Abstract
We present the case of an 8‐year‐old girl with type 1 diabetes who developed severe allergic contact dermatitis by using FreeStyle® Libre. A patch test was carried out using a piece of the adhesive part of the sensor of the FreeStyle® Libre, and subsequently by using the potential contact allergens in the sensor, isobornyl acrylate. She reacted positively to the adhesive part of the sensor, and also reacted positively to isobornyl acrylate with three different concentrations, 0.1%, 0.05% and 0.01%, over 48‐h, 72‐h and 7‐day periods. The FreeStyle® Libre is a useful and less invasive device that can be used for continuous glucose monitoring in patients with diabetes. In contrast to the remarkable advantages, allergic contact dermatitis caused by isobornyl acrylate in the adhesive part of FreeStyle® Libre is one of the potential adverse events.
Keywords: Adverse event, FreeStyle® Libre, Isobornyl acrylate
Introduction
The FreeStyle® Libre (Abbott Diabetes Care, Witney, UK), a flash glucose monitoring system, has recently been used for continuous subcutaneous analysis of glucose profiles in patients with diabetes. This innovative medical device does not require daily repeated finger pricks, and provides continuous and dynamic interstitial glucose levels presenting as ambulatory glucose profiles1. The FreeStyle® Libre consists of a sensor, affixed on the upper arm with an adhesive film, which analyzes real‐time interstitial glucose levels through a central catheter inserted in the subcutaneous tissue. The sensor and catheter are applied for up to 14 days with an applicator. Subcutaneous glucose concentrations are subsequently obtained by scanning the sensor with a reader. It can replace the classical capillary blood glucose meters and help improve patients' attitudes towards glycemic management. However, we experienced a case wherein a pediatric patient using FreeStyle® Libre developed severe allergic contact dermatitis, which was caused by the adhesive part of the sensor. Isobornyl acrylate (IBOA; CAS no.5888‐33‐5) originates from the sensor itself and then migrates to the adhesive part of the sensor2. We found IBOA as a relevant and causative contact allergen in this case. Several Caucasian cases were previously described in the dermatological literature2, but this is the first case of a Japanese pediatric patient with type 1 diabetes showing allergic contact dermatitis caused by IBOA using the FreeStyle® Libre.
Case Report
An 8‐year‐old girl with type 1 diabetes was under regular treatment with multiple daily injections of insulin; she managed her blood glucose levels with a traditional capillary blood glucose meter. She switched to a flash glucose monitoring system (the FreeStyle® Libre) in 2018. She wore the FreeStyle® Libre on her left arm; the next day, she noticed redness and eruptions just beneath the adhesive part of the film, whereas the central needle opening remained unaffected. The redness and eruptions shortly aggravated (Figure 1); finally, the patient visited the Department of Pediatrics, Nihon University Hospital, Tokyo, Japan. She used the FreeStyle® Libre on a trial basis at a diabetic summer camp for 4 days carried out 5 months earlier. She had no skin reaction at the summer camp and also never experienced any skin reactions from any medical devices, such as insulin pump or infusion sets. We referred her to the Department of Dermatology at our hospital to determine the potential causative allergen leading to allergic dermatitis.
Figure 1.
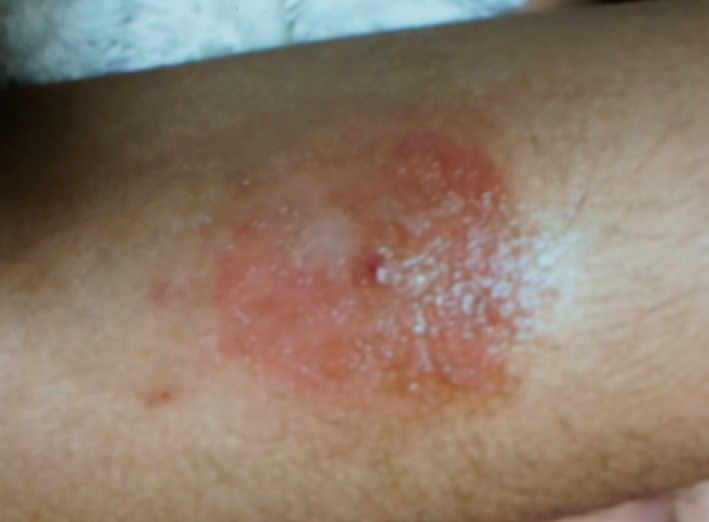
Allergic contact dermatitis caused by wearing the FreeStyle® Libre.
A patch test was initially carried out using a piece of the adhesive part of the sensor of the FreeStyle® Libre, and subsequently by using the potential contact allergen in the sensor, IBOA, on the basis of literature2. IBOA raw material was used for the patch test in three different concentrations, 0.1% (No. 1), 0.05% (No. 2) and 0.01% (No. 3), over 48‐h, 72‐h and 7‐day periods. The occlusion time was a 48‐h period. Patch testing was carried out with the adhesive part of the sensor, and with acetone solutions of IBOA material, applied on the upper arm. The chamber of this patch test was a Torii chamber (Torii Medical Plaza, Tokyo, Japan). The affected patient reacted positively with (++) to the adhesive part of the sensor. She also reacted positively with (+ or ++) to IBOA with all concentrations in the 48‐h (Figure 2), 72‐h and 7‐day periods. Acetone (No. 4) and Vaseline, used for the test chamber, (No. 5) as the control material provoked no reaction in the patient.
Figure 2.
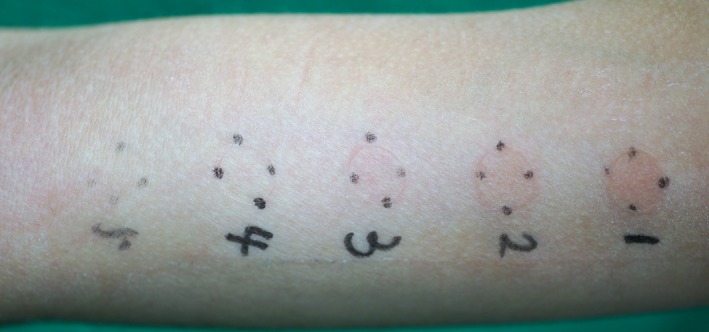
Positive patch test to isobornyl acrylate in the concentrations of 0.1% (No. 1; +), 0.05% (No. 2; +) and 0.01% (No. 3; +) over a 48‐h period. Acetone (No. 4) and Vaseline (No. 5) were negative as controls.
Informed consent was obtained from the patient's parents for publication of this case report and the accompanying images.
Discussion
In the present study, we report the case of a child with allergic contact dermatitis caused by IBOA in FreeStyle® Libre. IBOA is likely released from the glue used to join the top and bottom part of the sensor, and subsequently migrates into the other parts of the sensor. The chemical analyses indicated that the adhesive patch itself might not have been the actual source of IBOA. Herman et al.2 first reported that IBOA was a cause of allergic contact dermatitis as an adverse event of FreeStyle® Libre in 15 pediatric and adult patients with diabetes. They concluded that an allergic reaction to IBOA is strongly suspected to be the cause of severe contact dermatitis in patients using FreeStyle® Libre, and little to no cross‐reactivity with other acrylates was to be expected. We showed that IBOA strongly reacted in the patch test, which definitely showed IBOA as the potential allergen.
IBOA is an acrylate monomer used in plasticizers, coating and ultraviolet‐cured glues3. The first two cases with allergic contact dermatitis caused by IBOA were reported by Busschote et al.4 in 1995. The patients were being treated with insulin pumps, and IBOA was present in the ultraviolet‐cured glues that were used to fix the needle into the plastic stand. The third case with allergic contact dermatitis caused by IBOA was reported by Christoffers et al.5 and another case was described by Kanerva et al.6
The FreeStyle® Libre is a useful and less invasive device that can be used for continuous glucose monitoring in patients with diabetes. It has various merits, in that it changes patients' attitude towards diabetic management and improves their quality of life. In contrast to the remarkable advantages, allergic contact dermatitis caused by IBOA in the adhesive part of FreeStyle® Libre is one of the potential adverse events. We demonstrated this adverse event by patch testing. Two recent reports also described contact dermatitis caused by IBOA in the Omnipod insulin pump7, 8. In contrast, other unknown contact allergens might play a role as well.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2019; 10: 1382–1384
References
- 1. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, et al Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet 2016; 388: 2254–2263. [DOI] [PubMed] [Google Scholar]
- 2. Herman A, Aerts O, Baeck M, et al Allergic contact dermatitis caused by isobornyl acrylate in FreeStyle® Libre, a newly introduced glucose sensor. Contact Dermatitis 2017; 77: 367–373. [DOI] [PubMed] [Google Scholar]
- 3. Foti C, Romita P, Rigano L, et al Isobornyl acrylate: an impurity in alkyl glucosides. Cutan Ocul Toxicol 2016; 35: 115–119. [DOI] [PubMed] [Google Scholar]
- 4. Busschots AM, Meuleman V, Poesen N, et al Contact allergy to components of glue in insulin pump infusion sets. Contact Dermatitis 1995; 33: 205–206. [DOI] [PubMed] [Google Scholar]
- 5. Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth) acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis 2013; 69: 86–92. [DOI] [PubMed] [Google Scholar]
- 6. Kanerva L, Jolanki R, Leino T, et al Occupational allergic contact dermatitis from 2‐hydroxyethyl methacrylate and ethylene glycol dimethacrylate in a modified acrylic structural adhesive. Contact Dermatitis 1995; 33: 84–89. [DOI] [PubMed] [Google Scholar]
- 7. Oppel E, Högg C, Summer B, et al Isobornyl acrylate contained in the insulin patch pump OmniPod as the course of severe allergic contact dermatitis. Contact Dermatitis 2018; 79: 178–180. [DOI] [PubMed] [Google Scholar]
- 8. Raison‐Peyron N, Mowitz M, Bonardel N, et al Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis 2018; 79: 76–80. [DOI] [PubMed] [Google Scholar]


