnitine palmitoyl transferase 1 a
- CYP
Cytochrome P 450
- EET
Epoxyeicosatrienoic acid
- Fabp4
Fatty acid binding protein 4
- Fasn
Fatty acid synthase
- Fatp
Fatty acid transporter protein
- Foxa2
Forkhead box protein A2
- HETE
Hydroxyeicosatetraenoic acid
- HFD
High fat diet
- HODE
Hydroxyoctadecadienoic acid
- Pepck1
Phosphoenolpyruvate carboxykinase 1
- Pparα
Peroxisome proliferator activated receptor alpha
- Pparγ
Peroxisome proliferator activated receptor gamma
- PXR
Pregnane X receptor
- Srebp1
Sterol regulatory element-binding protein 1
- WT
Wildtype
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare
References
- [1].Janesick A, Blumberg B, Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity, Birth Defects Res C Embryo Today, 93 (2011) 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization, Global Database on Body Mass Index, http://apps.who.int/bmi/index.jsp, 2011.
- [3].Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN, Obesity prevalence from a European perspective: a systematic review, BMC Public Health, 8 (2008) 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sharp D, Environmental toxins, a potential risk factor for diabetes among Canadian Aboriginals, Int J Circumpolar Health, 68 (2009) 316–326. [DOI] [PubMed] [Google Scholar]
- [5].LeBlanc GA, Norris DO, Kloas W, Kullman SW, Baldwin WS, Greally JM, Detailed Review Paper on the State of the Science on Novel In Vitro and In Vivo Screening and Testing Methods and Endpoints for Evaluating Endocdrine Disruptors Series on Testing & Assessment: No. 178, Organisation for Economic Co-operation and Development, Paris, 2012, pp. 213. [Google Scholar]
- [6].Grun F, Blumberg B, Minireview: the case for obesogens, Mol Endocrinol, 23 (2009) 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baillie-Hamilton PF, Chemical toxins: a hypothesis to explain the global obesity epidemic, J Altern Complement Med, 8 (2002) 185–192. [DOI] [PubMed] [Google Scholar]
- [8].Zanger UM, Schwab M, Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation, Pharmacol Ther, 138 (1) (2013) 103–141. [DOI] [PubMed] [Google Scholar]
- [9].Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR, Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos, Drug Metab Dispos, 35 (2007) 189–193. [DOI] [PubMed] [Google Scholar]
- [10].Waxman DJ, Interactions of hepatic cytochromes P-450 with steroid hormones: Regioselectivity and stereoselectivity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression, Biochem Pharmcol, 37 (1988) 71–84. [DOI] [PubMed] [Google Scholar]
- [11].Arnold C, Konkel A, Fischer R, Schunck WH, Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids, Pharmacol Rep, 62(3) (2010) 536–547. [DOI] [PubMed] [Google Scholar]
- [12].Yoshinari K, Takagi S, Sugatani J, Miwa M, Changes in the expression of cytochromes P450 and nuclear receptors in the liver of genetically diabetic db/db mice, Biol Pharm Bull, 29 (2006) 1634–1638. [DOI] [PubMed] [Google Scholar]
- [13].Deol P, Evans JR, Dhahbi J, Chellappa K, Han DS, Spindler S, Sladek FM, Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver, PLoS One, 10(7) (2015) e0132672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spruiell K, Richardson RM, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA, Role of pregnane X receptor in obesity and glucose homeostasis in male mice, J Biol Chem, 289 (2014) 3244–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, Moore DD, Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease, Proc Natl Acad Sci U S A, 106 (2009) 18831–18836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tzameli I, Pissios P, Schuetz EG, Moore DD, The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR, Mol Cell Biol, 20 (2000) 2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baldwin WS, Roling JA, A concentration addition model for the activation of the constitutive androstane receptor by xenobiotic mixtures, Toxicol Sci, 107 (2009) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM, An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway, Cell, 92 (1998) 73–82. [DOI] [PubMed] [Google Scholar]
- [19].Finn RD, Henderson CJ, Scott CL, Wolf CR, Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway, Biochem J, 417 (2009) 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ma Y, Liu D, Activation of pregnane X receptor by pregnenolene 16 alpha carbonitrile prevents high-fat diet-induced obesity in AKR/J mice, PLoS One, 7(6) (2012) e38734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dussault I, Yoo H-D, Lin M, Wang E, Fan M, Batta A, Salen G, Erickson SK, Forman BM, Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance, Proc Natl Acad Sci USA, 100 (2003) 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD, The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism, Nature, 407 (2000) 920–923. [DOI] [PubMed] [Google Scholar]
- [23].Hernandez JP, Mota LC, Baldwin WS, Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation, Curr Pharmacog Personal Med, 7 (2009) 81–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dehal SS, Kupfer D, Metabolism of the proestrogenic pesticide methoxychlor by hepatic P450 monooxygenases in rats and humans. Dual pathways involving novel ortho ring-hydroxylation by CYP2B, Drug Metab Dispos, 22 (1994) 937–946. [PubMed] [Google Scholar]
- [25].Grun F, Blumberg B, Endocrine disrupters as obesogens, Mol Cell Endocrinol, 304 (2009) 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Auguet T, Berlanga A, Guiu-Jurado E, Martinez S, Porras JA, Aragones G, Sabench F, Hernandez M, Aguilar C, Sirvent JJ, Castillo DD, Richart C, Altered fatty acid metabolism-related gene expression in liver from morbidly obese women with non-alcoholic fatty liver disease, In J of Mol Med, 15 (2014) 22173–22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Patoine D, Levac X, Pilote S, Drolet B, Simrad C, Decreased CYP3A4 expression and activity in guinea pig models of diet-induced metabolic syndrome: is fatty liver infiltration involved?, Drug Metab Dispos, 41 (2013) 952–957. [DOI] [PubMed] [Google Scholar]
- [28].Dostalek M, Court MH, Yan B, Akhlaghi F, Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus, Br. J. Pharmacol, 163(5) (2011) 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Patoine D, Petit M, Pilote S, Picard F, Drolet B, Simrad C, Modulation of Cyp3a expression and activity in mice models of type 1 and type 2 diabetes, Pharmacol Res Perspect., 2 (2014) e00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guengerich FP, Update information on human P450s., Drug Metab Dispos, 34 (2002) 7–15. [DOI] [PubMed] [Google Scholar]
- [31].Bylund J, Kunz T, Valmsen K, Oliw EH, Cytochromes P450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with rat liver microsomes, J Pharmacol Exp Ther, 284 (1998) 51–60. [PubMed] [Google Scholar]
- [32].Ayajiki K, Okamura T, Fujioka H, Imaoka S, Funae Y, Toda N, Involvement of CYP3A-derived arachidonic acid metabolite(s) in responses to endothelium-derived K+ channel opening substance in monkey lingual artery, Br J Pharmacol, 128(3) (1999) 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ruparel S, Hargreaves KM, Eskander M, Rowan S, de Almeida JF, Roman L, Henry MA, Oxidized linoleic acid metabolite-cytochrome P450 system (OLAM-CYP) is active in biopsy samples from patients with inflammatory dental pain, Pain, 154 (2013) 2363–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Snider NT, Kornilov AM, Kent UM, Hollenberg PF, Anadamide metabolism by human liver and kidney microsomal cytochrome p450 enzymes to form hydroxyeicosatetrenoic and epoxyeicosatrienoic acid ethanolamides, J Pharm Exp Ther, 321 (2007) 590–597. [DOI] [PubMed] [Google Scholar]
- [35].Snider NT, Walker VJ, Hollenberg PF, Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications, Pharmacol. Rev, 62 (2010) 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pratt-Hyatt M, Zhang H, Snider NT, Hollenberg PF, Effects of a commonly occuring genetic polymorphism of human CYP3A4 (I118V) on the metabolism of anandamide, Drug Metab Dispos, 38 (2010) 2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wojnowski L, Kamdem LK, Clinical implications of CYP3A polymorphisms, Expert Opin Drug Metab Toxicol, 2 (2006) 171–182. [DOI] [PubMed] [Google Scholar]
- [38].Won CS, Oberlies NH, Paine MF, Mechanisms underlying food-drug interactions: inhibition of intestinal metabolism and transport, Pharmacol Ther, 136 (2012) 186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Spruiell K, Jones DZ, Cullen JM, Awumey EM, Gonzalez FJ, Gyamfi MA, Role of human pregnane X receptor in high fat diet-induced obesity in pre-menopausal female mice, Biochem Pharmacol, 89(3) (2014) 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Scheer N, Ross J, Kapelyukh Y, Rode A, Wolf CR, Invivo responses of the human and murine pregnane x receptor to dexamethasone in mice, Drug Metab Dispos, 38(7) (2010) 1046–1053. [DOI] [PubMed] [Google Scholar]
- [41].Hasegawa M, Kapelyukh Y, Tahara H, Seibler J, Rode A, Krueger S, Lee DN, Wolf CR, Scheer N, Quantitative prediction of human pregnane X receptor and cytochrome P450 3A4 mediated drug-drug interaction in a novel multiple humanized mouse line., Mol.Pharm, 80(3) (2011) 518–528. [DOI] [PubMed] [Google Scholar]
- [42].Van der Hoeven TA, Coon MJ, Preparation and properties of partially purified cytochrome P450 and NADPH-cytochrome P450 reductase from rabbit liver microsomes, J Biol Chem, 249 (1974) 6302–6310. [PubMed] [Google Scholar]
- [43].Damiri B, Holle E, Yu X, Baldwin WS, Lentiviral-mediated RNAi knockdown yields a novel mouse model for studying Cyp2b function, Toxicol Sci, 125 (2012) 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hernandez JP, Chapman LM, Kretschmer XC, Baldwin WS, Gender Specific Induction of Cytochrome P450s in Nonylphenol-Treated FVB/NJ Mice, Toxicol Appl Pharmacol, 216 (2006) 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Muller PY, Janovjak H, Miserez AR, Dobbie Z, Processing of gene expression data generated by quantitative real-time RT-PCR, Biotechniques, 32 (2002) 1372–1379. [PubMed] [Google Scholar]
- [46].Kumar R, Mota LC, Litoff EJ, Rooney JP, Boswell WT, Courter E, Henderson CM, Hernandez JP, Corton JC, Moore DD, Baldwin WS, Compensatory changes in CYP expression in three different toxicology mouse models: CAR-null, Cyp3a-null, and Cyp2b9/10/13-null mice, PLoS One, 12(3) (2017) e0174355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Isaac G, Jeannotte R, Esch SW, Welti R, New mass-spectrometry-based strategies for lipids, Genetic Engineering:principles and methods, 28 (2007) 129–157. [DOI] [PubMed] [Google Scholar]
- [48].Sengupta N, Gerard PD, Baldwin WS, Perturbations in polar lipids, starvation survival and reproduction following exposure to unsaturated fatty acids or environmental toxicants in Daphnia magna, Chemosphere, 144 (2016) 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hafner M, Rezen T, Rozman D, Regulation of hepatic cytochromes p450 by lipids and cholesterol, Curr Drug Metab, 12 (2011) 173–185. [DOI] [PubMed] [Google Scholar]
- [50].Rakshandehroo M, Hooiveld G, Muller M, Kersten S, Comparative analysis of Gene regulation by the transcription factor ppar-alpha between mouse and human, PLoS One, 4(8) (2009) e6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ayala-Sumuano J-T, Velez-delValle C, Beltran-Langarica A, Marsch-Moreno M, Cerbon-Solorzano J, Kuri-Harcuch W, Srebf1a is a key regulator of transcriptional control for adipogenesis, Sci. Rep, 1 (2011) 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH, NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse, Am J Physiol Endocrinol Metab, 297 (2009) E849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM, Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models, J Endocrinol, 222 (2014) G13–G25. [DOI] [PubMed] [Google Scholar]
- [54].Adrogue HJ, Eknoyan G, Suki WK, Diabetic ketoacidosis: role of the kidney in the acid-base homeostasis re-evaluated, Kidney Int, 25 (1984) 591–598. [DOI] [PubMed] [Google Scholar]
- [55].Datar J, Hunde AR, Kim WK, Taylor CG, Zahradka P, Suh M, Lipid Metabolism is Closely Associated with Normal Testicular Growth Based on Global Transcriptome Profiles in Normal and Underdeveloped Testis of Obese Zucker (fa/fa) Rats, Lipids, (2017). [DOI] [PubMed] [Google Scholar]
- [56].Nigro E, Scudiero O, Monaco M, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A, New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases, BioMed Research International, 2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xu C, Wang Z, Liu G, Li X, XIe G, Xia C, Zhang HY, Metabolic characteristics of the liver of diary cows during ketosis based on comparative proteomics, Asian-Aust. J. Anim. Sci, 21 (2008) 1003–1010. [Google Scholar]
- [58].Levene AP, Kudo HK, Armstrong MJ, Thursz MR, Gedroyc WM, Anstee QM, Goldin RD, Quantifying hepatic steatosis-more than meets the eye, Histopathology, 60 (2012) 971–981. [DOI] [PubMed] [Google Scholar]
- [59].Gautam S, Singh P, Singh M, Roy S, Rawat JK, Yadav RK, Devi U, Gupta PS, Saraf SA, Kaithwas G, Rifaximin, a pregnane X receptor (PXR) activator regulates apoptosis in a murine model of breast cancer, RSC Adv., 8 (2018) 3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hashita T, Sakuma T, Akada M, Nakajima A, Yamahara H, Ito S, Takesako H, Nemoto N, Forkhead box A2-mediated regulation of female-predominant expression of the mouse Cyp2b9 gene, Drug Metab Dispos, 36 (2008) 1080–1087. [DOI] [PubMed] [Google Scholar]
- [61].Jarukamjorn K, Sakuma T, Yamamoto M, Ohara A, Nemoto N, Sex-associated expression of mouse hepatic and renal CYP2B enzymes by glucocorticoid hormones, Biochem Pharmacol, 62 (2001) 161–169. [DOI] [PubMed] [Google Scholar]
- [62].Hernandez JP, Mota LC, Huang W, Moore DD, Baldwin WS, Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR), Toxicology, 256 (2009) 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mota LC, Barfield C, Hernandez JP, Baldwin WS, Nonylphenol-mediated CYP induction is PXR-dependent: The use of humanized mice and human hepatocytes suggests that hPXR is less sensitive than mouse PXR to nonylphenol treatment, Toxicol Appl Pharmacol, 252 (2011) 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Anakk S, Huang W, Staudinger JL, Tan K, Cole TJ, Moore DD, Strobel HW, Gender dictates the nuclear receptor mediated regulation of CYP3A44, Drug Metab Dispos, 35 (2006) 36–42. [DOI] [PubMed] [Google Scholar]
- [65].Wolbold R, Klein K, Burk O, Nussler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM, Sex is a major determinant of CYP3A4 expression in human liver, Hepatology, 38 (2003) 978–988. [DOI] [PubMed] [Google Scholar]
- [66].Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G, Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells, Diabetologia, 48(1) (2005) 132–139. [DOI] [PubMed] [Google Scholar]
- [67].Sweeney G, Garg RR, Ceddia RB, Li D, Ishiki M, Somwar R, Foster l.J., Neilson PO, Prestwich GD, Rudich A, Klip A, Intracellular delivery of phosphatidylinositol (3,4,5)-triphosphate causes incorporation of glucose transporter 4 into the plasma membrane of muscle and fat cells without increasing glucose uptake, J Biol Chem, 279(31) (2004) 32233–32242. [DOI] [PubMed] [Google Scholar]
- [68].Meidenbauer JJ, Ta N, Seyfried TN, Influence of a ketogenic diet, fish-oil and calorie restriction on plasma metabolites and lipids in C57BL/6J mice, Nutr Metab, (2014) 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K, Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome, J Clin Invest, 116 (2006) 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yao R, Yasuoka A, Kamei A, Ushiama S, Kitagawa Y, Rogi T, Shibata H, Abe K, Misaka T, Nuclear receptor-medited alleviation of alcoholic fatty liver by polyphenols contained in alcoholic beverages, PLoS One, 9(2) (2014) e87142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ajmo JM, Liang X, Rogers CQ, Pennock B, You M, Resveratol alleviates alcoholic fatty liver in mice, Am J Physiol Gastrointest Liver Physiol, 295(4) (2008) G833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tarn J, Godlewski G, Earley BJ, Zhou L, Jourdan T, Szanda G, Cinar R, Kunos G, Role of adiponectin in the metabolic effects of cannabinoid type 1 receptor blockade in mice with diet-induced obesity., Am J Physiol Endocrinol Metab, 306(4) (2014) E457–E468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kwatra D, Buddha B, Vadlapudi AD, Vadlapatla RK, Pal D, Mitra AK, Transfected MDCK cell line with enhanced expression of Cyp3a4 and P-glycoprotein as a model to study their role in drug transport and metabolism, Mol. Pharmaceutics, 9(7) (2012) 1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Murunga AN, Miruka DO, Driver C, Nkomo FS, Cobongela SZZ, Owira PMO, Grapefruit derived flavonoid naringin improves ketoacidosis and lipid peroxidation in type 1 diabetes rat model, PLoS One, 11(4) (2016) e0153241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD, Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action, Adv Nutr, 5(4) (2014) 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Murphy MM, Barraj LM, Rampersaud GC, Consumption of grapefruit is associated with higher nutrient intakes and diet quality among adults, and more favorable anthropometrics in women, NHANES 2003–2008, Food Nutr Res, 58 (2014) 22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Auguet T, Guiu-Jurado E, Berlanga A, Terra X, Martinez S, Porras JA, Ceausu A, Sabench F, Hernandez M, Aguilar C, Sirvent JJ, Castillo DD, Richart C, Downregulation of lipogenesis and fatty acid oxidation in the subcutaneous adipose tissue of morbidly obese women., Obesity, 22 (2014) 2032–2038. [DOI] [PubMed] [Google Scholar]
- [78].Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Jr CS, Huang LS, Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis, J Biol Chem, 283(19) (2008) 13087–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Montgomery MK, Osborne B, Brown SHJ, Small L, Mitchell TW, Cooney GJ, Turner N, Contrasting metabolic effects of medium-versus long chain fatty acids in skeletal muscle, J of Lipid Res, 54 (2013) 3322–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Odaib AA, Shneider BL, Bennett MJ, Pober BR, Reyes-Mugica M, Friedman AL, Suchy FJ, Rinaldo P, A defect in the transport of long-chain fatty acids associated with acute liver failure, N Engl J Med, 339 (1998) 1752–1757. [DOI] [PubMed] [Google Scholar]
- [81].Choi S, Snider AJ, Sphingolipids in high fat diet and obesity-related diseases, Mediators Inflamm, 2015 (2015) 520618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Iqbal J, Walsh M, Hammad SM, Hussain MM, Sphingolipids and lipoproteins in health and metabolic disorders, Trends Endocrinol Metab, 28 (2017) 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shimizu K, Ida T, Tsutsui H, Asai T, Otsubo K, Oku N, Anti-obesity effect of phosphatidylinositol on diet-induced obesity in mice, J. Agric. Food Chem, 58 (2010) 11218–11225. [DOI] [PubMed] [Google Scholar]
- [84].Xie Z, Houkai L, Wang K, Lin J, wang Q, Zhao G, Jia W, Zhang Q, Analysis of transcriptome and metabolome profiles alterations in fatty liver induced by high-fat diet in rat, Metabolism, 59 (2010) 554–560. [DOI] [PubMed] [Google Scholar]
- [85].Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Takayanagi R, Nakamuta M, Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease, In J of Mol Med, 20 (2007) 351–358. [PubMed] [Google Scholar]
- [86].Vallim T, Salter AM, Regulation of hepatic gene expression by saturated fatty acids, Prostaglandins Leukot Essent Fatty Acids, 82 (2010) 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Anstee QM, Goldin RD, Mouse models in non-alcoholic fatty liver disease and steatohepatitis research, Int J Exp Pathol, 87 (2006) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kishi R, Sata F, Katakura Y, Wang R-S, Nakajima T, Effects of pregnancy, age and sex in the metabolism of styrene in rat liver in relation to the regulation of cytochrome P450 enzymes, J Occup Health, 47 (2005) 49–55. [DOI] [PubMed] [Google Scholar]
- [89].Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS, Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells, J Biol Chem, 276(6) (2001) 4365–4372. [DOI] [PubMed] [Google Scholar]
- [90].Zhang Y, Lei T, Huang JF, Wang SB, Zhou LL, Yang ZQ, Chen XD, The link between fibroblast growth factor 21 and sterol regulatory element binding protein 1c during lipogenesis in hepatocytes, Mol Cell Endocrinol, 342 (2011) 41–47. [DOI] [PubMed] [Google Scholar]
- [91].Knebel B, Haas J, Hartwig S, Jacob S, Kollmer C, Nitzgen U, Muller-Wieland D, Kotzka J, Liver-specific expression of transcriptionally active SREBP-1c is associated with fatty liver and increased visceral fat mass, PLoS One, 7(2) (2012) e31812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thompson SJ, Askari A, Bishop-Bailey D, Anti-inflammatory effects of Epoxyeicosatrienoic acids, International Journal of Vascular Medicine, 2012 (2012) 605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhang X, Gao S, Niu J, Li P, Deng J, Xu S, Wang Z, Wang W, Kong D, Li C, Cannabinoid 2 Receptor agonist Improves Systemic Sensitivity to Insulin in High-Fat Diet/Streptozotocin-Induced Diabetic Mice, Cell Physiol Biochem, 40(5) (2016) 1175–1185. [DOI] [PubMed] [Google Scholar]
- [94].Brizzi A, Cascio MG, Frosini M, Ligresti A, Aiello F, Biotti I, Brizzi V, Pertwee RG, Corelli F, Di Marzo V, Resorcinol-sn-glycerol derivatives: novel 2-arachidonoglycerol mimetics endowed with high affinity and selectivity for cannabinoid type 1 receptor, J Med Chem, 54(24) (2011) 8278–8288. [DOI] [PubMed] [Google Scholar]
- [95].Snider NT, Nast JA, Tesmer L, Hollenberg PF, A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist, Mol Pharmacol, 75(4) (2009) 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. File 1: Timeline of the procedures performed during the eight weeks of High-Fat Diet (HFD) treatment.
Suppl. File 2: Primer sequences used to determine changes in gene expression by qPCR.
Suppl. File 3: Feed consumption rate of female (A) and male (B) WT and Cyp3a-null mice. Feed consumed was measured every other day during the entire eight-week period. Data are presented as mean ± SEM per week. There are no differences in feed consumption between genotypes. Statistical significance was determined by Student’s t-tests (n = 7 – 8).
Suppl. File 4: Fasting blood glucose and insulin levels. Blood glucose concentrations were determined during weeks 2,4 and 6 (A-C) as described in Materials and Methods. Blood insulin concentrations were determined using ELISA (D) kit purchased from EMD Millipore Corporation (EMB Millipore Corporation, Billerica MA USA). Mice were fasted 4–5hr during weeks 2,4 and 6 and fasting plasma glucose levels determined each time by tail bleed and serum fasting insulin determined using blood collected by tail bleed during week 6. Data are presented as mean blood plasma ± SEM. Statistical significance was determined by Student’s t-tests (n = 7 – 8).
Suppl. File 5: Principal component analysis (PCA) indicates gender differences in hepatic lipid species. Hepatic lipid species are primarily influenced by gender not the lack of Cyp3a with the exception of a few SM, PS and PI species.
Suppl. File 6: Relative change in polar lipid concentrations after eight-weeks of HFD treatment on Cyp3a-null and WT mice.
Suppl. File 7: Compensatory changes in hepatic CYP gene expression after eight weeks of HFD treatment in Cyp3a-null mice. Immunoblots were performed and quantified as described previously with β -actin as the reference protein [62]. β-actin was used as the reference protein (Sigma Aldrich, St.Louis MO).