Abstract
INTRODUCTION
Prognostic thresholds for 30-day major adverse cardiac events (MACE) have been studied for high-sensitivity troponin T (hsTnT) in patients with suspected acute coronary syndrome (ACS), but there is limited data on the prognostic performance of hsTnT for one-year MACE.
METHODS
We prospectively measured hsTnT (in ng/mL up to two decimal places) at 0, 2 and 7 hours for patients presenting with symptoms suggestive of ACS to our emergency department from March 2010 to April 2013. We assessed the prognostic performance of hsTnT cut-offs for 30-day and one-year MACE, and the utility of delta-hsTnT in predicting MACE.
RESULTS
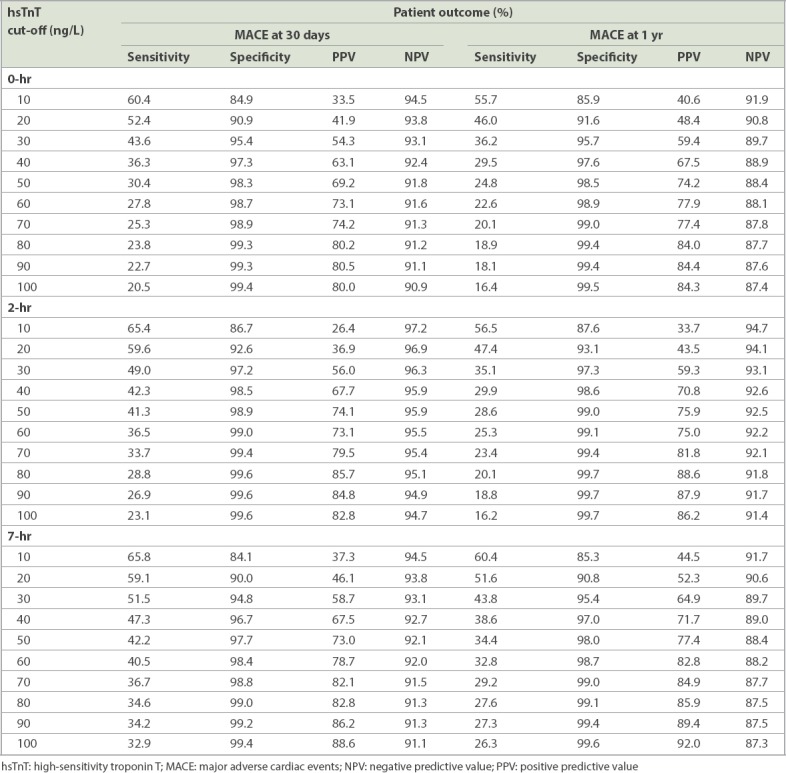
Among 2,444 patients studied, 273 (11.2%) developed MACE (including index MACE) by 30 days and 359 (14.7%) patients developed MACE at one year. The suggested hsTnT cut-off for 30-day MACE was ≥ 10 ng/L at 0 hour (positive predictive value [PPV] 33.5%, negative predictive value [NPV] 94.5%) and 7 hours (PPV 37.3%, NPV 94.5%), and ≥ 20 ng/L at 2 hours (PPV 36.9%, NPV 96.9%). For one-year MACE, the suggested cut-off was also ≥ 10 ng/L at all readings. Plasma hsTnT ≥ 30 ng/L at any reading gave PPV > 54% and NPV > 93% for 30-day MACE. Absolute 0–2 hour and 2–7 hour delta-hsTnT ≥ 10 ng/L gave PPV > 50% for 30-day and one-year MACE.
CONCLUSION
Patients with 0-, 2- or 7-hour hsTnT ≥ 30 ng/L and 0–2 hour delta-hsTnT ≥ 10 ng/L had PPV > 50% for 30-day and one-year MACE, and should be investigated thoroughly.
Keywords: acute coronary syndrome, chest pain, troponin
INTRODUCTION
Chest pain is a common presenting complaint to the emergency department (ED). Identifying dangerous causes of chest pain, such as acute coronary syndrome (ACS), can be a diagnostic challenge.(1) With the new high-sensitivity troponin assays, low cardiac troponin concentrations are detected with improved precision, allowing for earlier diagnosis of acute myocardial infarction (MI).(2,3) While, by definition, highly sensitive troponin assays do not detect elevations in unstable angina,(4) small rises are identified in many non-ACS presentations, such as chronic renal impairment, septic shock and arrhythmias.(5) Likewise, highly sensitive assays detect troponin elevations in pulmonary embolism(6) or congestive cardiac failure,(7) which may present with symptoms similar to ACS.
While elevated troponin levels are associated with poorer prognosis,(8,9) regardless of the underlying aetiology, optimal thresholds for prognosticating future major adverse cardiac events (MACE) have not been well evaluated. Similarly, while the change in troponin levels at different time points (delta) may be associated with poorer outcomes, such as mortality,(10) exact cut-offs have not been defined.
The prevalence and incidence of MI and coronary heart disease varies among presenting populations. In the past, rates of coronary heart disease were lower in Asia as compared to developed countries, but this is changing in advanced economies, such as Singapore, reflecting changes in urbanisation and lifestyle and concurrent worsening cardiovascular risk profiles in Southeast Asia.(11) The incidence of MI has been increasing in Singapore, with the age-standardised incidence rate increasing from 208.9 per 100,000 population in 2007 to 220.8 per 100,000 population in 2016.(12) This increase will affect the prevalence of ACS within ED presentations and influence the diagnostic and prognostic performance of tests, such as high-sensitivity troponin assays. Therefore, there is a need to assess test performance in the specific context of an affluent 21st century Southeast Asian population.
Currently, the diagnosis of acute MI is made on the basis of detection of a rise and/or fall of cardiac biomarker values with at least one value above the 99th percentile upper reference limit, together with symptoms of ischaemia, new changes on electrocardiography suggestive of ischaemia, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality, or identification of intracoronary thrombus.(5) For example, the accepted threshold to define an elevated high-sensitivity troponin T (hsTnT) (Roche Diagnostics, Penzberg, Germany) is 14 ng/L for the diagnosis of MI. This represents the 99th percentile of the normal population reference range and, at this value, the assay has less than a 10% coefficient of variation (CV) (13 ng/L), and the limit of detection is 5 ng/L. The 99th percentile upper reference limit varies from population to population. This remains one of the challenges of the high-sensitivity assays, as there is no consensus on early or long-term prognostic post-presentation MACE-defined cut-off levels.(13)
This study aimed to identify optimal values of hsTnT for the prediction of 30-day and one-year MACE in the setting of patients in Singapore presenting with symptoms suggestive of ACS, and to investigate the utility of both absolute and relative changes (delta) in hsTnT at 0–2, 2–7 and 0–7 hours for the risk stratification of patients for MACE.
METHODS
This was a prospective observational convenience sample cohort study of patients presenting to the ED at Singapore General Hospital, Singapore, from March 2010 to April 2013 with symptoms suggestive of ACS (e.g. chest pain or angina equivalent). The study site was the ED at Singapore General Hospital, which is a 1,597-bed, tertiary-care hospital. This ED sees approximately 350 patients per day and accounts for about 16% of ED attendances in Singapore’s public hospitals.(14) Patients were recruited only from Monday to Friday, from 0800 to 2100 hours, taking into account the availability of research coordinators. Patients were included if they were aged 25 years and above, provided informed written consent, and had a chief complaint consistent with suspected ACS. Exclusion criteria included an ED diagnosis of ST-elevation MI (STEMI) and a history of end-stage renal failure. Patients who did not have any data on cardiac troponin obtained as part of standard care as well as those lost to follow-up were also excluded.
Our standard operating procedures required patients with chest pain or symptoms suggestive of ACS, but with no diagnostic electrocardiography changes, to undergo continuous cardiac monitoring and a standard eight-hour observation protocol in the emergency cardiac care unit at our ED. Serial 12-lead electrocardiography and serum cardiac troponin were obtained at 0, 2 and 7 hours for these patients. At the time of study, hsTnT was used and reported to up to two decimal places in ng/mL at our institution. We defined an abnormal hsTnT as 30 ng/L, based on a previous study that identified a cut-off of 0.03 ng/mL or 30 ng/L for conventional cardiac troponin T (cTnT) (Troponin T 4th generation assay; Roche Diagnostics, Penzberg, Germany; 99th percentile upper reference and lower detection limit: 0.01 ng/mL, 10% CV precision: 0.03 ng/mL) giving a sensitivity of 88%, specificity of 97%, positive predictive value (PPV) of 39% and negative predictive value (NPV) of 100% for MI, and a sensitivity of 35%, specificity of 99%, PPV of 72% and NPV of 93% for the diagnosis of ACS at index visit.(15) The lowest value reported from our laboratory translated to an hsTnT level < 5 ng/L. Similarly, a hsTnT reading of 10 ng/L translated to a value of 5–14 ng/L, a reading of 20 ng/L translated to a value of 15–24 ng/L, 30 ng/L translated to 25–34 ng/L, 40 ng/L translated to 35–44 ng/L, 50 ng/L translated to 45–54 ng/L, and so forth.
During monitoring, patients who subsequently developed chest pain consistent with myocardial ischaemia, or had electrocardiography changes or an elevated troponin level were admitted to the inpatient cardiology service. Patients discharged from the ED were followed up by a cardiologist within 30 days for review and to assess if his/her symptoms were likely to be due to coronary artery disease. Patients at intermediate risk, as determined clinically by the attending physician, underwent stress nuclear myocardial perfusion imaging within three days, either during observation in the emergency cardiac care unit or as an outpatient.
Research staff collected a standardised data set on each participant. This included demographic variables, such as age and past medical history, current medications, presenting signs and symptoms, test results, all interventions and outcomes. Patients were followed up for a year via telephone and/or through assessing medical records.
The primary outcome for analyses was MACE within 30 days of the index visit, inclusive of MACE at the index visit, defined as any of the following: cardiac death; ventricular fibrillation; MI; critical stenosis found on coronary angiography (≥ 50% for the left main coronary artery stenosis or ≥ 70% for epicardial vessel stenosis); and emergency cardiac revascularisation procedures (e.g. coronary artery bypass graft, percutaneous coronary intervention). The secondary outcome was MACE at one year.
The 30-day and one-year diagnoses and outcomes were independently adjudicated by an emergency medicine attending physician and an attending cardiologist based on the case records, which included investigation results and data on troponin collected during the index visit and up to one year of follow-up. Where inter-reviewer differences with respect to adjudicated outcomes were found, discussion was held between the two reviewers to reach a consensus. The outcome of acute MI was defined according to the third universal definition,(5) and unstable angina was defined as the presence of ischaemic symptoms in the absence of elevated troponin level with subsequent or prior evidence of ischaemia on coronary angiography, stress echocardiography, stress electrocardiography or myocardial perfusion imaging. The other predefined diagnoses for the patient’s index visit were that of aortic dissection, pulmonary embolism, pericarditis, gastrointestinal diagnosis (including gastritis, peptic ulcer disease and gastric carcinoma), and non-cardiac or non-specific chest pain. These diagnoses followed the International Classification of Diseases, tenth revision.(16) Patients who were discharged without further evaluation and without MACE at one year were defined as having non-cardiac chest pain.
Statistical analysis was carried out using IBM SPSS Statistics version 20 (IBM Corp, Armonk, NY, USA). Univariate analysis was performed to identify variables associated with MACE, including variables such as age, gender, significant risk factors (e.g. history of hypertension, dyslipidaemia, diabetes mellitus and smoking), laboratory values (e.g. serum creatinine and estimated glomerular filtration rate), history of ischaemic heart disease and percutaneous coronary intervention and/or coronary artery bypass graft, and current medications, including the use of protective drugs (e.g. statins, beta-blockers and antiplatelets). Categorical variables were compared using Fisher’s exact test or chi-square test, as appropriate, and continuous variables were analysed with Mann-Whitney U test or Student’s t-test. Logistic regression analysis was done using variables with p < 0.1 from the univariate analysis to identify variables independently associated with MACE. Receiver operating characteristic (ROC) curves were derived using hsTnT levels at 0, 2 and 7 hours post presentation. Optimal cut-offs were derived from the Youden index. This study was approved by the institutional review board at SingHealth, Singapore.
RESULTS
A total of 2,546 patients were recruited. We excluded 102 patients from the final analysis for the following reasons: 47 patients due to diagnosis of STEMI in the ED; 25 patients for end-stage renal failure; three patients due to missing troponin data; and 27 patients who were lost to follow-up. A total of 2,444 patients were included in the final analysis population, of whom 1,634 (66.9%) patients were men (median age 55 [interquartile range (IQR) 47–64] years). Table I shows the demographics and patient characteristics of the study population.
Table I.
Characteristics of patients with 30-day and one-year MACE (n = 2,444).
In our study group, 273 (11.2%) patients developed 30-day MACE, of whom 153 (6.3%) patients had MI within 30 days. There were 359 (14.7%) patients who had MACE within one year, among whom 179 (7.3%) patients developed MI.
A total of 1,415 (57.9%) patients had all of the 0-, 2- and 7-hour hsTnT evaluations performed. A small proportion of patients had only 0-hour (n = 446; 18.2%); 0-hour and 2-hour (n = 117; 4.8%); and 0-hour and 7-hour hsTnT but no 2-hour hsTnT (n = 466; 19.1%) evaluations performed.
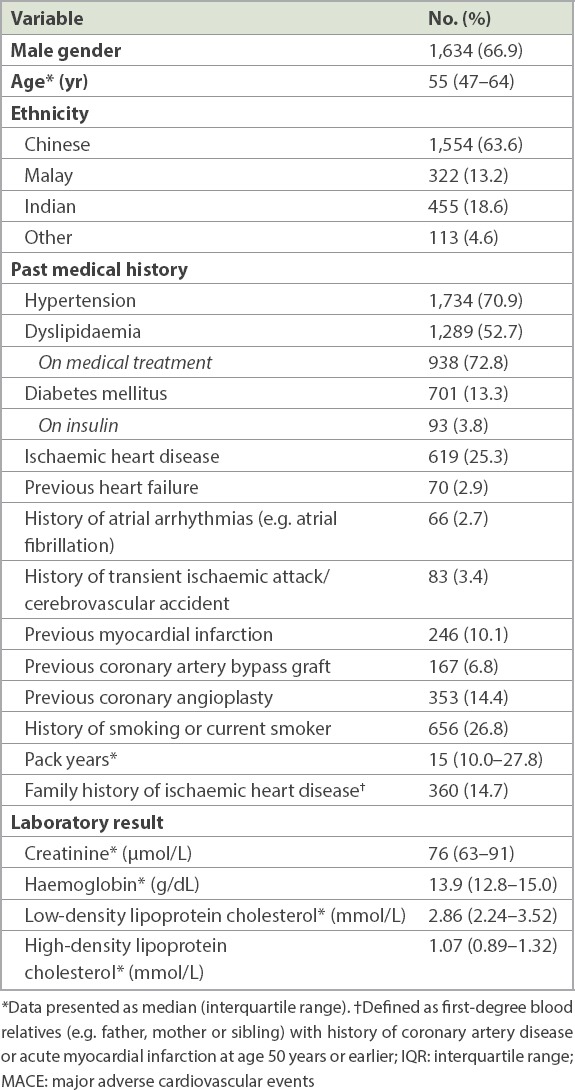
For 30-day MACE, hsTnT readings taken at 0, 2 and 7 hours were used for ROC analyses that yielded area under the curve (C-statistics) of 0.751, 0.790 and 0.783, respectively. Fig. 1 displays the ROC curves for 0-, 2- and 7-hour hsTnT with respect to 30-day MACE. The optimal cut-off by Youden index for hsTnT was 10 ng/L for 0-hour, 20 ng/L for 2-hour and 10 ng/L for 7-hour hsTnT predicting 30-day MACE. The cut-off of hsTnT of 30 ng/L consistently gave a PPV > 50% for 0-, 2- and 7-hour hsTnT for 30-day MACE. Table II provides the sensitivity, specificity, PPV and NPV of a selected range of hsTnT cut-off levels at 0, 2 and 7 hours for 30-day and one-year MACE.
Fig. 1.
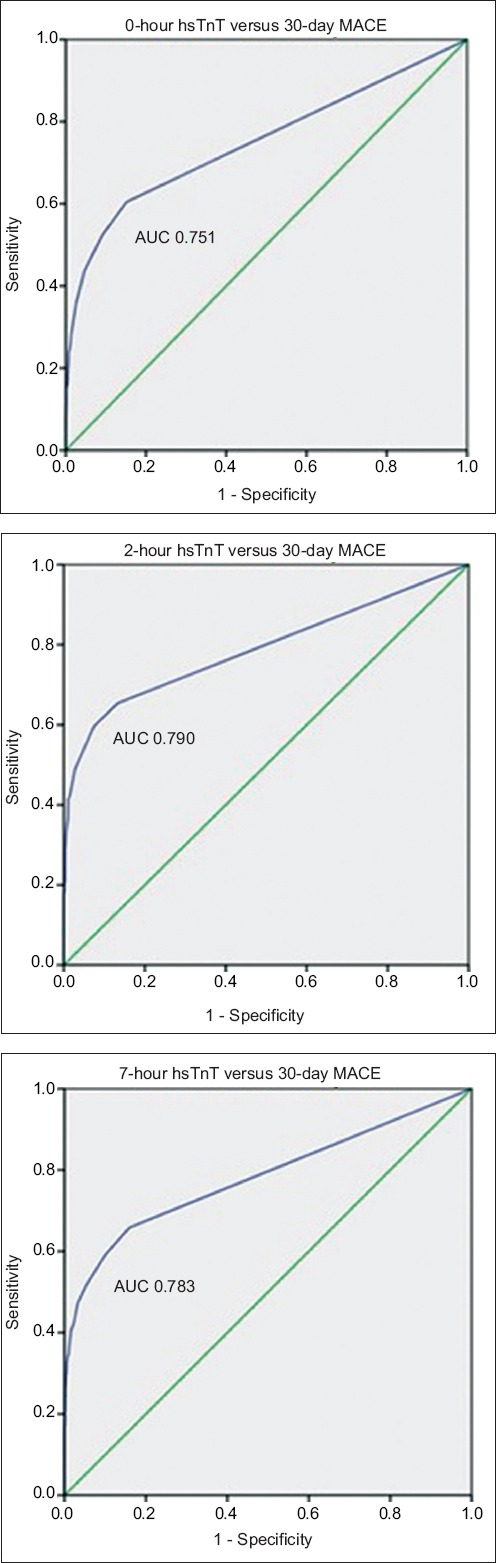
Charts show receiver operating characteristic curves for 0-, 2- and 7-hr hsTnT with respect to 30-day MACE. AUC: area under the curve; hsTnT: high-sensitivity troponin T; MACE: major adverse cardiac events
Table II.
hsTnT cut-off levels at presentation and their association with 30-day and 1-year MACE.
For the secondary outcome of one-year MACE, the ROC curve for hsTnT at presentation yielded C-statistics of 0.726, 0.739 and 0.753 for 0-, 2- and 7-hour hsTnT, respectively. Fig. 2 displays the ROC curves for 0-, 2- and 7-hour hsTnT with respect to the prediction of one-year MACE. The optimal cut-off by Youden index for hsTnT was also 10 ng/L for 0-, 2- and 7-hour hsTnT for one-year MACE.
Fig. 2.
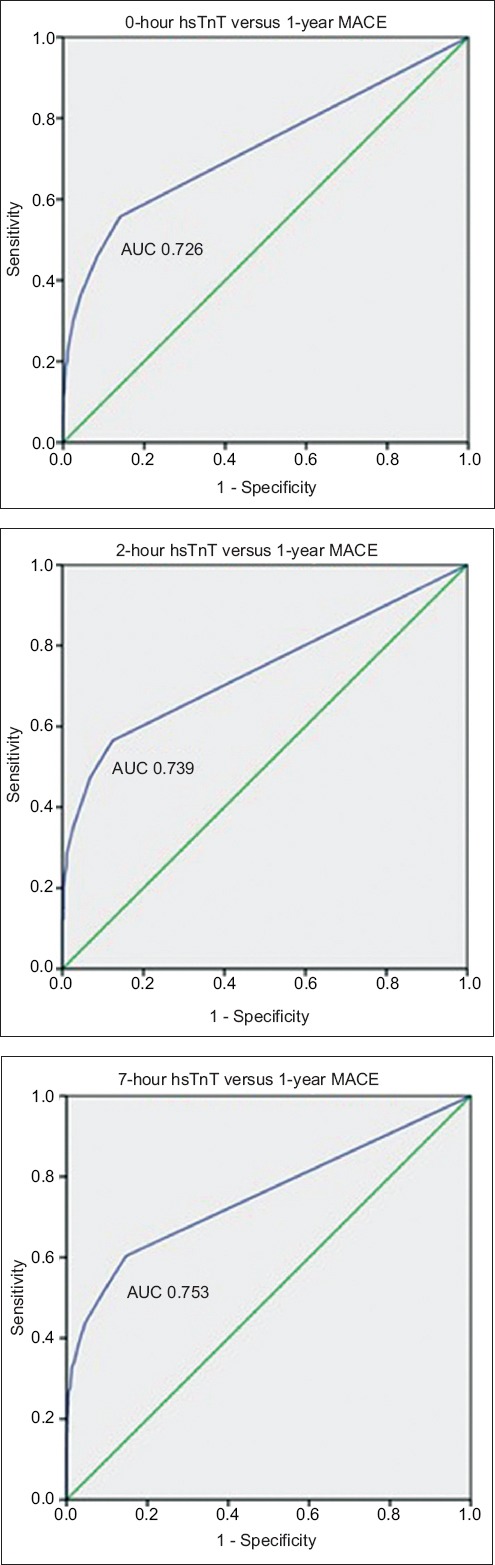
Charts show receiver operating characteristic curves for 0-, 2- and 7-hr hsTnT with respect to one-year MACE. AUC: area under the curve; hsTnT: high-sensitivity troponin T; MACE: major adverse cardiac events
A total of 552 (22.6%) patients had one or more troponin readings ≥ 10 ng/L. This was associated with both 30-day MACE (odds ratio [OR] 10.3, 95% confidence interval [CI] 7.83–13.64, p < 0.001; sensitivity 67.8%, specificity 83.1%, PPV 33.5%, NPV 95.4%) and one-year MACE (OR 8.62, 95% CI 6.76–10.99, p < 0.001; sensitivity 61.8%, specificity 84.2%, PPV 40.2%, NPV 92.8%). Out of these 552 patients, 185 (33.5%) patients developed 30-day MACE and 222 (40.2%) patients developed MACE within one year. There were 1,892 (77.4%) patients with hsTnT < 5 ng/L for all readings, of whom 88 (4.7%) patients had 30-day MACE and 137 (7.2%) patients had one-year MACE.
The cut-off of hsTnT at 50 ng/L, which corresponded to conventional troponin T (Roche Diagnostics) of 30 ng/L (10% CV precision of assay), gave PPVs of approximately 70% for 30-day and one-year MACE (Table II). There were 42 (25.3%) patients with hsTnT ≥ 50 ng/L at any one reading (i.e. at 0, 2 or 7 hours) who did not have one-year MACE. The final presenting diagnosis for these 42 patients comprised three patients with unstable angina, 11 patients with decompensated heart failure, one patient with aortic dissection, one patient with pulmonary embolism, one patient with dyspepsia, one patient with decompensated mitral regurgitation, three patients with sepsis/infection and 21 patients with non-cardiac chest pain. As compared to the study population, this group of patients who did not have 30-day or one-year MACE had a higher median age (66 years) when compared with that of patients having 30-day or one-year MACE (55 years). The median serum creatinine was also higher among patients who did not have 30-day or one-year MACE (median [IQR] 123 [95–215] µmol/L) when compared to the study population (median [IQR] 76 [63–91] µmol/L). Correspondingly, estimated glomerular filtration rate was lower among patients who did not have 30-day or one-year MACE (median [IQR] 40.2 [20.9–73.2] mL/min) when compared to the study population (median [IQR] 88.3 [66.6–112.8] mL/min). For the three patients with unstable angina, the elevation in hsTnT was attributed to other factors, such as chronic kidney disease, that lead to a higher value of hsTnT at baseline.
Table III shows the number of patients with true and false positive results for 30-day and one-year MACE at various hsTnT cut-offs. In our study population, when we used a 0-hour hsTnT cut-off of 10 ng/L instead of 30 ng/L, this picked up an additional 46 patients for 30-day MACE (16.8% of 30-day MACE patients) and additional 70 patients for one-year MACE (19.5% of one-year MACE patients). However, there were 228 (46.2% of patients with 0-hour hsTnT ≥ 10 ng/L) more patients with false positive results for 30-day MACE and 204 (41.4% of patients with 0-hour hsTnT ≥ 10 ng/L) more false positive patients for one-year MACE when using a 0-hour hsTnT cut-off of 10 ng/L instead of 30 ng/L, indicating that these patients may not have needed urgent cardiac investigations or intervention. The ratio of one false positive per one additional one-year MACE identified was 2.91.
Table III.
Patients with true positive and false positive results for 30-day and 1-year MACE at the various hsTnT cut-offs.
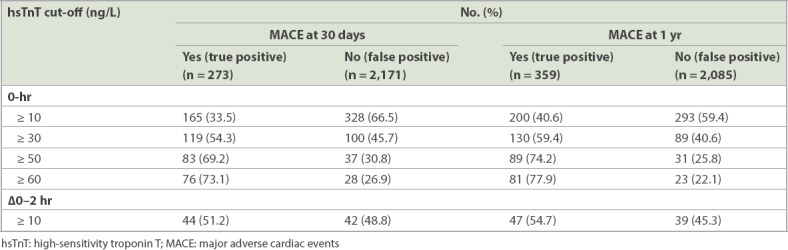
When compared to a 0-hour hsTnT cut-off of 50 ng/L, a cut-off of 30 ng/L picked up an additional 36 patients for 30-day MACE (13.2% of 30-day MACE patients) and an additional 41 patients for one-year MACE (11.4% of one-year MACE patients). In addition, there were only 63 (28.8% of patients with 0-hour hsTnT ≥ 30 ng/L) more patients with false positive results for 30-day MACE and 58 (26.5% of patients with 0-hour hsTnT ≥ 30 ng/L) more patients with false positive results for one-year MACE when using 0-hour hsTnT cut-off of 30 ng/L instead of 50 ng/L. The ratio of one false positive per one additional one-year MACE identified was 1.41.
Table IV shows the sensitivity, specificity, PPV and NPV of absolute delta-hsTnT for patients with 30-day and one-year MACE. For absolute 0–2 hour delta-hsTnT, the C-statistic was 0.829 for 30-day MACE and 0.789 for one-year MACE. A total of 86 patients had positive absolute 0–2 hour delta-hsTnT ≥ 10 ng/L, which gave a PPV of 51.2% and NPV of 95.9% for 30-day MACE, and PPV of 54.7% and NPV of 92.5% for one-year MACE. For absolute 2–7 hour delta-hsTnT, the C-statistics was 0.918 for 30-day MACE and 0.878 for one-year MACE. A positive absolute 2–7 hour delta-hsTnT ≥ 10 ng/L gave PPVs of over 55% for 30-day and one-year MACE.
Table IV.
Absolute delta-hsTnT and association with 30-day and 1-year MACE.
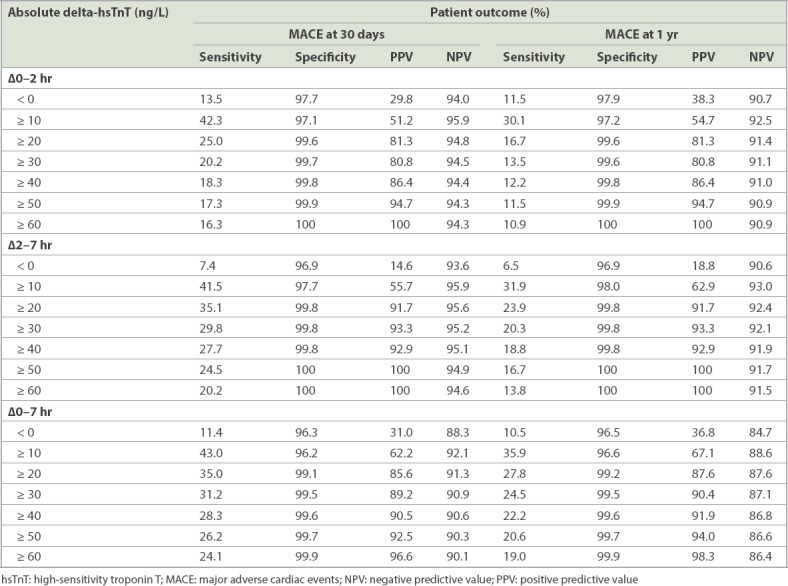
In our study population, 54 patients had both 0-hour hsTnT ≥ 10 ng/L and a positive absolute 0–2 hour delta-hsTnT ≥ 10 ng/L result, out of whom 35 patients had 30-day MACE and 36 patients had one-year MACE. By combining both 0-hour hsTnT ≥ 10 ng/L and positive absolute 0–2 hour delta-hsTnT ≥ 10 ng/L, there was a sensitivity of 12.8%, specificity of 99.1%, PPV of 64.8% and NPV of 90.0% for 30-day MACE, and a sensitivity of 10.0%, specificity of 99.1%, PPV of 66.7% and NPV of 86.5% for one-year MACE.
Negative delta-hsTnT at 0–2 hours (i.e. delta-hsTnT < 0 ng/L; 30-day MACE: OR 6.60, 95% CI 3.41–12.8, p < 0.001; one-year MACE: OR 6.08, 95% CI 3.29–11.2) and 2–7 hours (30-day MACE: OR 2.51, 95% CI 1.10–5.76, p = 0.025; one-year MACE: OR 2.22, 95% CI 1.05–4.68, p = 0.033) were associated with 30-day and one-year MACE. Patients with negative delta-hsTnT at 0–2 hours were found to have a higher 0-hour hsTnT (median 20 ng/L) when compared to the whole study population (median < 5 ng/L). Similarly, patients with negative delta-hsTnT at 2–7 hours had a higher 2-hour hsTnT (median 20 ng/L) when compared to those who did not (median < 5 ng/L).
A total of 1,698 (69.5%) patients had no change in their hsTnT values (delta-hsTnT = 0 for 0–2 hours and 2–7 hours, excluding those who had only a single hsTnT value taken at presentation). Out of these patients, 1,506 (88.7%) patients had hsTnT < 5 ng/L, whereas 192 (11.3%) patients had hsTnT ≥ 10 ng/L. Out of these 192 patients with no change in hsTnT but with a baseline hsTnT ≥ 10 ng/L, 33 (17.2%) patients had 30-day MACE (OR 4.02, 95% CI 2.58–6.25, p < 0.001; sensitivity 30.8%, specificity 90.0%, PPV 17.2%, NPV 95.1%) and 51 (26.6%) patients had one-year MACE (OR 4.29, 95% CI 2.96–6.23, p < 0.001; sensitivity 30.4%, specificity 90.8%, PPV 26.6%, NPV 92.2%).
The C-statistics for relative delta 0–2 hour hsTnT and 2–7 hour hsTnT was 0.637 and 0.669, respectively, for 30-day MACE, and 0.589 and 0.628, respectively, for one-year MACE. This was significantly lower than values for both absolute delta-hsTnT and hsTnT when used alone.
After multivariate logistic regression analysis, hsTnT at 0 hour ≥ 10 ng/L (30-day MACE: OR 3.52, 95% CI 2.01–6.14; one-year MACE: OR 3.78, 95% CI 2.33–6.13) and 0–2 hour delta-hsTnT ≥ 10 ng/L (30-day MACE: OR 8.94, 95% CI 4.68–17.1; one-year MACE: OR 6.03, 95% CI 3.25–11.2) were both shown to be independently associated with 30-day and one-year MACE. Additional variables that were independently associated with 30-day MACE were history of current or previous smoking (OR 2.25, 95% CI 1.29–3.93), presence of diaphoresis (OR 2.13, 95% CI 1.22–3.72), radiation of pain to both arms (OR 6.96, 95% CI 2.05–23.7) and serum low-density lipoprotein cholesterol levels ≥ 3.4 mmol/L (OR 2.70, 95% CI 1.58–4.63). Other variables associated with one-year MACE after multivariate logistic regression analysis were history of current or previous smoking (OR 2.07, 95% CI 1.26–3.40), presence of diaphoresis (OR 2.04, 95% CI 1.24–3.34), radiation of pain to both arms (OR 6.30, 95% CI 2.04–19.5) and symptoms relieved by rest (OR 3.78, 95% CI 2.33–6.13).
DISCUSSION
While several studies have evaluated the prognostic value of troponin for the prediction of 30-day MACE,(17-20) the data on its use for predicting one-year MACE is not as well established. In our study population, elevated levels of hsTnT were powerfully predictive of MACE up to one year after the index event. A cut-off for 0-, 2-, or 7-hour hsTnT results of 30 ng/L for predicting 30-day MACE gave a PPV of over 50%. This suggests that patients presenting to the ED with possible ACS and any hsTnT reading ≥ 30 ng/L should be further investigated prior to discharge (e.g. using coronary angiography, if there are no contraindications), as they would have > 50% chance of MACE in the subsequent 30 days, with a 0-hour hsTnT ≥ 30 ng/L giving a PPV of 54.3% and 59.4% for 30-day and one-year MACE, respectively. Those with hsTnT ≥ 10 ng/L but < 30 ng/L should be further evaluated with less invasive tests, such as computed tomography coronary angiography or stress nuclear myocardial perfusion imaging. For patients with 0-hour hsTnT ≥ 10 ng/L, PPV was 33.5% for 30-day MACE and 40.6% for one-year MACE. This cut-off of 10 ng/L correlates with the 10% CV precision of 13 ng/L for hsTnT.(21) It is similar to the current European Society of Cardiology guidelines,(22) which advise the use of a ‘rule in’ of 0-hour hsTnT > 52 ng/L or 0–1 hour delta-hsTnT ≥ 5 ng/L, and a ‘rule out’ of 0-hour hsTnT < 5 ng/L or 0-hour hsTnT together with 0–1 hour delta-hsTnT < 3 ng/L for the diagnosis of non-STEMI (NSTEMI). Patients who are neither in the ‘rule in’ nor the ‘rule out’ category may require further investigations, such as coronary angiography for those with high clinical suspicion, and stress myocardial perfusion imaging or computed tomography coronary angiography for those with low-to-intermediate probability.(22,23)
While the cut-offs that we attained for 30-day MACE had NPVs of over 94%, we were unable to identify a cut-off value to effectively rule out 30-day or one-year MACE based on single readings of hsTnT alone due to low sensitivity. However, it could be seen that hsTnT levels as low as 10 ng/L were associated with MACE at 30 days and even up to one year. As conventional fourth-generation troponin T assay levels cannot be directly converted to hsTnT levels (i.e. fourth-generation troponin T of 30 ng/L [10% CV] corresponds approximately to hsTnT of 52 ng/L), levels of troponin detected by the hsTnT assay may be undetectable when the fourth-generation assay is used. High-sensitivity troponin should thus be used instead of conventional troponin as it allows for a greater proportion of patients at risk of MACE to be identified for further intervention. In the ED, the use of high-sensitivity troponin may not only be used as a marker to rule out acute disease but should also be considered of prognostic value for future events, even for patients with detectable levels below the 99th percentile or with stable low-level elevations, who may need to be carefully followed up.(24)
Elevated cardiac troponin level is associated with worse prognosis for adverse events, such as mortality and MACE in various settings.(25-27) A lower 10% CV for hsTnT (10% CV 13 ng/L) versus conventional troponin T (10% CV 0.03 ng/mL [equivalent to hsTnT of 52 ng/L]) may lead to a better prognostic value of the test, as previously undetected levels of troponin T are now detectable. Patients with undifferentiated chest pain who were discharged from the ED were found to have a lower incidence of 30-day MACE after hsTnT was used for their evaluation, when compared to conventional troponin, postulating that more at-risk patients were identified using hsTnT and hence admitted to hospital for further follow-up.(18) The use of high-sensitivity reporting (actual reported level > 3 ng/L when compared with conventional reporting, where only actual levels ≥ 30 ng/L are reported) has also been associated with reduction in one-year deaths or new/recurrent ACS among patients with hsTnT levels < 30 ng/L as opposed to standard reporting (high-sensitivity reporting: 2.6% vs. standard reporting: 4.4%; p = 0.050), suggesting that even differences in hsTnT < 30 ng/L may have an impact on prognosis, although the trial showed that high-sensitivity reporting had no significant difference on 12-month mortality or angiography among the entire study population.(28) Detectable levels of hsTnT have also been associated with increased risk of death and cardiovascular outcomes among patients who present to the ED with chest pain and stable troponin levels.(29)
As a rule-out strategy, hsTnT alone is still not sufficient for the purposes of identifying patients with high risk of MACE, and should be combined with assessment of other clinical factors.(30) Variables identified from multivariate logistic regression analysis as independent predictors of MACE other than hsTnT include: presentation with history of radiation of pain to both arms, pain relieved by rest, presence of diaphoresis, history of smoking and serum low-density lipoprotein cholesterol levels ≥ 3.4 mmol/L. Alternative strategies also include investigating the use of hsTnT when used with other biomarkers, such as soluble isoform of suppression of tumorigenicity 2 (sST2), which has been shown to be useful in prognosticating 30-day cardiac mortality among ED patients with chest pain.(31)
While the rise and fall of cardiac biomarkers is one of the components in the diagnosis of MI,(5) the link between delta-hsTnT and future MACE is less established. Among patients with NSTEMI, relative delta-hsTnT of 20% was linked to higher long-term mortality, and 30-day mortality was similar across quartiles of relative delta-hsTnT.(32) Another study found that using an algorithm that incorporated both hsTnT and delta-hsTnT values based on current guidelines (with cut-offs of delta-hsTnT at 1 hour < 3 ng/L for ‘rule out’ and ≥ 5 ng/L for ‘rule in’)(23) together with clinical history and electrocardiography findings, when compared to using hsTnT and delta-hsTnT values alone, had a higher sensitivity for ruling out patients with 30-day MACE (troponin only: 87.6%, extended algorithm: 97.5%, p < 0.001).(33)
From our study, no 2-hour delta provided adequate sensitivity and NPV to allow discharge without further evaluation by stress testing; absolute delta-hsTnT ≥ 10 ng/L at 0–2 hours and 2–7 hours had high PPV for both 30-day and one-year MACE, and patients exceeding these delta values should be investigated early for underlying cardiovascular pathology and treated accordingly.
Our study was not without limitations. This was a prospective observational study and was prone to selection bias. We tried to overcome this shortcoming by collecting information from a large database of patients. Moreover, it was held at a single centre in Singapore and the results may not be generalisable internationally. However, as troponin cut-offs may differ from community to community, these values may be helpful and applicable specifically to the Singapore population.
Another limitation was that based on our institutional protocol, hsTnT was reported as ng/mL up to two decimal places instead of three, as previously mentioned in the Methods section. Hence, the loss of the last decimal place may have reduced the sensitivity of the test, and minute differences that may have possibly affected delta-hsTnT levels might have remained undetected. We also chose to report our findings in ng/L instead of ng/mL as they were originally reported, as the convention is for hsTnT to be interpreted in ng/L. In addition, while every patient had hsTnT done at presentation, not all patients had hsTnT done at 2 and 7 hours, as the tests were done at the discretion of the attending physician.
Despite this, to our knowledge, no other previous study has delineated the sensitivity, specificity, PPV and NPV of the different hsTnT cut-off levels and their association with MACE, specifically in an Asian population. Moreover, we were able to extract general trends regarding the use of hsTnT and its prognostic value with MACE.
In addition, this study included patients with chest pain or symptoms suggestive of ACS, the latter of which may be subjective and dependent on the attending physician. However, as ACS or cardiac chest pain may present atypically, this study may be a better reflection of real-life instances where the cause of symptoms (e.g. shortness of breath or diaphoresis) may not be very clear.
Finally, we lost 27 patients to follow-up. As Singapore has nationalised healthcare with a single payer, it is unlikely that any patient had an undetectable demise. It is much more likely that patients lost to follow-up in our study had simply left the country.
In conclusion, for patients presenting to the ED with symptoms suggestive of ACS, hsTnT is a powerful predictor of both 30-day and one-year MACE. Serum hsTnT ≥ 10 ng/L emerged as the suggested cut-off for 0- and 7-hour hsTnT for 30-day MACE, and the suggested cut-off for 0-, 2- and 7-hour hsTnT for one-year MACE. Meanwhile, 20 ng/L was the suggested cut-off for 2-hour hsTnT for 30-day MACE. However, for these values of hsTnT, PPV only ranged from 33.5% to 44.5%. Patients with 0-hour hsTnT ≥ 30 ng/L or a delta-hsTnT ≥ 10 ng/L had a PPV > 50% for 30-day and one-year MACE and should be investigated thoroughly. Serum hsTnT alone is not sensitive enough to rule out 30-day or one-year MACE and should be used in combination with other clinical factors and/or investigations.
ACKNOWLEDGEMENTS
This study was funded by the SingHealth Foundation Research grant (SHF/FG403P/2008) and National University of Singapore.
REFERENCES
- 1.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. New Engl J Med. 2000;342:1163–70. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 2.Mueller C, Giannitsis E, Christ M, et al. TRA PID-AMI Investigators. Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med. 2016;68:76–87 e4. doi: 10.1016/j.annemergmed.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. New Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 4.Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–93. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–67. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 6.Lankeit M, Friesen D, Aschoff J, et al. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010;31:1836–44. doi: 10.1093/eurheartj/ehq234. [DOI] [PubMed] [Google Scholar]
- 7.Peacock WF, 4th, De Marco T, Fonarow GC, et al. ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. New Engl J Med. 2008;358:2117–26. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 8.Yiu KH, Lau KK, Zhao CT, et al. Predictive value of high-sensitivity troponin-I for future adverse cardiovascular outcome in stable patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2014;13:63. doi: 10.1186/1475-2840-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenevier-Gobeaux C, Bonnefoy-Cudraz É, Charpentier S, et al. SFBC SFC SFMU 'Troponins'Workgroup. High-sensitivity cardiac troponin assays:answers to frequently asked questions. Arch Cardiovasc Dis. 2015;108:132–49. doi: 10.1016/j.acvd.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Apple FS, Smith SW, Pearce LA, Murakami MM. Delta changes for optimizing clinical specificity and 60-day risk of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome utilizing the ADVIA Centaur TnI-Ultra assay. Clin Biochem. 2012;45:711–3. doi: 10.1016/j.clinbiochem.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Meng Khoo C, Tai ES. Trends in the incidence and mortality of coronary heart disease in asian pacific region:the Singapore experience. J Atheroscler Thromb. 2014;21(Suppl 1):S2–8. doi: 10.5551/jat.21_sup.1-s2. [DOI] [PubMed] [Google Scholar]
- 12.National Registry of Diseases Office, Health Promotion Board, Singapore. Singapore Myocardial Infarction Registry Annual Report 2016. 3 Apr 2018. [Accessed May 30, 2018]. Available at: https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/smir-web-report-2016.pdf?sfvrsn=0 .
- 13.Collinson PO, Heung YM, Gaze D, et al. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin Chem. 2012;58:219–25. doi: 10.1373/clinchem.2011.171082. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Health Singapore. Attendances at Emergency Medicine Departments. [Accessed May 30, 2018]. Available at: https://www.moh.gov.sg/resources-statistics/healthcare-institution-statistics/attendances-at-emergency-medicine-departments .
- 15.Januzzi JL, Jr, Bamberg F, Lee H, et al. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation. 2010;121:1227–34. doi: 10.1161/CIRCULATIONAHA.109.893826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. International Classification of Diseases-10. 2016. [Accessed May 30, 2018]. Available at: http://apps.who.int/classifications/icd10/browse/2016/en .
- 17.Cullen L, Mueller C, Parsonage WA, et al. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol. 2013;62:1242–9. doi: 10.1016/j.jacc.2013.02.078. [DOI] [PubMed] [Google Scholar]
- 18.Nejatian A, Omstedt Å, Höijer J, et al. Outcomes in patients with chest pain discharged after evaluation using a high-sensitivity troponin T assay. J Am Coll Cardiol. 2017;69:2622–30. doi: 10.1016/j.jacc.2017.03.586. [DOI] [PubMed] [Google Scholar]
- 19.Mokhtari A, Lindahl B, Schiopu A, et al. A 0-hour/1-hour protocol for safe, early discharge of chest pain patients. Acad Emerg Med. 2017;24:983–92. doi: 10.1111/acem.13224. [DOI] [PubMed] [Google Scholar]
- 20.Pickering JW, Than MP, Cullen L, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection:a collaborative meta-analysis. Ann Intern Med. 2017;166:715–24. doi: 10.7326/M16-2562. [DOI] [PubMed] [Google Scholar]
- 21.Roche Diagnostics. Elecsys®Troponin T high sensitive (TnT-hs) [Accessed May 30, 2018]. Available at: http://www.cobas.com/home/product/clinical-and-immunochemistry-testing/elecsys-troponin-t-hs-tnt-hs.html .
- 22.Roffi M, Patrono C, Collet JP, et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation:Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 23.Lim SH, Anantharaman V, Sundram F, et al. Stress myocardial perfusion imaging for the evaluation and triage of chest pain in the emergency department:a randomized controlled trial. J Nucl Cardiol. 2013;20:1002–12. doi: 10.1007/s12350-013-9736-9. [DOI] [PubMed] [Google Scholar]
- 24.Bonaca MP. Time for a new strategy for high-sensitivity troponin in the emergency department. J Am Coll Cardiol. 2017;70:2237–9. doi: 10.1016/j.jacc.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Eggers KM, Lind L, Ahlström H, et al. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. Eur Heart J. 2008;29:2252–8. doi: 10.1093/eurheartj/ehn327. [DOI] [PubMed] [Google Scholar]
- 26.Latini R, Masson S, Anand IS, et al. Val-He FT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–9. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Hojo Y, Hirose M, et al. High-sensitivity troponin T is a prognostic marker for patients with aortic stenosis after valve replacement surgery. J Cardiol. 2013;61:342–7. doi: 10.1016/j.jjcc.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Chew DP, Zeitz C, Worthley M, et al. Randomized comparison of high-sensitivity troponin reporting in undifferentiated chest pain assessment. Circ Cardiovasc Qual Outcomes. 2016;9:542–53. doi: 10.1161/CIRCOUTCOMES.115.002488. [DOI] [PubMed] [Google Scholar]
- 29.Roos A, Bandstein N, Lundbäck M, et al. Stable high-sensitivity cardiac troponin T levels and outcomes in patients with chest pain. J Am Coll Cardiol. 2017;70:2226–36. doi: 10.1016/j.jacc.2017.08.064. [DOI] [PubMed] [Google Scholar]
- 30.McRae AD, Innes G, Graham M, et al. Undetectable concentrations of a Food and Drug Administration-approved high-sensitivity cardiac troponin T assay to rule out acute myocardial infarction at emergency department arrival. Acad Emerg Med. 2017;24:1467–77. doi: 10.1111/acem.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marino R, Magrini L, Orsini F, et al. GREAT NETWORK. Comparison between soluble ST2 and high-sensitivity troponin I in predicting short-term mortality for patients presenting to the emergency department with chest pain. Ann Lab Med. 2017;37:137–46. doi: 10.3343/alm.2017.37.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjurman C, Larsson M, Johanson P, et al. Small changes in troponin T levels are common in patients with non-ST-segment elevation myocardial infarction and are linked to higher mortality. J Am Coll Cardiol. 2013;62:1231–8. doi: 10.1016/j.jacc.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 33.Mokhtari A, Borna C, Gilje P, et al. A 1-h combination algorithm allows fast rule-out and rule-in of major adverse cardiac events. J Am Coll Cardiol. 2016;67:1531–40. doi: 10.1016/j.jacc.2016.01.059. [DOI] [PubMed] [Google Scholar]


