Abstract
Aims/Introduction
Sodium–glucose cotransporter 2 (SGLT‐2) inhibitors improve blood glucose control, as well as reducing bodyweight by promoting urinary glucose excretion. The weight loss is less than expected from urinary glucose loss, however, likely because of an increase in food intake. To investigate whether SGLT‐2 inhibitors might increase appetite by affecting related hormones, we examined the effects of the SGLT‐2 inhibitor, ipragliflozin, including those on appetite‐regulating hormones, in individuals with suboptimally controlled type 2 diabetes.
Materials and Methods
The present prospective, multicenter, open‐label study was carried out with 96 patients with a body mass index of ≥22 kg/m2 who were treated with ipragliflozin (50 mg/day) for 16 weeks. Parameters including glycated hemoglobin level, bodyweight, circulating leptin and active ghrelin concentrations, and appetite as assessed with a visual analog scale were measured before and during treatment.
Results
Both glycated hemoglobin level (from 7.9 ± 0.8 to 7.1 ± 0.7%) and bodyweight (from 75.2 ± 12.6 to 72.6 ± 12.4 kg) were significantly decreased after treatment for 16 weeks. The fasting serum leptin level was significantly decreased after 2 weeks (from 19.5 ± 13.1 to 18.1 ± 12.4 ng/mL) and remained decreased up to 16 weeks, even after adjustment for bodyweight, whereas the plasma active ghrelin level showed no significant change. The visual analog scale score for hunger was significantly increased at 2 and 8 weeks.
Conclusions
The present results suggest that ipragliflozin improved glycemic control and reduced bodyweight, but also reduced serum leptin levels and might thereby have increased appetite.
Keywords: Appetite, Ipragliflozin, Leptin
Introduction
Inhibitors of sodium–glucose cotransporter 2 (SGLT‐2) promote urinary glucose excretion by selectively blocking SGLT‐2 activity in proximal tubules of the kidney. SGLT‐2 inhibitors thus ameliorate hyperglycemia, dyslipidemia and insulin resistance; reduce bodyweight; and attenuate β‐cell exhaustion1, 2, 3. However, several clinical trials have shown that the effect of these agents on bodyweight is smaller than would be expected from the increase in urinary glucose excretion4, 5. In rats and other rodents, as well as humans, SGLT‐2 inhibitors have been shown to increase overall food intake in addition to increasing urinary glucose excretion, likely accounting for such attenuation of the weight‐reducing effect6, 7, 8. However, few studies have examined the mechanism underlying this change in appetite during treatment with SGLT‐2 inhibitors9. Therefore, we have here evaluated the efficacy of the SGLT‐2 inhibitor, ipragliflozin, in patients with type 2 diabetes, as well as investigated its effects on hormones related to appetite regulation.
Methods
Study participants
Individuals with suboptimally controlled type 2 diabetes mellitus who attended Kobe University Hospital or other participating medical centers between April 2015 and October 2017, and who satisfied all eligibility criteria and met none of the exclusion criteria were recruited to the study. The eligibility criteria included: (i) a diagnosis of type 2 diabetes; (ii) age 20–75 years; (iii) failure to achieve sufficient glycemic control after ≥12 weeks of diet and exercise therapy, or treatment with oral hypoglycemic agents, insulin or glucagon‐like peptide‐1 receptor agonists; (iv) a glycated hemoglobin (HbA1c) level of ≥6.5% and <9.5%; (v) a body mass index (BMI) of ≥22 kg/m2; and (vi) provision of written informed consent before participation in the study. The exclusion criteria were as follows: (i) a diagnosis of type 1 or other specific type of diabetes other than type 2; (ii) a history of severe ketosis, diabetic coma or diabetic precoma in the previous 6 months; (iii) the presence of severe infection, scheduled or recent surgery, or recent serious trauma; (iv) severe hepatic dysfunction (serum aspartate aminotransferase or alanine aminotransferase levels of ≥2.5‐fold the upper limit of normal); (v) renal dysfunction (serum creatinine level of ≥1.5 mg/dL in men or ≥1.3 mg/dL in women); (vi) ongoing steroid therapy; (vii) gastrectomy (including partial or total gastrectomy, but excluding endoscopic mucosal resection); (viii) a history of stroke, myocardial infarction or other major cardiovascular complication requiring hospitalization in the previous 6 months; (ix) previous treatment with SGLT‐2 inhibitors; (x) pregnancy, breast‐feeding or the chance of becoming pregnant; (xi) a history of hypersensitivity to ipragliflozin; and (xii) deemed unfit for any other reason by attending physicians.
Methods
The present study was designed as a multicenter, open‐label, prospective, interventional, investigator‐initiated clinical trial (SOAR‐KOBE Study), with Kobe University Graduate School of Medicine, Kobe, Japan, as the principal study center. The study protocol was approved by the institutional review boards of the participating centers; the study was carried out in accordance with the Declaration of Helsinki and its amendments, and written informed consent was obtained from all participants. Participants were treated with ipragliflozin (Suglat; Astellas Pharma Inc., Tokyo, Japan) at a dose of 50 mg/day after breakfast for 16 weeks. Concomitant medications are shown in Table 1. Changes to treatment were not permitted during the study period in principle. Blood samples were collected at baseline and at predetermined time intervals.
Table 1.
Clinical parameters for the study participants at baseline
| Age (years) | 57.2 ± 11.0 |
| Sex (male/female) | 47/49 |
| Disease duration (years) | 12.2 ± 8.3 |
| HbA1c (%) | 7.9 ± 0.8 |
| Glycated albumin (%) | 19.2 ± 3.8 |
| FPG (mg/dL) | 158.1 ± 37.8 |
| Bodyweight (kg) | 75.2 ± 12.6 |
| BMI (kg/m2) | 28.7 ± 4.3 |
| Waist circumference (cm) | 97.8 ± 10.8 |
| BP (mmHg) | 132.1 ± 14.2/77.0 ± 11.3 |
| AST (IU/L) | 28.6 ± 16.1 |
| ALT (IU/L) | 34.6 ± 26.5 |
| Serum creatinine (mg/dL) | 0.7 ± 0.2 |
| Total‐C (mg/dL) | 175.5 ± 31.7 |
| LDL‐C (mg/dL) | 106.4 ± 28.2 |
| HDL‐C (mg/dL) | 53.0 ± 13.5 |
| Triglyceride (mg/dL) | 154.9 ± 113.5 |
| Active ghrelin (fmol/mL) | 11.2 ± 8.2 |
| Leptin (ng/mL) | 19.3 ± 13.1 |
| Leptin (ng/mL)/bodyweight (kg) | 0.3 ± 0.2 |
| Antidiabetic medications | n (%) |
|---|---|
| Sulfonylurea | 32 (34.4) |
| Glinide | 1 (1.1) |
| Metformin | 78 (83.9) |
| Alpha‐glucosidase inhibitor | 24 (25.8) |
| Thiazolidinedione | 3 (3.2) |
| DPP‐4 inhibitor | 61 (63.5) |
| GLP‐1 receptor agonist | 19 (20.4) |
| Bolus insulin | 5 (5.4) |
| Basal insulin | 23 (24.7) |
Total n = 96. Data are the mean ± standard deviation or medians (25–75% quartiles). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; DPP‐4; dipeptidyl peptidase, FPG, fasting plasma glucose; GLP‐1; glucagon like peptide‐1; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol, LDL‐C, low‐density lipoprotein cholesterol.
The study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR) as UMIN000015478, was carried out with funding by Astellas Pharma Inc., and was monitored by a third party (DOT WORLD, Tokyo, Japan) to ensure compliance with ethical guidelines for medical research involving human subjects and with the study protocol, as well as to ensure that the data were collected accurately and completely.
Data collection
Clinical data (sex, age, height, bodyweight, BMI, waist circumference and blood pressure while sitting) were collected at baseline. Blood and urine samples were obtained after the participants had fasted overnight. The levels of HbA1c, glycated albumin and plasma glucose, as well as serum total cholesterol, low‐density lipoprotein cholesterol, triglyceride, high‐density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase and creatinine were measured with commercial kits at 0, 4, 8, 12 and 16 weeks, and those of serum leptin and plasma active ghrelin were measured at 0, 2, 4, 8 and 16 weeks. To measure plasma active ghrelin levels, 2 mL of blood was collected directly into a tube containing aprotinin (NP‐EA0205; TERUMO Corporation, Tokyo, Japan); collected samples were immediately centrifuged at 1,670 g for 10 min at 4°C. The isolated plasma samples were treated with one‐tenth of its volume of 1 N HCl, and the tubes were rocked gently. The samples were stored in a freezer at −80°C. To measure serum leptin levels, 3 mL of blood was collected with vacuum tubes containing serum separating agent (VP‐AS073K; NIPRO Corporation, Osaka, Japan). After leaving to stand 30 min at room temperature, collected samples were centrifuged at 1,670 g for 10 min. Isolated serum samples were stored in a freezer at −20°C. These prepared samples were analyzed by LSI Medience Corporation (Tokyo, Japan). The plasma active ghrelin levels were measured by an automated enzyme immunoassay (Emax Plus Microplate Reader; Molecular Devices, San Jose, CA, USA) with Active Ghrelin ELISA kit (SCETI K.K., Tokyo, Japan). The serum leptin levels were measured by AccuFLEX γ‐7010 (Hitachi Aloka Medical Ltd., Tokyo, Japan) with HUMAN LEPTIN RIA KIT (Linco Rearch Inc., St. Charles, MO, USA). Appetite was assessed with a visual analog scale (VAS) that consisted of eight items: hunger, fullness, prospective food consumption, satiety and the desire to eat something sweet, savory, salty or fatty10. The results were recorded by the participants at home after an overnight fast, and before breakfast at 0, 2, 8 and 16 weeks.
Rationale for sample size
Previous efficacy and safety trials of SGLT‐2 inhibitors with the change in HbA1c level as the primary end‐point were carried out with 100–150 participants in each group11, 12. A trial of the reliability of a VAS for appetite was carried out with 55 participants10. On the basis of these previous studies and taking participant dropout into account, we selected a target sample size of 150 participants.
Statistical analysis
The primary end‐point of the study was the change in HbA1c level between baseline and 16 weeks. Secondary end‐points included changes in fasting plasma glucose level, bodyweight, serum lipid levels, blood pressure, the levels of hormones related to appetite regulation (serum leptin and plasma active ghrelin) and VAS scores for appetite. The results in Table 1 are presented as the mean ± standard deviation. Intergroup differences of normally or non‐normally distributed data were tested for significance with the unpaired Student's t‐test or Mann–Whitney U‐test, respectively. Within‐group comparisons of time series data were carried out by one‐way analysis of variance (anova). The relationship between two variables was assessed with Spearman's correlation coefficient.
A P value of <0.05 was considered statistically significant. All statistical analysis was carried out with SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient recruitment and baseline characteristics
A total of 104 participants were enrolled in the study. Of these individuals, one failed to satisfy the eligibility, while two others met the exclusion criteria. Ipragliflozin was therefore administered to 101 patients with type 2 diabetes. Four of these patients deviated from the study protocol, whereas one was excluded from analysis because of the progression of anemia. A total of 96 participants thus completed the study and were included in the analysis (Figure 1). The baseline characteristics of these 96 patients are shown in Table 1.
Figure 1.
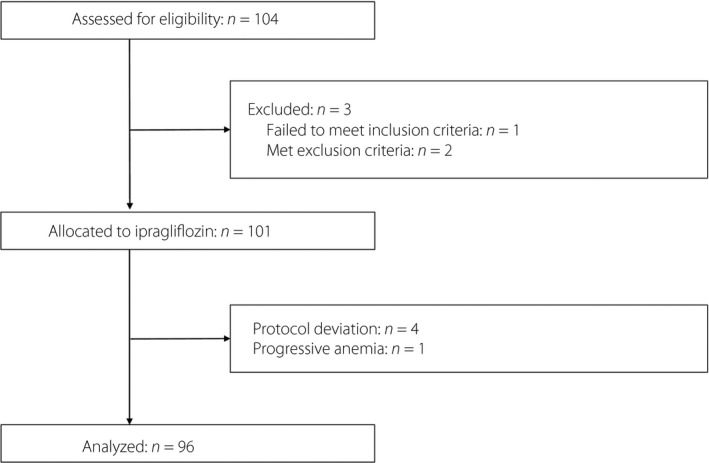
Flow diagram of participant recruitment.
Clinical parameters
The HbA1c level was significantly decreased at 4 weeks after the initiation of ipragliflozin (50 mg/day) treatment, and it continued to decline until 12 weeks, after which it remained the same (baseline 7.9 ± 0.8%, 16 weeks 7.1 ± 0.7%, P < 0.01; Figure 2a). The fasting plasma glucose level also decreased significantly over 16 weeks of ipragliflozin treatment from 158.1 ± 37.8 mg/dL at baseline to 129.1 ± 29.9 mg/dL (P < 0.01). Bodyweight was significantly decreased at 2 weeks and continued to decrease gradually up to 16 weeks (baseline 75.2 ± 12.6 kg, 16 weeks 72.6 ± 12.4 kg, P < 0.01; Figure S1a).
Figure 2.
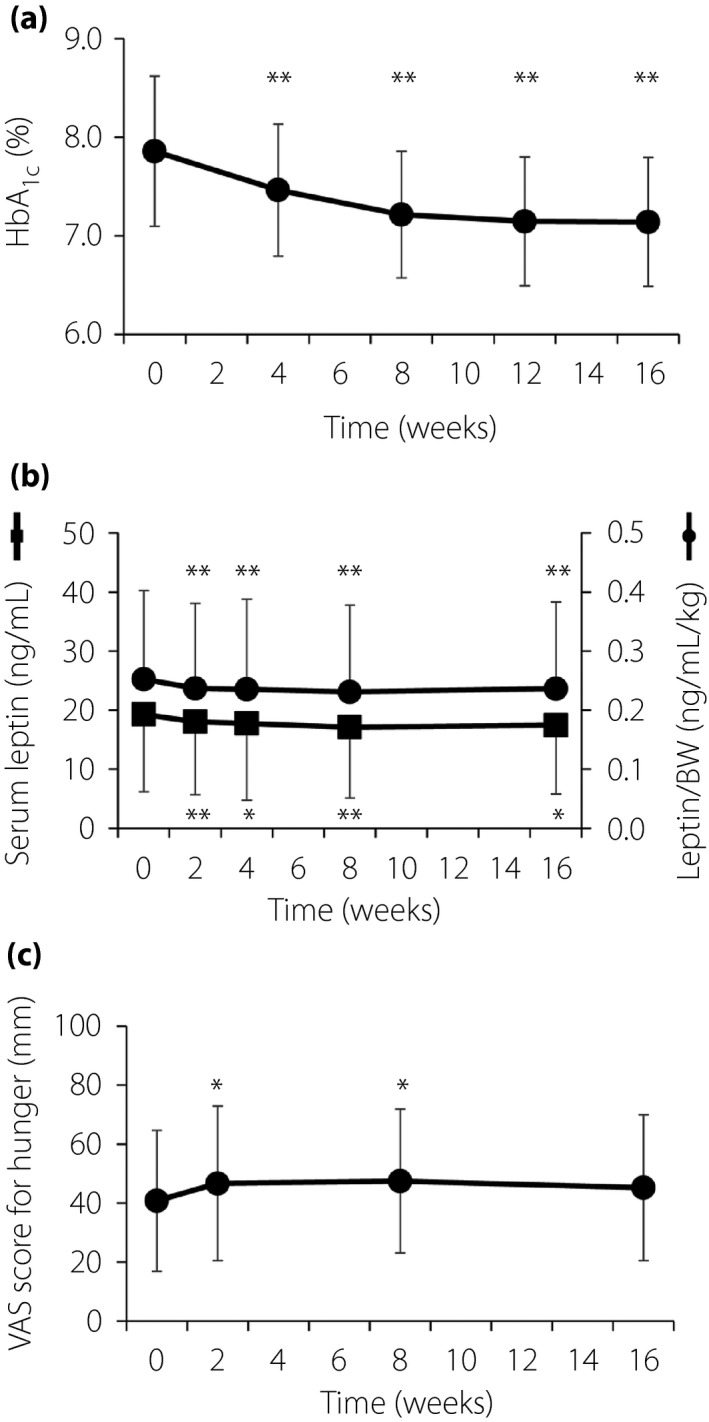
(a) Time course of glycated hemoglobin (HbA1c) level during treatment with ipragliflozin. Data are the mean ± standard deviation (n = 94). **P < 0.01 versus baseline (time 0) by one‐way analysis of variance. (b) Time course of serum leptin concentration (n = 94) and the leptin/bodyweight (BW) ratio (n = 92) during treatment with ipragliflozin. Data are the mean ± standard deviation. *P < 0.05, **P < 0.01 versus baseline (time 0) by one‐way analysis of variance. (c) Time course of the visual analog scale (VAS) score for hunger (0: not hungry at all, 100: never been more hungry) during treatment with ipragliflozin. Data are the mean ± standard deviation (n = 91). *P < 0.05 versus baseline (time 0) by one‐way analysis of variance.
Glycated albumin, BMI and waist circumference were also significantly decreased during the course of treatment (glycated albumin baseline 19.2 ± 3.8%, 16 weeks 16.4 ± 2.6%, P < 0.01; BMI baseline 28.7 ± 4.3 kg/m2, 16 weeks 27.7 ± 4.3 kg/m2, P < 0.01; waist circumference baseline 97.8 ± 10.8 cm, 16 weeks 95.5 ± 10.8 cm; P < 0.01). Although the change of bodyweight for 16 weeks was not related to basal serum leptin level (r = −0.17, P = 0.09), it was significantly related to the change of serum leptin level (r = 0.45, P < 0.01). The changes in HbA1c level and bodyweight over the 16‐week treatment period showed a significant positive correlation (r = 0.32, P < 0.01; Figure S1b). We divided the study participants into two groups according to whether or not they showed an improvement in HbA1c level between at baseline and at 16 weeks. The HbA1c improvement group showed a significantly higher HbA1c level at baseline compared with the non‐improvement group (7.9 ± 0.7% vs 7.2 ± 0.6%, P < 0.01). However, age, bodyweight, serum leptin level and plasma active ghrelin level at baseline did not differ between these two groups (Table S1). A similar analysis for groups of patients with or without an improvement in bodyweight between at baseline, and at 16 weeks showed no significant differences in baseline characteristics between the two groups (Table S1).
Appetite and hormones related to appetite regulation
The serum leptin levels were significantly decreased at 2 weeks after treatment onset (baseline 19.3 ± 13.1 ng/mL, 2 weeks 18.1 ± 12.4 ng/mL, P < 0.01), and remained at a similar reduced level thereafter (16 weeks 17.5 ± 11.7 ng/mL, P = 0.02 vs baseline). Serum leptin levels adjusted for bodyweight showed a similar temporal pattern (Figure 2b). Whereas basal serum leptin levels were not correlated with age (data not shown), those were higher in women than men (women 26.3 ± 14.2 ng/mL, men 12.7 ± 7.3 ng/mL, P < 0.01). Therefore, using one‐way analysis of covariance, we adjusted the basal leptin levels by sex, and found that the adjusted leptin levels were also decreased significantly at 2 weeks after treatment onset and remained at a similar level of reduction. Bodyweight‐adjusted serum leptin levels also showed a similar temporal pattern after the adjustment of sex (data not shown). The plasma concentration of active ghrelin did not change significantly during the treatment period (baseline 11.2 ± 8.2 fmol/mL, 16 weeks 12.6 ± 10.0 fmol/mL, P = 0.44; Figure S2).
Changes in VAS scores for appetite are shown in Table S2. The hunger score was increased significantly after 2 weeks (baseline 39.9 ± 23.5 mm, 2 weeks 45.8 ± 26.2 mm, P = 0.04) and remained increased at 8 weeks, although the increase of the score at 16 weeks was not significant (Figure 2c). No other item of the VAS score changed significantly during the study period. The changes in the hunger score and serum leptin level at 2 weeks after treatment onset were not significantly correlated (Figure S1c). Concomitant medications had no influence on either VAS or the serum levels of hormones after examination by two‐way analysis of variance (data not shown).
Other clinical parameters
Changes in other clinical parameters are shown in Table S3. Systolic blood pressure decreased significantly from 132.2 ± 14.7 mmHg at baseline to 127.4 ± 13.0 mmHg at 16 weeks (P < 0.01). Serum aspartate aminotransferase decreased significantly from 28.6 ± 16.1 U/L at baseline to 23.5 ± 11.5 U/L at 16 weeks (P < 0.01), whereas alanine aminotransferase decreased significantly from 34.6 ± 26.5 to 26.3 ± 18.1 U/L (P < 0.01). The serum creatinine level increased significantly from 0.71 ± 0.22 mg/dL at baseline to 0.73 ± 0.22 mg/dL at 16 weeks (P = 0.03). Finally, the high‐density lipoprotein cholesterol concentration increased significantly from 53.0 ± 13.6 mg/dL at baseline to 55.8 ± 15.5 mg/dL at 16 weeks (P < 0.01).
Adverse events
Adverse events were reported for 12 participants (11.5%), with those in eight patients being attributed to ipragliflozin (Table S4). Three of these eight events were urinary tract or genital infection, including one relatively severe case, all of which were improved by treatment with no sequela. Three of the eight events were mild hypoglycemia.
Discussion
The present results show that the addition of ipragliflozin to the treatment of Japanese non‐lean individuals with suboptimally controlled type 2 diabetes led to a significant reduction in HbA1c level, fasting plasma glucose concentration and bodyweight. The effects of ipragliflozin were evident as early as 2 weeks after treatment onset and were sustained for up to 16 weeks, consistent with the results of previous studies13, 14, 15. The beneficial effects of ipragliflozin on blood pressure, lipid levels and hepatic function observed in the present study are also consistent with previous findings16, 17.
We examined the effects of ipragliflozin on appetite with the use of a VAS and by the measurement of hormones related to appetite regulation. The reproducibility of the VAS for appetite has been verified in several studies10, 18. Leptin influences appetite, and regulates bodyweight and energy balance19, 20, with leptin supplementation having been shown to reduce bodyweight and BMI in leptin‐deficient adults or overweight men21, 22. Obese individuals have been suggested to have leptin resistance, which attenuates the anorexigenic effect of the hormone despite the presence of increased serum levels23, 24. In contrast, ghrelin, also known as the “hunger hormone,” is a gastrointestinal hormone that stimulates appetite25. We found that administration of ipragliflozin resulted in a significant reduction in the fasting serum leptin level at 2 weeks after treatment onset, whereas it had no effect on the plasma concentration of active ghrelin at any time during the study period. Consistent with the present results, one small‐sized study reported that postprandial active ghrelin levels did not change after SGLT‐2 inhibitor administration in type 2 diabetes26. Serum leptin levels have been found to be positively correlated with bodyweight27, and thus have been evaluated after the adjustment with bodyweight28 or with BMI29, 30. We thus examined serum leptin levels adjusted for bodyweight to evaluate whether the changes in the leptin level are solely dependent on the changes in bodyweight. Even after such adjustment, the serum leptin level was significantly reduced at 2 weeks after treatment initiation. Of note, the score for the hunger item in the VAS for appetite was significantly increased at 2 weeks after the onset of ipragliflozin administration. The score of items other than hunger in the VAS examination did not change in time course analysis. Given that desire for something sweet, savory, salty or fatty did not change, it is possible that ipragliflozin does not affect food preference. Neither fullness nor satiety was altered in the current VAS analysis. Given that we carried out the VAS examination after overnight fast, this setting might reduce the sensitivity for fullness and satiety. Furthermore, the present results suggest that ipragliflozin reduced the serum leptin concentration and might thereby have increased appetite. SGLT‐2 inhibitors have been found to reduce serum leptin levels31, 32, 33, but a reduction in the circulating leptin concentration adjusted for bodyweight has not previously been reported. The present study thus suggests that ipragliflozin might have reduced the circulating leptin concentration by an unknown mechanism. The absence of a significant correlation between the change in serum leptin level and the change in the VAS score for hunger might have been due to a lack of synchronization between the two indicators or to the effects of other hormones. The apparent return of appetite to the baseline level by 16 weeks despite a continuing reduction in the serum leptin level might have been attributable to resolution of leptin resistance as a consequence of bodyweight reduction. The study period of this trial was relatively short. A longer‐term interventional trial would provide more useful information about the effect of SGLT‐2 inhibitor on bodyweight, glycemic profile, appetite and its related hormones.
As far as we are aware, the present study is the first to simultaneously examine a VAS for appetite and circulating levels of hormones related to appetite regulation after administration of an SGLT‐2 inhibitor. Our study was not without limitations, however. First, the study was designed as a single‐arm trial, with the lack of a control group, making it impossible to rule out potential effects of unknown confounders. Second, the sample size was small. Third, we did not measure the actual food intake of the participants. We therefore cannot rule out the possibility that the results obtained with the VAS for appetite might not reflect actual food intake. Fourth, the appetite‐regulating hormone levels, which could change after taking meals, were measured only in a fasting state. Further investigations will be required to overcome these limitations of the present study.
In conclusion, the results of the present trial suggest that ipragliflozin improves blood glucose levels and other clinical parameters in patients with suboptimally controlled type 2 diabetes, but that it also reduces the serum leptin concentration, with this latter effect possibly explaining the observed increase in appetite.
Disclosure
KS has received lecture fees from MSD and Astellas Pharma Inc. YO has received research support from MSD and Astellas Pharma Inc., and lecture fees from MSD and Astellas Pharma Inc. YH has received research support from MSD and Astellas Pharma Inc., and lecture fees from MSD and Astellas Pharma Inc. YT has received research support from MSD and Astellas Pharma Inc., and lecture fees from MSD. WO has received research support from MSD and Astellas Pharma Inc., and lecture fees from MSD and Astellas Pharma Inc. TO has received lecture fees from MSD and Astellas Pharma Inc. YK has received lecture fees from MSD. KH has received lecture fees from MSD and Astellas Pharma Inc. TM has received lecture fees from MSD and Astellas Pharma Inc. MK has received lecture fees from MSD and Astellas Pharma Inc. AT has received lecture fees from MSD and Astellas Pharma Inc. All other authors, HM, TY, NO, AS, HK and KY, declare no conflict of interest.
Supporting information
Figure S1 | (a) Time course of body weight during treatment with ipragliflozin. Data are the mean ± standard deviation (n = 92). **P < 0.01 versus baseline (time 0) by one‐way analysis of variance. Where the number of patients differed from the number of patients in the full analysis set, the number is given in parentheses. (b) Correlation between changes in glycated hemoglobin (HbA1c) level and bodyweight from baseline to 16 weeks after the onset of ipragliflozin treatment (n = 95). Where the number of patients differed from the number of patients in the full analysis set, the number is given in parentheses. (c) Correlation analysis for changes in the visual analog scale score for hunger and the serum leptin concentration corrected by bodyweight between baseline and 2 weeks after the onset of ipragliflozin treatment (n = 92). Where the number of patients differed from the number of patients in the full analysis set, the number is given in parentheses.
Figure S2 | Time course of plasma active ghrelin concentration (n = 64) during treatment with ipragliflozin.
Table S1 | Comparison of parameters at baseline between patients who did or did not show an improvement in glycated hemoglobin level or bodyweight after treatment with ipragliflozin for 16 weeks
Table S2 | Changes in visual analog scale scores (mm) for appetite in the study participants (n = 96) during treatment with ipragliflozin for 16 weeks
Table S3 | Changes in clinical parameters for the study participants (n = 96) during treatment with ipragliflozin for 16 weeks
Table S4 | Summary of treatment‐emergent adverse events and selected treatment‐emergent adverse events and of interest in the study patients (n = 104)
Acknowledgments
We thank all the patients, SOAR‐Kobe study investigators and staff at participating sites for their contributions to this study. This study was funded by Astellas Pharma Inc. The company was not involved in study design, patient selection, data aggregation/analysis, interpretation of results or preparation of the manuscript.
Principal investigators at the participating centers are as follows: Kazuhiko Sakaguchi (Kobe University Hospital, Obara Hospital), Takeshi Ohara (Hyogo Brain and Heart Center), Yasuo Kuroki (Kobe Century Memorial Hospital), Kenta Hara (Kita‐harima Medical Center), Tomokazu Matsuda (Kaisei Hospital), Minoru Kishi (Nishiwaki Municipal Hospital), Akihiko Takeda (Shinko Hospital) and Kazuki Yokota (Yokota Medical Clinic).
J Diabetes Investig 2019; 10: 1254–1261
Clinical Trial Registry University Hospital Medical Information NetworkUMIN000015478
References
- 1. Kamezaki M, Kusaba T, Komaki K, et al Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep 2018; 8: 4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 3. Ferrannini E, Muscelli E, Frascerra S, et al Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamartina L, Borget I, Mirghani H, et al Surgery for neck recurrence of differentiated thyroid cancer: outcomes and risk factors. J Clin Endocrinol Metab 2017; 102: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 5. Vasilakou D, Karagiannis T, Athanasiadou E, et al Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 6. Iuchi H, Sakamoto M, Matsutani D, et al Time‐dependent effects of ipragliflozin on behaviour and energy homeostasis in normal and type 2 diabetic rats: continuous glucose telemetry analysis. Sci Rep 2017; 7: 11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devenny JJ, Godonis HE, Harvey SJ, et al Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet‐induced obese (DIO) rats. Obesity 2012; 20: 1645–1652. [DOI] [PubMed] [Google Scholar]
- 8. Ferrannini G, Hach T, Crowe S, et al Energy balance after sodium‐glucose cotransporter 2 inhibition. Diabetes Care 2015; 38: 1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masuda T, Watanabe Y, Fukuda K, et al Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Renal Physiol 2018; 315: F653–F664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flint A, Raben A, Blundell JE, et al Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000; 24: 38–48. [DOI] [PubMed] [Google Scholar]
- 11. Osonoi T, Nakamoto S, Saito M, et al Efficacy of ipragliflozin as monotherapy or as add‐on therapy with other oral antidiabetic medications for treating type 2 diabetes in Japanese patients with inadequate glycemic control: a subgroup analysis based on patient characteristics. J Diabetes Investig 2018; 9: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ji L, Ma J, Li H, et al Dapagliflozin as monotherapy in drug‐naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther 2014; 36: 84–100. [DOI] [PubMed] [Google Scholar]
- 13. Kashiwagi A, Takahashi H, Ishikawa H, et al A randomized, double‐blind, placebo‐controlled study on long‐term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long‐term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab 2015; 17: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kashiwagi A, Kazuta K, Goto K, et al Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2015; 17: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishihara H, Yamaguchi S, Nakao I, et al Efficacy and safety of ipragliflozin as add‐on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi‐centre, randomized, placebo‐controlled, double‐blind study. Diabetes Obes Metab 2016; 18: 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komiya C, Tsuchiya K, Shiba K, et al Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS ONE 2016; 11: e0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura T, Sanada J, Shimoda M, et al Switching from low‐dose thiazide diuretics to sodium‐glucose cotransporter 2 inhibitor improves various metabolic parameters without affecting blood pressure in patients with type 2 diabetes and hypertension. J Diabetes Investig 2018; 9: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stubbs RJ, Hughes DA, Johnstone AM, et al The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand‐held computerized systems for temporal tracking of appetite ratings. Br J Nutr 2000; 84: 405–415. [DOI] [PubMed] [Google Scholar]
- 19. Boguszewski CL, Paz‐Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol Pol 2010; 61: 194–206. [PubMed] [Google Scholar]
- 20. Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015; 148: 1219–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Licinio J, Caglayan S, Ozata M, et al Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin‐deficient adults. Proc Natl Acad Sci U S A 2004; 101: 4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huckshorn CJ, Westerterp‐Plantenga MS, Saris WH. Peglated human recombinant leptin (PEG‐OB) causes additional weight loss in severely energy‐restricted, overweight men. Am J Clin Nutr 2003; 77: 771–776. [DOI] [PubMed] [Google Scholar]
- 23. Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav 2007; 91: 486–498. [DOI] [PubMed] [Google Scholar]
- 24. Shintani T, Higashi S, Suzuki R, et al PTPRJ inhibits leptin signaling, and induction of PTPRJ in the hypothalamus is a cause of the development of leptin resistance. Sci Rep 2017; 7: 11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care 2013; 16: 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ueno H, Nakazato H, Ebihara E, et al Effects of ipragliflozin on postprandial glucose metabolism and gut peptides in type 2 diabetes: a pilot study. Diabetes Ther 2018; 9: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Considine RV, Sinha MK, Heiman ML, et al Serum immunoreactive‐leptin concentrations in normal‐weight and obese humans. N Engl J Med 1996; 334: 292–295. [DOI] [PubMed] [Google Scholar]
- 28. Romero‐Figueroa Mdel S, Garduño‐García Jde J, Duarte‐Mote J, et al Insulin and leptin levels in obese patients with and without breast cancer. Clin Breast Cancer 2013; 13: 482–485. [DOI] [PubMed] [Google Scholar]
- 29. Iacobellis G, Ribaudo MC, Zappaterreno A, et al Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol 2005; 62: 487–491. [DOI] [PubMed] [Google Scholar]
- 30. Mogul HR, Freeman R, Nguyen K, et al Carbohydrate modified diet & insulin sensitizers reduce body weight & modulate metabolic syndrome measures in EMPOWIR (enhance the metabolic profile of women with insulin resistance): a randomized trial of normoglycemic women with midlife weight gain. PLoS ONE 2014; 9: e108264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fukuda T, Bouchi R, Terashima M, et al Ipragliflozin reduces epicardial fat accumulation in non‐obese type 2 diabetic patients with visceral obesity: a pilot study. Diabetes Ther 2017; 8: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Packer M. Do sodium‐glucose co‐transporter‐2 inhibitors prevent heart failure with a preserved ejection fraction by counterbalancing the effects of leptin? A novel hypothesis Diabetes Obes Metab 2018; 20: 1361–1366. [DOI] [PubMed] [Google Scholar]
- 33. Garvey WT, Van Gaal L, Leiter LA, et al Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 2018; 85: 32–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | (a) Time course of body weight during treatment with ipragliflozin. Data are the mean ± standard deviation (n = 92). **P < 0.01 versus baseline (time 0) by one‐way analysis of variance. Where the number of patients differed from the number of patients in the full analysis set, the number is given in parentheses. (b) Correlation between changes in glycated hemoglobin (HbA1c) level and bodyweight from baseline to 16 weeks after the onset of ipragliflozin treatment (n = 95). Where the number of patients differed from the number of patients in the full analysis set, the number is given in parentheses. (c) Correlation analysis for changes in the visual analog scale score for hunger and the serum leptin concentration corrected by bodyweight between baseline and 2 weeks after the onset of ipragliflozin treatment (n = 92). Where the number of patients differed from the number of patients in the full analysis set, the number is given in parentheses.
Figure S2 | Time course of plasma active ghrelin concentration (n = 64) during treatment with ipragliflozin.
Table S1 | Comparison of parameters at baseline between patients who did or did not show an improvement in glycated hemoglobin level or bodyweight after treatment with ipragliflozin for 16 weeks
Table S2 | Changes in visual analog scale scores (mm) for appetite in the study participants (n = 96) during treatment with ipragliflozin for 16 weeks
Table S3 | Changes in clinical parameters for the study participants (n = 96) during treatment with ipragliflozin for 16 weeks
Table S4 | Summary of treatment‐emergent adverse events and selected treatment‐emergent adverse events and of interest in the study patients (n = 104)


