Abstract
It is strongly suggested that dipeptidyl peptidase‐4 inhibitors are associated with increased risk of bullous pemphigoid onset, especially in the elderly. Heightened clinical vigilance for bullous pemphigoid associated with dipeptidyl peptidase‐4 inhibitor use is required in daily diabetes care.
Bullous pemphigoid (BP) is a rare autoimmune blistering skin disorder, which involves an immune response directed against hemidesmosomal proteins of basal keratinocytes, BP antigen 180 (BP180) and BP antigen 230 (BP230). It manifests as polymorphic pruritus, blisters, erosions and crusts on the skin. It has been classically known that the development of BP can be induced by certain medications, such as diuretics, β‐blockers and antibiotics1. Recently, the possible association of dipeptidyl peptidase‐4 (DPP‐4) inhibitors with BP has become an issue2, 3, 4. DPP‐4 inhibitors are widely used therapy for type 2 diabetes in the Asian population, including the elderly, because of their general safety and efficacy5. To clarify potential mechanisms of DPP‐4 inhibitors that might cause BP and to develop strategies to screen patients susceptible to BP associated with DPP‐4 inhibitors are emerging tasks for healthcare professionals and scientists working in the field of diabetes.
Soon after retrospective analyses of pharmacovigilance databases from Europe, and warning case reports with multiple DPP‐4 inhibitors from Asia were published1, 2, an increasing number of BP cases associated with DPP‐4 inhibitor use have been reported in clinical settings. However, it has been difficult to clarify the causality of DPP‐4 inhibitors in these BP cases due to the involvement of multiple comorbidities, including diabetes, polypharmacy and an aged population, such as are frequently seen in BP patients. Furthermore, type 2 diabetes is commonly treated with DPP‐4 inhibitors together with a combination of medications that might provoke susceptibility in the elderly. Recently, Arai et al.4 found a significant reporting odds ratio (ROR) for DPP‐4 inhibitors in a disproportionality analysis using the Japanese Adverse Event Report (JADER) database. In that study, the RORs for DPP‐4 inhibitors remained statistically significant after excluding individuals who were receiving other antihyperglycemic drugs or possible causal agents for BP, whereas the RORs for other antihyperglycemic drugs were found to be insignificant after excluding individuals receiving DPP‐4 inhibitors. Furthermore, Kawaguchi et al. also showed a higher prevalence of BP in over 9,000 patients receiving DPP‐4 inhibitors compared with that in the general population (DPP‐4 inhibitors vs reported spontaneous incident rate: 0.0859% vs 0.0021–0.0066%)6. Taken together with other case–control studies suggesting that antidiabetes drugs other than DPP‐4 inhibitors are not associated with an increased risk of BP7, these studies strongly suggest that DPP‐4 inhibitors uniquely increase the risk of BP.
Focusing on the clinical features and risks of BP associated with DPP‐4 inhibitors, many reports suggest advanced age (>60–70 years) as a risk factor of BP associated with DPP‐4 inhibitor use3, 4, 6. Regarding sex, some reports found that men tend to show more susceptibility3, although other reports showed no significant relationship between sex and BP onset4. The latency period for BP from the introduction of DPP‐4 inhibitors varied, and was found to be relatively long (8–79 months)2, 3, 6. In addition, compared with conventional BP, BP associated with DPP‐4 inhibitor use might tend to present as non‐inflammatory, with fewer erythematous and generally mild skin symptoms. Although the autoantibody against the extracellular non‐collagenous 16A domain of hemidesmosomal collagen XVII (BP180‐NC16A) has been clinically used for diagnosis of BP and severity evaluation, BP associated with DPP‐4 inhibitor use might be more likely to show negative for the autoantibody against BC180‐NC16A6, while non‐negligible cases of BP harboring positive anti‐BC180‐NC16A antibody with DPP‐4 inhibitors are clinically observed2. In those cases with positive anti‐BP180‐NC16A antibody, skin symptoms reportedly tend to be mild, and the titers of the antibody tend to be lower6.
Although the underlying pathophysiological mechanism of BP associated with DPP‐4 inhibitor use remains unclear, the clinical features suggest a mechanism distinct from BP cases not associated with DPP‐4 inhibitor use. The high ratio of negative autoantibody against BP180‐NC16A suggests a mechanism involving antibody targeting other parts of BP180 proteins, or even a different mechanism that does not involve BP180 antibody. Actually, autoantibody targeting the whole lesion of BP180 was detected in some individuals with a non‐inflammatory type of BP. The former hypothesis is supported by the finding that the antibody titers to full‐length BP180 were found to be similar between BP associated with DPP‐4 inhibitor use and conventional BP patients, whereas those of BP180‐NC16A were significantly lower in BP associated with DPP‐4 inhibitor use8. The high ratio of cases with non‐inflammatory skin symptoms is thought to be consistent with a decrease in eosinophils infiltrating into the skin of patients with BP associated with DPP‐4 inhibitor use compared with conventional BP. Furthermore, possible different risks of BP onset in the case of different DPP‐4 inhibitors is intriguing. As previously shown, estimated risks for induction of BP can differ among DPP‐4 inhibitors; BP onset occurred most frequently in patients treated with vildagliptin in several studies3, 4, 6, and, in a study based on the JADER database, linagliptin, teneligliptin and vildagliptin showed higher RORs than other DPP‐4 inhibitors3. These results suggest that lower substrate selectivity for DPP‐4 inhibitors can be associated with the pathophysiology of the disease, which accords with the finding of no difference of DPP‐4 expression levels between BP skin samples with and without DPP‐4 inhibitor use. In addition, different specificity of DPP‐4 inhibitors against DPP‐8 and DPP‐9 might underlie differing immunological reactions in the skin, although the physiological functions of DPP‐8 and DPP‐9 are still not elucidated. Furthermore, an association between human leukocyte antigen (HLA) types and mucous membrane pemphigoid has been suggested. HLA‐DQB1*03:01 was found to be a biomarker for genetic susceptibility to BP associated with DPP‐4 inhibitor use in Japanese patients8. Ujiie et al.8 showed that 86% of cases with non‐inflammatory BP associated with DPP‐4 inhibitor use carried HLA‐DQB1*03:01. In BP associated with DPP‐4 inhibitor use, the frequency of carriers of HLA‐DQB1*03:01 was found to be significantly higher than in the Japanese general population control group (odds ratio 27.6, 95% confidence interval 8.0–94.8, P‐value 5.86 × 10−11), and the frequency of HLA‐DQB1*03:01 carriers was significantly higher than in DPP‐4 inhibitor‐tolerant control patients with type 2 diabetes who were exposed to DPP‐4 inhibitors for at least 2 years (odds ratio 13.3, 95% confidence interval 3.5–50.5, P‐value 2.13 × 10−5). Furthermore, they also showed that HLA‐DQB1*03:01 had a closer relationship with the non‐inflammatory type rather than the inflammatory type of BP associated with DPP‐4 inhibitor use. In that study, HLA‐DQB1*03:01 showed 86% sensitivity and 69% specificity when the allele was applied as a risk predictor for non‐inflammatory BP associated with DPP‐4 inhibitor use in the Japanese population. The usefulness of HLA‐DQB1*03:01 evaluation should be further examined in large‐scale studies among Japanese patients with type 2 diabetes carrying this allele, as well as in other racial populations.
Finally, treatment of BP associated with DPP‐4 inhibitor use should be noted. Topical and oral corticosteroids are usually used for DPP‐4 inhibitor‐associated BP in the same manner as with conventional BP treatment. In severe cases, intravenous immunoglobulin administration can be an option2. Noteworthy on this topic, a favorable impact of discontinuing DPP‐4 inhibitors with affected patients has been reported2 and seems to be effective (Figure 1). Prompt withdrawal of the antidiabetic agents can avoid exacerbation of skin symptoms, and often bring the patients into remission after management both with and without corticosteroid use2. In our experience, it takes at least 2 weeks after withdrawal to achieve complete remission. In contrast, some studies have argued that discontinuing DPP‐4 inhibitors should not be considered essential, as no exacerbation of BP was observed in certain cases in which DPP‐4 inhibitors were continued after BP onset6. However, the clinical features of such cases have not been clarified, and further studies are required. Considering the unfavorable effect of systemic corticosteroids on glycemic control and the limited appropriate options of antidiabetic agents in the elderly, early diagnosis of BP as well as optional, but prompt, withdrawal of the candidate agents are important. As alternative diabetic agents, any other drugs can be selected in consideration of BP causality7, while paying attention to possible hypoglycemia.
Figure 1.
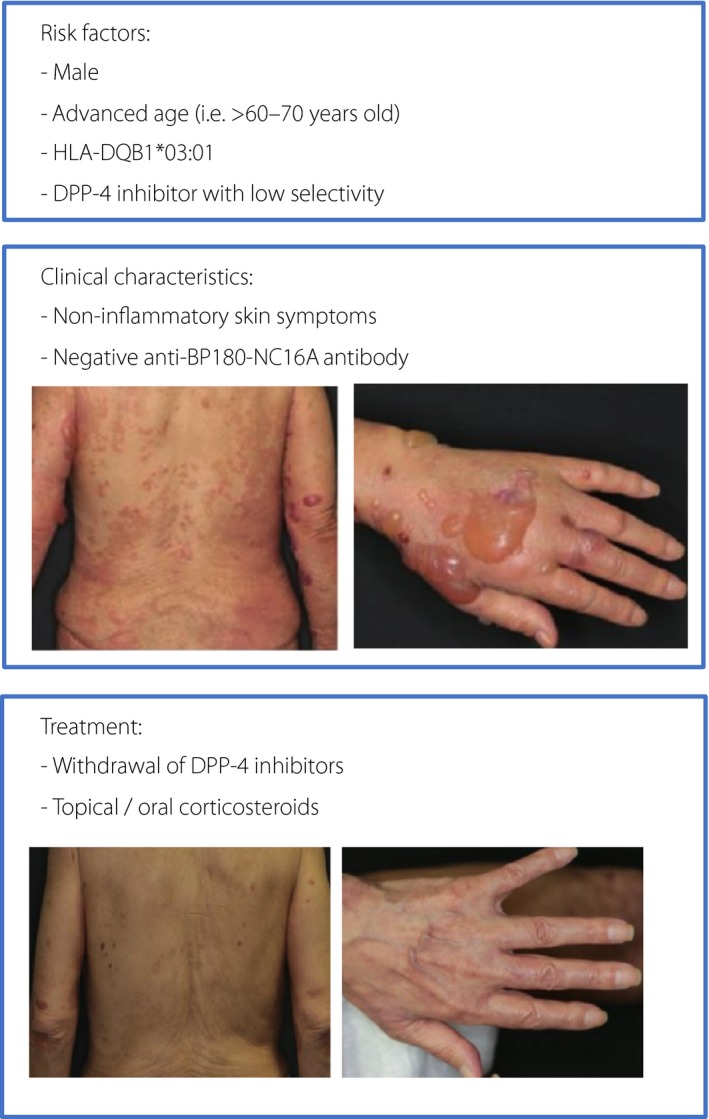
Risk factors, clinical characteristics and treatment of bullous pemphigoid (BP) associated with dipeptidyl peptidase‐4 (DPP‐4) inhibitor use. BP180‐NC16A, non‐collagenous 16A domain of BP antigen 180; HLA, human leukocyte antigen.
In summary, it is strongly suggested that DPP‐4 inhibitors are associated with increased risk of BP onset, especially in the elderly. BP associated with DPP‐4 inhibitor use is a distinct disorder from conventional BP; non‐inflammatory skin symptoms and negative anti‐BP180‐NC16A antibody are more common in BP associated with DPP‐4 inhibitor use (Figure 1). Furthermore, HLA‐DQB1*03:01 is a possible biomarker for genetic susceptibility to BP associated with DPP‐4 inhibitor use in Japanese patients. In addition, withdrawal of the agents can be a favorable option as treatment of BP. Finally, heightened clinical vigilance for BP associated with DPP‐4 inhibitor use is required in daily diabetes care.
Disclosure
DY received clinical commissioned/joint research grants from Nippon Boehringer Ingelheim, Eli Lilly, Taisho‐Toyama, MSD, Takeda, Ono and Novo Nordisk Pharma. NI received clinical commissioned/joint research grants from Mitsubishi Tanabe, AstraZeneca, Astellas and Novartis Pharma, and scholarship grants from Takeda, MSD, Ono, Sanofi, Japan Tobacco Inc., Mitsubishi Tanabe, Novartis, Boehringer Ingelheim, Kyowa Kirin, Astellas and Daiichi‐Sankyo. TM declares no conflict of interest.
References
- 1. Feliciani C, Joly P, Jonkman MF, et al Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol 2015; 172: 867–877. [DOI] [PubMed] [Google Scholar]
- 2. Yoshiji S, Murakami T, Harashima SH, et al Bullous pemphigoid associated with dipeptidyl peptidase‐4 inhibitors: a Report of Five Cases. J Diabetes Investig 2018; 9: 445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Béné J, Moulis G, Bennani I, et al Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case–noncase study in the French Pharmacovigilance Database. Br J Dermatol 2016; 175: 296–301. [DOI] [PubMed] [Google Scholar]
- 4. Arai M, Shirakawa Jun, Konishi H, et al Bullous pemphigoid and dipeptidyl peptidase 4 inhibitors: a disproportionality analysis based on the Japanese Adverse Drug Event Report Database. Diabetes Care 2018; 41: e130–e132. [DOI] [PubMed] [Google Scholar]
- 5. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016; 7: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawaguchi Y, Shimauchi R, Nishibori N, et al Dipeptidyl peptidase 4 inhibitors: a retrospective study of 168 pemphigoid and 9,304 diabetes mellitus patients. J Diabetes Investig 2019; 10: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varpuluoma O, Försti AK, Jokelainen J, et al Oral diabetes medications other than dipeptidyl peptidase 4 inhibitors are not associated with bullous pemphigoid: a Finnish nationwide case‐control study. J Am Acad Dermatol 2018; 79: 1034–1038. [DOI] [PubMed] [Google Scholar]
- 8. Ujiie H, Muramatsu K, Mushiroda T, et al HLA‐DQB1*03:01 as a biomarker for genetic susceptibility to bullous pemphigoid induced by DPP‐4 inhibitors. J invest Dermatol 2018; 138: 1201–1204. [DOI] [PubMed] [Google Scholar]


