Abstract
Background
Substantial evidence supports the use of inexpensive β-AR antagonists (beta blockers) against a variety of cancers, and the β-AR antagonist propranolol was recently approved by the European Medicines Agency for the treatment of soft tissue sarcomas. Prospective and retrospective data published by our group and others suggest that non-selective β-AR antagonists are effective at reducing proliferative rates in breast cancers, however the mechanism by which this occurs is largely unknown.
Methods
In this study, we measured changes in tumor proliferation and apoptosis in a late stage breast cancer patient treated with neoadjuvant propranolol. We expounded upon these clinical findings by employing an in vitro breast cancer model, where we used cell-based assays to evaluate propranolol-mediated molecular alterations related to cell proliferation and apoptosis.
Results
Neoadjuvant propranolol decreased expression of the pro-proliferative Ki-67 and pro-survival Bcl-2 markers, and increased pro-apoptotic p53 expression in a patient with stage III breast cancer. Molecular analysis revealed that β-AR antagonism disrupted cell cycle progression and steady state levels of cyclins. Furthermore, propranolol treatment of breast cancer cells increased p53 levels, enhanced caspase cleavage, and induced apoptosis.
Conclusion
Collectively, these data provide support for the incorporation of β-AR antagonists into the clinical management of breast cancer, and elucidate a partial molecular mechanism explaining the efficacy of β-AR antagonists against this disease.
Keywords: Breast cancer, Propranolol, Beta blockade, Beta adrenergic receptor, Proliferation, Apoptosis
At a glance of commentary
Scientific background on the subject
Use of beta adrenergic antagonists (beta blockers) has shown clinical efficacy against various tumor types. Retrospective analysis has correlated the use of beta blockers with decreased tumor proliferation in early phase breast cancer, though it is largely unknown if similar effects occur in late stage breast cancer and what mechanism/s drives this effect.
What this study adds to the field
This data presented in this study demonstrates that use of the beta blocker propranolol reduces the proliferative index of a late stage breast cancer. This data suggests that prospective clinical studies with larger patient numbers should be performed to accurately evaluate the efficacy of beta blockers against breast cancer.
The beta adrenergic receptors (β-ARs) are a class of G-protein coupled receptors (GPCRs) that are targets of the catecholamines epinephrine and norepinephrine. Stimulation of these receptors induces sympathetic nervous system responses, most notably the fight-or-flight reaction. β-ARs were first detected in breast cancer nearly thirty years ago [1], [2], and it has recently been reported that β-ARs are over-expressed in breast cancer relative to non-diseased breast epithelium [3]. Pre-clinical studies using breast cancer cell lines have correlated β-AR antagonist (beta blocker) treatment to decreased cell proliferation and migration [3], [4], [5], [6], [7], [8], [9]. Studies using animal tumor models have yielded similar results, with β-AR antagonists resulting in decreased breast cancer growth, reductions in metastasis to the brain, bone, and other organs, and improved host survival [6], [10], [11], [12], [13]. Moreover, combinations of β-AR antagonists with standard anti-cancer treatments exhibit improved pre-clinical results for breast cancer compared to single treatments [5], [14].
Several retrospective studies have sought to correlate the use of β-AR antagonists with clinical outcomes in breast cancer. In these studies, β-AR antagonists were associated with reduced tumor proliferation rates, decreased mortality rates, decreased metastatic development, longer disease free survival, and reduced tumor recurrence [3], [12], [14], [15], [16]. In contrast, one retrospective study has reported that use of β-AR antagonists has no effect on breast cancer patient survival [17]. In prospective clinical settings, the non-selective β-AR antagonist propranolol significantly reduced the tumor proliferation rate of early stage breast cancer [3] and improved immune biomarker profiles when combined with COX-2 inhibitors [18].
The mechanisms by which beta blockers inhibit the proliferation rate of breast cancer cells and potentially improve clinical outcomes are largely unknown, however a handful of studies have provided some insight into these processes. Propranolol treatment of breast cancer cell lines decreases MAPK, HSP70, and inducible nitric oxide synthase activity, as well as increases interleukin 10 and RANKL expression [3], [11]. Propranolol exerts effects on breast cancer metabolism through post-transcriptional downregulation of hexokinase 2 to inhibit glucose metabolism [19]. Hexokinase 2, an enzyme often overexpressed in tumor cells, plays a critical role in tumor initiation and development, and leads to negative clinical outcomes [20], [21]. Norepinephrine-mediated β-AR stimulation in breast cancer cells enhances cancer cell adhesion to microvascular endothelial cells [22], suggesting that this signaling pathway may play a role in the early steps of the metastatic cascade through interactions with the vascular system. Supporting this possibility, β-AR antagonism inhibits capillary network formation and greatly exacerbates the anti-angiogenic effects of paclitaxel [5].
In the current study, propranolol was prospectively administered to a late stage breast cancer patient in a neoadjuvant setting. Changes in tumor cell proliferation rates, mitogenic markers, and apoptotic markers were evaluated before and after β-AR antagonist administration. Propranolol-mediated alterations in tumor biomarkers were corroborated at the molecular level using a breast cancer cell line.
Materials and methods
Neoadjuvant treatment of a late stage breast cancer patient with propranolol
Clinical studies presented in this report were performed following approval of the Texas Tech University Health Sciences Center Institutional Review Board. A female patient, age 44, presented at the Texas Tech Breast Care Center with a stage IIIA, 6.5 cm left breast invasive lobular carcinoma (T3N1M). Tumor tissue from the diagnostic biopsy was collected for analysis. The patient was immediately prescribed 1.5 mg/kg/day propranolol for 18 days and then a tapering dose over the subsequent 7 days. After the treatment period, the tumor was removed through a radical mastectomy and tumor tissue was collected for analysis.
Immunohistochemistry (IHC)
Tumor tissue was collected and processed from both the patient's diagnostic biopsy (pre-treatment) and the radical mastectomy (post-treatment). IHC for Ki-67 was performed at the University Medical Center's Pathology facilities according to their in-house procedures. The Ki-67 index was blindly quantified in both the pre- and post-treatment tissues by counting 10 fields under a microscope, with each field composed of 250 + nuclei. The Ki-67 index was calculated as the (number of Ki-67 positive nuclei/total number of nuclei) × 100. For IHC staining of total p53 and Bcl-2 proteins, slides were deparaffinized, rehydrated, and stained using the rabbit specific HRP/DAB (ABC) Detection IHC Kit (ab#64261) or the mouse specific HRP/DAB (ABC) Detection IHC Kit (ab#64259) according to the manufacturer's protocols. Antibodies included: anti-p53 (total) (Cell Signaling #2527) and anti-Bcl-2 (Abcam #ab196495).
Cell culture and treatment
MDA-MB-231 breast cancer cells (ATCC #HTB-26) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. Cells were maintained in a water-jacketed CO2 incubator at 37 °C. DL-propranolol hydrochloride (Acros Organics) and doxorubicin hydrochloride (Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO) and were administered as indicated for each experiment. As a solvent control, DMSO was tested at the same concentration (1% v/v) as contained in the experimental samples. Also, untreated cells were used to establish the background of dead cells provoked by factors inherent to the cell cultures and cell manipulation.
Flow cytometry
MDA-MB-231 cells were collected via trypsinization and resuspended in PBS. Nuclear isolation medium (NIM)-DAPI solution (Beckman Coulter) was added to the cell suspensions and analyzed by a Gallios flow cytometer (Beckman Coulter) according to previously described methods [23]. Determination of the main phases of the cell cycle subpopulations (G0/G1, S, and G2/M), as well as the occurrence of cells experiencing DNA fragmentation (sub-G0/G1; apoptotic subpopulation), was performed as previously described [23]. The percentage of each cell cycle phase distributions was determined by using Kaluza flow cytometry software (Beckman Coulter). Consistently, twenty thousand cells were measured in each condition.
Immunoblotting
Lysates were subjected to SDS-PAGE, and transferred to polyvinylidene fluoride membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). Membranes were blocked in Tris-buffered saline plus 3% bovine serum albumin and 0.05% Tween-20, and incubated with the following antibodies as indicated for each experiment: anti-cyclin A (Cell Signaling #4656), anti-cyclin B1 (Cell Signaling #4138), anti-cyclin D1 (Cell Signaling #2978), anti-cyclin D2 (Cell Signaling #3741), anti-cyclin E1 (Cell Signaling #4129), anti-cyclin E2 (Cell Signaling #4132), anti-p53 (total) (Cell Signaling #2527), anti-phospho-p53 (S9) (Cell Signaling #9288), anti-phospho-p53 (S15) (Cell Signaling #9286), anti-phospho-p53 (S20) (Cell Signaling #9287), anti-phospho-p53 (S37) (Cell Signaling #9289), anti-phospho-p53 (S46) (Cell Signaling #2521), anti-phospho-p53 (S392) (Cell Signaling #9281), cleaved caspase 3 (Cell Signaling #9664), cleaved caspase 6 (Cell Signaling #9761), cleaved caspase 9 (Cell Signaling #7237), and anti-beta actin (Santa Cruz Biotech #sc8432). Each primary antibody was detected with an appropriate 1:1000 HRP-conjugated secondary antibody, subjected to Supersignal West Dura Extended Duration Substrate (ThermoFisher Scientific), and digitally captured using a GE Image Quant Las4000 imaging system.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde, permeabilized with Triton X-100, and incubated with the anti-p53 antibody (Cell Signaling #2527), rhodamine-conjugated phalloidin (Cytoskeleton Inc), or Hoechst 33342 (Thermo Fisher Scientific). Appropriate fluorescent-conjugated secondary antibodies were used for primary antibody detection. Single plane high-resolution digital fluorescent images were captured using an LSM 700 confocal microscope (Zeiss) equipped with a 40x immersion oil objective and assisted with Zen 2009 software (Zeiss); for each channel, 1-airy unit (AU) pinhole setting was utilized as previously described [24].
Live/dead staining
Cells were stained with Apopxin Green Indicator, 7-AAD, and CytoCalcein Violet 450 using the Apoptosis/Necrosis Detection Kit (Abcam #ab176749) according to the manufacturer's instructions.
Statistical analysis
The Mann-Whitney rank sum test was performed in GraphPad Prism version 7 for [Fig. 2, Fig. 3B, F]. One-way ANOVA was performed in GraphPad Prism version 7 for [Fig. 2, Fig. 4C]. Differences were considered statistically significant if the p < 0.01.
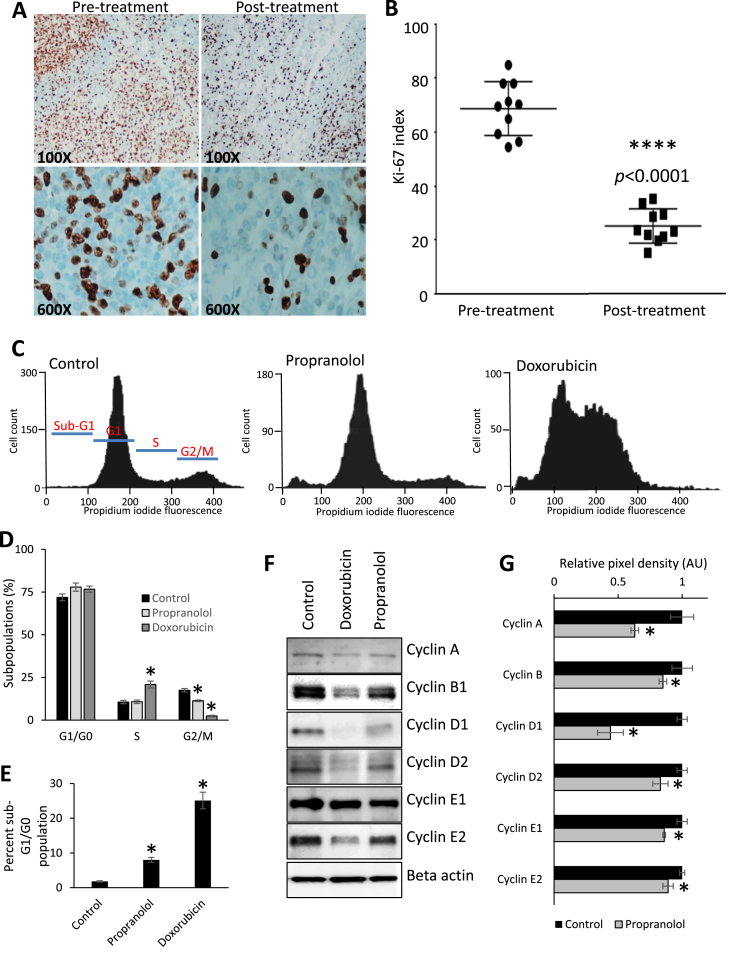
Fig. 2.
Propranolol decreases the mitogenic potential of breast cancer cells. (A & B) IHC and graphical representation for Ki-67 performed on tissues from the diagnostic biopsy (pre-treatment) and surgical resection (post-treatment). Brown staining indicates Ki-67 antigenicity. Ten random vision fields were counted per condition. (C & D) DAPI staining of MDA-MB-231 breast cancer cells was analyzed via flow cytometry after treatment with vehicle control or with 40 μM propranolol for 24 h. Three μM doxorubicin was added as a positive experimental control. (E) Quantification of the sub-G1/G0 cell sub-population from the flow cytometry analysis. Cell cycle populations (sub-G1, G1, S, G2/M) are marked in red in the control flow cytometry graph. (F & G) Immunoblotting and quantification of cyclin protein levels in MDA-MB-231 cells treated with vehicle control or 40 μM propranolol. Three μM doxorubicin was added as a positive experimental control. For quantification, cyclins were normalized relative levels of actin. AU indicates arbitrary units.
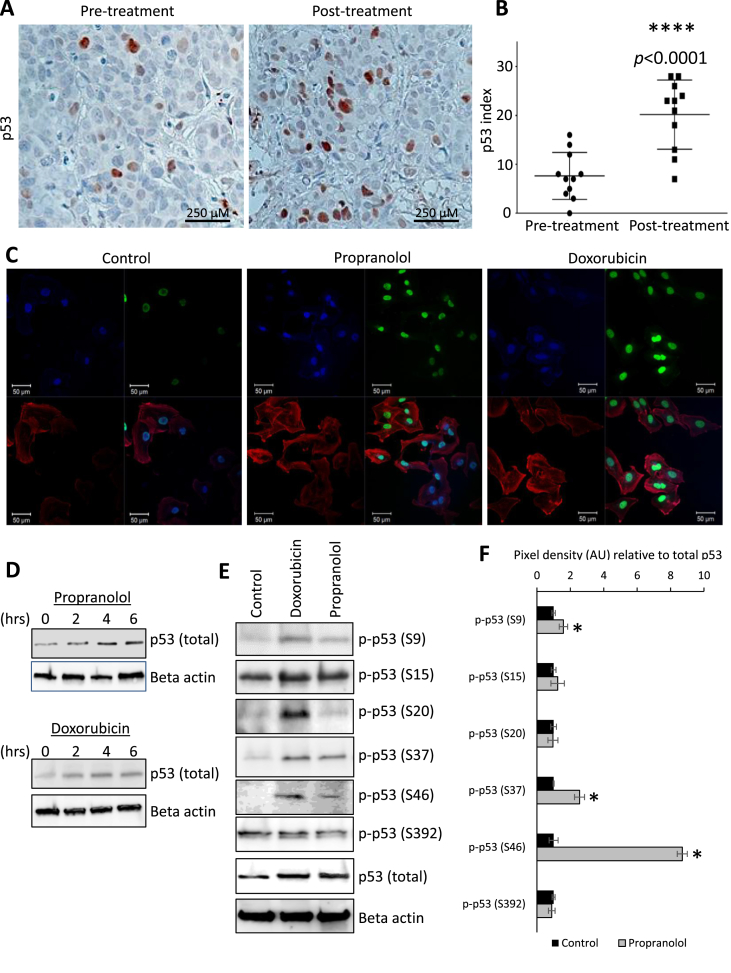
Fig. 3.
Propranolol modulates p53 steady state protein levels and post-translational modifications. (A & B) IHC and graphical representation for p53 protein performed on tissues from the diagnostic biopsy (pre-treatment) and surgical resection (post-treatment). Brown staining indicates p53 antigenicity. Ten random vision fields were counted per condition. (C) Immunofluorescent staining for p53 (green), Hoechst 33342 nuclear counterstain (blue), and rhodamine-conjugated phalloidin as an actin counterstain (red) in MDA-MB-231 cells treated with vehicle control or 40 μM propranolol. Three μM doxorubicin was added as a positive experimental control. (D) Immunoblotting for p53 protein levels in MDA-MB-231 cells treated with 40 μM propranolol over a 6 h time course. Three μM doxorubicin was added as a positive experimental control. (E & F) Immunoblotting of p53 phosphorylation events in MDA-MB-231 cells treated with vehicle control or 40 μM propranolol for 6 h. Three μM doxorubicin was added as a positive experimental control. For quantification, phospho-p53 was normalized relative levels of both total p53 and actin. AU indicates arbitrary units.
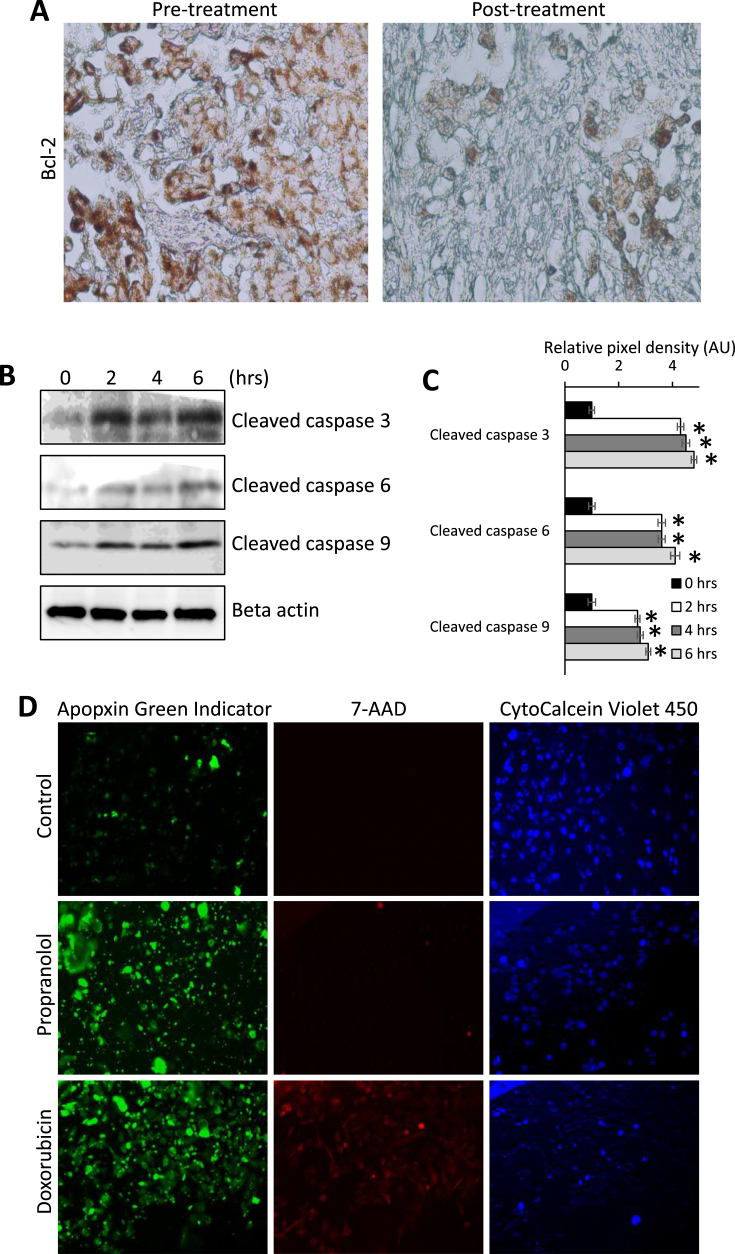
Fig. 4.
Propranolol induces apoptosis in breast cancer cells. (A) IHC for Bcl-2 protein performed on tissues from the diagnostic biopsy (pre-treatment) and surgical resection (post-treatment). Brown staining indicates Bcl-2 antigenicity. (B & C) Immunoblotting of cleavage caspase products in MDA-MB-231 cells treated 40 μM propranolol over a 6 h time course. For quantification, cleaved caspase products were normalized relative levels of beta-actin. AU indicates arbitrary units. (D) MDA-MD-231 cells were treated with vehicle control, 40 μM propranolol, or 3 μM doxorubicin as a positive control for 6 h. Cells were stained with the apoptotic marker Apopxin Green Indicator (green), the necrotic marker 7-AAD (red), and CytoCalcein Violet (blue) as a nuclear counterstain. Images were collected using confocal microscopy.
Results
Neoadjuvant treatment of a late stage breast cancer patient with propranolol
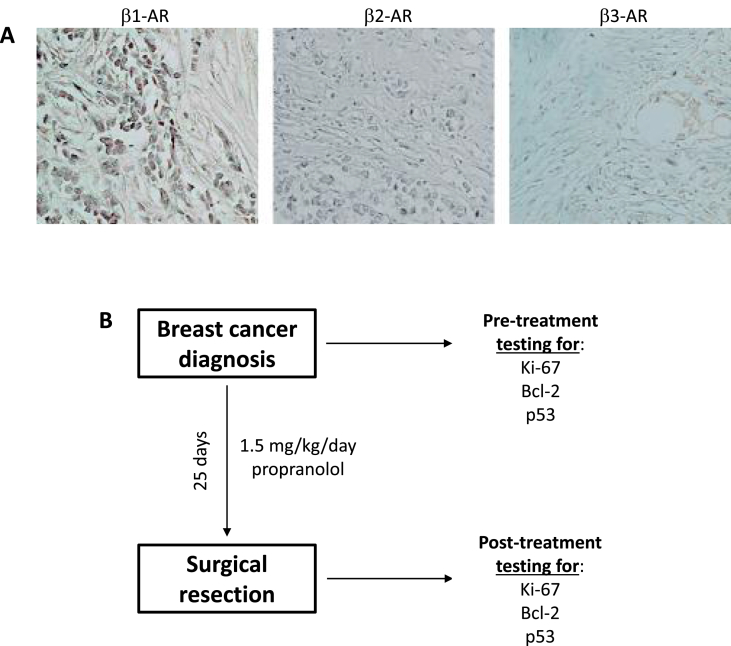
We have previously reported that administration of propranolol to an early stage breast cancer patient resulted in a decreased tumor proliferative index [3]. To prospectively evaluate if beta blockade could reduce the proliferation rate of a late stage breast tumor in a neoadjuvant setting, propranolol was administered to a patient diagnosed with a 6.5 cm invasive lobular carcinoma that was estrogen and progesterone receptor positive, HER2-neu negative, and expressed β1-AR, β2-AR, and β3-AR. The treatment regime is outlined in [Fig. 1].
Fig. 1.
Neoadjuvant treatment of a breast cancer patient with propranolol. (A) Immunohistochemical staining of breast cancer tissue from the patient discussed in this study for β1-AR, β2-AR, and β3-AR proteins. Purple/brown staining indicated positivity for the antigen tested. (B) Flow diagram showing the treatment and tumor tissue collection schemes for the stage III breast cancer patient.
Propranolol alters breast cancer cell cycle progression
IHC for Ki-67 was performed on the diagnostic biopsy (before treatment with propranolol) and on the tumor tissue from the radical mastectomy (after 25 days of propranolol treatment). The Ki-67 proliferative indices at the time of diagnostic biopsy and surgical removal were 68 ± 3% (mean ± SEM) and 25 ± 2% (mean ± SEM), respectively [Fig. 2A and B]. This equated to an approximately 2.7-fold reduction in tumor proliferative index after propranolol treatment (p < 0.0001). Substantial concordance has been reported for Ki-67 indices between diagnostic biopsy and surgical resection [25], therefore an alteration in tumor proliferation, as observed in this patient, can be attributed to propranolol administration with reasonable confidence. We corroborated this finding using the MDA-MB-231 breast cancer cell line, which was originally isolated from a metastatic pleural effusion. Cell cycle analysis was performed using flow cytometry in control and propranolol (40 μM) treated breast cancer cells (24 h treatment, as substantial changes in cell viability were visually observed at this time). In our previously published study examining the effects of beta blockade on MDA-MB-231 cells, we demonstrated that the EC50 for propranolol in this cell line was approximately 78 μM [3]. In the current study, we treated MDA-MB-231 cells with a lower dose of propranolol for multiple reasons: 1) the EC50 of propranolol established in our previous publication is not a physiologically attainable plasma concentration in patients; 2) dosing of patients with chemotherapeutic compounds generally involves low dose/longer treatment regimens that are not well recapitulated in lethality testing associated with in vitro cell culture systems; and 3) we sought to examine the acute molecular effect of sub-lethal doses of propranolol on the breast cancer cells. The anthracycline chemotherapy drug, doxorubicin (3 μM), was used as a positive experimental control for this and all subsequent in vitro experiments. Addition of propranolol reduced the percentage of the cell population residing in the G2/M phase of the cell cycle [Fig. 2C and D], and increased the sub-G1 cell population, representing dead or dying cells [Fig. 2C and E]. Moreover, the expression of multiple cyclins was reduced following 24 h propranolol treatment [Fig. 2F and G], further demonstrating the impact of β-AR antagonism on breast cancer cell proliferation.
Propranolol enhances p53 activation in breast cancer cells
IHC for total p53 was performed on the diagnostic biopsy and tumor tissue from the radical mastectomy in the late stage breast cancer patient. The p53 indices at the time of diagnostic biopsy and surgical removal were 8 ± 2% (mean ± SEM), and 20 ± 2% (mean ± SEM), respectively [Fig. 3A and B]. This equated to an approximately 2.5-fold increase in p53 protein expression after propranolol treatment (p < 0.0001). These data were corroborated by immunofluorescent staining of MDA-MB-231 cells for total p53, revealing a marked increase in p53 protein levels by 6 h post-treatment with propranolol [Fig. 3C]. Immunoblotting of the breast cancer cells showed similar propranolol-mediated increases in total p53 levels between two to 6 h post-treatment [Fig. 3D]. Using immunoblotting, we evaluated specific post-translational modifications that occur on the p53 protein following 6 h propranolol treatment of MDA-MB-231 cell lysates. Phosphorylation events were normalized based on levels of actin and total p53, revealing that propranolol increased the phosphorylation of p53 on residues S9, S37, and S46 [Fig. 3E and F].
Propranolol induces apoptosis in breast cancer cells
IHC for the pro-survival protein, Bcl-2, was performed on the diagnostic biopsy and tumor tissue from the radical mastectomy in the late stage breast cancer patient. Decreased Bcl-2 staining intensity was observed after propranolol administration. We then treated MDA-MB-231 cells with a time course of propranolol up to 6 h and evaluated changes in cleaved caspase levels using immunoblotting. Our analysis revealed that propranolol markedly increased the levels of caspase 3, 6, and 9 cleavage products as early as 2 h post treatment [Fig. 4B and C]. MDA-MB-231 cells treated for 6 h with control or propranolol were stained with the apoptotic and necrotic markers, Apopxin Green Indicator and 7-AAD, respectively, revealing substantial apoptotic increases in propranolol treated cells [Fig. 4D]. At the time point evaluated, we observed no visible increases in necrosis for the propranolol treated cells [Fig. 4D].
Discussion
In a retrospective analysis of over 400 patients, we previously reported that use of non-selective β-AR antagonists was associated with decreased proliferation of early stage breast cancer [3]. While we did not observe an effect on late stage breast cancer patients in this study, these observations may have been confounded due to an inability to control β-AR antagonist dosing or length of administration in a retrospective setting. Therefore, we sought to evaluate, in a controlled prospective manner, whether propranolol could inhibit tumor growth in a late stage breast cancer patient. Our findings revealed that neoadjuvant propranolol reduced the proliferative index of a stage III breast tumor by 2.7-fold. We then corroborated these findings at the molecular level, showing that propranolol disrupted the cell cycle distribution of breast cancer populations and altered the protein expression of the cell cycle regulatory proteins cyclin A, D1, E1, and E2. Decreased proliferation in response to β-AR antagonists has been reported across multiple tumor cell types, and is accompanied by disruption of mitogenic signal transduction cascades including AKT, MAPK, and cAMP [3], [26], [27], [28], [29]. These β-AR antagonist-mediated changes in tumor proliferation rates and mitogenic signaling cascades may contribute to decreased breast cancer metastasis and recurrence, as well as improved overall patient survival demonstrated in a number of clinical studies [12], [14], [16], [30], [31], [32].
In addition to decreased Ki-67 antigenicity, we observed an increase in pro-apoptotic p53 protein expression and a decrease in pro-survival Bcl-2 protein expression in late stage breast cancer tissue following neoadjuvant propranolol administration. Moreover, β-AR antagonism increased the sub-G1 cell cycle population of breast cancer cells, suggesting that blocking β-AR signaling leads to breast cancer cell death. Indeed, our live/dead assay indicated that propranolol enhanced apoptosis as indicated by higher Apopxin staining in propranolol treated cells relative to the control. We corroborated these findings at the molecular level by showing that propranolol stabilized p53 protein levels in breast cancer cells, and enhanced the phosphorylation of p53 regulatory sites including S9, S37, and S46. Stabilization of p53 by DNA damage or cell stress has been extensively shown to trigger multiple anti-proliferative and pro-apoptotic signaling cascades [33]. DNA damage induces phosphorylation of p53 at S9 and S37, while phosphorylation at the S46 residue regulates the ability of p53 to induce apoptosis [34], [35]. Moreover, propranolol led to increased levels of cleaved initiator caspase 9 and execution caspases 3 and 6 in breast cancer cells, further indicating that propranolol treatment leads to programmed cell death. Similar effects on cell survival and apoptotic signaling have been observed in previous studies across other tumor types, including propranolol-induced caspase activation, increases in p53 steady state protein levels, and enhanced cell death [28], [36], [37], [38], [39], [40]. We have previously reported propranolol selectivity against breast cancer cells, while this treatment relatively spares non-diseased mammary epithelial cells [3]. Our understanding of the mechanism behind selectivity of propranolol is currently very limited, whereas mechanism(s) have been addressed to some degree in infantile hemangiomas. While it has been reported that propranolol inhibits cell proliferation of both infantile hemangioma and normal endothelial cells in culture [27], selectivity has been observed in infantile hemangioma cells. β-AR antagonism induces apoptosis in hemangioma endothelial cells, yet spares hemangioma-derived stem cells in part through a differential regulation of Akt and other survival regulators [41], as well as a propensity for propranolol to selectively promote adipogenesis in hemangioma-derived stem cells [42]. Further studies are necessary to understand the mechanisms behind the selectivity of β-AR antagonism against diseased cells.
While the current study focused exclusively on the effects of propranolol on mitogenic and apoptotic signaling regulators in breast cancer, studies from other tumor types suggest that β-AR antagonism may disrupt a variety of processes. For instance, propranolol interferes with several steps during angiogenesis including cell proliferation and formation of capillary tubules [43], [44]. β-AR antagonism also modulates the expression and activation of angiogenic signaling pathways including angiopoietin/TIE2, vascular endothelial growth factor (VEGF), and hypoxia inducible factor (HIF) [27], [28], [39], [45], [46], [47]. Propranolol exhibits a biphasic effect on vascular resistance, with lower (10–20 mg/kg/day) and higher (20 + mg/kg/day) doses inducing vasoconstriction and vasodilation, respectively [48]. This same study showed that propranolol affects tumor arteriogenesis, but not capillary density in melanoma tumor models [48]. In both breast and neuroblastoma models, propranolol has been shown to increase the treatment efficacy of chemotherapy by potentiating anti-angiogenic effects [5], suggesting that combination treatments with propranolol may be more effective at decreasing vascular networks than either treatment alone. An emerging area of research where β-AR antagonism may substantially impact the oncogenic process is regulation of tumor immunity. Catecholamine activation of β-ARs has long been known to suppress innate and cellular immunity by decreasing interleukin (IL)-12 production and increasing IL-10 secretion, thus shifting the immune reaction away from a beneficial T helper cell 1 (TH1) response [49], [50]. A recent study in melanoma has demonstrated that propranolol is capable of reducing infiltration of immunosuppressive myeloid cells into the tumor and increasing infiltration of cytotoxic lymphocytes [51]. As the ability of tumors to foster an immunosuppressive microenvironment is a major impediment to current immunotherapy strategies, the addition of propranolol with this class of anti-cancer therapeutics may help shift the balance toward a more desirable immunoreactive state within the tumor.
Conclusions
Collectively, our data suggest that incorporation of β-AR antagonists may decrease tumor proliferation not only in localized breast tumors, as we previously demonstrated [3], but also in advanced stages of disease. β-ARs are expressed across many different cancer types [52] and preclinical and clinical efficacy has been reported following inhibition of these receptors across a large number of diverse cancers [5], [9], [12], [15], [16], [18], [28], [29], [31], [48], [51], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]. The propranolol-mediated, anti-cancer mechanisms reported in the current study using breast cancer models may translate into a much broader group of cancers, paving the way for an inexpensive and non-toxic drug to be incorporated as an adjuvant to current treatment regimens.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This project was supported by grants to ZN (CPRIT RP120528), ZN and BAB (TTUHSC seed grant #533701), and RJA and AVR (NIMHD RCMI 5G12MD007592).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Marchetti B., Spinola P.G., Plante M., Poyet P., Follea N., Pelletier G. Beta-adrenergic receptors in DMBA-induced rat mammary tumors: correlation with progesterone receptor and tumor growth. Breast Cancer Res Treat. 1989;13:251–263. doi: 10.1007/BF02106575. [DOI] [PubMed] [Google Scholar]
- 2.Badino G.R., Novelli A., Girardi C., Di Carlo F. Evidence for functional beta-adrenoceptor subtypes in CG-5 breast cancer cell. Pharmacol Res. 1996;33:255–260. doi: 10.1006/phrs.1996.0036. [DOI] [PubMed] [Google Scholar]
- 3.Montoya A., Amaya C.N., Belmont A., Diab N., Trevino R., Villanueva G. Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. 2017;8:6446–6460. doi: 10.18632/oncotarget.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iseri O.D., Sahin F.I., Terzi Y.K., Yurtcu E., Erdem S.R., Sarialioglu F. beta-Adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharmaceut Biol. 2014;52:1374–1381. doi: 10.3109/13880209.2014.892513. [DOI] [PubMed] [Google Scholar]
- 5.Pasquier E., Ciccolini J., Carre M., Giacometti S., Fanciullino R., Pouchy C. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797–809. doi: 10.18632/oncotarget.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rico M., Baglioni M., Bondarenko M., Laluce N.C., Rozados V., Andre N. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8:2874–2889. doi: 10.18632/oncotarget.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slotkin T.A., Zhang J., Dancel R., Garcia S.J., Willis C., Seidler F.J. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2000;60:153–166. doi: 10.1023/a:1006338232150. [DOI] [PubMed] [Google Scholar]
- 8.Szewczyk M., Richter C., Briese V., Richter D.U. A retrospective in vitro study of the impact of anti-diabetics and cardioselective pharmaceuticals on breast cancer. Anticancer Res. 2012;32:2133–2138. [PubMed] [Google Scholar]
- 9.Wilson J.M., Lorimer E., Tyburski M.D., Williams C.L. beta-Adrenergic receptors suppress Rap1B prenylation and promote the metastatic phenotype in breast cancer cells. Cancer Biol Ther. 2015;16:1364–1374. doi: 10.1080/15384047.2015.1070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdin A.A., Soliman N.A., Saied E.M. Effect of propranolol on IL-10, visfatin, Hsp70, iNOS, TLR2, and survivin in amelioration of tumor progression and survival in Solid Ehrlich Carcinoma-bearing mice. Pharmacol Rep PR. 2014;66:1114–1121. doi: 10.1016/j.pharep.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Campbell J.P., Karolak M.R., Ma Y., Perrien D.S., Masood-Campbell S.K., Penner N.L. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choy C., Raytis J.L., Smith D.D., Duenas M., Neman J., Jandial R. Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: the potential benefit of perioperative beta-blockade. Oncol Rep. 2016;35:3135–3142. doi: 10.3892/or.2016.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloan E.K., Priceman S.J., Cox B.F., Yu S., Pimentel M.A., Tangkanangnukul V. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D., Yang Z., Wang T., Yang Z., Chen H., Hu Y. beta2-AR signaling controls trastuzumab resistance-dependent pathway. Oncogene. 2016;35:47–58. doi: 10.1038/onc.2015.58. [DOI] [PubMed] [Google Scholar]
- 15.Barron T.I., Connolly R.M., Sharp L., Bennett K., Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 16.Powe D.G., Voss M.J., Zanker K.S., Habashy H.O., Green A.R., Ellis I.O. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardwell C.R., Pottegard A., Vaes E., Garmo H., Murray L.J., Brown C. Propranolol and survival from breast cancer: a pooled analysis of European breast cancer cohorts. Breast Cancer Res BCR. 2016;18:119. doi: 10.1186/s13058-016-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaashua L., Shabat-Simon M., Haldar R., Matzner P., Zmora O., Shabtai M. Perioperative COX-2 and beta-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;23:4651–4661. doi: 10.1158/1078-0432.CCR-17-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang F., Ma W., Ma X., Shao Y., Yang W., Chen X. Propranolol inhibits glucose metabolism and 18F-FDG uptake of breast cancer through posttranscriptional downregulation of hexokinase-2. J Nucl Med. 2014;55:439–445. doi: 10.2967/jnumed.113.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra K.C., Hay N. Hexokinase 2 as oncotarget. Oncotarget. 2013;4:1862–1863. doi: 10.18632/oncotarget.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patra K.C., Wang Q., Bhaskar P.T., Miller L., Wang Z., Wheaton W. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strell C., Niggemann B., Voss M.J., Powe D.G., Zanker K.S., Entschladen F. Norepinephrine promotes the beta1-integrin-mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROalpha release. Mol Cancer Res MCR. 2012;10:197–207. doi: 10.1158/1541-7786.MCR-11-0130. [DOI] [PubMed] [Google Scholar]
- 23.Robles-Escajeda E., Das U., Ortega N.M., Parra K., Francia G., Dimmock J.R. A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell Oncol. 2016;39:265–277. doi: 10.1007/s13402-016-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sierra-Fonseca J.A., Najera O., Martinez-Jurado J., Walker E.M., Varela-Ramirez A., Khan A.M. Nerve growth factor induces neurite outgrowth of PC12 cells by promoting Gbetagamma-microtubule interaction. BMC Neurosci. 2014;15:132. doi: 10.1186/s12868-014-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricci M.D., Calvano Filho C.M., Oliveira Filho H.R., Filassi J.R., Pinotti J.A., Baracat E.C. Analysis of the concordance rates between core needle biopsy and surgical excision in patients with breast cancer. Revista da Associacao Medica Brasileira. 2012;58:532–536. [PubMed] [Google Scholar]
- 26.Munabi N.C., England R.W., Edwards A.K., Kitajewski A.A., Tan Q.K., Weinstein A. Propranolol targets hemangioma stem cells via cAMP and mitogen-activated protein kinase regulation. Stem Cells Trans Med. 2016;5:45–55. doi: 10.5966/sctm.2015-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiles J., Amaya C., Pham R., Rowntree R.K., Lacaze M., Mulne A. Propranolol treatment of infantile hemangioma endothelial cells: a molecular analysis. Exp Therap Med. 2012;4:594–604. doi: 10.3892/etm.2012.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stiles J.M., Amaya C., Rains S., Diaz D., Pham R., Battiste J. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C., Chen X., Zeng W., Peng C., Huang G., Li X. Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget. 2016;7:68314–68327. doi: 10.18632/oncotarget.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Childers W.K., Hollenbeak C.S., Cheriyath P. Beta-blockers reduce breast cancer recurrence and breast cancer death: a meta-analysis. Clin Breast Cancer. 2015;15:426–431. doi: 10.1016/j.clbc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Parada-Huerta E., Alvarez-Dominguez T., Uribe-Escamilla R., Rodriguez-Joya J., Ponce-Medrano J.D., Padron-Lucio S. Metastasis risk reduction related with beta-blocker treatment in Mexican women with breast cancer. Asian Pac J Cancer Prev. 2016;17:2953–2957. [PubMed] [Google Scholar]
- 32.Raimondi S., Botteri E., Munzone E., Cipolla C., Rotmensz N., DeCensi A. Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: systematic review and meta-analysis. Int J Cancer. 2016;139:212–219. doi: 10.1002/ijc.30062. [DOI] [PubMed] [Google Scholar]
- 33.Zilfou J.T., Lowe S.W. Tumor suppressive functions of p53. Cold Spr Harb Persp Biol. 2009;1:a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bode A.M., Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 35.Saito S., Goodarzi A.A., Higashimoto Y., Noda Y., Lees-Miller S.P., Appella E. ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J Biol Chem. 2002;277:12491–12494. doi: 10.1074/jbc.C200093200. [DOI] [PubMed] [Google Scholar]
- 36.Albinana V., Villar Gomez de Las Heras K., Serrano-Heras G., Segura T., Perona-Moratalla A.B., Mota-Perez M. Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J Rare Dis. 2015;10:118. doi: 10.1186/s13023-015-0343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji Y., Li K., Xiao X., Zheng S., Xu T., Chen S. Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J Pediatr Surg. 2012;47:2216–2223. doi: 10.1016/j.jpedsurg.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Wolter J.K., Wolter N.E., Blanch A., Partridge T., Cheng L., Morgenstern D.A. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget. 2014;5:161–172. doi: 10.18632/oncotarget.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolter N.E., Wolter J.K., Enepekides D.J., Irwin M.S. Propranolol as a novel adjunctive treatment for head and neck squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2012;41:334–344. [PubMed] [Google Scholar]
- 40.Zhang D., Ma Q., Shen S., Hu H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist's anticancer effect in pancreatic cancer cell. Pancreas. 2009;38:94–100. doi: 10.1097/MPA.0b013e318184f50c. [DOI] [PubMed] [Google Scholar]
- 41.Kum J.J., Khan Z.A. Propranolol inhibits growth of hemangioma-initiating cells but does not induce apoptosis. Pediatr Res. 2014;75:381–388. doi: 10.1038/pr.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma X., Zhao T., Ouyang T., Xin S., Ma Y., Chang M. Propranolol enhanced adipogenesis instead of induction of apoptosis of hemangiomas stem cells. Int J Clin Exp Pathol. 2014;7:3809–3817. [PMC free article] [PubMed] [Google Scholar]
- 43.Annabi B., Lachambre M.P., Plouffe K., Moumdjian R., Beliveau R. Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol Res. 2009;60:438–445. doi: 10.1016/j.phrs.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Lamy S., Lachambre M.P., Lord-Dufour S., Beliveau R. Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vasc Pharmacol. 2010;53:200–208. doi: 10.1016/j.vph.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Chim H., Armijo B.S., Miller E., Gliniak C., Serret M.A., Gosain A.K. Propranolol induces regression of hemangioma cells through HIF-1alpha-mediated inhibition of VEGF-A. Ann Surg. 2012;256:146–156. doi: 10.1097/SLA.0b013e318254ce7a. [DOI] [PubMed] [Google Scholar]
- 46.Hajighasemi F., Hajighasemi S. Effect of propranolol on angiogenic factors in human hematopoietic cell lines in vitro. Iran Biomed J. 2009;13:223–228. [PubMed] [Google Scholar]
- 47.Zhang L., Mai H.M., Zheng J., Zheng J.W., Wang Y.A., Qin Z.P. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol. 2014;7:48–55. [PMC free article] [PubMed] [Google Scholar]
- 48.Maccari S., Buoncervello M., Rampin A., Spada M., Macchia D., Giordani L. Biphasic effects of propranolol on tumour growth in B16F10 melanoma-bearing mice. Br J Pharmacol. 2017;174:139–149. doi: 10.1111/bph.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charmandari E., Tsigos C., Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 50.Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 51.Jean Wrobel L., Bod L., Lengagne R., Kato M., Prevost-Blondel A., Le Gal F.A. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget. 2016;7:77825–77837. doi: 10.18632/oncotarget.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rains S.L., Amaya C.N., Bryan B.A. Beta-adrenergic receptors are expressed across diverse cancers. Oncoscience. 2017;4:95–105. doi: 10.18632/oncoscience.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armaiz-Pena G.N., Allen J.K., Cruz A., Stone R.L., Nick A.M., Lin Y.G. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. doi: 10.1038/ncomms2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chow W., Amaya C.N., Rains S., Chow M., Dickerson E.B., Bryan B.A. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated beta-blockade. JAMA Dermatol. 2015;151:1226–1229. doi: 10.1001/jamadermatol.2015.2554. [DOI] [PubMed] [Google Scholar]
- 55.Daguze J., Saint-Jean M., Peuvrel L., Cassagnau E., Quereux G., Khammari A. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016;2:497–499. doi: 10.1016/j.jdcr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dal Monte M., Casini G., Filippi L., Nicchia G.P., Svelto M., Bagnoli P. Functional involvement of beta3-adrenergic receptors in melanoma growth and vascularization. J Mol Med. 2013;91:1407–1419. doi: 10.1007/s00109-013-1073-6. [DOI] [PubMed] [Google Scholar]
- 57.Diaz E.S., Karlan B.Y., Li A.J. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol. 2012;127:375–378. doi: 10.1016/j.ygyno.2012.07.102. [DOI] [PubMed] [Google Scholar]
- 58.Filippi L., Tamburini A., Berti E., Perrone A., Defilippi C., Favre C. Successful propranolol treatment of a kaposiform hemangioendothelioma apparently resistant to propranolol. Pediatr Blood Canc. 2016;63:1290–1292. doi: 10.1002/pbc.25979. [DOI] [PubMed] [Google Scholar]
- 59.Grytli H.H., Fagerland M.W., Fossa S.D., Tasken K.A. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635–641. doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Grytli H.H., Fagerland M.W., Fossa S.D., Tasken K.A., Haheim L.L. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate. 2013;73:250–260. doi: 10.1002/pros.22564. [DOI] [PubMed] [Google Scholar]
- 61.Lamkin D.M., Sung H.Y., Yang G.S., David J.M., Ma J.C., Cole S.W. alpha2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology. 2015;51:262–270. doi: 10.1016/j.psyneuen.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemeshow S., Sorensen H.T., Phillips G., Yang E.V., Antonsen S., Riis A.H. beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomark Prev. 2011;20:2273–2279. doi: 10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melhem-Bertrandt A., Chavez-Macgregor M., Lei X., Brown E.N., Lee R.T., Meric-Bernstam F. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pasquier E., Andre N., Street J., Chougule A., Rekhi B., Ghosh J. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87–95. doi: 10.1016/j.ebiom.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasquier E., Street J., Pouchy C., Carre M., Gifford A.J., Murray J. beta-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 2013;108:2485–2494. doi: 10.1038/bjc.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pramanik R., Gogia A., Malik P.S., Gogi R. Metastatic primary angiosarcoma of the breast: can we tame it the metronomic way. Indian J Med Paediatr Oncol. 2017;38:228–231. doi: 10.4103/ijmpo.ijmpo_156_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sereni F., Dal Monte M., Filippi L., Bagnoli P. Role of host beta1- and beta2-adrenergic receptors in a murine model of B16 melanoma: functional involvement of beta3-adrenergic receptors. Naunyn Schmiedeberg’s Arch Pharmacol. 2015;388:1317–1331. doi: 10.1007/s00210-015-1165-7. [DOI] [PubMed] [Google Scholar]
- 68.Udumyan R., Montgomery S., Fang F., Almroth H., Valdimarsdottir U., Ekbom A. Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res. 2017;77:3700–3707. doi: 10.1158/0008-5472.CAN-17-0108. [DOI] [PubMed] [Google Scholar]
- 69.Watkins J.L., Thaker P.H., Nick A.M., Ramondetta L.M., Kumar S., Urbauer D.L. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121:3444–3451. doi: 10.1002/cncr.29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wnorowski A., Sadowska M., Paul R.K., Singh N.S., Boguszewska-Czubara A., Jimenez L. Activation of beta2-adrenergic receptor by (R,R')-4'-methoxy-1-naphthylfenoterol inhibits proliferation and motility of melanoma cells. Cell Signal. 2015;27:997–1007. doi: 10.1016/j.cellsig.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]