Abstract
Aims/Introduction
Diabetes prevalence in China has increased, but the trend in gestational diabetes mellitus prevalence is unclear. The objective of the present study was to examine the prevalence of gestational diabetes in Xiamen, China, and its association with maternal risk factors.
Materials and Methods
This linked‐database cohort study used the Medical Birth Registry of Xiamen. Between 1 March 2011 and 30 March 2018, 78,572 women who were diagnosed with gestational diabetes mellitus (GDM) were enrolled in the study. Maternal factors associated with the prevalence of GDM were examined using multivariate logistic regression.
Results
A total of 13,738 (17.6%) pregnant women were diagnosed with GDM according to the International Association of Diabetes and Pregnancy Study Groups criteria. GDM prevalence ranged from 15.5% (2012) to 19.9% (2017). Increasing age was associated with GDM; women aged >40 years versus those aged >25 years had an adjusted odds ratio (OR) of 5.91 (95% confidence interval [CI] 4.202–8.314). A positive correlation was observed between weight and GDM risk; obese women versus normal‐weight women had an adjusted OR of 2.508 (95% CI 2.253–2.792). Family history of diabetes and hypertension were more commonly observed among women with GDM. Multivariate analysis showed that family history of diabetes (OR 1.101, 90% CI 1.028–1.180), weight gain during early pregnancy (OR 1.087, 90% CI 1.052–1.124) and systolic blood pressure (OR 1.015, 90% CI 1.011–1.020) were risk factors associated with GDM incidence.
Conclusions
GDM affects 17.6% of all pregnant women in Xiamen. Age and maternal obesity were major contributors to GDM. The trend of GDM risk remained stable during the study.
Keywords: Family history of diabetes, Gestational diabetes mellitus, Prevalence
Introduction
Gestational diabetes mellitus (GDM) is one of the most common metabolic complications during pregnancy. According to the International Diabetes Federation, global GDM occurs in approximately 14% of all pregnant women, ranging from 9% in Africa, 12.6% in North America and 21% in Southeast Asia in 20171. Given the increasing prevalence of obesity, advancing maternal age and a greater number of people living a sedentary lifestyle, the increase in GDM is not unexpected. Recently, a study in Europe reported a GDM prevalence at nearly 40% in obese women.
In China, a previous study based in Tianjin city has reported the GDM prevalence at 2.3% in 19992, 6.8% in 20083 and 9.3% in 20124. Of note, the prevalence of GDM in Beijing in 2013 was reported to be as high as 19.7%5. According to a Chinese nationwide study including 17,186 pregnant women in 13 hospitals, the incidence of GDM is estimated to be 17.5%6. However, there were some deficiencies in these previous studies. First of all, diagnostic criteria differed across these studies. Second, the estimated incidence of GDM was based on data collected from limited hospitals in certain areas. Furthermore, the studies were applied in relatively small sample sizes. Accurate and up‐to‐date data on the incidence of hyperglycemia in pregnancy in China are lacking.
The present study aimed to determine the accurate prevalence of GDM in the study population, calculating trends in the prevalence of GDM and analyzing its associated risk factors.
Methods
Ethics statement
Ethics approval for this study was obtained from the review board of the First Affiliated Hospital of Xiamen University. The study conforms to the provisions of the Declaration of Helsinki in 1995 (as revised in Fortaleza, Brazil, October 2013).
Study design and population
The study population in the present cohort study was recruited from the Medical Birth Registry of Xiamen (MBRX) between 1 March 2011 and 30 March 2018, and was linked by individual record linkages to the Xiamen Citizen Health Information System using the person‐unique identification number assigned to each citizen at birth in Xiamen, China. The MBRX was established in 2007, and is based on compulsory notification of all live‐ and stillbirths from 12 weeks of gestation. The MBRX contains information on maternal characteristics (maternal age, education, body mass index [BMI], obstetric history, etc.); pregnancy, labor and delivery characteristics (gestational diabetes, gestational weight gain, gestational age at delivery, hypertension in pregnancy, etc.); and birth outcomes (fetal and neonatal death, birthweight, Apgar score at 5 min, etc.). From 2011 through 2018, 279,992 births were recorded in this registry. The present study was restricted to 78,572 pregnant women with singleton births who underwent a 75‐g oral glucose tolerance test at 24–28 weeks of gestation. Women with pre‐existing diabetes mellitus were excluded.
Outcome measures
The primary outcome measure was the incidence of GDM based on laboratory screening testing. A routine fasting plasma glucose test to rule out previously undiagnosed diabetes was carried out at the time of booking or during the first trimester. A plasma glucose reading of ≥7.0 mmol/L was considered diagnostic of diabetes. Between 24 and 28 weeks of gestation, a diagnostic 2‐h 75‐g oral glucose tolerance test was carried out for all pregnant women. GDM was diagnosed according to the 2014 National Health and Family Planning Commission of the People's Republic of China criteria when one of the following plasma glucose values was reached or exceeded: 0 h, 5.1 mmol/L; 1 h, 10.0 mmol/L; or 2 h, 8.5 mmol/L. The test was considered valid even if it was carried out after 28 weeks.
Statistical analysis
The rates of GDM were calculated and examined for temporal trends for screenings carried out between 2011 and 2018. The statistical significance of temporal trends was assessed using a logistic regression model with screening as the outcome and the year as the only predictor. The quantitative parameters followed a normal distribution and were expressed as mean ± standard deviation (SD), and tested using independent t‐tests. Pearson's χ2‐test and Fisher's exact test were applied to examine the differences between the groups for categorical parameters. The association of a GDM diagnosis with other factors was examined using multivariable binary logistic regression. A total of 16 variables were included in the logistic regression model using the conditional forward method. All reported P‐values were two‐tailed, and P < 0.05 was established as the level of significance. The following variables were included in the analysis: maternal age, pre‐pregnancy BMI, BMI gain before 13 weeks, education level, family history of diabetes, family history of hypertension, menarche and hepatitis B antigen status. Data analysis was carried out using Python 3.6 and SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA), for the Windows x64‐based system.
Results
A total number of 279,992 birth events from 1 March 2011 to 30 March 2018, were recorded in the MBRX (Figure 1). Patients with duplicate reports, uncertain test project name and uncertain pregnancy were excluded. Overall, 78,572 mothers who gave birth in Xiamen, China, during the examined years from 2011 to 2018, and were diagnosed with gestational diabetes mellitus according to the 75‐g oral glucose tolerance test, were enrolled. The average age of the mothers was 28.4 ± 4.2 years. The average pre‐gestational BMI was 21.0 ± 2.9 kg/m2. A total of 13,738 (17.6%) pregnant women were diagnosed with GDM.
Figure 1.
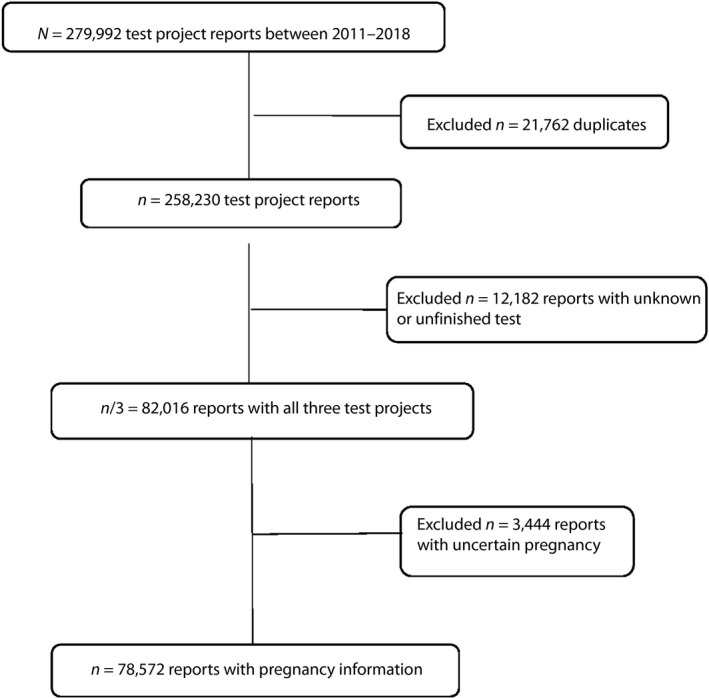
Flow chart of the study population.
As shown in Table. 1, basic information of GDM patients was compared with the non‐GDM population. The GDM population were older (mean age 30.1 years in those with GDM vs 28.0 years in those without GDM), with higher BMI (mean, 22.1 vs 20.8 kg/m2), younger age of menarche (≤11 vs ≥14 years) and lower education level (≤9 vs >9 years). Family history of diabetes mellitus and hypertension were more common observed in women with GDM than those without. As shown in Figure 2, recording the annual crude and adjusted incidences of GDM, the age‐ and BMI‐adjusted incidence of GDM ranged from 17.5% in 2012 to 17.7% in 2017, displaying a relatively stable trend during the study period.
Table 1.
Clinical characteristics of participants with and without gestational diabetes
| n | Non‐GDM n (%) | GDM n (%) | P‐value | |
|---|---|---|---|---|
| n | 78,572 | 64,726 (82.4) | 13,846 (17.6) | |
| Age, years (SD) | 28.0 ± 4.0 | 30.1 ± 4.5 | <0.001* | |
| n | 77,968 | 64,230 (82.4) | 13,738 (17.6) | |
| <25 | 12,167 | 11,079 (17.2) | 1,114 (8.1) | <0.001* |
| 25–29 | 39,616 | 33,881 (52.7) | 5,735 (41.7) | |
| 30–34 | 19,045 | 14,651 (22.8) | 4,394 (32.0) | |
| 35–39 | 6,140 | 4,075 (6.3) | 2,065 (15.0) | |
| ≥40 | 1,000 | 570 (0.9) | 4,30 (3.1) | |
| BMI, kg/m2 (SD) | 20.8 ± 2.74 | 22.1 ± 3.18 | <0.001* | |
| n | 73,498 | 60,655 (82.5) | 12,843 (17.5) | |
| <18.5 | 13,532 | 12,094 (19.9) | 1,438 (11.2) | <0.001** |
| 18.5–24.9 | 49,305 | 41,119 (67.8) | 8,186 (63.7) | |
| 25.0–27.9 | 8,930 | 6,329 (10.4) | 2,601 (20.3) | |
| ≥28.0 | 1,731 | 1,113 (4.8) | 618 (1.8) | |
| Education (years) | ||||
| n | 68,066 | 56,080 (82.4) | 11,986 (17.6) | |
| ≤9 | 16,900 | 13,715 (24.5) | 3,185 (26.6) | <0.001** |
| >9 | 51,166 | 42,365 (75.5) | 8,801 (73.4) | |
| Family history of diabetes | ||||
| n | 73,670 | 60,798 (82.5) | 12,872 (17.5) | |
| Yes | 1,825 | 1,295 (2.1) | 5,30 (4.1) | <0.001** |
| No | 71,845 | 59,503 (97.9) | 12,342 (95.9) | |
| Family history of hypertension | ||||
| n | 73,670 | 60,798 (82.5) | 12,872 (17.5) | |
| Yes | 3,849 | 3,045 (5.0) | 804 (6.2) | <0.001** |
| No | 69,821 | 57,753 (95.0) | 12,068 (93.8) | |
| Age of menarche (years) | ||||
| n | 70,278 | 57,906 (82.4) | 12,372 (17.6) | |
| ≤11 | 1,052 | 861 (1.5) | 191 (1.5) | <0.001** |
| 12 | 6,206 | 5,061 (8.7) | 1,145 (9.3) | |
| 13 | 17,300 | 14,070 (24.3) | 3,230 (26.1) | |
| ≥14 | 45,720 | 37,914 (65.5) | 7,806 (63.1) | |
| HbsAg | ||||
| n | 44,814 | 36,812 (82.1) | 8,002 (17.9) | |
| Positive | 4,766 | 3,830 (11.7) | 936 (10.4) | <0.001** |
| Negative | 40,048 | 32,982 (88.3) | 7,066 (89.6) | |
GDM, gestational diabetes mellitus; HbsAg, hepatitis B surface antigen.
*P‐value derived from t test, **P‐value derived from Chi‐square test.
Figure 2.
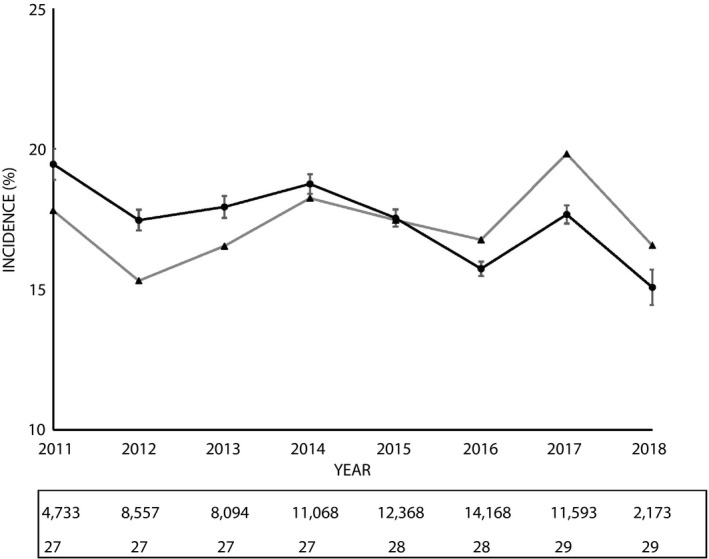
Trends in gestational diabetes. Annual crude and adjusted incidence of gestational diabetes, number of pregnancy and annual median age of woman. ▲, crude incidence; ●, age‐ and body mass index‐adjusted incidence, and 95% confidence interval.
GDM‐associated risk factors
The associated risk factors for GDM were analyzed based on 78,572 pregnant women. “GDM diagnosis” (0 = normal, 1 = GDM) was used as a dependent variable to carry out the logistic regression analysis in the present study. A total of 16 independent variables were selected by the enter method. Approximately 13,738 participants without missing information were included in the analysis. There were nine continuous variables: age, education, pre‐pregnancy BMI, weight gain during the early stage of pregnancy, weight gain during the mid‐term stage of pregnancy, systolic/diastolic blood pressure, age of menarche and pregnancy interval. Four categorical variables, including family histories of diabetes and hypertension, hepatitis B surface antigen, and tocolytic agent, were included. The grouping of categorical variables used in logistic regression analysis is shown in Table 2. Age and pre‐pregnant BMI were positively associated with the risk of GDM, and increased with successive groups. Women aged >40 years showed a sevenfold higher risk of being diagnosed with GDM than women aged <25 years. Compared with the reference group (BMI 18.5–24.9 kg/m2), the odds ratio (OR) was 2.789 (confidence interval [CI] 2.521–3.086) in the obese group (BMI ≥28.0 kg/m2) and 0.597 (CI 0.563–0.634) in the underweight group (BMI <18.5 kg/m2). Women with a family history of diabetes had a 1.973‐fold risk of GDM compared with women without. Weight gain in early pregnancy was a significant risk factor, in which circumstance, a 1‐unit increase in BMI >14 increased the risk by 15.6% (1.156‐fold risk), whereas a 1‐kg increase in weight gain in early pregnancy increased the risk by 9.0% (1.090‐fold risk). Blood pressure was also a risk factor for GDM. The risk of GDM increased by 2.7% (1.027‐fold risk) and by 2.3% (1.023‐fold risk) with a 1‐mmHg increase in systolic and diastolic blood pressure, respectively. Women with a longer interpregnancy interval (>36 months) had a higher risk of 1.521 (95% CI 1.259–1.836) compared with women with an interpregnancy interval <12 months.
Table 2.
Crude odds ratios with 95% confidence interval of gestational diabetes mellitus by univariate logistic regression analysis
| Odds ratio | 95% CI | β | SE | P‐value | |
|---|---|---|---|---|---|
| Age (years) | 1.124 | 1.119–1.129 | 0.117 | 0.002 | <0.001 |
| <25 | 1.000 | ||||
| 25–29 | 1.679 | 1.570–1.797 | 0.518 | 0.035 | <0.001 |
| 30–34 | 2.976 | 2.774–3.192 | 1.09 | 0.036 | <0.001 |
| 35–39 | 5.028 | 4.636–5.453 | 1.615 | 0.041 | <0.001 |
| ≥40 | 7.485 | 6.510–8.606 | 2.013 | 0.071 | <0.001 |
| BMI (kg/m2) | 1.156 | 1.149–1.163 | 0.145 | 0.003 | <0.001 |
| <18.5 | 0.597 | 0.563–0.634 | −0.515 | 0.03 | <0.001 |
| 18.5–24.9 | 1.000 | ||||
| 25–27.9 | 2.064 | 1.961–2.173 | 0.725 | 0.026 | <0.001 |
| ≥28 | 2.789 | 2.521–3.086 | 1.026 | 0.052 | <0.001 |
| Education (years) | |||||
| ≤9 | 1.000 | ||||
| >9 | 0.895 | 0.855–0.936 | −111 | 0.023 | <0.001 |
| Family history of diabetes | 1.973 | 1.780–2.187 | 0.68 | 0.053 | <0.001 |
| Family history of hypertension | 1.264 | 1.166–1.369 | 0.234 | 0.041 | <0.001 |
| Age of menarche (years) | |||||
| ≤11 | 1.000 | ||||
| 12 | 1.020 | 0.861–1.208 | 0.02 | 0.086 | 0.82 |
| 13 | 1.035 | 0.881–1.216 | 0.034 | 0.082 | 0.677 |
| ≥14 | 0.928 | 0.792–1.088 | −0.075 | 0.081 | 0.357 |
| HbsAg | 1.141 | 1.057–1.231 | 0.132 | 0.039 | 0.001 |
| Tocolytic agent | 1.243 | 1.163–1.328 | 0.217 | 0.034 | <0.001 |
| Pregnancy interval (months) | |||||
| <12 | 1.000 | ||||
| 12–<24 | 1.037 | 0.843–1.275 | 0.036 | 0.105 | 0.732 |
| 24–<36 | 1.248 | 1.011–1.540 | 0.222 | 0.107 | 0.039 |
| ≧36 | 1.521 | 1.259–1.836 | 0.419 | 0.096 | <0.001 |
| Weight gain in early pregnancy (kg) | 1.090 | 1.057–1.124 | 0.086 | 0.016 | <0.001 |
| SBP (mmHg) | 1.027 | 1.024–1.030 | 0.027 | 0.001 | <0.001 |
| DBP (mmHg) | 1.023 | 1.021–1.025 | 0.023 | 0.001 | <0.001 |
| Fetus times | |||||
| 1 | 1.000 | ||||
| ≥2 | 1.509 | 1.45–1.571 | 0.412 | 0.02 | <0.001 |
CI, confidence interval; DBP, diastolic blood pressure; HbsAg, hepatitis B surface antigen; SBP, systolic blood pressure; SE, standard error.
Because each risk related to GDM found by univariate analysis was closely interrelated, multivariate analysis to identify independent risk factors for the incidence of GDM was carried out. Using the enter method, five variables (Table 3) were preserved in the equation and 11 were removed. The variables that were removed included the education level, family history of hypertension, age of menarche, hepatitis B antigen status, interpregnancy interval, weight gain in mid‐term pregnancy and diastolic blood pressure, as these showed no statistical correlation with the GDM diagnosis. Age, pre‐pregnancy BMI, family history of diabetes, weight gain in early pregnancy and systolic blood pressure showed a statistically significant correlation, and these five variables were considered to be risk factors for GDM. After multivariable adjustment (as shown in Table 3), a striking correlation was observed between the prevalence of GDM and maternal age, as well as BMI group. A positive correlation was observed between the prevalence of GDM and age; 9.2%, <25 years; 14.5%, 25–29 years; 23.0%, 30–34 years; 33.6%, 35–39 years; and 43.0%, >40 years. Similarly, the GDM incidence increased with an increase in BMI. The BMI‐stratified rates of GDM prevalence were 10.6%, underweight; 16.6%, normal weight; 29.1%, overweight; and 35.7%, obese. As shown in Table 3, family history of diabetes (OR 1.101, 90% CI 1.028–1.180), weight gain in early stage of pregnancy (OR 1.087, 90% CI 1.052–1.124) and systolic blood pressure (OR 1.015, 90% CI 1.011–1.020) were additional associated risk factors of the incidence of GDM according to multivariate analysis.
Table 3.
Adjusted odds ratios with associated 95% confidence interval for gestational diabetes mellitus by using multivariate logistic regression analysis
| β | SE | P | OR (95% CI) | |
|---|---|---|---|---|
| Age range (years) | <0.001 | |||
| <25 | Reference | |||
| 25–29 | 0.411 | 0.114 | <0.001 | 1.508 (1.205; 1.887) |
| 30–34 | 0.861 | 0.116 | <0.001 | 2.366 (1.884; 2.971) |
| 35–39 | 1.248 | 0.125 | <0.001 | 3.485 (2.727; 4.452) |
| ≥40 | 1.747 | 0.174 | <0.001 | 5.736 (4.075; 8.075) |
| BMI category | <0.001 | |||
| 18.5–24.9 | Reference | |||
| <18.5 | −0.381 | 0.076 | <0.001 | 0.683 (0.588; 0.793) |
| 25–27.9 | 0.606 | 0.068 | <0.001 | 1.833 (1.603; 2.096) |
| ≥28 | 0.74 | 0.146 | <0.001 | 2.097 (1.575; 2.790) |
| History of diabetes | 0.458 | 0.108 | <0.001 | 1.581 (1.280; 1.952) |
| Weight gain in early pregnancy | 0.084 | 0.017 | <0.001 | 1.088 (1.053; 1.125) |
| Systolic blood pressure | 0.015 | 0.002 | <0.001 | 1.015 (1.010; 1.020) |
CI, confidence interval; OR, odds ratios; SE, standard error. All variables adjusted for education level, family history of hypertension, age of menarche, hepatitis B antigen status, inter‐pregnancy interval, weight gain in mid‐term pregnancy and diastolic blood pressure.
Discussion
The current study is the first large population‐based study to examine recent trends in the prevalence of GDM in China, in which the incidence of GDM was calculated to be 17.6%, and its associations with maternal factors were analyzed. The prevalence was strongly correlated with maternal age and the pre‐pregnancy bodyweight. Furthermore, family history of diabetes, weight gain during the early stage of pregnancy and systolic blood pressure were independent risks for GDM. Of note, the present study showed that the increased incidence of GDM in Xiamen city from 2012 to 2017 is not the same as that of type 2 diabetes mellitus, which has been increasing rapidly in recent years. We speculated that lifestyle disparities, growing incidence of obesity and older age of pregnant women might have contributed to the high prevalence of GDM7. One study showed that the onset of GDM might be shaped by early‐life exposure to poor nutrition; that is, under‐ or overnutrition, and/or epigenetics based on the Developmental Origins of Health and Disease theory8. Interestingly, the annual incidence of GDM was varied. We have no clear causes for such a discrepancy of annual incidence. It was suggested that factors for this difference might be maternal lifestyle. We did not track whether or not the pregnant women's lifestyle changed, which is a flaw of the present study. Furthermore, the number and age of pregnant women varied from year to year. In addition, the number of second births varied from year to year, which could lead to increased incidence of GDM. Furthermore, some studies reported that the incidence of GDM was associated with seasonal changes9, 10, 11.
GDM reported in the present study based in Xiamen was higher compared with previous studies in other cities in China2, 3, 4, and Western countries including the USA, Europe and Australia at 9.2, 5.4 and 5.7%, respectively12, 13, 14. A meta‐analysis investigating the GDM prevalence in Eastern and Southeast Asia showed the highest prevalence of GDM in Vietnam and Singapore, where approximately one in five mothers was diagnosed GDM, in which study, the incidence of GDM in China was reported to be 11.91%15. The 17.6% prevalence of GDM for all pregnant women in the present study is comparable to rates reported in Southeast Asia. The incidence of GDM remained relatively stable during the period examined in the current study, which could be partially explained by there not being an increase in the age of mothers and average BMI during the study. Furthermore, the incidence of GDM remained relatively stable over the 7‐year period after adjustment for age and BMI.
As noted in previous studies, maternal age, pre‐pregnancy BMI, weight gain in early pregnancy and family history of diabetes were identified as common risk factors for GDM6, 16, 17. The present study confirmed that these risk factors played a role in pregnant Chinese women developing GDM. Further research is required to study the optimal pre‐pregnancy BMI and weight gain during early pregnancy to lower the risk of GDM among Chinese women. A previous study showed an association between short and long interpregnancy intervals and adverse perinatal outcomes18. The current analysis showed a positive association with an interpregnancy interval of >36 months in the univariate analysis; however, this did not persist after adjustment for other risk factors, such as age and obesity. Thus, it was suggested that additional factors should be involved in the relationship between the interpregnancy interval and GDM. A low level of education has been reported to be a risk factor for GDM19 and diabetes. A previous study showed that people without a college education had a 57% higher risk of diabetes than those with a college or higher education20. In the present study, educational level was negatively correlated with GDM risk, and this might play a role through its effect on lifestyle.
The strengths of the present study include analyzing GDM universally, which was true population‐based. Although it has been controversial whether to screen for GDM in all pregnant women or selectively in women at high risk of developing type 2 diabetes mellitus in other countries, it is recommended to be carried out for all pregnant women in China. However, no study has been published about the actual incidence rate or the trend for GDM in China. The present study reports novel findings on the rate and the risk factors associated with GDM in an economically developed, medium‐sized city in China. The primary limitation of the present study is that it was a local, regional study.
In conclusion, in the present population‐based study of pregnant women using a universal screening test for GDM accompanied with various risk factors examined, the overall incidence of GDM was 17.6% in 78,572 enrolled pregnant women in Xiamen, China. The prevalence of GDM remained stable over the duration of the study. The age and pre‐pregnancy BMI were strongly associated with GDM. Family history of diabetes, weight gain in early pregnancy and systolic blood pressure were independent risks for GDM.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by Xiamen Diabetes Institute, Xiamen, China. We thank all the doctors, nurses, technicians and patients involved at our center for their dedication to the study.
J Diabetes Investig 2019; 10: 1358–1364
References
- 1. Hyperglycemia in pregnancy In: International Diabetes Federation . IDF Diabetes Atlas, 8th edn Brussels, Belgium: International Diabetes Federation, 2017. [Google Scholar]
- 2. Yang X, Hsu‐Hage B, Zhang H, et al Gestational diabetes mellitus in women of single gravidity in Tianjin City, China. Diabetes Care 2002; 25: 847–851. [DOI] [PubMed] [Google Scholar]
- 3. Zhang F, Dong L, Zhang CP, et al Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med 2011; 28: 652–657. [DOI] [PubMed] [Google Scholar]
- 4. Leng J, Shao P, Zhang C, et al Prevalence of gestational diabetes mellitus and its risk factors in Chinese pregnant women: a prospective population‐based study in Tianjin, China. PLoS One 2015; 10: e0121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu WW, Yang HX, Wang C, et al High prevalence of gestational diabetes mellitus in Beijing: effect of maternal birth weight and other risk factors. Chin Med J 2017; 130: 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu WW, Yang HX, Wei YM, et al Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 2013; 36: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simjak P, Cinkajzlova A, Anderlova K, et al The role of obesity and adipose tissue dysfunction in gestational diabetes mellitus. J Endocrinol 2018; 238: R63–R77. [DOI] [PubMed] [Google Scholar]
- 8. Barker DJ. The origins of the developmental origins theory. J Intern Med 2007; 261: 412–417. [DOI] [PubMed] [Google Scholar]
- 9. Chiefari E, Pastore I, Puccio L, et al Impact of seasonality on gestational diabetes mellitus. Endocr Metab Immune Disord Drug Targets 2017; 17: 246–252. [DOI] [PubMed] [Google Scholar]
- 10. Moses RG, Wong VC, Lambert K, et al Seasonal changes in the prevalence of gestational diabetes mellitus. Diabetes Care 2016; 39: 1218–1221. [DOI] [PubMed] [Google Scholar]
- 11. Katsarou A, Claesson R, Ignell C, et al Seasonal pattern in the diagnosis of gestational diabetes mellitus in Southern Sweden. J Diabetes Res 2016; 2016: 8905474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007‐2010. Prev Chronic Dis 2014; 11: E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chamberlain C, Joshy G, Li H, et al The prevalence of gestational diabetes mellitus among Aboriginal and Torres Strait Islander women in Australia: a systematic review and meta‐analysis. Diabetes Metab Res Rev 2015; 31: 234–247. [DOI] [PubMed] [Google Scholar]
- 14. Eades CE, Cameron DM, Evans JMM. Prevalence of gestational diabetes mellitus in Europe: a meta‐analysis. Diabetes Res Clin Pract 2017; 129: 173–181. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen CL, Pham NM, Binns CW, et al Prevalence of Gestational Diabetes Mellitus in Eastern and Southeastern Asia: a Systematic Review and Meta‐Analysis. J Diabetes Res 2018; 2018: 6536974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Cianni G, Volpe L, Lencioni C, et al Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract 2003; 62: 131–137. [DOI] [PubMed] [Google Scholar]
- 17. Savona‐Ventura C, Vassallo J, Marre M, et al group M‐Gs: a composite risk assessment model to screen for gestational diabetes mellitus among Mediterranean women. Int J Gynaecol Obstet 2013; 120: 240–244. [DOI] [PubMed] [Google Scholar]
- 18. Zhu BP, Rolfs RT, Nangle BE, et al Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med 1999; 340: 589–594. [DOI] [PubMed] [Google Scholar]
- 19. Anna V, van der Ploeg HP, Cheung NW, et al Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care 2008; 31: 2288–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang W, Lu J, Weng J, et al Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. [DOI] [PubMed] [Google Scholar]


