Abstract
Emerging data indicate that the nervous system plays an important role in carcinogenesis. However, more studies are required to help further elucidate the mechanisms involved in the neural regulation of carcinogenesis. Some recent findings describing the neural regulatory mechanisms of action in prostate cancer, pancreatic cancer and hepatocellular carcinoma are discussed, with a focus on the sympathetic, parasympathetic, and sensory neuronal elements of the nervous system. Norepinephrine, which is released by the sympathetic nervous system and binds to the beta-adrenergic receptor, regulates cellular responses in both normal and tumor cells. It has also been shown that the destruction of sensory neurons can prevent or at least slow pancreatic cancer. Cortisol, the main stress hormone, is also discussed and how it could potentially be involved in hepatocellular carcinoma development. The importance of studying other signaling molecules in the nervous system, such as oxytocin and its receptor, the oxytocin receptor, and how they might be involved in carcinogenesis when aberrantly expressed is highlighted. This is an area of study which clearly needs further investigation. A clearer understanding of the detailed mechanisms of how the nervous system is involved in carcinogenesis could potentially aid in the identification of novel biomarkers and development of novel preventative and therapeutic strategies in various cancers.
Keywords: Neural regulation, Prostate, Pancreatic cancer, Hepatocellular carcinoma, Oxytocin, Oxytocin receptor
Core tip: Increasing evidence points to the importance of neural regulatory mechanisms in carcinogenesis. However, these mechanisms are not fully understood. A better understanding of these mechanisms could lead to prevention, early detection, and novel therapeutic strategies in various cancers. Consequently, this area of study warrants further investigation.
INTRODUCTION
The nervous system plays an important role in maintaining homeostasis in peripheral organs by facilitating cross-talk between these organs and the brain. Emerging data from several pre-clinical and clinical studies have suggested that this neural regulation is involved in cancer progression and therapeutic resistance in many peripheral organs when it gets altered[1]. Additionally, psychosocial studies indicate that there are alterations observed in brain activities of neuromediators and neuroendocrine hormones in patients with solid tumors[1-3].
The sympathetic and parasympathetic divisions of the autonomic nervous system directly innervate most distal organs and facilitate tissue homeostasis in them by releasing neurotransmitters such as catecholamines and acetylcholine (Ach). Both the sympathetic and parasympathetic divisions of the autonomic nervous system have been shown to regulate tumor cell growth, migration, and invasiveness. When the sympathetic nervous system (SNS), which mediates the flight-or-flight stress responses gets activated, it releases the neurotransmitter, norepinephrine (NE) via its nerve fibers[4,5]. NE and cortisol (the major stress hormone) bind to the beta-adrenergic receptor or the intracellular glucocorticoid receptor, respectively, to trigger cellular responses[6]. Cortisol release has been linked to the development and progression of various cancers[7-11]. Ach can bind to nicotinic and muscarinic receptors which are expressed on tumor and stromal cells in the tumor microenvironment[1,12].
While more is known about the emerging role of the nervous system in cancer progression, fewer studies have been done on elucidating its role in cancer initiation.
CURRENT FINDINGS
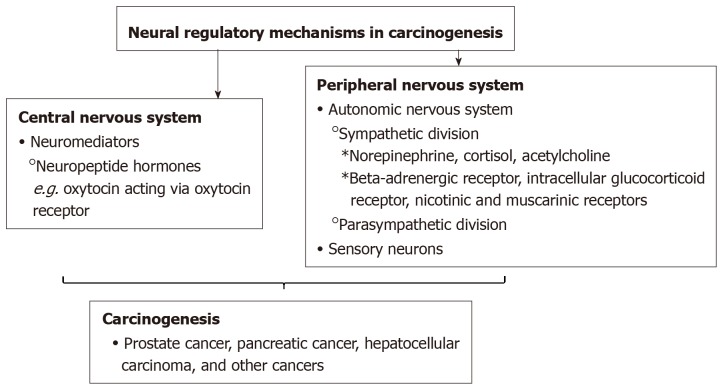
There is an emerging understanding of the neural regulatory mechanisms in carcinogenesis (Figure 1). Previously, some studies have proposed that solid tumors lacked innervation. However, newer studies have demonstrated the process of neoneurogenesis in which nerves infiltrate solid tumors. Specifically, some studies have shown that nerves play a role in the etiology of prostate, breast, and pancreatic solid tumors[13-15].
Figure 1.
Neural regulatory mechanisms in carcinogenesis.
Magnon et al[13] conducted a study using mouse models and showed that prostate cancer development was regulated by the formation of autonomic nerve fibers in the prostate gland. Prostate cancer development was inhibited by genetic deletion of neurotransmitter activity, stromal b2- and b3-adrenergic receptors. Additionally, in a retrospective blinded study conducted by this group using 43 prostate adenocarcinoma specimens, it was found that the denser the amount of sympathetic and parasympathetic nerve fibers in the tumor microenvironment, the poorer the patient outcomes[13]. The SNS nerve fibers provide NE which acts on b2- and b3-adrenergic receptors (Adrb2, Adrb3) expressed on stromal cells and plays a role in the development and progression of prostate tumors. Additionally, nerve fibers from the parasympathetic nervous system provide tumors with Ach, which promotes prostate tumor growth and metastasis[13].
Previous studies have shown that gastric tumorigenesis is enhanced by activity of the SNS[16-18]. Like the prostate, the pancreas is heavily innervated by the autonomic nervous system. Saloman et al[19] have suggested that pancreatic cancer could be prevented or slowed via the destruction of some sensory neurons. Sensory neurons densely populate pancreatic tumors and the stimulation of these neurons have been shown to advance inflammation[19,20]. Such inflammation is believed to initiate tumors by creating a conducive environment[19,20].
Wu et al[11] suggested that cortisol plays a role in hepatobiliary carcinoma (HCC) development. In HCC patients, serum levels of cortisol have been shown to be higher than in healthy individuals. Additionally, HCC cell cultures exposed to cortisol has been shown to repress the expression of p53 by upregulating the expression of Bcl2L12, a suppressor of p53[11].
CONCLUSION
The findings thus far suggest the emergence of a potentially critical role of the nervous system in carcinogenesis. This requires further investigation. It would be interesting to study how other signaling molecules which have traditionally been associated with nervous system function, but recently been implicated in carcinogenesis, play a role in neural regulation in carcinogenesis. For example, many neuropeptides are aberrantly expressed in cancer cells. One recently discovered example is oxytocin. Oxytocin is produced by hypothalamic neurons and has multiple roles in the central nervous system. Apart from its well-known functions in the female reproductive system (milk ejection), oxytocin has more recently been shown to play roles in stress and trust, anxiety, social interaction and bonding, and parental care, as well as on neuropsychiatric disorders linked to such social behaviors[21,22]. Even further, emerging findings are linking the aberrant expression of oxytocin and its receptor, the oxytocin receptor to various cancers[23-27]. A better understanding of the detailed mechanisms of the role nerves and neural mediators play in carcinogenesis, could lead to the identification of novel biomarkers and development of novel preventative, early detection, or therapeutic strategies for various cancers.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Invited Manuscript
Peer-review started: April 30, 2019
First decision: August 2, 2019
Article in press: August 14, 2019
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM S-Editor: Tang JZ L-Editor: A E-Editor: Qi LL
Contributor Information
Trisheena Harricharran, Department of Biological Sciences, Hunter College of the City University of New York, New York, NY 10065, United States; The Graduate Center Departments of Biology and Biochemistry, the City University of New York, New York, NY 10016, United States; Joan and Sanford I Weill Department of Medicine, Weill Cornell Medicine, Cornell University, New York, NY 10065, United States; Hunter College Center for Cancer Health Disparities Research, New York, NY 10065, United States.
Olorunseun O Ogunwobi, Department of Biological Sciences, Hunter College of the City University of New York, New York, NY 10065, United States; The Graduate Center Departments of Biology and Biochemistry, the City University of New York, New York, NY 10016, United States; Joan and Sanford I Weill Department of Medicine, Weill Cornell Medicine, Cornell University, New York, NY 10065, United States; Hunter College Center for Cancer Health Disparities Research, New York, NY 10065, United States. ogunwobi@genectr.hunter.cuny.edu.
References
- 1.Keskinov AA, Tapias V, Watkins SC, Ma Y, Shurin MR, Shurin GV. Impact of the Sensory Neurons on Melanoma Growth In Vivo. PLoS One. 2016;11:e0156095. doi: 10.1371/journal.pone.0156095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green McDonald P, O'Connell M, Lutgendorf SK. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun. 2013;30 Suppl:S1–S9. doi: 10.1016/j.bbi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tashiro M, Kubota K, Itoh M, Yoshioka T, Yoshida M, Nakagawa Y, Bereczki D, Sasaki H. Hypometabolism in the limbic system of cancer patients observed by positron emission tomography. Psychooncology. 1999;8:283–286. doi: 10.1002/(SICI)1099-1611(199907/08)8:4<283::AID-PON384>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Deborde S, Wong RJ. How Schwann cells facilitate cancer progression in nerves. Cell Mol Life Sci. 2017;74:4405–4420. doi: 10.1007/s00018-017-2578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015;75:1777–1781. doi: 10.1158/0008-5472.CAN-14-3180. [DOI] [PubMed] [Google Scholar]
- 6.Cole SW. New challenges in psycho-oncology: Neural regulation of the cancer genome. Psychooncology. 2018;27:2305–2309. doi: 10.1002/pon.4838. [DOI] [PubMed] [Google Scholar]
- 7.Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30 Suppl:S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Fabre B, Grosman H, Gonzalez D, Machulsky NF, Repetto EM, Mesch V, Lopez MA, Mazza O, Berg G. Prostate Cancer, High Cortisol Levels and Complex Hormonal Interaction. Asian Pac J Cancer Prev. 2016;17:3167–3171. [PubMed] [Google Scholar]
- 9.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–1881. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrepf A, Thaker PH, Goodheart MJ, Bender D, Slavich GM, Dahmoush L, Penedo F, DeGeest K, Mendez L, Lubaroff DM, Cole SW, Sood AK, Lutgendorf SK. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53:256–267. doi: 10.1016/j.psyneuen.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu W, Liu S, Liang Y, Zhou Z, Bian W, Liu X. Stress Hormone Cortisol Enhances Bcl2 Like-12 Expression to Inhibit p53 in Hepatocellular Carcinoma Cells. Dig Dis Sci. 2017;62:3495–3500. doi: 10.1007/s10620-017-4798-1. [DOI] [PubMed] [Google Scholar]
- 12.Asare GA, Bronz M, Naidoo V, Kew MC. Synergistic interaction between excess hepatic iron and alcohol ingestion in hepatic mutagenesis. Toxicology. 2008;254:11–18. doi: 10.1016/j.tox.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs JT. Cancer. Prostate cancer takes nerve. Science. 2013;341:134–135. doi: 10.1126/science.1241776. [DOI] [PubMed] [Google Scholar]
- 15.Ventura S, Evans BA. Does the autonomic nervous system contribute to the initiation and progression of prostate cancer? Asian J Androl. 2013;15:715–716. doi: 10.1038/aja.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, Katoh H. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65:164–170. doi: 10.1002/(sici)1096-9098(199707)65:3<164::aid-jso4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, Shimokawa T, Akita K, Tanaka S. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003;27:225–229. doi: 10.1097/00006676-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol Cancer. 2003;2:15. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, Rhim AD, Davis BM. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci USA. 2016;113:3078–3083. doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan JD, Peng RY, Wang Y, McVey DC, Vigna SR, Liddle RA. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938–G946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 21.Scerbo MJ, Gerdes JM. Bonding With β-Cells-A Role for Oxytocin in Glucose Handling. Diabetes. 2017;66:256–257. doi: 10.2337/dbi16-0053. [DOI] [PubMed] [Google Scholar]
- 22.Lerman B, Harricharran T, Ogunwobi OO. Oxytocin and cancer: An emerging link. World J Clin Oncol. 2018;9:74–82. doi: 10.5306/wjco.v9.i5.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Cancer. 1997;72:340–344. doi: 10.1002/(sici)1097-0215(19970717)72:2<340::aid-ijc23>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Fu S, Chen Q, Gu M, Zhou J, Liu C, Chen Y, Wang Z. The function of oxytocin: a potential biomarker for prostate cancer diagnosis and promoter of prostate cancer. Oncotarget. 2017;8:31215–31226. doi: 10.18632/oncotarget.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mankarious A, Dave F, Pados G, Tsolakidis D, Gidron Y, Pang Y, Thomas P, Hall M, Karteris E. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. Int J Oncol. 2016;48:1805–1814. doi: 10.3892/ijo.2016.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindblad M, García Rodríguez LA, Chandanos E, Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br J Cancer. 2006;94:136–141. doi: 10.1038/sj.bjc.6602906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner HG, Michaud DS, Colditz GA, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12:433–438. [PubMed] [Google Scholar]