Abstract
Humanin (HN) is a mitochondrial peptide that exhibits cytoprotective actions against various stresses and diseases. HN has been shown to induce the phosphorylation of AMP-activated protein kinase (AMPK), which is a negative regulator of receptor activator of nuclear factor-κB ligand (RANKL). However, the role of HN in osteoclastogenesis or other skeletal disorders remains unknown. Here, we examined whether HN regulates osteoclastogenesis via AMPK activation using bone marrow-derived macrophage (BMM) cultures. Our results show that HN inhibited RANKL-induced osteoclast formation and reduced the expression of genes involved in osteoclastogenesis, including nuclear factor of activated T-cells cytoplasmic 1, osteoclast-associated receptor, cathepsin K, and tartrate-resistant acid phosphatase. Moreover, HN increased the levels of phosphorylated AMPK protein; compound C, an AMPK inhibitor, recovered HN-induced osteoclast differentiation. In addition, we found that HN significantly decreased the levels of RANKL-induced reactive oxygen species in BMMs. Therefore, these results indicate that HN plays an important role in osteoclastogenesis and may function as an inhibitor of bone disorders via AMPK activation.
Keywords: AMP-activated protein kinase, Humanin, Osteoclastogenesis, Receptor activator of nuclear factor-κB, ligand
INTRODUCTION
Bone is a mineralized connective tissue that is made up of cells, vessels, and calcium-containing crystals; it provides shape, protection, and support for body structures [1]. Bone undergoes continuous remodeling for healing and adaptation. Bone remodeling is continuously replaced by new tissue, requires interactions between bone-resorbing osteoclasts and bone-forming osteoblasts [2,3]. An imbalance between these cell types can result in several bone diseases, including Paget's diseases, osteoporosis, and osteopetrosis [4].
Osteoclasts are derived from hematopoietic cells of a mononuclear lineage and mediate bone loss. Both osteoclast formation and function are regulated by macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) [5]. M-CSF is critical for the proliferation of osteoclast progenitors, whereas RANKL binds to its receptor (RANK) in osteoclast precursors, inducing osteoclast differentiation [1]. Thus, the osteoclast remains a potential therapeutic target for the treatment of osteoporosis and other resorptive bone diseases.
AMP-activated protein kinase (AMPK) is a cellular energy sensor in eukaryotes that is activated when intracellular ATP production decreases [6]. Previous studies have indicated that AMPK is a negative regulator of RANKL-induced osteoclast differentiation [7,8]. Moreover, the mitochondria-derived peptide (MDP), MOTS-c, suppresses ovariectomy-induced bone loss via AMPK activation [9]. MDPs are retrograde signaling molecules that include humanin (HN), MOTS-c, and small humanin-like peptides. HN, the first MDP discovered, is a potential therapeutic agent for Alzheimer's disease [10]. In pancreatic β-cells, HN induces the phosphorylation of AMPK-like MOTS-c [11]. HN also regulates cellular processes via the CNTFR/WSX-1/gp130 trimeric receptor. Interestingly, activation of this receptor leads to stimulation of the STAT3 signaling pathway, which plays a negative role in regulating osteoclastogenesis [12,13]. In addition, HN protein levels in skeletal muscle increase after resistance training in the men with prediabetes and patients with coronary endothelial dysfunction have reduced levels of HN [14,15]. These studies suggest a physiological significance to study the role of HN in various conditions, but endogenous regulation of HN under physiological conditions in bone is not yet established.
Previous studies report that stimulation of bone marrow-derived macrophages (BMMs) by RANKL transiently induces the production of intracellular reactive oxygen species (ROS). Moreover, ROS mediate RANK signaling during osteoclast differentiation [16,17]. HN is known to protect against mitochondrial dysfunction induced by endoplasmic reticulum stress. HN directly inhibits oxidative stress at the mitochondrial membrane [18]. However, no studies have examined the relationship between HN and osteoclasts. We therefore investigated the regulatory mechanism by which HN exerts its effects on RANKL-induced osteoclast differentiation.
METHODS
Reagents
HN were synthesized by Anygen (Gwangju, Korea) and were characterized as having > 99% purity by high-performance liquid chromatography. The amino acid sequence of HN was followed as: Met Ala Pro Arg Gly Phe Ser Cys Leu Leu Leu Leu Thr Ser Glu Ile Asp Leu Pro Val Lys Arg Arg Ala. The peptides were dissolved in double-distilled H2O and aliquots were frozen at −20℃. Cell culture media, alpha-minimum essential medium (α-MEM) was obtained from GE Healthcare Life Sciences (Marlborough, MA, USA). Fetal bovine serum (FBS) and antibiotic-antimycotic were purchased from Gibco (Grand Island, NY, USA). M-CSF was purchased from PeproTech (Rocky Hill, NJ, USA) and RANKL was purchased from R&D Systems (Minneapolis, MN, USA). Compound C was purchased from Cayman Chemical (Ann Arbor, MI, USA). Specific antibody against NFATc1 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), Tubulin was obtained from Developmental Studies Hybridoma Bank (Iowa City, IA, USA), and Actin was obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against AMPK, phosphor (p)-AMPK were obtained from Cell Signaling (Beverly, MA, USA).
Cell culture
Mouse bone marrow cells were isolated from 4–6 week old male ICR mice (Nara Biotech, Seoul, Korea) by flushing the femurs and tibias with α-MEM. The cells were cultured overnight in α-MEM with 10% FBS and 1% antibiotic-antimycotic reagent and incubated in 5% CO2 incubator. Non-adherent cells were collected and seeded on adequate number of plates with M-CSF. After 3 days, non-adherent cells were washed out with fresh media, and the adherent cells were used as BMMs. M-CSF was treated at 30 ng/ml and RANKL was treated at 50 ng/ml concentration in α-MEM.
Tartrate-resistant acid phosphatase (TRAP) staining
BMMs were seeded in 48-well plates at a concentration of 3 × 104 cells per well and cultured in α-MEM containing 10% FBS with M-CSF, RANKL and with or without different concentrations of HN (0, 1, 5 µM). The culture medium was replaced every 2 days. After 6 days, a TRAP staining was performed to confirm the cell differentiation rate. TRAP+ cells were stained using a Leukocyte Acid Phosphate Assay Kit (Sigma-Aldrich) by following the manufacturer's procedure. TRAP+ multinucleated cells (containing ≥ 3 nuclei) were counted.
Cell viability assay
BMMs were cultured in the medium with 30 ng/ml of M-CSF and 50 ng/ml of RANKL with or without HN at indicated concentrations in a 96-well plate. CellTiter 96 AQueous One Solution (Promega, Madison, WI, USA) was used to determine the cell viability following the manufacturer's instructions. Cell viability was measured at the wavelength of 490 nm using a 96-well plate reader.
Western blot
Cellular proteins were extracted in RIPA buffer (Tech & Innovation, Seoul, Korea) and the amounts were measured by using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were separated with 8% or 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore Corp., Bedford, MA, USA). The membranes were blocked for 1 h at room temperature with 5% nonfat skim milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) and then probed with specific antibodies in 5% BSA in TBS-T overnight at 4℃. The primary specific antibodies used were as follows: NFATc1 (1 : 750), AMPK (1 : 2,000), p-AMPK (1 : 2,000), Actin (1 : 1,000), and Tubulin (1 : 5,000). Membranes were then incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies. The antigen-antibody complexes were detected with an ECL Prime Western Blotting Detection Reagent (Amersham Biosciences, Piscataway, NJ, USA). Quantification was performed by densitometry using ImageJ (National Institutes of Health, https://imagej.nih.gov/ij/).
Real-time PCR analysis
Total RNA was isolated by using Trizol reagent (Thermo Fisher Scientific) according to the manufacturer's instruction. cDNA was reverse transcribed from 2 µg of RNA using the MultiScribe Reverse Transcriptase (Applied Biosystems, Foster City, CA, USA). PCR amplification was performed in a 20-µl reaction mix containing cDNA, 0.2 µM primers, and 10 µl of SensiFAST SYBR Hi-ROX Kit (Bioline, London, UK). Gene expression levels were measured using real-time RT-PCR with an StepOnePlus Real-Time PCR System (Applied Biosystems). The relative amount of mRNA normalized to HPRT was calculated using the deltadelta method. The following primers were used: NFATc1 forward: 5′-CAAGTCTCACCACAGGGCTCACTA-3′, reverse: 5′-GCGTGAGAGGTTCATTCTCCAAGT-3′; osteoclast-associated receptor (OSCAR) forward: 5′-CCTAGCCTCATACCCCCAG-3′, reverse: 5′-CGTTGATCCCAGGAGTCACAA-3′; cathepsin K (CTSK) forward: 5′-AAGAAGACTCACCAGAAGCA-3′, reverse: 5′-TCCAGGTTATGGGCAGAGATT-3′; TRAP forward: 5′-GCAACATCCCCTGGTATGTG-3′, reverse: 5′-GCAAACGGTAGTAAGGGCTG-3′; HPRT forward: 5′-GCCTAAGATGAGCGCAAGTTG-3′, reverse: 5′-TACTAGGCAGATGGCCACAGG-3′.
ROS generation assay
The cells were seeded in 12-well plate with cover glass at a density of 0.15 × 106 / well. ROS was detected using the fluorescent probe 5-(and 6)-carboxy-2′,7′-dichlorofluorescin diacetate (DCF-DA). The cells were exposed to 10 µM DCF-DA for 20 min and examined with a laser-scanning confocal microscope (model LSM 510; Carl Zeiss, Jena, Germany) with a green fluorescent protein filter set. The mean relative fluorescence intensity for each field was measured with a Zeiss vision system and averaged.
Statistical analysis
Data were expressed as mean ± standard deviation from at least 3 independent experiments. Statistical significance was determined by using a paired Student's t-test. Statistical significance was set at p < 0.05 level.
RESULTS
HN inhibits RANKL-induced osteoclast differentiation
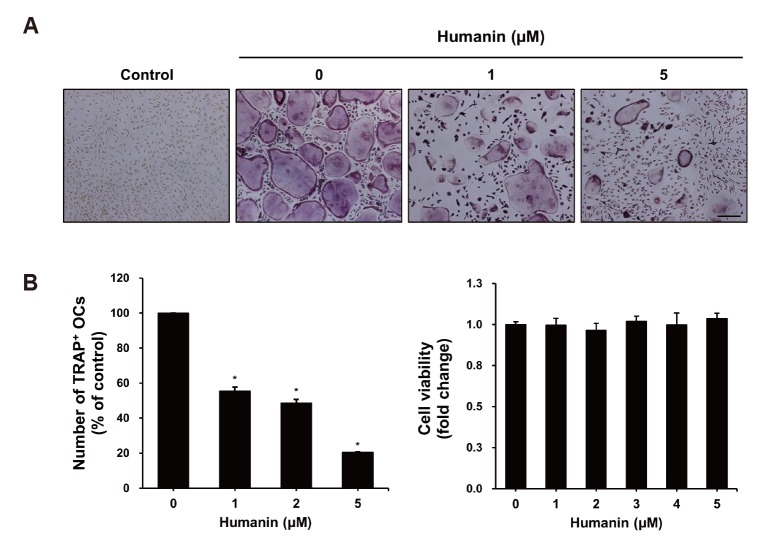
To investigate the effects of HN on RANKL-induced osteoclast differentiation, BMMs were incubated with the indicated concentrations of HN in the presence of 30 ng/ml M-CSF and 50 ng/ml RANKL for 5 days. HN treatment of BMMs dramatically inhibited the formation of TRAP+ osteoclasts (Fig. 1A). The number of TRAP+ osteoclasts (≥ 3 nuclei) was decreased by HN in a dose-dependent manner. In addition, using an MTS assay, we observed that there were no cytotoxic effects if the concentration of HN used was less than 5 µM (Fig. 1B). These results demonstrate that HN suppresses osteoclast differentiation.
Fig. 1. Effects of humanin (HN) on receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation.
(A) Bone marrow-derived macrophages (BMMs) were cultured in the presence of macrophage colony-stimulating factor (M-CSF) (30 ng/ml), RANKL (50 ng/ml), and the indicated concentrations of HN for 6 days. Cells were stained for tartrate-resistant acid phosphatase (TRAP). Scale bar, 500 µm. (B) Numbers of TRAP+ multinucleated cells were counted (≥ 3 nuclei). BMMs were seeded into 96-well plates and incubated with various concentrations of HN. Cell viability was measured by the MTS assay. OCs, osteoclasts.*p < 0.05 compared with control.
HN inhibits osteoclast-specific gene expression
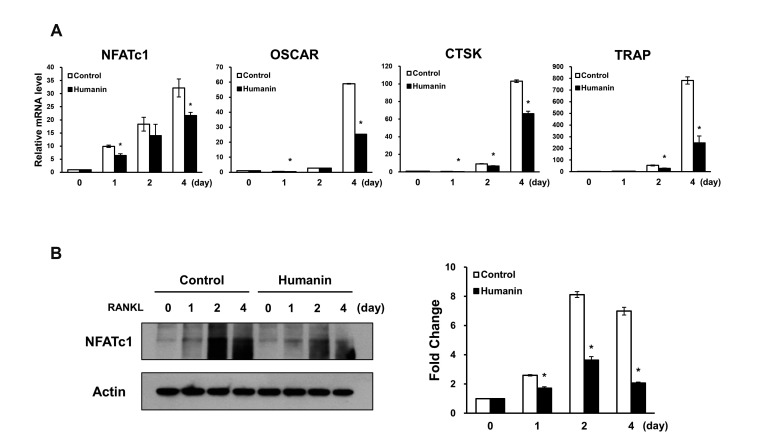
To further assess the inhibitory activity of HN on osteoclastogenesis, we assessed the mRNA expression levels of osteoclast markers. Results of RT-PCR analysis revealed that HN caused a reduction in the mRNA levels of NFATc1, which is an important nuclear transcriptional factor in osteoclastogenesis. Furthermore, the mRNA levels of molecules downstream of NFATc1, such as OSCAR, CTSK, and TRAP, were significantly reduced by HN during osteoclast differentiation (Fig. 2A). Next, we investigated NFATc1 protein levels during osteoclast differentiation; the protein levels of NFATc1 were significantly reduced in HN-treated osteoclasts (Fig. 2B). Together, these results suggest that HN has the potential to suppress osteoclast formation.
Fig. 2. Effects of humanin on the mRNA and protein levels of osteoclastogenesis-related genes.
(A) The mRNA levels of receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation markers were evaluated by quantitative RT-PCR. (B) NFATc1 protein levels were examined by Western blot analyses. OSCAR, osteoclast-associated receptor; CTSK, cathepsin K; TRAP, tartrate-resistant acid phosphatase. *p < 0.05 compared with control.
HN increases p-AMPK protein levels during osteoclast differentiation
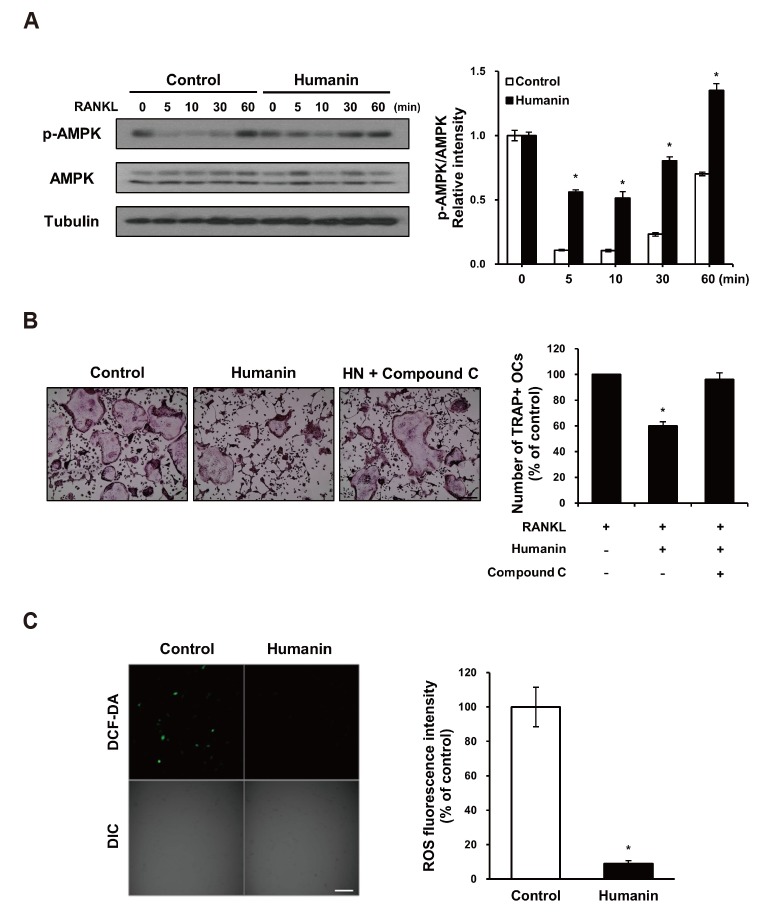
Based on previous findings that HN induces the phosphorylation of AMPK [11] and that AMPK is a negative regulator of RANKL-induced osteoclast differentiation [7,8], we investigated whether AMPK links osteoclastogenesis with HN. HN treatment significantly induced the phosphorylation of AMPK protein and increased the p-AMPK:AMPK ratio during RANKL-induced osteoclastogenesis (Fig. 3A). To confirm whether AMPK signaling regulates HN-induced osteoclast differentiation, we used compound C, a known AMPK inhibitor [19]. The number of TRAP+ osteoclasts cultured with compound C recovered to the level of control that treated with RANKL only (Fig. 3B). These results suggest that HN suppresses osteoclast differentiation via AMPK activation.
Fig. 3. Effects of humanin (HN) on AMP-activated protein kinase (AMPK) activation during osteoclast differentiation and receptor activator of nuclear factor-κB ligand (RANKL)-induced reactive oxygen species production in bone marrow-derived macrophages (BMMs).
(A) Western blot analysis of phosphor (p)-AMPK and AMPK during RANKL-induced osteoclastogenesis in untreated or HN-treated BMMs. The p-AMPK:AMPK ratio was calculated. (B) BMMs were treated with or without HN, and compound C, and stained for tartrate-resistant acid phosphatase (TRAP). Numbers of TRAP+ multinucleated cells were counted (≥ 3 nuclei). Scale bar, 500 µm. (C) BMMs were treated with RANKL (50 ng/ml) for 10 min and loaded with 10 µM dichlorofluorescein diacetate (DCF-DA). Data are expressed relative to the value for control BMMs. Scale bar, 100 µm. OCs, osteoclasts. *p < 0.05 compared with control.
HN decreases ROS release during osteoclast differentiation
RANKL increases the levels of intracellular ROS in BMMs [16]. We thus assessed whether HN could affect ROS production in osteoclast precursors after RANKL treatment. RANKL-induced ROS production, as measured using DCF-DA, was significantly diminished in HN-treated BMMs (Fig. 3C). These data indicate that RANKL-generated ROS production in osteoclast precursors is downregulated by HN.
DISCUSSION
HN, a novel MDP, was discovered by screening a cDNA library obtained from the brain of an Alzheimer's disease patient in 2001 [10]. HN acts as an extracellular ligand for a trimeric receptor consisting of CNTFR, the cytokine receptor WSX-1, and the transmembrane glycoprotein gp130 [20]. Via this trimeric receptor, HN regulates important cellular processes such as STAT3 activation, which is a negative regulator of osteoclast differentiation [13]. Moreover, HN protects cells from oxidative stress and hypoxia and is a promising therapeutic agent for the treatment of cardiovascular and Alzheimer's diseases in previous studies [21]. However, the role of HN in osteoclastogenesis remains unknown. In the present study, we found that HN inhibited osteoclast differentiation in BMMs (Fig. 1A).
The skeleton is continuously remodeled via bone resorption by osteoclasts and bone formation by osteoblasts [2]. An imbalance in these cell types is a cause of bone diseases such as Paget's diseases, osteoporosis, and osteopetrosis [3,22]. Osteoclasts are multinucleated cells that are specialized in bone resorption and derived from hematopoietic cells of a mononuclear lineage [23]. Osteoclast differentiation is initiated by M-CSF and RANKL, which activate osteoclastic transcription factors [24]. M-CSF promotes the proliferation and survival of osteoclast precursors, and RANKL, which is expressed by osteoblasts, regulates the signaling pathway for osteoclast precursors to differentiate into osteoclasts [25]. RANKL/RANK binding initiates tumor necrosis factor receptor-associated factor 6 and downstream signaling pathways that include nuclear factor-κB, c-Jun N-terminal kinase, and extracellular signal-regulated kinase [26,27]. Additionally, NFATc1 is a downstream target of RANK and a master regulator of osteoclast differentiation [28,29]. NFATc1 regulates osteoclast-specific genes, including OSCAR, CTSK, and TRAP [30]. Briefly, NFATc1 cooperates with PU.1 and microphthalmia-associated transcription factor to elicit effects on the CTSK and OSCAR promoters [31]. In addition, the NFAT:AP-1 complex activates TRAP [32]. NFATc1 is an essential regulator, induced by RANKL signaling, of osteoclast differentiation. We thus confirmed the inhibition of NFATc1 protein and mRNA expression in HN-treated BMMs (Fig. 2). RANKL stimulation induced a signaling pathway that leads to generation of ROS during osteoclastogenesis [16,17]. Additionally, AMPK was known to suppress ROS in acute myeloid leukemia cells obtained from bone marrow [33]. Therefore, these findings and our data (Fig. 3C) suggest that ROS increased by RANKL is reduced via AMPK activation in HN treated BMMs and HN may have a protective role in osteoclast differentiation.
AMPK is a central regulator of cellular energy homeostasis and is activated by the depletion of intracellular ATP [34]. AMPK has been studied as a possible therapeutic target for preventing diabetes and cancer over the last decade. For example, AMPK mediates the tumor-suppressor effects of LKB1, and its activation improves insulin sensitivity and glucose homeostasis [35]. Moreover, as AMPK consists of an α catalytic subunit (α1 and α2), a β subunit (β1 and β2), and a regulatory γ subunit (γ1, γ2, and γ3) [36], the deletion of the AMPK α and β subunits in mice has been reported to decrease bone mass in vivo. Additionally, a number of in vitro studies of AMPK have been conducted in bone cells [9,37,38]. Thus, AMPK is activated by osteoclast differentiation and plays a negative role in TRAP+ osteoclast formation. Further, compound C has been used as a selective inhibitor of AMPK in cellular systems [19]. Our results show that the number of TRAP+ osteoclasts cultured with compound C recovered to the level of control (Fig. 3B). These results suggest that HN suppresses osteoclast differentiation via AMPK activation.
In this study, we demonstrated that HN inhibited osteoclast differentiation and osteoclast-specific expression of the NFATc1, OSCAR, CTSK, and TRAP genes. Furthermore, our results revealed that HN increased the phosphorylation of AMPK in BMMs, suggesting that HN suppresses RANKL-induced osteoclast differentiation via AMPK activation. Therefore, our findings support the hypothesis that HN is potential therapeutic target for the treatment of bone loss in osteoporosis and bone diseases caused by osteoclasts.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF. 2016R1A5A2008630).
Footnotes
Author contributions: N.K. designed the study and performed experiments. K.W.K. contributed to data interpretation. D.M.S. drafted and revised the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol. 2008;61:577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- 2.Charles JF, Aliprantis AO. Osteoclasts: more than ‘bone eaters’. Trends Mol Med. 2014;20:449–459. doi: 10.1016/j.molmed.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erkhembaatar M, Gu DR, Lee SH, Yang YM, Park S, Muallem S, Shin DM, Kim MS. Lysosomal Ca2+ signaling is essential for osteoclastogenesis and bone remodeling. J Bone Miner Res. 2017;32:385–396. doi: 10.1002/jbmr.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 6.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeyabalan J, Shah M, Viollet B, Chenu C. AMP-activated protein kinase pathway and bone metabolism. J Endocrinol. 2012;212:277–290. doi: 10.1530/JOE-11-0306. [DOI] [PubMed] [Google Scholar]
- 8.Lee YS, Kim YS, Lee SY, Kim GH, Kim BJ, Lee SH, Lee KU, Kim GS, Kim SW, Koh JM. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone. 2010;47:926–937. doi: 10.1016/j.bone.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Ming W, Lu G, Xin S, Huanyu L, Yinghao J, Xiaoying L, Chengming X, Banjun R, Li W, Zifan L. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem Biophys Res Commun. 2016;476:412–419. doi: 10.1016/j.bbrc.2016.05.135. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci U S A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin Q, Jin J, He F, Zheng Y, Li T, Zhang Y, He J. Humanin promotes mitochondrial biogenesis in pancreatic MIN6 β-cells. Biochem Biophys Res Commun. 2018;497:292–297. doi: 10.1016/j.bbrc.2018.02.071. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab. 2013;24:222–228. doi: 10.1016/j.tem.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Welte T, Troiano N, Maher SE, Fu XY, Bothwell AL. Osteoporosis with increased osteoclastogenesis in hematopoietic cell-specific STAT3-deficient mice. Biochem Biophys Res Commun. 2005;328:800–807. doi: 10.1016/j.bbrc.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Gidlund EK, von Walden F, Venojärvi M, Risérus U, Heinonen OJ, Norrbom J, Sundberg CJ. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol Rep. 2016;4:e13063. doi: 10.14814/phy2.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol. 2013;304:H393–H397. doi: 10.1152/ajpheart.00765.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010;285:6913–6921. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sreekumar PG, Hinton DR, Kannan R. Endoplasmic reticulum-mitochondrial crosstalk: a novel role for the mitochondrial peptide humanin. Neural Regen Res. 2017;12:35–38. doi: 10.4103/1673-5374.198970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Chhipa RR, Nakano I, Dasgupta B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther. 2014;13:596–605. doi: 10.1158/1535-7163.MCT-13-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka M, Hashimoto Y. Humanin and the receptors for humanin. Mol Neurobiol. 2010;41:22–28. doi: 10.1007/s12035-009-8090-z. [DOI] [PubMed] [Google Scholar]
- 21.Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YM, Kim MS, Son A, Hong JH, Kim KH, Seo JT, Lee SI, Shin DM. Alteration of RANKL-induced osteoclastogenesis in primary cultured osteoclasts from SERCA2+/− mice. J Bone Miner Res. 2009;24:1763–1769. doi: 10.1359/jbmr.090420. [DOI] [PubMed] [Google Scholar]
- 23.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 24.Feng X, Teitelbaum SL. Osteoclasts: new insights. Bone Res. 2013;1:11–26. doi: 10.4248/BR201301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teitelbaum SL. Osteoclasts, integrins, and osteoporosis. J Bone Miner Metab. 2000;18:344–349. doi: 10.1007/s007740070007. [DOI] [PubMed] [Google Scholar]
- 26.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son A, Kim MS, Jo H, Byun HM, Shin DM. Effects of inositol 1,4,5-triphosphate on osteoclast differentiation in RANKL-induced osteoclastogenesis. Korean J Physiol Pharmacol. 2012;16:31–36. doi: 10.4196/kjpp.2012.16.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 29.Park B, Yang YM, Choi BJ, Kim MS, Shin DM. Activation of G proteins by aluminum fluoride enhances RANKL-mediated osteoclastogenesis. Korean J Physiol Pharmacol. 2013;17:427–433. doi: 10.4196/kjpp.2013.17.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation. J Bone Metab. 2014;21:233–241. doi: 10.11005/jbm.2014.21.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma SM, Bronisz A, Hu R, Patel K, Mansky KC, Sif S, Ostrowski MC. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem. 2007;282:15921–15929. doi: 10.1074/jbc.M609723200. [DOI] [PubMed] [Google Scholar]
- 32.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 33.Saito Y, Chapple RH, Lin A, Kitano A, Nakada D. AMPK protects leukemia-initiating cells in myeloid leukemias from metabolic stress in the bone marrow. Cell Stem Cell. 2015;17:585–596. doi: 10.1016/j.stem.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48:e224. doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 37.Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007;8:51. doi: 10.1186/1471-2121-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS, Koh JT. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48:885–893. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]