Abstract
Aims/Introduction
The present study analysis was carried out to evaluate the safety and efficacy of tofogliflozin, a sodium–glucose cotransporter 2 inhibitor, in Japanese patients with type 2 diabetes mellitus in real‐world clinical practice.
Materials and Methods
This was a 3‐year non‐interventional observational study of patients with type 2 diabetes mellitus newly administered tofogliflozin who were uncontrolled on current therapy. We carried out a 12‐week interim analysis of tofogliflozin as part of 3‐year post‐marketing surveillance study. The incidence of adverse drug reactions was evaluated as a safety end‐point. As efficacy end‐points, glycated hemoglobin and bodyweight were evaluated.
Results
A total of 6,897 patients were enrolled. Tofogliflozin significantly reduced mean changes from baseline glycated hemoglobin (−0.63%, P < 0.0001) and bodyweight (−2.02 kg, P < 0.0001). The change in glycated hemoglobin and bodyweight reductions in response to tofogliflozin was consistently observed in all body mass index subgroups. Adverse drug reactions occurred in 345 of 6,712 patients (5.14%). There was a low incidence of adverse drug reactions known to be associated with sodium–glucose cotransporter 2 inhibitors, and they were reported as non‐serious. The incidences of polyuria/pollakiuria were higher in patients aged ≥65 years than <65 years, and were significantly different among estimated glomerular filtration rate subgroups. Urinary tract and genital infections occurred more frequently in women than in men.
Conclusions
Tofogliflozin was well tolerated, and no emerging new safety concerns were observed. Tofogliflozin significantly improved glycemic control with no impact on bodyweight gain. The short‐term administration of tofogliflozin is considered to have a favorable benefit–risk profile in Japanese patients with type 2 diabetes mellitus.
Keywords: Post‐marketing study, Tofogliflozin, Type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus is a complex metabolic disorder characterized by an imbalance between insulin secretion and insulin activity associated with chronic hyperglycemia1. The primary aim of diabetes management is to control blood glucose as close as possible to the normal level, and thereby prevent the onset and the progression of micro‐ and macrovascular complications associated with hyperglycemia2, 3, 4, 5, 6. Comprehensive consideration of patient characteristics, such as age, obesity and complications, and the profile of glucose‐lowering agents, such as efficacy/safety and mechanism of action of glucose‐lowering agents, are important for selecting appropriate therapeutic options for patients with type 2 diabetes mellitus. Currently, insulin secretagogues, thiazolidinediones, biguanides and α‐glucosidase inhibitors as oral antidiabetic drugs, as well as insulin products and glucagon‐like peptide‐1 receptor agonists, are widely used for the treatment of type 2 diabetes mellitus in many countries including Japan7, 8, 9. However, despite the large options to manage diabetes, a large portion of patients is currently uncontrolled10. In addition, it is well known that unfavorable side‐effects, such as relatively high incidence of hypoglycemia events11 and weight gain12, are observed in the treatment of patients with type 2 diabetes mellitus. Therefore, new types of glucose‐lowering agents aimed at improving glycemic control, without increasing bodyweight and with a good safety profile, are required for the long‐term treatment of type 2 diabetes mellitus patients. Recently, new oral sodium‐glucose co‐transporter 2 (SGLT2) inhibitors have been approved as a novel therapeutic option for type 2 diabetes mellitus13, 14. SGLT2 inhibitors improve glycemic control by increasing urinary glucose excretion through inhibition of renal glucose re‐absorption, regardless of insulin secretion14, 15, 16, 17. Also, SGLT2 inhibitors are considered to have the potential to address some of the remaining unmet needs to lower the risk of hypoglycemia and weight gain associated with treatment with existing glucose‐lowering agents, such as insulin products12, 13. Therefore, SGLT2 inhibitors are expected to serve as new therapeutic options for type 2 diabetes mellitus.
Tofogliflozin, an SGLT2 inhibitor, was approved in Japan as a treatment for type 2 diabetes mellitus in 201418, 19. The good efficacy and safety profiles of tofogliflozin were shown in Japanese patients with type 2 diabetes mellitus in previous studies, including randomized trials20, 21, 22. As urinary glucose excretion depends on the degree of estimated glomerular filtration rate (eGFR), diabetes patients with renal impairment are less likely to respond to SGLT2 inhibitors23. In “Recommendations on appropriate usage of SGLT2 inhibitors” issued by a Japanese expert committee in May 2016, it was recommended that careful attention be paid to serious adverse drug reactions (ADRs) of SGLT2 inhibitors, including hypoglycemia, urinary tract and genital infections, ketoacidosis, dehydration, and skin disorders24. However, as there is only limited information from clinical trials on SGLT2 inhibitors in Japanese patients, and clinical experience with long‐term treatment using SGLT2 inhibitors is limited as compared with existing glucose‐lowering agents, further information on the long‐term safety and efficacy of tofogliflozin in a large number of Japanese patients in real‐world clinical practice is required for achieving optimal management of type 2 diabetes mellitus.
The present analysis was carried out to evaluate the early safety and efficacy of tofogliflozin at 12 weeks after starting treatment in Japanese patients with type 2 diabetes mellitus.
Methods
Patients and survey method
The Japanese Study of Tofogliflozin with Type 2 Diabetes Mellitus Patients/Long Term (J‐STEP/LT) was designed as a prospective, 3‐year observational and multicenter post‐marketing study, and was co‐sponsored by the manufacturers (Sanofi K.K. and Kowa Company, Ltd.) to evaluate the safety and efficacy of two tofogliflozin hydrate products, Apleway® 20‐mg tablet (Sanofi K.K., Tokyo, Japan)18 and DEBERZA® 20‐mg tablet (Kowa Company, Ltd., Nagoya, Japan)19. The overall study period was planned from September 2014 through February 2019. As a part of this long‐term study, we evaluated the early safety and efficacy of tofogliflozin at 12 weeks after the initiation of treatment. This study was carried out using a centralized registration system for patients with type 2 diabetes mellitus newly treated with tofogliflozin. A total of 6,897 patients were enrolled between September 2014 and November 2015.
The study was carried out in accordance with the ethical principles of the Declaration of Helsinki and Good Post‐marketing Study Practice without intervening in the dosage and administration of tofogliflozin. The patients’ consent and approval of study protocol by each participating center's institutional review board in Good Post‐marketing Study Practice are not required, and we did not obtain patients’ consent. The approved dosages and administration in the package of Apleway® 20‐mg tablet18 and DEBERZA® 20‐mg tablet19 are described as follows: “normally, tofogliflozin (20 mg, once daily) in adults is orally administered once daily before or after breakfast.”
Survey items
The safety and efficacy data were collected from patients using an Electronic Data Capture system in a post‐marketing study. Demographic and baseline characteristics collected were sex, age, duration of diabetes, glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), plasma C‐peptide, height and bodyweight, plasma creatinine (Cr) for calculating eGFR (mL/min/1.73 m2) = 194 × Cr−1.094 × age−0.287 (men) or 194 × Cr−1.094 × age−0.287 × 0.739 (women), complications, pre‐treatment with glucose‐lowering agents, daily doses and treatment duration of tofogliflozin, and concomitant glucose‐lowering and non‐glucose‐lowering agents. We also collected the information on incidences of ADRs including those defined as special interest (hypoglycemia, polyuria/pollakiuria, volume depletion‐related events, urinary tract infection [UTI], genital infection and skin disorders), laboratory test values including eGFR, vital signs (systolic and diastolic blood pressures, and pulse rate) and urinalysis results, as well as efficacy data (HbA1c, FPG, plasma C‐peptide, bodyweight and waist circumference) after tofogliflozin treatment.
Safety
The incidence of ADRs, including those of special interest, clinical laboratory data, vital signs and urinalysis, was evaluated as safety end‐points. ADRs were defined as adverse events that were considered to be related to the investigational drug. Multiple occurrences of the same events in one patient were counted only once. The incidence of ADRs, the period of ADR occurrence, severity and outcomes of administering the investigational drug were collected from the initiation of tofogliflozin treatment until 12 weeks after the initiation of treatment. The causal relationship of the investigational drug with adverse events and the severities of these events were assessed by the investigators at each facility. Individual ADRs were coded according to the Medical dictionary for regulatory activities (MedDRA) version 20.0, and classified according to System Organ Class and Preferred Term. Clinical laboratory (hematological and biochemical) values, vital signs and urinalysis results were evaluated at baseline, and at 4 and 12 weeks after the initiation of tofogliflozin treatment, or at the last observation carried forward (LOCF). In subgroup analysis, the influences of age, sex and eGFR at baseline on the incidences of hypoglycemia, polyuria/pollakiuria, volume depletion‐related events, UTI, genital infection and skin disorders defined as ADRs of special interest were evaluated.
Efficacy
The efficacy of tofogliflozin was evaluated based on mean changes from baseline in HbA1c, FPG, plasma C‐peptide, bodyweight, waist circumference, body mass index (BMI) and eGFR. These efficacy parameters were measured at baseline, and at 4 and 12 weeks after the initiation of tofogliflozin treatment, or at the LOCF. In the subgroup analyses, the influences of baseline eGFR and BMI on HbA1c and bodyweight after tofogliflozin treatment were evaluated at the LOCF.
Statistical analyses
The safety analysis population was defined as all patients for whom electronic case report forms were collected, excluding those with no visits after the first administration of tofogliflozin. The efficacy analysis population included all patients in the safety analysis population, excluding those with no efficacy data. The mean differences in HbA1c, FPG, plasma C‐peptide, bodyweight, waist circumference, BMI and eGFR from the baseline to the LOCF were analyzed using the paired t‐test. In subgroup analyses, patient background factors that affected ADRs of special interest were analyzed using Fisher's exact test, which was used when background factors to be studied were recorded using nominal scales, and the Cochran–Armitage test for those of an ordinal scale. All the tests were carried out with a significance level of 5%. The statistical analyses were calculated using statistical analysis software (SAS) version 9.3 (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Patient disposition
The patient disposition is shown in Figure 1. A total of 6,897 Japanese patients at 1,258 study sites were enrolled, and electronic case report forms were collected from 6,818 patients. In total, 6,712 patients, excluding 106 patients (enrolment failure in 64 patients and no visit after the first treatment in 42 patients), were evaluated as the safety analysis population, and 6,321 patients, excluding 391 patients lacking efficacy data, were evaluated as the efficacy analysis population. A total of 641 patients (9.55%, no duplicate patients) in the 6,712 safety analysis population discontinued tofogliflozin treatment. The main reasons for treatment discontinuation were adverse events (208 patients, 3.10%), no visit (163 patients, 2.43%), patient choice (140 patients, 2.09%) and insufficient or no response (83 patients, 1.24%).
Figure 1.

Patient disposition. eCRFs, electronic case report forms.
Patient characteristics
The patient characteristics of the safety analysis population are shown in Table 1. Of 6,712 patients, 60.8% were men. The mean age ± standard deviation (SD) was 56.9 ± 12.2 years, and 1,896 patients (28.3%) were aged ≥65 years. The mean duration of diabetes ± SD was 8.20 ± 6.40 years. The mean BMI ± SD was 28.7 ± 5.02 kg/m2, and a BMI of ≥25 kg/m2 was observed in 4,329 patients (64.5%). The mean HbA1c at baseline was 8.00 ± 1.48%. The mean eGFR ± SD at baseline was 82.5 ± 22.4 mL/min/1.73 m2, and the proportion of patients with eGFR of <90 mL/min/1.73 m2 was 48.3%. As for diabetic complications, the rates of patients with diabetic retinopathy, nephropathy and neuropathy were 7.97, 21.0 and 9.24%, respectively. A total of 5,221 patients (77.8%) received concomitant glucose‐lowering agents. The majority of patients used oral antidiabetic drugs, including mainly dipeptidyl peptidase‐4 inhibitors (55.8%), biguanides (40.6%) and sulfonylureas (26.6%), and the mean number of concomitant oral antidiabetic drugs per patient at baseline was 2.0 ± 1.0. Insulin products, glucagon‐like peptide‐1 receptor agonists and diuretics were used by 11.2, 3.31 and 6.26% of patients, respectively. The baseline demographic and metabolic characteristics for the efficacy analysis population were similar to those of the safety analysis population (data not shown).
Table 1.
Baseline demographic and metabolic characteristics (safety analysis population)
| Patient characteristic | n | (%) |
|---|---|---|
| Total no. patients | 6,712 | 100.0 |
| Sex | ||
| Men | 4,083 | 60.8 |
| Women | 2,629 | 39.2 |
| Age (years) | ||
| Mean ± SD | 56.9 ± 12.2 | |
| <18 | 2 | 0.03 |
| 18 to <65 | 4,814 | 71.7 |
| ≥65 | 1,896 | 28.3 |
| 65 to <75 | 1,404 | 20.9 |
| ≥75 | 492 | 7.33 |
| Unknown | 0 | 0.00 |
| Duration of diabetes (years) | ||
| Mean ± SD | 8.20 ± 6.40 | |
| <1 | 271 | 4.04 |
| 1 to <10 | 2,288 | 34.1 |
| ≥10 | 1,409 | 21.0 |
| Unknown | 2,744 | 40.9 |
| BMI at baseline (kg/m2) | ||
| Mean ± SD | 28.7 ± 5.02 | |
| <22.0 | 331 | 4.93 |
| 22.0 to <25.0 | 878 | 13.1 |
| 25.0 to <30.0 | 2,410 | 35.9 |
| ≥30.0 | 1,919 | 28.6 |
| Unknown | 1,174 | 17.5 |
| HbA1c at baseline (%) | ||
| Mean ± SD | 8.00 ± 1.48 | |
| <6.5 | 623 | 9.28 |
| 6.5 to <7.0 | 948 | 14.1 |
| 7.0 to <8.0 | 2,136 | 31.8 |
| ≥8.0 | 2,704 | 40.3 |
| Unknown | 301 | 4.48 |
| eGFR at baseline (mL/min/1.73 m2) | ||
| Mean ± SD | 82.5 ± 22.4 | |
| <30 | 13 | 0.19 |
| 30 to <45 | 148 | 2.21 |
| 45 to <60 | 538 | 8.02 |
| 60 to <90 | 2,544 | 37.9 |
| ≥90 | 1,706 | 25.4 |
| Unknown | 1,763 | 26.3 |
| Complications | ||
| Diabetic complications | ||
| Yes | 1,879 | 28.0 |
| Diabetic retinopathy | 535 | 7.97 |
| Diabetic nephropathy | 1,410 | 21.0 |
| Diabetic neuropathy | 620 | 9.24 |
| Hepatic diseases | ||
| Yes | 1,966 | 29.3 |
| Renal disease | ||
| Yes | 584 | 8.70 |
| Cardio‐ and cerebrovascular diseases | ||
| Yes | 687 | 10.2 |
| Cardiovascular diseases | 550 | 8.19 |
| Cerebrovascular diseases | 124 | 1.85 |
| Concomitant glucose‐lowering agents | ||
| No | 1,434 | 21.4 |
| Yes | 5,221 | 77.8 |
| OADs | ||
| Mean ± SD | 2.0 ± 1.0 | |
| DPP‐4 inhibitors | 3,745 | 55.8 |
| Biguanides | 2,728 | 40.6 |
| Sulfonylureas | 1,782 | 26.6 |
| Rapid‐acting insulin secretagogues | 310 | 4.62 |
| Thiazolidinediones | 870 | 13.0 |
| α‐glucosidase inhibitors | 753 | 11.2 |
| SGLT2 inhibitors | 0 | 0.00 |
| Others | 2 | 0.03 |
| Insulin products | 754 | 11.2 |
| GLP‐1 receptor agonists | 222 | 3.31 |
| Diuretics | 420 | 6.26 |
BMI, body mass index; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; HbA1c, glycated hemoglobin; OADs, oral antidiabetic drugs; SD, standard deviation; SGLT2, sodium‐glucose co‐transporter 2.
Safety
The incidences of all ADRs and all ADRs of special interest are shown in Tables 2 and 3, respectively. ADR was reported in 345 (5.14%) of 6,712 patients. The most common ADRs of special interest (indicated by † in Tables 2 and 3) were pollakiuria (38 patients, 0.57%), followed by hypoglycemia (25 patients, 0.37%), cystitis (21 patients, 0.31%), pruritus genital and dehydration (16 patients each, 0.24%), and UTI (14 patients, 0.21%). Most ADRs were non‐serious, and serious ADRs were reported in 30 patients (0.45%). The serious ADRs were UTI and acute myocardial infarction (3 patients each, 0.04%), and dehydration and hypoglycemia (2 patients each, 0.03%). Of 25 patients (0.37%) who experienced hypoglycemia, including two patients (0.03%) with a serious event, eight patients (0.12%) and eight patients (0.12%) were co‐treated with insulin products and sulfonylureas at the occurrence of hypoglycemia, respectively. The information regarding the severity of hypoglycemia, patient outcome and concomitant use of antidiabetic drugs at the occurrence of hypoglycemia in 25 patients with hypoglycemia (including two patients with serious hypoglycemia) is shown in Table S1. Also, of 24 patients with volume depletion‐related events, two patients (0.03%) were co‐treated with diuretics. Three patients (0.04%) died during the study period, but these deaths (suicide [two patients] and pancreatic cancer [one patient]) were considered by the investigators to be unrelated to tofogliflozin. Furthermore, ADRs leading to treatment discontinuation are shown in Table S2.
Table 2.
Overall summary of the incidences of adverse drug reactions (safety analysis population)
| Incidence of ADRs‡ | ||
|---|---|---|
| Non‐serious and serious | Serious | |
| Total no. patients | 6,712 | |
| Total no. patients with ADRs | 345 | 30 |
| Incidence rate of ADRs | 5.14% | 0.45% |
| SOC | ||
|---|---|---|
| PT | Incidence of ADRs, n (%) | |
| Infections and infestations | 78 (1.16) | 6 (0.09) |
| Cystitis† | 21 (0.31) | 1 (0.01) |
| Pneumonia | 1 (0.01) | 1 (0.01) |
| Pyelonephritis acute† | 1 (0.01) | 1 (0.01) |
| Sepsis† | 1 (0.01) | 1 (0.01) |
| Septic shock† | 1 (0.01) | 1 (0.01) |
| Urinary tract infection† | 14 (0.21) | 3 (0.04) |
| Vulvovaginal candidiasis† | 9 (0.13) | – |
| Genital infection† | 12 (0.18) | – |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (0.01) | 1 (0.01) |
| Pancreatic carcinoma | 1 (0.01) | 1 (0.01) |
| Immune system disorders | 3 (0.04) | 1 (0.01) |
| Anaphylactic shock | 1 (0.01) | 1 (0.01) |
| Metabolism and nutrition disorders | 45 (0.67) | 4 (0.06) |
| Dehydration† | 16 (0.24) | 2 (0.03) |
| Hypoglycemia† | 25 (0.37) | 2 (0.03) |
| Psychiatric disorders | 3 (0.04) | 1 (0.01) |
| Insomnia | 1 (0.01) | 1 (0.01) |
| Nervous system disorders | 23 (0.34) | 4 (0.06) |
| Brain stem infarction | 1 (0.01) | 1 (0.01) |
| Cerebral hemorrhage | 1 (0.01) | 1 (0.01) |
| Depressed level of consciousness† | 1 (0.01) | 1 (0.01) |
| Dizziness | 9 (0.13) | – |
| Lacunar infarction | 1 (0.01) | 1 (0.01) |
| Cardiac disorders | 16 (0.24) | 7 (0.10) |
| Acute myocardial infarction | 3 (0.04) | 3 (0.04) |
| Angina pectoris | 1 (0.01) | 1 (0.01) |
| Cardiac failure | 2 (0.03) | 1 (0.01) |
| Cardiac failure congestive | 1 (0.01) | 1 (0.01) |
| Myocardial infarction† | 1 (0.01) | 1 (0.01) |
| Gastrointestinal disorders | 25 (0.37) | 2 (0.03) |
| Melaena | 2 (0.03) | 1 (0.01) |
| Pancreatitis chronic | 1 (0.01) | 1 (0.01) |
| Hepatobiliary disorders | 12 (0.18) | 2 (0.03) |
| Chronic hepatitis | 1 (0.01) | 1 (0.01) |
| Hepatic function abnormal | 8 (0.12) | 1 (0.01) |
| Skin and subcutaneous tissue disorders | 34 (0.51) | 1 (0.01) |
| Rash† | 9 (0.13) | – |
| Skin ulcer† | 1 (0.01) | 1 (0.01) |
| Renal and urinary disorders | 63 (0.94) | 3 (0.04) |
| Nocturia† | 7 (0.10) | – |
| Pollakiuria† | 38 (0.57) | – |
| Urinary retention | 1 (0.01) | 1 (0.01) |
| Renal impairment | 3 (0.04) | 1 (0.01) |
| Acute kidney injury† | 1 (0.01) | 1 (0.01) |
| Reproductive system and breast disorders | 26 (0.39) | – |
| Pruritus genital† | 16 (0.24) | – |
| Congenital, familial and genetic disorders | 1 (0.01) | 1 (0.01) |
| Phimosis | 1 (0.01) | 1 (0.01) |
| General disorders and administration site conditions | 19 (0.28) | – |
| Malaise | 7 (0.10) | – |
| Investigations | 32 (0.48) | 1 (0.01) |
| Blood potassium decreased | 1 (0.01) | 1 (0.01) |
| Urine ketone body present | 7 (0.10) | – |
Individual adverse drug reactions (ADRs) were coded according to Medical Dictionary for Regulatory Activities version 20.0 and classified according to System Organ Class (SOC) and Preferred Term (PT). †ADRs of special interest. ‡Seven or more patients (≥0.1%) or one or more patients with serious ADR.
Table 3.
Incidence of all adverse drug reactions of special interest (safety analysis population)
| ADRs of special interest | Incidence of ADRs, n (%) |
|---|---|
| Non‐serious and serious | |
| Total number of patients | 6,712 |
| Hypoglycemia | 25 (0.37) |
| Hypoglycemia | 25 (0.37) |
| Polyuria/pollakiuria | 50 (0.74) |
| Pollakiuria | 38 (0.57) |
| Nocturia | 7 (0.10) |
| Polyuria | 4 (0.06) |
| Urine output increased | 1 (0.01) |
| Volume depletion‐related events | 24 (0.36) |
| Dehydration | 16 (0.24) |
| Thirst | 6 (0.09) |
| Depressed level of consciousness | 1 (0.01) |
| Dry mouth | 1 (0.01) |
| Myocardial infarction | 1 (0.01) |
| Acute kidney injury | 1 (0.01) |
| Urinary tract infection | 40 (0.60) |
| Cystitis | 21 (0.31) |
| Urinary tract infection | 14 (0.21) |
| Urethritis | 2 (0.03) |
| Cystitis‐like symptom | 2 (0.03) |
| Pyelonephritis acute | 1 (0.01) |
| Sepsis | 1 (0.01) |
| Septic shock | 1 (0.01) |
| Genital infection | 56 (0.83) |
| Pruritus genital | 16 (0.24) |
| Genital infection | 12 (0.18) |
| Vulvovaginal candidiasis | 9 (0.13) |
| Balanoposthitis | 4 (0.06) |
| Vulvar erosion | 3 (0.04) |
| Genital candidiasis | 3 (0.04) |
| Vaginal infection | 2 (0.03) |
| Vulvitis | 2 (0.03) |
| Candida infection | 2 (0.03) |
| Balanitis candida | 1 (0.01) |
| Genital herpes | 1 (0.01) |
| Prostatitis | 1 (0.01) |
| Vulvovaginal pruritus | 1 (0.01) |
| Genital infection female | 1 (0.01) |
| Skin disorders | 32 (0.48) |
| Rash | 9 (0.13) |
| Pruritus | 5 (0.07) |
| Eczema | 5 (0.07) |
| Dermatitis | 2 (0.03) |
| Rash erythematous | 2 (0.03) |
| Rash pruritic | 2 (0.03) |
| Drug eruption | 1 (0.01) |
| Erythema | 1 (0.01) |
| Seborrhoeic dermatitis | 1 (0.01) |
| Skin disorder | 1 (0.01) |
| Skin erosion | 1 (0.01) |
| Skin ulcer | 1 (0.01) |
| Urticaria | 1 (0.01) |
| Pruritus generalized | 1 (0.01) |
Individual adverse drug reactions (ADRs) were coded according to Medical Dictionary for Regulatory Activities version 20.0 and classified according to System Organ Class and Preferred Term.
In subgroup analyses, the influences of age, sex and eGFR at baseline on the incidences of ADRs of special interest were evaluated (Figure 2). A higher incidence of polyuria/pollakiuria was observed in patients aged ≥65 years than in patients aged <65 years, and the difference in the incidence was significant (P = 0.0174). There were no significant differences in the incidences of hypoglycemia, volume depletion‐related events, UTI, genital infection and skin disorders between patients aged <65 years and ≥65 years. UTI and genital infection were more common in women than in men (P < 0.0001), but no significant differences were observed in other ADRs of special interest between men and women. There was a significant difference in the incidence of polyuria/pollakiuria among eGFR subgroups (P = 0.0414), and the incidence tended to be higher in patients with eGFR <60 mL/min/1.73 m2 than patients with eGFR ≥60 mL/min/1.73 m2. There were no significant differences in the incidence of other ADRs of special interest among eGFR subgroups.
Figure 2.

Incidences of adverse drug reactions (ADRs) of special interest by baseline age, sex and estimated glomerular filtration rate (eGFR) after tofogliflozin treatment. (a) Age subgroups. (b) Sex subgroups. (c) eGFR subgroups. The statistical analysis in (a) and (b) was determined by Fisher's exact test, and the analysis in (c) was determined by the Cochran–Armitage test. Numbers of patients are in parentheses. NS, not significant.
Detailed clinical laboratory testing and urinalysis results are shown in Table S3. Increases in hemoglobin, hematocrit, Na, Cl, Mg, phosphorus levels and high‐density lipoprotein cholesterol, and decreases in total cholesterol, non‐high‐density lipoprotein cholesterol, triglyceride and uric acid levels from baseline to the LOCF were observed (P = 0.0330 to P < 0.0001).
Efficacy
The effect of tofogliflozin on HbA1c, FPG, bodyweight, BMI and eGFR is shown in Table 4. Tofogliflozin significantly reduced HbA1c and FPG, and mean changes from baseline to the LOCF in HbA1c and FPG were −0.63 ± 1.11% (P < 0.0001) and −31.9 ± 59.2 mg/dL (P < 0.0001), respectively. Bodyweight, waist circumference, BMI and eGFR were also decreased after tofogliflozin treatment, and the mean changes from baseline were −2.02 ± 2.57 kg (P < 0.0001), −0.75 ± 0.95 kg/m2 (P < 0.0001) and −1.49 ± 11.0 mL/min/1.73 m2 (P < 0.0001) for bodyweight, BMI and eGFR, respectively.
Table 4.
Effect of tofogliflozin on glycated hemoglobin, fasting plasma glucose, bodyweight, body mass index and estimated glomerular filtration rate
| Items | At baseline | 4 weeks | 12 weeks | LOCF | Change from baseline to LOCF | Paired t‐test |
|---|---|---|---|---|---|---|
| HbA1c (%) | 8.00 ± 1.48 (6,120) | 7.61 ± 1.22 (4,997) | 7.34 ± 1.13 (5,469) | 7.38 ± 1.17 (6,137) | −0.63 ± 1.11 (5,973) | P < 0.0001 |
| FPG (mg/dL) | 170.0 ± 64.4 (3,232) | 142.8 ± 47.0 (2,487) | 137.7 ± 45.4 (2,738) | 138.3 ± 46.0 (3,291) | −31.9 ± 59.2 (2,884) | P < 0.0001 |
| Bodyweight (kg) | 77.9 ± 16.7 (5,312) | 76.7 ± 16.5 (4,445) | 75.9 ± 16.2 (4,659) | 76.0 ± 16.4 (5,241) | −2.02 ± 2.57 (5,011) | P < 0.0001 |
| BMI (kg/m2) | 28.9 ± 5.01 (4,893) | 28.5 ± 4.94 (4,112) | 28.1 ± 4.86 (4,270) | 28.2 ± 4.91 (4,785) | −0.75 ± 0.95 (4,624) | P < 0.0001 |
| eGFR (mL/min/1.73 m2) | 82.5 ± 22.4 (4,727) | 79.3 ± 22.5 (3,306) | 81.0 ± 22.0 (3,869) | 80.9 ± 22.3 (4,622) | −1.49 ± 11.0 (4,079) | P < 0.0001 |
Values presented as mean ± standard deviation. Numbers of patients are in parentheses. BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; LOCF, last observation carried forward; HbA1c, glycated hemoglobin.
In the subgroup analyses, the influences of baseline eGFR and BMI on HbA1c and bodyweight in patients treated with tofogliflozin were evaluated (Figure 3). Tofogliflozin produced clinically meaningful reductions in HbA1c ranging from 0.30 to 0.88% in patients with baseline eGFRs 30 to <45 (−0.30 ± 1.15%, P = 0.0029), 45 to <60 (−0.45 ± 1.08%, P < 0.0001), 60 to <90 (−0.56 ± 1.06%, P < 0.0001) and ≥90 mL/min/1.73 m2 (−0.88 ± 1.24%, P < 0.0001), respectively, but did not reduce HbA1c in 13 patients with eGFR <30 mL/min/1.73 m2 (0.05 ± 0.76%, P = 0.8033; Figure 3a). Tofogliflozin significantly reduced bodyweight from baseline in all eGFR subgroups (−1.56 to −2.06 kg, P = 0.0031 to P < 0.0001; Figure 3b). Tofogliflozin produced clinically meaningful reductions in HbA1c ranging from 0.50 to 0.65% in all subgroups regardless of baseline BMI (each P < 0.0001; Figure 3c). Tofogliflozin significantly reduced bodyweight from baseline in all BMI subgroups (−1.18 to −2.42 kg, each P < 0.0001; Figure 3d).
Figure 3.
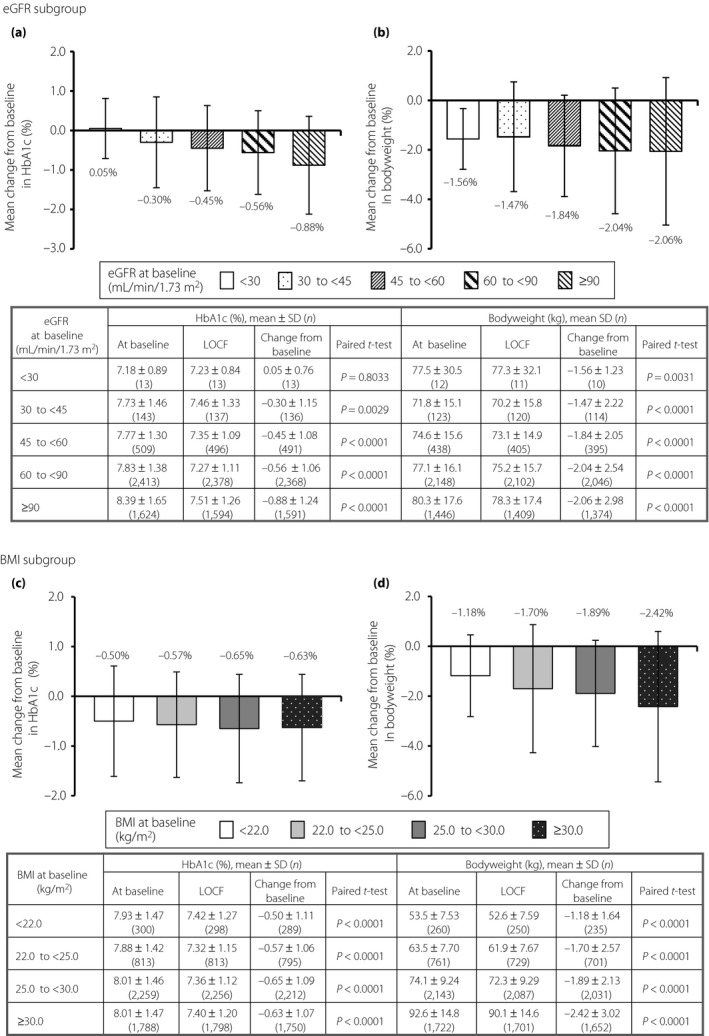
The influences on glycated hemoglobin (HbA1c) and bodyweight by estimated glomerular filtration rate (eGFR) and body mass index (BMI) after tofogliflozin treatment. (a,b) Influences of baseline eGFR on HbA1c and bodyweight, respectively. (c,d) Influences of baseline BMI on HbA1c and bodyweight, respectively. Numbers of patients are in parentheses. LOCF, last observation carried forward; SD, standard deviation.
Discussion
The present study analysis was carried out to evaluate the early safety and efficacy of tofogliflozin at 12 weeks after starting treatment in Japanese patients with type 2 diabetes mellitus in real‐world clinical practice. Tofogliflozin was well tolerated, and the occurrences of confirmed hypoglycemia, polyuria/pollakiuria, volume depletion‐related events, UTI, genital infection and skin disorders defined as ADRs of special interest were generally similar to those seen in response to tofogliflozin or other SGLT2 inhibitors in previous studies17, 20, 21, 22, 25, 26, 27, 28. Unexpected safety concerns related to tofogliflozin were not identified. Clinically meaningful reductions in HbA1c, FPG and bodyweight were observed in patients treated with tofogliflozin.
As for safety, the most common ADR of special interest observed in the present study analysis was pollakiuria, followed by hypoglycemia, cystitis, UTI, pruritus genital and dehydration. These ADRs were already known to be associated with tofogliflozin and other SGLT2 inhibitors in previous studies17, 20, 21, 22, 25, 26, 27, 28. The incidence of confirmed hypoglycemia in these studies was reported to be low due to their insulin‐independent actions29. The incidence of hypoglycemia was also low in the present analysis, reported in just 25 patients (0.37%), including two patients (0.03%) with a serious event, regardless of age, sex or eGFR at baseline. Most of these patients had been concomitantly treated with insulin products (8 patients, 0.12%) and sulfonylureas (8 patients, 0.12%), which might have contributed to hypoglycemia induction. Similarly, the incidence rates of hypoglycemia occurring with other SGLT2 inhibitors, such as dapagliflozin, empagliflozin and canagliflozin monotherapy, were generally low, but an increased incidence of hypoglycemia was observed when SGLT2 inhibitors were given in combination with insulin products or sulfonylureas17. Therefore, when tofogliflozin is used in combination with insulin products or sulfonylureas, dose reductions of these glucose‐lowering agents to minimize the risk of hypoglycemia are recommended18, 19.Pollakiuria was the most common ADR observed in the present study analysis (38 patients, 0.57%), and the incidence of polyuria/pollakiuria was higher in patients aged ≥65 years than those aged <65 years, and/or tended to be higher in patients with eGFR <60 mL/min/1.73 m2 than in correspondent other subgroups. However, we could not draw definitive conclusions regarding the effects of low eGFR on polyuria/pollakiuria because of the small number of incidences (eGFR 30 to <45 mL/min/1.73 m2, 2 patients; and eGFR 45 to <60 mL/min/1.73 m2, 9 patients).Volume depletion‐related events were observed in 24 patients (0.36%), and no significant differences in these events were observed among any of the subgroups based on age, sex or eGFR at baseline. In the subgroup analysis of the Japanese post‐marketing study, ipragliflozin significantly increased the incidence rates of volume depletion with advanced age (≥75 years), low BMI, impaired renal function and diuretic use30. In the present study, the incidence rates of volume depletion were higher in tofogliflozin‐treated patients with low eGFR (30 to <90 mL/min/1.73 m2) than in those with higher eGFR (≥90 mL/min/1.73 m2). The effects of impaired renal function and diuretic use on volume depletion could not draw definitive conclusions because of the small number of incidences (ADRs with renal impairment: 3 patients; and diuretic use in those with volume depletion: 2 patients). The effects of age on volume depletion also could not draw definitive conclusions because of the small sample sizes and different subgroup. Considering that 420 patients in total had been concomitantly treated with diuretics, but the majority of these patients did not experience volume depletion‐related events, diuretics were unlikely to have much impact on the induction of such events. More UTIs and genital infections were observed in women than in men after tofogliflozin treatment, and these findings were generally similar to those of tofogliflozin and other SGLT2 inhibitors obtained in previous studies17, 27, 28. Based on these safety results, tofogliflozin was considered to be well tolerated. However, as elderly diabetes patients have reduced renal function and delayed recognition of dehydration symptoms, such as thirst, compared with younger patients, close safety monitoring of these patients after tofogliflozin treatment might be required18, 19.
As for efficacy, clinically meaningful reductions of HbA1c, FPG and bodyweight in response to SGLT2 inhibitors have been reported in type 2 diabetes mellitus patients from both Japan and Western countries17, 18, 19, 20, 21, 22. In the present study analysis, significant reductions in HbA1c, FPG and bodyweight with tofogliflozin were observed at 12 weeks after starting treatment, being comparable with those obtained with tofogliflozin in 1,506 Japanese elderly diabetes patients in a prior 12‐week interim analysis27. Therefore, tofogliflozin is expected as a new glucose‐lowering agent achieving not only glycemic control, but also weight reduction. Obesity is particularly well known as one of the major risk factors for the development of type 2 diabetes mellitus 31, and the rising prevalence rates of type 2 diabetes mellitus are associated with the global increases in overweight and obese patients32. In Japan, the percentages of men and women who are overweight/obese (BMI ≥25 kg/m2) according to a report from the Ministry of Health, Labour and Welfare in 2015 were 29.5 and 19.2%, respectively33. Therefore, control of the disease by treatment in terms of not only glycemic level, but also bodyweight, is important for the management of type 2 diabetes mellitus. In the subgroup analysis by BMI, there were significant reductions in mean changes from baseline in HbA1c and bodyweight after tofogliflozin treatment in all subgroups. These results show that tofogliflozin can contribute to the management of type 2 diabetes mellitus, especially for overweight/obese patients. As urinary glucose excretion depends on the degree of eGFR, diabetes patients with renal impairment are less likely to respond to SGLT2 inhibitors23, and canagliflozin is recommended for patients with eGFR of ≥45 mL/min/1.73 m2 34. In our subgroup, tofogliflozin significantly reduced HbA1c and bodyweight in all eGFR subgroups, except for those with severely reduced eGFR (<30 mL/min/1.73 m2). Overall, the efficacy results in the early assessment of the present study analysis suggest that tofogliflozin produces clinically meaningful improvements of glycemic control and bodyweight in patients with type 2 diabetes mellitus. Recently, the clinical benefit of several SGLT2 inhibitors on cardiovascular outcome in type 2 diabetes mellitus patients of has been shown in clinical studies, and consistent effects were also shown in a wide range of subgroups35, 36. In addition, the cardiovascular benefit of the SGLT2 inhibitors class was shown in real‐world practice37, 38. Considering the evidence stated above and results obtained in this analysis, which are similar to those of other SGLT2 inhibitors, tofogliflozin might also have clinical benefit on cardiovascular outcome. However, this would need to be confirmed in a cardiovascular outcome clinical trial of tofogliflozin.
The present study analysis had some limitations. J‐STEP/LT is an observational study carried out in a real‐world clinical practice. Therefore, the evidence level is not comparable with that of other randomized clinical studies carried out with the application of strict criteria for patient selection, dosage and administration of the investigational drug, and safety/efficacy evaluation points. Another limitation is that the observation period was limited to 12 weeks after the initiation of tofogliflozin treatment, but this long‐term (3 years) observational study is currently ongoing and, on completion, we plan to report the results of long‐term tofogliflozin treatment. Furthermore, as the present study was carried out in a real‐world clinical setting, the use/handling of concomitant drugs, such as sulfonylureas and insulin preparations, was not stipulated. Therefore, we could not rule out the possibility that HbA1c and weight change might have been affected by such concomitant drugs. We cannot draw definitive conclusions regarding the effects of impaired renal function and diuretic use on volume depletion because of the small sample size.
In conclusion, the safety and efficacy of tofogliflozin during 12 weeks after the initiation of treatment in 6,712 Japanese patients with type 2 diabetes mellitus was evaluated in a real‐world clinical practice by carrying out an early analysis of a long‐term post‐marketing study. Tofogliflozin reduced HbA1c and bodyweight significantly. ADRs observed in the present study analysis were generally similar to those of tofogliflozin and other SGLT2 inhibitors identified in previous studies, and no new safety concerns were found. The beneficial efficacy and safety profile of tofogliflozin might contribute to better management of type 2 diabetes mellitus, especially for overweight/obese patients, and provide a potential therapeutic option as monotherapy and combination therapy, especially with insulin treatment.
Disclosure
KU is a medical advisor for Kowa and Sanofi, and received honoraria for lectures from Sanofi, MSD, Taisho Toyama Pharmaceutical, Eli Lilly, Terumo, Arkray, Astellas Pharma, Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Novo Nordisk Pharma and Kissei Pharmaceutical. KK is a medical advisor for Sanwa Kagaku Kenkyusho, and received honoraria for lectures from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fujifilm Pharma, MSD, Novo Nordisk Pharma, Ono Pharmaceutical, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma and Taisho Toyama Pharmaceutical, and received scholarship grants from Boehringer Ingelheim, Taisho Toyama Pharmaceutical and Mitsubishi Tanabe Pharma. MS, HK and MT are employees of Sanofi K. K. SK, YK, RG, SF and HF are employees of Kowa Company, Ltd.
Supporting information
Table S1 ¦ Occurrence of hypoglycemia (adverse drug reactions).
Table S2 ¦ Occurrence of adverse drug reactions leading to treatment discontinuation.
Table S3 ¦ Clinical laboratory test values after treatment with tofogliflozin.
Acknowledgments
This study was funded by Sanofi K.K. and Kowa Company, Ltd. The authors thank all physicians who participated in this study. Medical writing support was provided by Honyaku Center Inc., and funded by Sanofi K.K. and Kowa Company, Ltd. All authors agreed to the content of the manuscript and its submission for publication.
J Diabetes Investig 2019; 10: 1272–1283
References
- 1. Japan Diabetes Society . Treatment guide for diabetes 2016–2017. Tokyo: Bunkodo Co., Ltd, 2016. Available from: http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2016-2017.pdf (Accessed August 7, 2018). [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 3. Holman RR, Paul SK, Bethel MA, et al 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 4. Shichiri M, Kishikawa H, Ohkubo Y, et al Long‐term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(Suppl 2): B21–B29. [PubMed] [Google Scholar]
- 5. Wake N, Hisashige A, Katayama T, et al Cost‐effectiveness of intensive insulin therapy for type 2 diabetes: a 10‐year follow‐up of the Kumamoto study. Diabetes Res Clin Pract 2000; 48: 201–210. [DOI] [PubMed] [Google Scholar]
- 6. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 7. Tahrani AA, Bailey CJ, Del Prato S, et al Management of type 2 diabetes: new and future developments in treatment. Lancet 2011; 378: 182–197. [DOI] [PubMed] [Google Scholar]
- 8. Esposito K, Chiodini P, Bellastella G, et al Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab 2012; 14: 228–233. [DOI] [PubMed] [Google Scholar]
- 9. Chaudhury A, Duvoor C, Reddy Dendi VS, et al Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 2017; 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casagrande SS, Fradkin JE, Saydah SH, et al The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013; 36: 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bramlage P, Gitt AK, Binz C, et al Oral antidiabetic treatment in type‐2 diabetes in the elderly: balancing the need for glucose control and the risk of hypoglycemia. Cardiovasc Diabetol 2012; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med 2003; 115(Suppl 8A): 42S–48S. [DOI] [PubMed] [Google Scholar]
- 13. Shyangdan DS, Uthman OA, Waugh N. SGLT‐2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta‐analysis. BMJ Open 2016; 6: e009417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: clinical data and mechanism of action. J Diabetes Investig 2014; 5: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012; 14: 5–14. [DOI] [PubMed] [Google Scholar]
- 16. Michel MC, Mayoux E, Vallon V. A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans. Naunyn Schmiedebergs Arch Pharmacol 2015; 388: 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther 2014; 8: 1335–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanofi K.K. Apleway, package insert, (Japanese). Available from: https://e-mr.sanofi.co.jp/-/media/EMS/Conditions/eMR/di/tenpu/apleway.pdf (Accessed December 17, 2018).
- 19. Kowa Company, Ltd . Deberza, package insert, (Japanese). Available from: https://www.kowa-souyaku.co.jp/upload/item/66/4-pt_175.pdf (Accessed December 17, 2018).
- 20. Terauchi Y, Tamura M, Senda M, et al Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J‐STEP/INS): results of a 16‐week randomized, double‐blind, placebo‐controlled multicentre trial. Diabetes Obes Metab 2017; 19: 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikeda S, Takano Y, Cynshi O, et al A novel and selective sodium‐glucose cotransporter‐2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2015; 17: 984–993. [DOI] [PubMed] [Google Scholar]
- 22. Tanizawa Y, Kaku K, Araki E, et al Long‐term safety and efficacy of tofogliflozin, a selective inhibitor of sodium‐glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open‐label, randomized controlled trials. Expert Opin Pharmacother 2014; 15: 749–766. [DOI] [PubMed] [Google Scholar]
- 23. Kasichayanula S, Chang M, Hasegawa M, et al Pharmacokinetics and pharmacodynamics of dapagliflozin, a novel selective inhibitor of sodium‐glucose co‐transporter type 2, in Japanese subjects without and with type 2 diabetes mellitus. Diabetes Obes Metab 2011; 13: 357–365. [DOI] [PubMed] [Google Scholar]
- 24. Committee of experts in the field: Inagaki N, Ueki K, Kaku K, et al. Recommendations for appropriate use of SGLT2i (revised on May 12, 2016) (Japanese). Available from: http://www.fa.kyorin.co.jp/jds/uploads/recommendation_SGLT2.pdf (Accessed August 7, 2018).
- 25. Shiba T, Ishii S, Okamura T, et al Efficacy and safety of empagliflozin in Japanese patients with type 2 diabetes mellitus: a sub‐analysis by body mass index and age of pooled data from three clinical trials. Diabetes Res Clin Pract 2017; 131: 169–178. [DOI] [PubMed] [Google Scholar]
- 26. Andrianesis V, Glykofridi S, Doupis J. The renal effects of SGLT2 inhibitors and a mini‐review of the literature. Ther Adv Endocrinol Metab 2016; 7: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Utsunomiya K, Shimmoto N, Senda M, et al Japanese study of tofogliflozin with type 2 diabetes mellitus patients in an observational study of the elderly (J‐STEP/EL): a 12‐week interim analysis. J Diabetes Investig 2016; 7: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seufert J. SGLT2 inhibitors – an insulin‐independent therapeutic approach for treatment of type 2 diabetes: focus on canagliflozin. Diabetes Metab Syndr Obes 2015; 8: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fioretto P, Giaccari A, Sesti G. Efficacy and safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in diabetes mellitus. Cardiovasc Diabetol 2015; 14: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terauchi Y, Yokote K, Nakamura I, et al Safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA‐ELDER): interim results of a post‐marketing surveillance study. Expert Opin Pharmacother 2016; 17: 463–471. [DOI] [PubMed] [Google Scholar]
- 31. Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997; 146: 214–222. [DOI] [PubMed] [Google Scholar]
- 32. CDC's Division of Diabetes Translation . Maps of trends in diagnosed diabetes and obesity. United States Diabetes Surveillance System. 2017. Available from: https://www.cdc.gov/diabetes/statistics/slides/maps_diabetesobesity_trends.pdf (Accessed August 7, 2018)
- 33. Ministry of Health, Labour and Welfare (2015) National Health and Nutrition Examination Survey in Japan. (Japanese) Available from: https://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/kekkagaiyou.pdf (Accessed August 7, 2018)
- 34. Khurana M, Vaidyanathan J, Marathe A, et al Canagliflozin use in patients with renal impairment‐Utility of quantitative clinical pharmacology analyses in dose optimization. J Clin Pharmacol 2015; 55: 647–656. [DOI] [PubMed] [Google Scholar]
- 35. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 36. Neal B, Perkovic V, Mahaffey KW, et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 37. Kosiborod M, Cavender MA, Fu AZ, et al Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation 2017; 136: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kosiborod M, Lam CSP, Kohsaka S, et al Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: The CVD‐REAL 2 study. J Am Coll Cardiol 2018; 71: 2628–2639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Occurrence of hypoglycemia (adverse drug reactions).
Table S2 ¦ Occurrence of adverse drug reactions leading to treatment discontinuation.
Table S3 ¦ Clinical laboratory test values after treatment with tofogliflozin.


