Abstract
Aims/Introduction
Peptides are considered to be quasi‐hormones and effective molecules for regulation of the cells function and prevention of metabolic disorders. Di‐ and tripeptides gastrointestinal absorption ability have been proposed to prevent diabetes progression.
Materials and Methods
Small peptides with different sequences of specific amino acids were synthesized based on a solid phase peptide synthesis protocol, and carnosine (A) and glutathione were examined for the prevention of diabetes induced by multiple low‐doses of streptozotocin in mice.
Results
The peptides A, Leu‐Gly (D) and Pro‐Pro showed preventive effects on blood glucose elevation and impairment of the signaling and performance of β‐cells. The β‐cell function assessed by immunofluorescence and blood glucose level in mice exposed to diabetes treated by the peptides A and D was similar to the normal mice. The peptide D prevented bodyweight loss caused by diabetes induction. The use of D and A peptides dramatically prevented the incidence of disruption in β‐cells signaling by maintaining the natural balance of intracellular Akt‐2 and cyclic adenosine monophosphate.
Conclusions
The results proved that peptide D (Leu‐Gly), named Hannaneh, inhibits the bodyweight loss caused by diabetes induction. The Hannaneh and carnosine dipeptides, with preservation of normal β‐cell signaling and anti dipeptidyl peptidase‐4 activity, prevented blood glucose increases in mice at risk of diabetes. These dipeptides might be regarded as the pharmaceutical agents for the prevention of diabetes.
Keywords: β‐Cell, Glucose, Peptide
Introduction
In recent years, there has been a peptides revolution as a result of newly discovered influences of peptides on different physiological systems. Small peptides are defined as quasi‐hormones and pharmaceutical agents; these bioactive molecules can modulate the cells’ physiological functions1. Bioactive small peptides can be absorbed from the intestine directly into bloodstream through the PepT1 H+/peptide cotransporter2. After enteric absorption, the pancreas is one of the important organs, as it is exposed to high concentrations of absorbed bioactive peptides. β‐Cells in the pancreas are one of the major cellular targets for bioactive peptides3, 4.
β‐Cells are very sensitive to apoptotic damage induced by oxidative and inflammatory induced compounds. Streptozotocin (STZ) as a chemical toxin binds to the glucose transporter 2 receptors on β‐cells5, 6. Administration of multiple low‐dose streptozotocin (MLDS) induces distortion of the β‐cells in conjunction with mononuclear cell infiltration and apoptosis by caspases enzymes, deoxyribonucleic acid mutilation, nitric oxide production and free radical generation7, 8. It is known that the STZ model of diabetes simulates the complications of human diabetes. Given the established similarities of some structural, functional and biochemical abnormalities to human disease, it is an appropriate model for assessing the mechanism of diabetes7. Although there is disagreement among researchers, the majority agree that MLDS‐generated mild hyperglycemia (200–400 mg/dL) in experimental mice causes insulin secretion that is similar to that of human type 2 diabetes9. However, there are conflicting reports indicating that administration of MLDS in the first days generates type 2 diabetes and in progression produces type 1 diabetes10, 11.
Glucagon‐like peptide‐1 (GLP‐1), a major incretin hormone released in response to nutrient intake and bloodstream amino acids and peptides, contributes >50% of postprandial glucose‐stimulated insulin release, and plays important roles in insulin secretion and maintaining normal glucose regulation12, 13. The durability of this hormone is transient, because it is rapidly cleared by the kidney and inactivated by cleavage at the N‐terminal by dipeptidyl peptidase‐4 (DPP‐4) enzyme14. Therefore, it is important for type 2 diabetes therapy to develop a potent DPP‐4 inhibitor, GLP‐1 stimulator and direct β‐cell‐signaling activator from natural sources, such as peptides15. The receptors for GLP‐1 are located in the pancreas of humans and mice. Previous studies have shown the proliferative and anti‐apoptotic effects of GLP‐1, leading to the expansion of the mass of β‐cells and improved glucose homeostasis in type 2 diabetes14, 16. The serine/threonine‐specific protein kinase Akt‐2, also known as protein kinase B, intracellular enzyme for β‐cells, plays an important role in signal transduction downstream of the insulin receptors. Activation of Akt‐2 signaling in mice constitutively overexpressing activated Akt‐2 and cyclic adenosine monophosphate (cAMP) as the second messenger for regulation of insulin exocytosis in β‐cells resulted in increased islet mass, largely owing to neogenesis, proliferation of β‐cells and improved glucose tolerance. Therefore, Akt‐2 might represent a potential target to improve β‐cell proliferation and survival18, 19. GLP‐1 might activate Akt‐2 signaling of the cAMP‐dependent pathways in β‐cells. cAMP signaling is one main factor in transducing GLP‐1‐mediated activities in β‐cells. In contrast, GLP‐1 has been shown to improve both β‐cell proliferation and survival through cAMP‐dependent stimulation of the cAMP in mice18, 19.
One of the main causes of diabetes development is linked to the oxidation phenomenon. Therefore, anti‐oxidative peptides might exert antidiabetic effects, particularly in the prediabetes stage20, 21. Some histidine‐containing peptides, particularly the dipeptide known as carnosine (β‐alanine–l‐histidine; peptide A), show anti‐oxidant activity20, 22. The tripeptide, glutathione (peptide B), exists in natural tissues, and is important in the regulation of the redox state and protection of cells from oxidative damage by the disposal of free radicals, which have a pathogenic effect in the chronic complications of diabetes23. Hydrophobic amino acids, such as proline (Pro) and leucine (Leu), especially in the C‐ and N‐terminals of peptides, have anti‐oxidative effects20. Pro is an essential amino acid in regulating gene expression, cell signaling and anti‐oxidative responses. This amino acid also modulates protein–protein interactions, thus playing important roles in many cell‐signaling pathways. Leu is an important branched‐chain amino acid owing to its most popular benefit in the metabolism. It activates the mechanistic target of rapamycin and cAMP pathways, leading to increased protein synthesis, specifically insulin, by β‐cells (as an insulin production stimulator), and prevents mitochondrial gene mutations (one of the causes of diabetes and obesity). Leu and its small peptides are known to stimulate insulin secretion from pancreatic β‐cells by serving as metabolic fuel and regulating cell metabolism. Furthermore, Leu peptides regulate the expression of key metabolic genes in β‐cells as a new opportunity for prevention of islet dysfunction and diabetes23, 24. The peptides of the glyproline family show various bioactivities, and the linear glyproline peptides are relatively stable (t 1/2 of Pro‐Gly‐Pro is >24 h)24, 25, 26, 28.
The objective of the present study was to investigate the anti‐diabetic effects of di‐ and tripeptides with different sequences consisting of three specific amino acids, including Pro, Gly and Leu.
Methods
Peptides synthesis
Experimental peptides were synthesized using a classical glass reaction vessel following the Fmoc solid‐phase peptide synthesis protocol29, 31. The 2‐CTC resin (specific for Pro and Gly peptides to prevent diketopiperazine formation) was swelled in dry dimethylformamide (DMF; this resin is extremely moisture‐sensitive) for 1 h. Then the DMF was filtered under vacuum. The first amino acid at the C‐terminal of the peptide was coupled in the presence of tetrafluoroborate and diisopropylethylamine in dry DMF by mixing with nitrogen gas for 2 h. After the completion of the coupling reaction (which was confirmed by Kaiser test30 for Leu and Gly, and isatin test for Pro29), the solution was filtered off and then the resin coupled with the first amino acid was washed with DMF (3 × 2 mL) and dichloromethane (DCM; 3 × 2 mL)35. In order to ensure that no unreacted sites remained on the resin, the resin was end‐capped (twice) using high‐performance liquid chromatography‐grade methanol MeOH. The N‐terminal Fmoc deprotection was carried out using piperidine in DMF (20% v/v, 10 mL, 2 × 30 min). The resin was washed with DMF (3 × 2 mL) and DCM (3 × 2 mL). The subsequently activated amino acids were coupled in a similar manner. The resin was washed (three times) with DMF and DCM, sequentially, at the end of all stages. After obtaining the required peptide sequence, the resin–peptide was dried under vacuum for 24 h. Fresh cleavage cocktail, composed of 5% trifluoroacetic acid (TFA) in DCM, was added to the resin–peptide. The mixture was shaken at room temperature for 2 h, then the resin was collected by filtration. The isolated peptide was washed with another 2 mL of cleavage cocktail. The combined filtrates containing 5% TFA in DCM and the peptides were evaporated to a minimum volume using a nitrogen evaporator. The resulting solution was lyophilized for 24 h. Purification of peptides was carried out by analytical reversed‐phase–high‐performance liquid chromatography using a Zorbax 300 SB‐C8 narrow bore column with a linear AB gradient (1% acetonitrile/min) at a flow rate of 0.25 mL/min, where eluent A was 0.2% aq. TFA, pH 2 and eluent B was 0.2% TFA in acetonitrile. Eventually, approximately 200 mg of each di‐ and tripeptide was obtained. The chemical structures of the seven most effective peptides that were produced and studied in the present research are shown in Figure 1.
Figure 1.
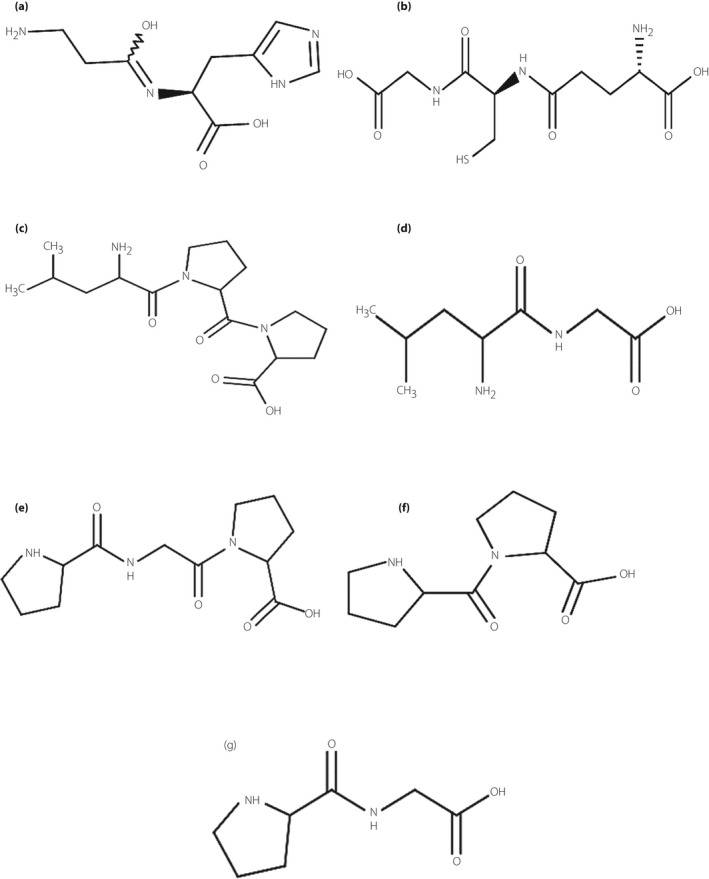
The chemical structures of the studied peptides. (a) Ala‐His (carnosine), (b) Glu‐Cys‐Gly (glutathione), (c) Leu‐Pro‐Pro, (d) Leu‐Gly, (e) Pro‐Gly‐Pro, (f) Pro‐Pro and (g) Pro‐Gly. These chemical structures were designed using Marvin Beans, 16.8.22.0 (Marvin Sketch) software for windows in a two‐dimensional manner.
Animals, experimental design and tested peptides
Male BALB/c mice (aged 9–10 weeks) were purchased from the Pasteur Institute of Tehran (Iran) and were housed in a controlled environment in a specific pathogen‐free animal facility at 22°C under a 12:12 h light : dark cycle at the Drug Applied Research Center, Tabriz University of Medical Sciences. The normal diet and water were offered ad libitum. After animal adaptation to the environment for 1 week, all mice were weighed, labeled and allocated to treatment groups on the basis of the average weight (35.4 ± 0.3 g; 10 male mice per group). After a week of re‐adaptation, the experiment was started. On the first day of the experiment, simultaneous injection of STZ and peptides was commenced. Diabetes was induced by MLDS injection on the left side of the intraperitoneal (i.p.) space. For this purpose, The STZ (S0130/Sigma; St. Louis, MO, USA) was dissolved in 0.1 mol/L citrate buffer (pH 4.5) and injected within 20 min of preparation at a dose of 50 mg/kg/day for five consecutive days. Coinciding with the STZ injection, each of the peptides was injected on the right side every day (peptides dissolved in dimethyl sulfoxide [DMSO], 3% in phosphate‐buffered saline [PBS] and injected at 20 mg/kg of bodyweight/day). Briefly, the experimental treatments were as follows: in the control group (CON), mice received bilateral i.p. injections of citrate buffer without STZ (left side) and PBS without peptide (right side). In the group exposed to diabetes (DI), the mice received bilateral i.p. injections of STZ (left side) and PBS without peptide (right side). The third group (DM) was mice exposed to diabetes and 3% DMSO that received bilateral i.p. injections of STZ (left side) and PBS + 3% DMSO without peptide (right side). The other groups of mice received peptides and were exposed to diabetes where each group received one of the peptides dissolved in 3% DMSO in the PBS, the mice with bilateral i.p. injections of STZ (left side) and one of the peptides + 3% DMSO in PBS (right side; seven groups for the seven reported peptides from A to G). In all groups, STZ was injected from 1 to 5 days and peptides were injected from 1 to 12 days continuously. The bodyweight of each of the labeled mice was recorded at three time points (1st, 6th and 12th days of the experiment).
In the present study, 15 peptides including Leu‐Pro‐Pro, Leu‐Pro, Leu‐Leu, Leu‐Leu‐Gly, Leu‐Gly, Leu‐Leu‐Leu; Pro‐Gly‐Pro, Pro‐Pro, Pro‐Leu‐Gly, Pro‐Gly, Pro‐Pro‐Pro; Gly‐Gly, Gly‐Gly‐Gly, Gly‐Pro‐Gly and Gly‐Leu‐Gly were prepared. These peptides were tested in three stages under the same environmental and nutritional conditions, and the same mice species. In each of the three experiments, there were three control groups (CON, DI and DM), and some of these 15 peptides were tested with a limited number of animals. Based on our preliminary screening experiments in the final stage (main experiment), a retest with five peptides, including Leu‐Pro‐Pro (peptide C), Leu‐Gly (peptide D), Pro‐Gly‐Pro (peptide E), Pro‐Pro (peptide F) and Pro‐Gly (peptide G), which showed promising antidiabetic effects along with the previously known effective peptides; that is, Glu‐Cys‐Gly (glutathione) and β‐alanine–l‐histidine (carnosine), were tested.
Glucometry, insulin, GLP‐1 and DPP‐4 assay
On the even days of the experiment (days 2, 4, 6, 8, 10 and 12), blood samples were obtained from the tail vein of each mouse in the fed state and glucose was measured using a glucometer (GALA, TD‐4277, 2016 approved by the US Food and Drug Administration) between 09.00 and 11.00 hours. Mice were considered diabetic when their fed blood glucose level was >200 mg/dL. Furthermore, the other blood samples (fed state) were taken from the orbital sinus at days 6 (09.00 hours) and 12 (11.00 hours) for measurement of insulin, GLP‐1 and DPP‐4 at a maximum of 30 min after peptide injection. For this purpose, obtained sera were measured by enzyme‐linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Awareness Technology Inc., Palm City, FL, USA), including an ELISA microplate reader (Stat Fax 2100) and an automatic ELISA plate washer (Stat Fax 2006) for mouse insulin (Sigma‐Aldrich, St.Louis, MO, USA), mouse GLP‐1 (Eastbiopharm Inc., Hangzhou, China) and mouse DPP‐1 (Eastbiopharm Inc.).
Dissection and isolation of mice pancreatic islets
At the end of the experiment, the pancreases were dissected from anesthetized (by ketamine and xylazine) mice. Islets were isolated from the pancreas based on the described protocol for collagenase digestion33. After being washed in Krebs–Ringer bicarbonate buffer solution with 0.2% bovine serum albumin, the islets were digested with 0.025% trypsin (Life Technologies, Carlsbad, CA, USA) for 5 min at 37°C and passed through a 400‐mm wire mesh. The cells were then plated on coverslips that were coated with poly‐l‐lysine and maintained at 37°C in a 5% CO2 incubator for 24 h32, 33.
Immunofluorescence imaging
The dissociated islet cells were fixed in 7% paraformaldehyde for 15 min and washed with 0.1 mol/L PBS with 0.3% Triton X‐100 for 30 min at 37°C. After incubation in 5% bovine serum albumin and 0.15% Triton X‐100 blocking solution for 1 h, the cells were incubated by primary antibodies for 1 h at 4°C, washed with PBS and incubated again with the appropriate fluorochrome‐conjugated antibodies for 1 h. Antibodies included a guinea pig anti‐insulin antibody (Abcam, Cambridge, UK), a mouse anti‐glucagon antibody (Abcam) and a mouse anti‐somatostatin antibody (Abcam). After being thoroughly washed with PBS, immunofluorescence images of the islets were obtained based on minimal background under a total internal reflection fluorescence microscope, because high background fluorescence might occur as a result of exocrine contamination33.
Akt‐2 and cAMP assay
Isolated islets were used to assay Akt‐2 and cAMP of β‐cells. The extracted biomass was diluted in 10× volume of 0.1 mol/L HCl, homogenized and centrifuged at 2,300 g for 5 min. After incubation, the surface liquid used for cAMP lysates was measured using the direct Cyclic AMP EIA kit (Assay Designs, Farmingdale, NY, USA) and then Akt2 (AKT2 ELISA Kit; My Biosource Inc., San Diego, CA, USA) according to the manufacturer's instructions33, 45.
Ethical Approval
Ministry of Health and Medical Education ethics committee (number 95.93, 2015) approved the present study.
Statistical analysis
All variables were checked for normality using the Kolmogorov–Smirnov test using SPSS, version 19.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov rest showed that all variables were normally distributed. Data were analyzed by one‐way (glucose data) or two‐way (hormone data) GLM procedure anova using SAS for Windows release 9.2 (SAS Institute Inc., Cary, NC, USA). Data are reported as the mean and standard deviation. The differences between means were studied by Duncan's multiple‐range test where P < 0.05 were reported as statistically significant.
Results
Table 1 presents the mean plasma glucose levels for the six time points of the experimental period and statistically compares these values between treatments. The glucometry of the second day of intervention shows that in the exposed to diabetes mice (DI), the glucose level was significantly elevated compared with the control (CON) group (111.6 vs 94.6 mg/dL; P < 0.01). The mice that received peptide E showed lower blood glucose levels than those in the DI and CON groups (P < 0.01). On the fourth day of the trial, the blood glucose level was higher than that of most groups in mice that were in the DM and peptide D groups (P < 0.05). Furthermore, the glucose level in the mice that received peptide E was lower than all other groups (P < 0.05). The mice that received peptides A, B, C and G showed lower blood glucose levels compared with the DI group at the 6th day. In addition to Table 1, the charts in Figure 2 show the effects of the peptides at days 10 and 12 of the experiment. At 10 days after the start of the trial and 5 days after MLDS protocol completion, the blood glucose was significantly higher in mice of the DI and DM groups compared with the CON group (174.8 and 177.3 vs 111.7 mg/dL; Figure 2a). Two diabetes‐exposed groups treated by peptides A and D had blood glucose levels similar to those of the CON group (116.4 and 112.0 vs 111.7 mg/dL). The mice that received other peptides showed intermediate blood glucose levels between the CON and DI groups. In other words, peptides A and D prevented hyperglycemia under the influence of STZ injection at this stage (Figure 2a). On the 12th day of the experiment, the mice in the DI and DM groups were completely diabetic (glucose levels 279.3 and 219.1 mg/dL, respectively). Also, the results (Table 1 and Figure 2b) showed that the difference between blood glucose levels in the DI and DM groups was significant at the end of the experiment (P < 0.01). The mice that received DMSO showed lower blood glucose levels than the DI group. The blood glucose levels of mice that received peptides A, D, E and F were statistically similar to those of the CON mice at the end of the experiment. It is clear that the differences between the glucose levels for the CON mice in the six time stages of glucometry were not significant (respectively, 94.6, 107.4, 120.3, 99.2, 111.7 and 118.7 mg/dL). The blood glucose levels in the DI mice increased with a steep slope during the six stages. A similar result was obtained for the DM group that received 3% DMSO in PBS, so that the mice in this group (DM) were diabetic by the 12th day of the trial (219.1 mg/dL). The differences between the means of blood glucose levels at the six glucometry times were not significant in mice that received peptides A, D and F (P > 0.05). This means that the use of any of these three peptides dramatically reduced the incidence of diabetes. However, the other peptides also effectively prevented the excessive increase of glucose levels until the 10th day of the trial. The results showed that the blood glucose levels of mice that received any of the peptides did not exceed 200 mg/dL (diabetic level), even on the last day.
Table 1.
Comparison of mean plasma glucose level between treatments at different time points
| Treatments | Time (days) | |||||
|---|---|---|---|---|---|---|
| Time stages | 2 | 4 | 6 | 8 | 10 | 12 |
| CON | 94.6 ± 1.7b | 107.4 ± 3.1abc | 120.3 ± 4.2abc | 99.2 ± 4.0a | 111.7 ± 4.5a | 118.7 ± 4.5a |
| DI | 111.6 ± 2.1cd | 113.9 ± 2.5bc | 146.4 ± 4.5cd | 159.8 ± 4.9b | 174.8 ± 6.8b | 279.3 ± 14.5d |
| DM | 104.4 ± 2.0bcd | 122.4 ± 2.4cd | 134.3 ± 3.2bcd | 133.3 ± 4.3ab | 177.3 ± 6.5b | 219.1 ± 12.1c |
| A | 101.1 ± 1.7bc | 94.3 ± 2.2ab | 98.0 ± 2.8a | 126.1 ± 4.0ab | 116.4 ± 4.3a | 138.8 ± 4.9ab |
| B | 117.9 ± 2.3d | 109.7 ± 3.1abc | 108.9 ± 3.5ab | 134.2 ± 4.4ab | 127.3 ± 5.0ab | 199.6 ± 8.1bc |
| C | 104.4 ± 1.8bcd | 113.2 ± 3.3bc | 121.0 ± 4.3abc | 143.4 ± 4.9b | 159.6 ± 5.7ab | 190.4 ± 7.2bc |
| D | 110.3 ± 1.9bcd | 138.2 ± 4.1d | 150.6 ± 4.6cd | 145.0 ± 4.8b | 112.0 ± 4.9a | 146.0 ± 5.1ab |
| E | 78.9 ± 1.4a | 86.4 ± 2.0a | 139.9 ± 4.0bcd | 144.8 ± 4.7b | 140.9 ± 5.5ab | 172.6 ± 8.1abc |
| F | 118.4 ± 2.0d | 109.6 ± 2.4abc | 130.0 ± 4.0abcd | 127.1 ± 3.9ab | 129.3 ± 4.9ab | 157.9 ± 5.7ab |
| G | 109.7 ± 2.1bcd | 101.7 ± 2.9abc | 121.4 ± 3.1abc | 139.3 ± 4.0ab | 132.8 ± 4.4ab | 186.8 ± 7.9bc |
| P‐value | 0.00 | 0.00 | 0.01 | 0.05 | 0.03 | 0.00 |
Mean ± standard deviation within each column with no common superscript letter differ significantly; n = 10 for each group. Plasma levels are mg/dL. CON, control group (only citrate buffer injection); DI, exposed to diabetes; DM, DI and 3% dimethyl sulfoxide as the solvent of the peptides (without of peptide); A, DM + Ala‐His (carnosine); B, DM + Glu‐Cys‐Gly (glutathione); C, DM + Leu‐Pro‐Pro; D, DM + Leu‐Gly; E, DM + Pro‐Gly‐Pro; F, DM + Pro‐Pro; G, DM + Pro‐Gly.
Figure 2.

The effect of the studied peptides administration on the blood glucose levels in diabetes‐induced mice at (a) day 10 and (b) day 12 of the experiment. The mean columns of each diagram with no common superscript letter differ significantly (P < 0.05; n = 10). CON, control group (only citrate buffer injection); DI, exposed to diabetes; DM, DI and 3% dimethyl sulfoxide as the solvent for the peptides (without a peptide); A, DM + Ala‐His (carnosine); B, DM + Glu‐Cys‐Gly (glutathione); C, DM + Leu‐Pro‐Pro; D, DM + Leu‐Gly; E, DM + Pro‐Gly‐Pro; F, DM + Pro‐Pro; G, DM + Pro‐Gly.
The bodyweight of the mice was determined at three time points (days 1, 6 and 12), and the results are shown in Figure 3. Owing to the initial control of the mice weight at the start of the experiment, the weight differences were not significant at the first day. On the 6th day of the experiment, the differences in their mean bodyweight between treatments were significant (P < 0.05). The induction of diabetes caused a reduction in bodyweight in the DI mice, whereas in the CON group the bodyweight was significantly elevated in ascending order from day 1 to day 12 (Figure 3). The injection of all peptides (except for peptides A, B and G) and also DMSO prevented bodyweight loss. Peptide D was the most efficient at preventing bodyweight loss.
Figure 3.
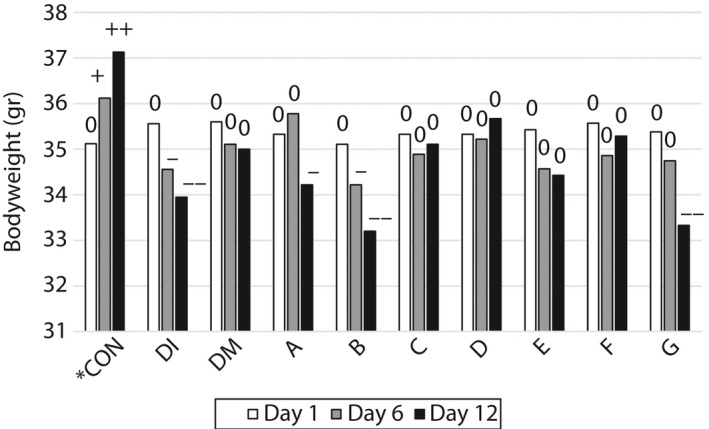
The effect of the administration of the studied peptides on the bodyweight of diabetes‐induced mice on day 1 (35.38 ± 0.27 g in all treatments), day 6 and day 12 of the experiment. 0, Non‐significant with the first day of the experiment; +, significant increase (P < 0.05); + +, very significant increase (P < 0.01); − , significant decrease (P < 0.05); − −, very significant decrease (P < 0.01; n = 10); CON, control group (only citrate buffer injection); DI, exposed to diabetes; DM, DI and 3% dimethyl sulfoxide as the solvent for the peptides (without a peptide); A, DM + Ala‐His (carnosine); B, DM + Glu‐Cys‐Gly (glutathione); C, DM + Leu‐Pro‐Pro; D, DM + Leu‐Gly; E, DM + Pro‐Gly‐Pro; F, DM + Pro‐Pro; G, DM + Pro‐Gly.
As shown in Figure 4, on the 6th day of the experiment, the blood insulin levels in the CON and DI groups were not statistically significant (5 and 5.1 mIU/L). Whereas the insulin levels in the mice that received peptides E and G (3.7 and 3.0 mIU/L, respectively), and especially peptides A and B (2.04 and 2.80 mIU/L, respectively), were lower. Data obtained on the 12th day for insulin levels showed that, in contrast to the 6th day, the insulin levels of the CON, DI and DM mice were lower (2.9, 2.8 and 2.5 mIU/L, respectively) than insulin levels in mice treated by peptides A, B and D (P < 0.05). It should be noted that as mice are nocturnal animals and eat overnight, the level of insulin in their blood is therefore higher in the early morning compared with later in the morning. This could be why there was an observed difference in insulin levels for the control group for the 6th and 12th days of the experiment.
Figure 4.
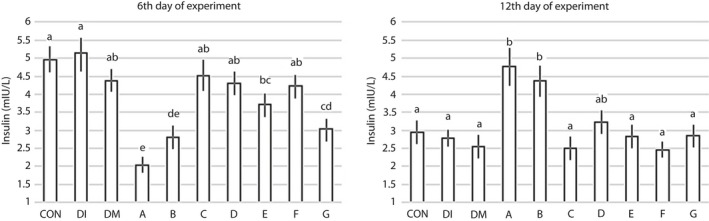
The effect of peptide administration on the plasma insulin level in diabetes‐induced mice on the 6th and 12th experimental days. The mean columns of each diagram with no common superscript letter differ significantly (P < 0.05; n = 10). CON, control group (only citrate buffer injection); DI, exposed to diabetes; DM, DI and 3% dimethyl sulfoxide as the solvent for the peptides (without a peptide); A, DM + Ala‐His (carnosine); B, DM + Glu‐Cys‐Gly (glutathione); C, DM + Leu‐Pro‐Pro; D, DM + Leu‐Gly; E, DM + Pro‐Gly‐Pro; F, DM + Pro‐Pro; G, DM + Pro‐Gly.
According to Table 2, the difference between the mean GLP‐1 levels in the CON and DI mice was significant on the 6th day (P < 0.05). The GLP‐1 levels were elevated in the DI group compared with the CON group 1 day after the end of the MLDS protocol. All peptides, except peptides B and G, prevented GLP‐1 increasing in DI mice on the 6th day. However, the difference in the GLP‐1 levels in the CON and DI groups was not significant on the 12th day. Furthermore, the mice that received peptides D or F and the DM group mice showed a decline in GLP‐1 amounts that was not significantly different compared with the CON and DI groups. Development of diabetes did not have an effect on DPP‐4 activity. In other words, there was no significant difference between the CON and DI groups on the 6th and 12th days of the experiment. Although the use of peptides, specifically A, D and F, caused a reduction in DPP‐4 activity on the 6th day of the experiment (Table 2).
Table 2.
Comparison of mean plasma glucagon‐like peptide‐1 level and dipeptidyl peptidase‐4 activity between treatments at two time points (6 and 12 days)
| Parameter | GLP‐1 (ng/L) | GLP‐1 (ng/L) | DPP‐4 (ng/mL) | DPP‐4 (ng/mL) |
|---|---|---|---|---|
| Time stages | 6 | 12 | 6 | 12 |
| CON | 332.5 ± 9.1ab | 303.4 ± 8.1abcd | 1.9 ± 0.0ab | 1.9 ± 0.0 |
| DI | 461.9 ± 15.4c | 286.0 ± 9.5abcd | 2.0 ± 0.1a | 1.9 ± 0.1 |
| DM | 339.1 ± 11.6ab | 255.3 ± 8.2ab | 1.6 ± 0.1abcd | 1.8 ± 0.1 |
| A | 277.8 ± 8.9a | 340.5 ± 9.4cd | 1.5 ± 0.1bcd | 1.8 ± 0.0 |
| B | 405.4 ± 12.4bc | 346.7 ± 10.0d | 1.6 ± 0.1abcd | 2.1 ± 0.1 |
| C | 350.8 ± 11.0ab | 275.0 ± 8.9abcd | 1.6 ± 0.1abcd | 1.8 ± 0.1 |
| D | 338.2 ± 9.1ab | 249.5 ± 8.6a | 1.5 ± 0.0cd | 1.8 ± 0.0 |
| E | 330.8 ± 9.3ab | 262.9 ± 9.8abc | 1.7 ± 0.0abcd | 1.9 ± 0.0 |
| F | 331.5 ± 9.7ab | 235.3 ± 9.1a | 1.4 ± 0.0d | 1.8 ± 0.0 |
| G | 387.2 ± 9.6bc | 336.6 ± 9.8bcd | 1.9 ± 0.0abc | 1.7 ± 0.0 |
| P‐value | 0.01 | 0.02 | 0.03 | 0.66 |
Mean ± standard deviation within each column with no common superscript letter differ significantly; n = 10 for each group. CON, control group (only citrate buffer injection); DI, exposed to diabetes; DM, DI and 3% dimethyl sulfoxide as the solvent of the peptides (without of peptide); DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; A, DM + Ala‐His (carnosine); B, DM + Glu‐Cys‐Gly (glutathione); C, DM + Leu‐Pro‐Pro; D, DM + Leu‐Gly; E, DM + Pro‐Gly‐Pro; F, DM + Pro‐Pro; G, DM + Pro‐Gly.
Figure 5 shows that Akt‐2 and cAMP levels of the isolated islets, as β‐cell‐signaling indicators, are affected (P < 0.05) by treatments. The induction of diabetes (DI) has been associated with a sharp decline in both indicators of the intracellular signaling of β‐cells compared with the control group. Using DMSO (DM) had no effect on disorder improvement cell signaling. The mice that received peptides B and E did not show any effect on Akt‐2 activity of the β‐cells. The other peptides, particularly peptides A and D, had a significant effect on Akt‐2 and prevented intracellular signaling impairment of β‐cells in the mice exposed to diabetes. The injection of the peptides C, E and G did not have any effect on cAMP levels. The mice that received peptides A and D had a higher concentration of intracellular cAMP in their β‐cells (P < 0.01). Whereas mice treated with peptides B and F were affected to a certain extent. Therefore, peptides A and D prevented any interference in cell signaling by STZ‐induced diabetes in mice. The immunofluorescence staining of the islets at the end of the experiment showed a normal islet architecture, with insulin‐producing β‐cells in green and glucagon‐producing alpha cells in red for the CON group (Figure 6). The severe destruction of endocrine cells with minimal hormonal content was observed in the islets of mice exposed to diabetes (Figure 6), similar cell‐signaling disorder (Figure 5), bodyweight loss (Figure 3) and glucose concentration elevation (Figure 2), but not similar blood insulin levels. The A and D peptides showed preventive effects against the incidence of diabetes. The blood glucose levels were similar in mice treated with peptides A and D on the 10th and 12th days. A significant (P < 0.05) anti‐DPP‐4 effect was observed on the 6th day, mainly for the mice groups receiving peptides F, D and A. As shown in Table 2, unlike the CON and DI groups, the DPP‐4 enzyme activity was elevated from days 6 to 12 in all peptide groups, and particularly in the F peptide group (P < 0.05). The injection of all the reported peptides had significant effects on the survival and secretion activity of the endocrine cells of the islets, especially the β‐cells. Of course, the role of the two peptides A and D was distinct from the others, which reaffirmed the role of these dipeptides for maintaining the normal survival and hormonal secretion of β‐cells in mice exposed to STZ‐induced diabetes.
Figure 5.
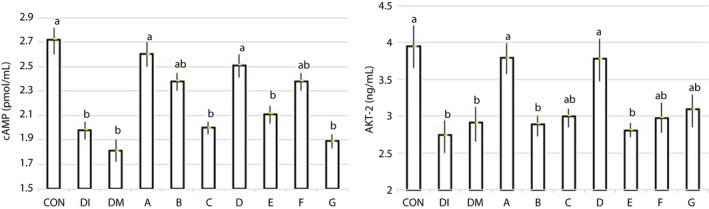
The effect of peptide administration on the intracellular Akt‐2 and cyclic adenosine monophosphate (cAMP) levels in diabetes‐induced mice at the end of the experiment. The mean columns of each diagram with no common superscript letter differ significantly (P < 0.05; n = 10). CON, control group (only citrate buffer injection); DI, exposed to diabetes; DM, DI and 3% dimethyl sulfoxide as the solvent for the peptides (without a peptide); A, DM + Ala‐His (carnosine); B, DM + Glu‐Cys‐Gly (glutathione); C, DM + Leu‐Pro‐Pro; D, DM + Leu‐Gly; E, DM + Pro‐Gly‐Pro; F, DM + Pro‐Pro; G, DM + Pro‐Gly.
Figure 6.
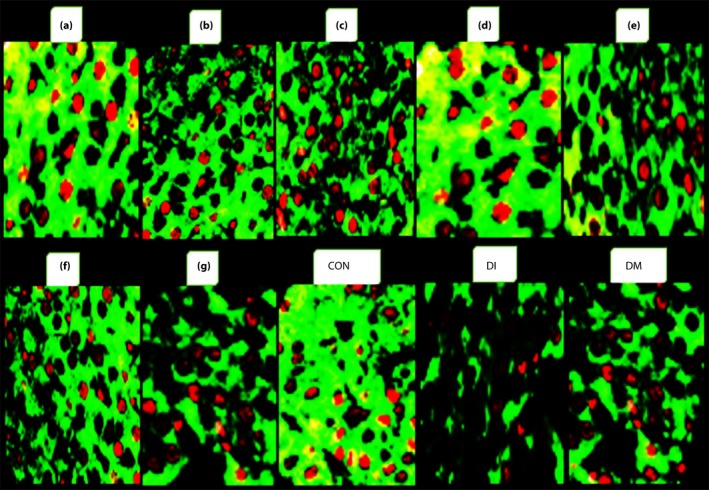
The effect of administration of the studied peptides. a, Ala‐His (carnosine); b, Glu‐Cys‐Gly (glutathione); c, Leu‐Pro‐Pro; d, Leu‐Gly; e, Pro‐Gly‐Pro; f, Pro‐Pro; g, Pro‐Gly compared with control (CON), exposed to diabetes (DI) and DI + 3% dimethyl sulfoxide (DM) on β‐cell function assessed by immunofluorescence staining of the islets on the 12th day of the experiment (the insulin content of the β‐cells is shown in green, and glucagon‐producing α‐cells are red; original magnification: ×1,000).
Discussion
Recent findings have shown the roles of small peptides as drug active agents that can modulate the physiological functions for the prevention of diabetes13, 44. The present study reports the preventive effects of small peptides in mice exposed to diabetes by hormonal (insulin and GLP‐1) and enzymatic (DPP‐4) communications involved in glucose homeostasis. Small peptides with insulinotropic and GLP‐1 tropic effects combat obesity and type 2 diabetes26. In vitro and in vivo studies highlighted the potential of the peptides referred to in the BIOPEP database for functioning antidiabetic effects, and recommended more in vivo studies for assessment of the type and sequences of these small active peptides37. In addition, the moderate effect of DMSO as an organic solvent on diabetes suppression has already been confirmed in previous studies36.
In the present study, it has been shown that the peptides produced from proline, glycine and leucine, especially Leu‐Gly and carnosine (β‐alanine–l‐histidine) dipeptides, caused a significant prevention of blood glucose elevation in mice at risk of diabetes. It has recently been reported that the tripeptides of glycine‐glycine‐leucine (Diapin) elevated the plasma insulin and GLP‐1 levels in diabetic mice30. In the present study, peptides A and D prevented the increase of GLP‐1. The results showed that peptides A and D have an anti‐DPP‐4 effect as a diabetes incidence mechanism of MLDS, as described by Lin et al.,8 and blood glucose elevation 7 days after the beginning of the experiment. Dietary amino acids and peptides can also induce insulin secretion through GLP‐1 stimulation mechanisms. Also, some cell‐permeable peptides as inhibitors of the c‐Jun NH2‐terminal kinase prevent cytokine‐induced β‐cell apoptosis. A wide range of short‐length peptides have been reported to possess DPP‐4 inhibitory activity, and are effective for stimulating insulin secretion and improving glycemic control in animal models and humans with type 2 diabetes33, 38.
The results of insulin measured on the 12th day showed that the elevated glucose levels of the diabetes exposed group had no relationship with insulin levels. The elevation of blood glucose levels promotes an increased demand on the pancreatic β‐cells to secrete insulin to bring the glucose content in the blood to a normal level. As this homeostasis mechanism reduces blood glucose during the prediabetes stage of diabetes development, chronic and persistent insulin resistance exposes the β‐cells to an excess of glucose, thereby promoting β‐cell dysfunction, failure and death. The β‐cells respond to many nutrients in the blood circulation, including glucose, other monosaccharaides, amino acids and peptides19, 33. Peptides A and D significantly caused insulin elevation compared with both the control and diabetes induced groups without increasing GLP‐1 and the depression of DPP‐4 on the 12th day. Therefore, the therapeutic agents that can halt or prevent pancreatic β‐cell failure will likely have a major impact on disease progression. The action mechanism of small peptides in target cells is conditioned by their binding to special membrane receptors and is able to influence physiological functions44. The peptide substances have been shown to be direct regulators or mediators of physiological processes38. The important factor for a peptide action is the stability of the peptide in the bloodstream. The glyproline family was the most stable in blood38, and the peptides containing branched chain amino acids, mainly leucine, induced an increase in GLP‐139. The endogenous peptides containing leucine stimulated insulin secretion in vitro and in vivo 40. In a study involving several animal models of insulin resistance, leucine‐containing peptides showed antidiabetic effects with a suppression of the elevation of blood glucose. It should be noted that all blood sampling and STZ administration in the present study were in the fed state. MLDS under fed or fasted conditions is equally effective in experimental hyperglycemia, and the fasting state before administration of MLDS is not required. Also, fasting might be an unnecessary step in the experimental design, and it is an unnecessary metabolic stress to the mice being studied40.
A significant increase in bodyweight in the control group was observed in the present experiment (Figure 3), whereas a marked depression in bodyweight was observed in the diabetes exposed group. Takeda et al.42 reported that bodyweight increased throughout the study in non‐diabetic control mice, whereas mice injected with STZ lost their bodyweight irrespective of the treatment.
The cellular nutrition and stimulation of β‐cell mitosis by these peptides can be a major factor in the prevention of the effects of STZ‐induced diabetes. In the present study, most of the synthesized peptides, especially Leu‐Gly, completely prevented bodyweight loss under the influence of diabetes development. Also, only dipeptide Leu‐Gly had the greatest protection against both glucose elevation and bodyweight loss. The authors have named this peptide “Hannaneh,” which means it is reliable. The present results showed that Hannaneh and carnosine can effectively prevent the rise of blood glucose elevation in the onset of diabetes. The Hannaneh peptide has mild anti‐DPP‐4 effects and stimulates the insulin secretion effects, probably based on immunofluorescence imaging.
Previous studies have shown that dipeptides have multiple physiological functions, including anti‐inflammation, anti‐oxidant and antidiabetic effects or increased diabetic wound healing43. Furthermore, it can be postulated that these dipeptides activate AMP‐protection kinase. AMP‐protection kinase has been identified as an important target in the prevention and treatment of obesity and type 2 diabetes43. It was reported that the synthetic di‐ and tripeptides containing glycine, such as glycine–leucine (reverse in N and C‐terminal of amino acid in comparison with peptide Hannaneh), are a strong stimulus for GLP‐1 and are involved in a two sensory mechanism (PEPT1 and calcium‐sensing) receptor21. Also, proline‐containing di‐ and tripeptides can cause a prophylactic antidiabetic effects in experimental insulin‐dependent diabetes mellitus in rats24.
Recent findings showed that Akt‐2 regulated β‐cell mass by modulating proliferation, cell size and apoptosis33, 34. GLP‐1 promotes islet cells survival through the second messenger, cAMP. cAMP, as a main regulator of β‐cell growth and survival, promotes insulin secretion and enhances the activation of the Akt‐2 in response to insulin for promoting cell survival. The cAMP level in the intracellular space has important effects on ion channels and exocytosis of insulin granules.
In the present study, peptides A and D prevented endocrine β‐cells injury and caused survival by reinforcing the intracellular signaling of Akt‐2 and cAMP in mice exposed to STZ oxidation and toxicity. There is a direct link between cell metabolism and the signaling cascade of β‐cells for insulin secretion, cell growth, differentiation and survival of the cell. This link is important in understanding β‐cell dysfunction with loss of insulin release in type 2 diabetes46. Impaired glucose‐induced cAMP formation has been reported in islets from diabetic animal models with reduced insulin secretion, and cAMP‐elevating agents have been found to ameliorate β‐cell function in diabetes patients. The key amino acids, Leu and Pro, and their small peptides play a crucial role in the mediation of the insulin secretion by modulating β‐cell signaling.
The tripeptide, glutathione, is the most important non‐enzymatic soluble intracellular anti‐oxidant, and has many metabolic functions in cellular metabolism, including attenuation of oxidative stress and inflammation45, 46. The authors have shown that carnosine and Hannaneh peptides promote β‐cell survival, and therefore Hannaneh represents a new and promising peptide for therapeutic and prevention strategies aimed at improving cell mass and function. In this present study, the Hannaneh peptide had multiple functions, including anti‐DPP‐4 and insulin secretion stimulator causing normal conditions in β‐cell signaling, and triggering blood glucose homeostasis and a balance in bodyweight for the prevention of diabetes induced by MLDS in mice model.
In conclusion, leucine‐glycine (Hannaneh) as a dipeptide with natural l‐amino acids and carnosine might show controlling effects on blood glucose homeostasis by protection of β‐ cell signaling and anti DPP‐4 activity, and also bodyweight loss curbing with Hannaneh, influenced by diabetes induction. Based on the new strategies for peptides delivery and the ability of the gastrointestinal system to directly absorb the dipeptides, Hannaneh and carnosine might gain some attraction as pharmaceutical agents to attenuate diabetes development.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The present study was a post‐doctoral research project (ID 95.93) funded by the Iran National Science Foundation (INSF; grant number 94004659), and Deputy of Research and Technology, Ministry of Health and Medical Education, Islamic Republic of Iran (grant number 10516), and was carried out at the Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
J Diabetes Investig 2019; 10: 1177–1188
References
- 1. Okorochenkov SA. Recent updates in peptide drugs delivery. Ann Transl Med Epidemiol 2016; 3: 1–2. [Google Scholar]
- 2. Miner‐Williams WM, Stevents BR, Moughan PJ. Are intact peptides absorbed from the healthy gut in the adult human? Nut Res Rev 2014; 27: 308–329. [DOI] [PubMed] [Google Scholar]
- 3. Oh Y, Jun H. Role of bioactive food components in diabetes prevention: effects on beta‐cell function and preservation. Nut Metabol Insights 2014; 7: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newsholme P, Mauricio K. Nutritional regulation of insulin secretion: implications for diabetes. Clin Biochem Rev 2012; 33: 35–47. [PMC free article] [PubMed] [Google Scholar]
- 5. Frode TS, Medeiros YS. Animal models to test drugs with potential antidiabetic activity. J Ethno Pharmacol 2007; 115: 173–183. [DOI] [PubMed] [Google Scholar]
- 6. Ardestani A, Paroni F, Azizi Z, et al MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat Med 2014; 20: 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eleazu CO, Eleazu KC, Chukwuma S, et al Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord 2013; 12: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin M, Yin B, Murphy B, et al Immune cell‐derived C3 is required for autoimmune diabetes induced by multiple low doses of streptozotocin. Diabetes 2010; 59: 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ventura‐Sobrevilla J, Boone‐Villa VD, Aguilar CN, et al Effect of varying vase and administration of streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol Soc 2011; 54: 5–9. [PubMed] [Google Scholar]
- 10. Arora S, Ojha Kumar S, Vohora D. Characterization of streptozotocin induced diabetes mellitus in swiss albino mice. Global J Pharmacol 2009; 3: 81–84. [Google Scholar]
- 11. Dal S, Sigrist S. The protective effect of antioxidants consumption on diabetes and vascular complications. Diabetes 2016; 24: 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fearch K, Torekof SS, Vistisen D, et al GLP‐1 response to oral glucose is reduced in prediabetes, screen‐detected type 2 diabetes, and obesity and influenced by sex: the addition‐pro study. Diabetes 2015; 64: 2513–2525. [DOI] [PubMed] [Google Scholar]
- 13. Velarde‐Salcedo AJ, Barrera‐Pacheco A, Lara‐Gonzalez S, et al Invitro inhibition of dipeptidyl peptidase‐4 by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem 2013; 136: 758–764. [DOI] [PubMed] [Google Scholar]
- 14. Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 2013; 62: 3316–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jao CL, Hung CC, Tung YS, et al The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase‐4 inhibitor for the monument of type 2 diabetes. BioMed 2015; 5: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JH, Pan JH, Cho HT, et al Black ginseng extract counteracts streptozotocin‐induced diabetes in mice. PLoS One 2016; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basu A, Gram Pederson M, Cobelli C. Prediabetes: evaluation of β‐cell function. Diabetes 2012; 61: 270–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puddu A, Sanguineti R, Mach F, et al Update on the protective molecular pathways improving pancreatic beta‐cell dysfunction. Mediators Inflamm 2013; 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rachel ES, Rohit BS, Yahui K, et al Glucose induces mouse b‐cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes 2016; 65: 981–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ying L, Jianmei Y. Research progress in structure‐activity relationship of bioactive peptides. J Med Food 2014; 18: 1–10. [DOI] [PubMed] [Google Scholar]
- 21. Diakogiannaki E, Pais R, Tolhurst G, et al Oligopeptides stimulate glucagon‐like peptide‐1 secretion in mice through proton‐coupled uptake and the calcium‐sensing receptor. Diabetologia 2013; 56: 2688–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klessens CQF, Baelde H, Hear ED. Tissue storage of dipeptides as protein guards against oxidative injury in patients with type 2 diabetes and its microvascular complications. Int Clin Pathol J 2015; 3: 1–4. [Google Scholar]
- 23. Mclennan S, Heffernan S, Wright L, et al Changes in hepatic glutathione metabolism in diabetes. Diabetes 1991; 40: 344–348. [DOI] [PubMed] [Google Scholar]
- 24. Lyapina LA, Myasoedov NF, Grigoreva ME, et al The modern concept of the regulatory role of peptides of the Glyproline family in the correction of hemostasis system function during development of diabetes mellitus. Anim Hum Physiol 2013; 40: 386–393. [PubMed] [Google Scholar]
- 25. Bakaeva ZV, Sangadzhieva AD, Sani S, et al Glyproline exert protective and repair‐promoting effects in the rat stomach: potential role of the cytokine GRO/CINC‐1. J Physiol Pharmacol 2016; 67: 253–260. [PubMed] [Google Scholar]
- 26. Jakubowicz D, Froy O. Biochemical and metabolic mechanism by which dietary whey protein may combat obesity and type 2 diabetes. J Nut Biochem 2013; 24: 1–5. [DOI] [PubMed] [Google Scholar]
- 27. Pedroso JAB, Zampieri TT, Jose Donato J. Reviewing the effects of L‐Leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients 2015; 7: 3914–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Guo K, LeBlanc RE, et al Increasing dietary leucine intake reduces diet‐induces obesity and improves glucose and cholesterol metabolism in mice via multimechanism. Diabetes 2007; 56: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 29. Merrifield RB. Solid phase peptide synthesis. I. The synthesis of a tetra peptide. J Am Chem Soc 1963; 85: 2149–2154. [Google Scholar]
- 30. Zhang J, Xue C, Zhu T, et al A tripeptide Diapin effectively lowers blood glucose levels in male type 2 diabetes mice by increasing blood levels of insulin and GLP‐1. PLoS One 2013; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mussa Farkhani S, Asoudeh Fard A, Zakeri‐Milani P, et al Enhancing antitumor activity of silver nanoparticles by modification with cell‐penetrating peptides. Artif Cells Nanomed Biotechnol 2016; 12: 1–7. [DOI] [PubMed] [Google Scholar]
- 32. Joshua CN, Nathan AT, Jamie WJ, et al A method for mouse pancreatic islet isolation and intracellular cAMP determination. J Vis Exp 2014; 88: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yi W, Chengsheng H, Wenzhen Z, et al An optical method to evaluate both mass and functional competence of pancreatic α‐ and β‐cells. J Cell Sci 2016; 129: 2462–2471. [DOI] [PubMed] [Google Scholar]
- 34. Shirani A, Shahbazi Mojarrad J, Mussa Farkhani S, et al The relation between thermodynamic and structural properties and cellular uptake of peptides containing tryptophan and arginine. Adv Pharm Bull 2015; 5: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaiser E, Colescott RL, Bossinger CD, et al Color test for detection of free terminal amino groups in the solid‐phase synthesis of peptides. Anal Biochem 1970; 34: 595–598. [DOI] [PubMed] [Google Scholar]
- 36. Klandorf H, Chirra AR, Degruccio A, et al Dimethyl sulfoxide modulation of diabetes onset in NOD mice. Diabetes 1989; 38: 194–197. [DOI] [PubMed] [Google Scholar]
- 37. Lin GJ, Sytwu HK, Yu JG, et al Dimethyl sulfoxide inhibits spontaneous diabetes and autoimmune recurrence in non‐obese diabetic mice by inducing differentiation of regulatory T cells. Toxicol Appl Pharmacol 2015; 282: 207–214. [DOI] [PubMed] [Google Scholar]
- 38. Kolomin T, Shadrina M, Slominsky P, et al A new generation of drugs: synthetic peptides based on natural regulatory peptides. Neurosci Med 2013; 4: 223–252. [Google Scholar]
- 39. Mansour A, Hosseini S, Larijani B, et al Nutrients related to GLP‐1 secretory ersponses. Nutrition 2013; 29: 813–820. [DOI] [PubMed] [Google Scholar]
- 40. Gaudel C, Nongonierma AB, Maher S, et al A whey protein hydrolysate promotes insulinotropic activity in a clonal pancreatic b‐cell line and enhances glycemic function in ob/ob mice. J Nut Disease 2013; 8: 1–6. [DOI] [PubMed] [Google Scholar]
- 41. Chaudhry Z, Morris DL, Moss DR, et al Streptozotocin is equally diabetogenic whether administered to fed or fasted mice. Lab Anim 2013; 7: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takeda Y, Fujita Y, Honjo J, et al Reduction of both beta cell death and alpha cell proliferation by dipeptidyl peptidase‐4 inhibition in a streptozotocin‐induced model of diabetes in mice. Diabetologia 2012; 55: 404–412. [DOI] [PubMed] [Google Scholar]
- 43. Soga M, Ohashi A, Taniguchi M, et al The di‐peptide Trp‐His activates AMP‐activated protein kinase and enhances glucose uptake independently on insulin in L6 myotubes. FEBS Open Biol 2014; 4: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tubbs E, Rieusset J. Metabolic a functions of ER‐mitochondria contact sites: role in metabolic diseases. J Mol Endocrinol 2017; 58: 87–106. [DOI] [PubMed] [Google Scholar]
- 45. Lynda E, Ernesto BM. Akt/PTEN: β‐cell mass and pancreas plasticity. Trends Endocrinol Metabol 2009; 20: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Newsholme P, Cruzat V, Arfuso F, et al Nutrient regulation of insulin secretion and action. J Endocrinol 2014; 221: R105–R120. [DOI] [PubMed] [Google Scholar]


