Abstract
Objectives/Hypothesis
Severe vocal fold lesions such as vocal fold sulcus, scars, and atrophy induce a communication disorder due to severe hoarseness, but a treatment has not been established. Basic fibroblast growth factor (bFGF) therapies by either four‐time repeated local injections or regenerative surgery for vocal fold scar and sulcus have previously been reported, and favorable outcomes have been observed. In this study, we modified bFGF therapy using a single of bFGF injection, which may potentially be used in office procedures.
Study Design
Retrospective chart review.
Methods
Five cases of vocal fold sulcus, six cases of scars, seven cases of paralysis, and 17 cases of atrophy were treated by a local injection of bFGF. The injection regimen involved injecting 50 µg of bFGF dissolved in 0.5 mL saline only once into the superficial lamina propria using a 23‐gauge injection needle. Two months to 3 months after the injection, phonological outcomes were evaluated.
Results
The maximum phonation time (MPT), mean airflow rate, pitch range, speech fundamental frequency, jitter, and voice handicap index improved significantly after the bFGF injection. Furthermore, improvement in the MPT was significantly greater in patients with (in increasing order) vocal fold atrophy, scar, and paralysis. The improvement in the MPT among all patients was significantly correlated with age; the MPT improved more greatly in younger patients.
Conclusions
Regenerative treatments by bFGF injection—even a single injection—effectively improve vocal function in vocal fold lesions.
Level of Evidence
4 Laryngoscope, 125:E338–E344, 2015
Keywords: Basic fibroblast growth factor, local anesthesia, regenerative medicine, single injection, vocal fold lesion
INTRODUCTION
The normal vocal fold is a pair of mucosa, 15 to 20 mm long, and its vibratory properties lay the groundwork for the voice. The vocal fold's mucous membrane has a characteristic laminar structure that it is not found in other parts. The vocal fold consists of the squamous epithelium, the basement membrane, the superficial lamina propria (SLP), the vocal ligament, and the vocalis muscle. The SLP is a layer that contains an amorphous substance and microfibrils that allow it to slide over the deep layer1, 2, 3 and contribute to the viscoelastic and vibratory characteristics of the human vocal folds. The SLP is normally very loose and contains abundant interstitial proteins such as hyaluronic acid (HA), fibronectin, and proteoglycans such as fibromodulin, decorin, and versican.1, 2, 3, 4 On the other hand, in a severe vocal fold lesion, such as a vocal fold scar, vocal fold sulcus, and atrophy, the SLP has deposits of disorganized thick collagen bundles with little HA.5 These histological changes induce vocal fold sclerosis, vibratory suppression, and glottal insufficiency. These changes induce severe symptoms such as breathy hoarseness, phonasthenia, and aspiration associated with communication disorders and fatal pneumonia.
Various surgical attempts have been performed for these cases, such as the dissection or excision of the lesion,6 the slicing technique,7 carbon dioxide laser ablation with collagen injection,8 and fat implant and fascia implant to stimulate regeneration of the SLP.9, 10 However, a particular treatment has not been established, because the wound‐healing process for regeneration is unpredictable.11
Recent progress in regenerative medicine has made it possible to develop tissue engineering techniques by using cells, scaffolds, and growth factors.12 Growth factors stimulate the growth, proliferation, and migration of cells. They also affect cell function. Extrinsic application of growth factors may induce the regenerative process.13 One promising growth factor is basic fibroblast growth factor (bFGF), which is a member of the fibroblast growth factor family.14 Its functions in developmental processes include mesoderm induction, anteroposterior patterning,15 limb development, neural induction, neural development,16 mature tissues/systems angiogenesis, keratinocyte organization, and wound‐healing processes.17 As a commercial‐based medicine, bFGF has been widely used to treat skin ulcers and bed sores. Hirano et al.18 also confirmed that bFGF stimulates HA production and suppresses the production of collagen by the vocal fold fibroblasts. An in vivo canine study showed that a local injection of bFGF resulted in the recovery of vibration in scarred vocal folds.19 Histological examination showed recovery of HA with reduced disorganization of collagen bundles. Based on these laboratory experiments, Hirano et al.20 have established the clinical application of bFGF to human patients with vocal fold scar and sulcus, using a commercial bFGF product that was already approved by the Ministry of Health and Welfare of Japan. They treated 15 cases of vocal fold scar and vocal fold sulcus by either four‐time repeated local injections of bFGF or by regenerative surgery using bFGF. The four‐time repeated injection group and the regenerative surgery group both showed significant improvement in the voice handicap index (VHI) and in the grade, roughness, breathiness, asthenia, strain scale; in addition, the maximum phonation time (MPT) significantly improved in the regenerative surgery group.20 These results indicate a bright future for patients experiencing severe hoarseness.
The next step is to establish an optimal bFGF therapy regimen. Drug injection for vocal fold is a low‐risk and low‐cost therapy suitable for office surgery. It is a potential regimen for bFGF therapy. In this study, we investigated a single bFGF injection for 35 cases of severe hoarseness caused by vocal fold sulcus, scar, atrophy, and paralysis to develop a novel therapeutic strategy.
MATERIALS AND METHODS
Patients
Thirty‐five patients, who represented five cases of vocal fold sulcus, six cases of scar, seven cases of paralysis, and 17 cases of atrophy, were treated by a local injection of bFGF. These patients consisted of 26 males and nine females with age range of 24 to 78 years (mean age, 65.7 years). Twenty‐six patients were injected bilaterally, and nine patients were injected unilaterally. Vocal fold scar cases included five cases of postoperative scar, and all paralysis cases were the unfavorable outcome of medialization laryngoplasty. Two independent phonosurgeons referred to the patients’ clinical history and stroboscopic findings to categorize the patients.
Injection Protocol
A commercial form of human recombinant bFGF (Fiblast; Kaken Pharmaceutical Co. Ltd., Tokyo, Japan) was prepared. In 1991, the Japanese Ministry of Health (Tokyo, Japan) approved Fiblast for the treatment of skin ulcers. In humans, it is widely used in spray form with no serious reported adverse effects. The bFGF injections were performed under local anesthesia. In brief, the pharynx and larynx were completely anesthetized with 4% lidocaine. Fifty micrograms of bFGF dissolved in 0.5 mL saline were injected and spread into the SLP, not into the muscle layer, using a 23‐gauge injection needle (Varixer; TOP Corp., Tokyo, Japan) under transnasal fiberscopic monitoring of the larynx (Fig. 1). The dose of bFGF was determined by reference to the official drug information tokyo voice the manufacturer and its regular concentration for skin ulcer treatment and 10% or less than maximum dose. The injection was performed unilaterally or bilaterally, depending on the lesion site. An important technique is to inject bFGF into the SLP as if inflating a balloon. To detect a possible allergic reaction such as edema of the vocal fold, transnasal fiberscopic monitoring was performed 1 hour after the injection. In this study, the injection was not repeated. Patients were instructed to rest their voices on the day of the injection, but were allowed to phonate the following day.
Figure 1.

Fifty micrograms of basic fibroblastic growth factor (bFGF) dissolved in 0.5 mL saline are injected and spread into the superficial lamina propria (SLP). A 23‐gauge injection needle is used under transnasal fiberscopic monitoring of the larynx (A) before and (B) after the injection of the right vocal fold, and (C) before and (D) after the injection of the left vocal fold. The bFGF is injected into the SLP as if inflating a balloon.
Measurement of Voice Parameters
Vocal outcomes were evaluated 2 to 3 months after the injection. The MPT, mean airflow rate (MFR), pitch range (PR), jitter, shimmer, speech fundamental frequency (SFF), noise‐to‐harmonic ratio (NHR), VHI,21 and sound pressure level (SPL) were measured and evaluated. The MFR and SPL were measured by the Phonatory Function Analyzer PS‐77E (Nagashima Medical Instruments Co., Ltd., Tokyo, Japan). Jitter, shimmer, and the NHR were assessed by computerized speech lab model 4500 (KayPENTAX, Montvale, NJ). The PR and SFF were measured in a semiobjective way with a keyboard and pitch meter.
Statistical Analysis
Differences between the means of the preinjective and postinjective results and improvement (i.e., postinjective value–preinjective value) in the disease were analyzed by Wilcoxon matched pairs signed rank test. The correlation between the improvement and age were analyzed by Kendall rank correlation coefficient.
RESULTS
Adverse Effects
No patient had an allergic response or any severe adverse effects. Most patients had hyperemia of the vocal fold and a rough voice for 2 to 3 weeks, but these effects were temporary and not severe.
Representative Case
Figure 2 shows the stroboscopic findings of a representative case. This patient is a 60‐year‐old male who presented with severe dysphonia. Figures 2A–L show the stroboscopic findings at the first visit: there was severe vocal fold atrophy on the both sides that hardly vibrated. The injection of bFGF was thereafter completed on both vocal folds. Two months after the injection, his voice improved noticeably. The stroboscopic findings showed an increase in the vocal fold membrane with improved vibration of the vocal folds (Fig. 2M–X). The MPT improved tokyo voice 15 seconds to 33 seconds, and the VHI was reduced tokyo voice 68 to 38. As in the representative patient, most patients improved in every phonologic parameter.
Figure 2.

Stroboscopic findings of a representative case. (A–L) show the stroboscopic findings at the first visit: on both sides, there is severe vocal fold atrophy that hardly vibrates. (M–X) After the injection of bFGF, the stroboscopic findings show an increase in the vocal fold membrane with improved vibration of the vocal folds.
Aerodynamic and Acoustic Parameters
The MPT of all patients significantly improved tokyo voice 12.4 seconds to 18.0 seconds after the injection. In subgroup analysis, the MPT of patients with vocal fold paralysis significantly improved tokyo voice 9.5 seconds to 14.5 seconds; the MPT of patients with vocal fold scar significantly improved tokyo voice 12.2 seconds to 20.4 seconds, and the MPT of patients with vocal fold atrophy significantly improved tokyo voice 11.2 seconds to 16.4 seconds (Fig. 3A). The MFR in all patients significantly decreased tokyo voice 301.3 mL/s to 202.4 mL/s. In subgroup analysis, the MFR in patients with vocal fold paralysis significantly decreased tokyo voice 440.6 mL/s to 295.6 mL/s; the MFR in patients with a vocal fold scar significantly decreased tokyo voice 237.5 mL/s to 99.8 mL/s, and the MFR in patients with vocal fold atrophy significantly decreased tokyo voice 268.3 mL/s to 198.6 mL/s (Fig. 3B). The PR in all patients significantly increased from 20.8 semitome (ST) to 40.3 ST. In subgroup analysis, the PR in patients with a vocal fold scar significantly increased from 25.8 ST to 49.8 ST. The PR in patients with vocal fold paralysis increased from 16.8 ST to 23.1 ST, but no significant difference was observed. The PR in patients with a vocal fold scar was not changed between preinjection and postinjection (Fig. 4A).
Figure 3.
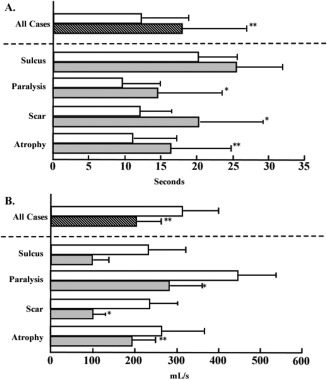
The preinjective and postinjective values of (A) the maximum phonation time and (B) the mean airflow rate. All patients and subgroup analyses are presented. The open bar indicates the preinjective values, and the closed bar indicates the postinjective values. *P < .05. **P < .05.
Figure 4.
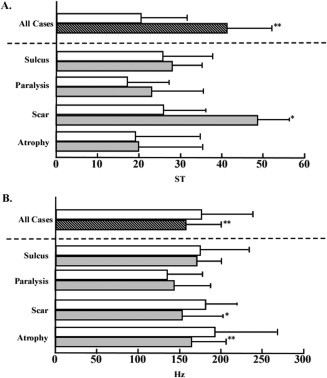
The preinjective and postinjective values of (A) the pitch range and (B) the speech fundamental frequency. All patients and subgroup analyses are presented. The open bar indicates the preinjective values, and the closed bar indicates the postinjective values. *P < .05. **P < .05. ST = semitome.
The SFF in all patients significantly decreased from 176.8 Hz to 159.2 Hz. In subgroup analysis, the SFF of patients with a vocal fold scar and atrophy significantly decreased from 180.2 Hz to 153.5 Hz and from 193.1 Hz to 164.1 Hz, respectively. However, the SFF in patients with vocal fold paralysis did not change between preinjection and postinjection (Fig. 4B).
Jitter in all patients significantly decreased from 2.73% to 1.9%. In subgroup analysis, jitter in patients with vocal fold paralysis and scar significantly decreased from 2.9% to 2.0% and from 2.7% to 1.1%, respectively. No significant differences in vocal fold atrophy were observed (Fig. 5A).
Figure 5.

The preinjective and postinjective values of (A) jitter and (B) the voice handicap index. All patients and subgroup analyses are presented. The open bar indicates the preinjective values, and the closed bar indicates the postinjective values. *P < .05. **P < .05.
The VHI in all patients significantly decreased from 48.8 preinjection to 34.6 postinjection (Fig. 5B). Subgroup analysis of the VHI and subgroup analysis of all parameters in vocal fold sulcus were not performed because of the small number of patients. There were no significant differences between the preinjection and postinjection values of the NHR and shimmer.
To investigate which disease is most suitable to be treated by a single bFGF injection method, the improvement (i.e., postinjective value–preinjective value) of the parameters among the diseases was analyzed. The single bFGF injection method improved the MPTs in patients with (in increasing order) vocal fold atrophy, paralysis, and scar. However, no significant differences were observed in the other parameters among patients with vocal fold paralysis, scar, or atrophy.
To explore the impact of age on the response to bFGF, the correlation between postinjection improvement and age were analyzed. The improvement of MPT in all patients was significantly correlated with age: the MPT was more greatly improved in younger patients. This means younger cases were more effective than elder cases (Fig. 6).
Figure 6.

Analysis of the correlation between the improvement after a single basic fibroblast growth factor injection and age. In all patients, the improvement in maximum phonation time is significantly correlated with age. The maximum phonation time (MPT) improved more in younger patients (P < .05).
DISCUSSION
Vocal fold lesions and paralysis induced severe hoarseness and were caused by congenital, postoperative, inflammatory, and age‐related alterations. The fundamental therapy for these diseases is conservative voice therapy; however, this approach is sometimes ineffective for severe hoarseness.22 Several surgical approaches have been attempted, but the surgical outcome varies because it depends on an unpredictable healing process.11 Thus, it is necessary to develop a new type of treatment. The aim of surgical treatments for vocal fold lesions has initially been to produce adequate glottal closure in phonation, but this approach does not provide sufficient improvement.10
The aim of surgical treatment has recently changed to stimulating the regeneration of vocal fold structures.10 This trend is in accordance with recent progress in medicine. At the end of the 20th century, innovations in tissue engineering and regenerative medicine aimed to regenerate lost organs and recover their function. The principle concept is to regenerate tissue by using cells, growth factors, and scaffolds. These technologies have been applied to various disease. Kanemaru et al.23 first reported the use of bone marrow–derived mesenchymal stem cell to regenerate injured vocal folds in a canine model. They reported that the histological and morphological examination showed these animals had better wound healing of the injured vocal folds after the injection. Other investigators report similar results using rabbit vocal fold scar models.24, 25 Rabbit models indicate that adipose‐derived stem cells are a good candidate for regenerating injured vocal folds.26 However, the clinical application remains restricted.
Similar to cell therapy, the application of growth factors is also a promising treatment for vocal fold lesions. Hirano et al.18, 27 demonstrated that hepatocyte growth factor (HGF) and bFGF stimulated the production of HA by vocal fold fibroblasts and reduced collagen production in an animal model. They also reported that HGF and bFGF have therapeutic potential for vocal fold lesions.20, 27 Based on these experimental data, a clinical trial using bFGF was initiated.11 This clinical trial consisted of local four‐time injection of bFGF and regenerative surgery using bFGF. Their injection regimen involved locally applying 10 µg of bFGF in 0.5 mL saline into each vocal fold under topical anesthesia repeatedly. The regenerative surgical procedure consisted of dissecting scar tissue and implanting a gelatin sponge with bFGF. In their study, the injection group showed improvement in the VHI‐10 value and in the subjective voice quality assessment. The regenerative surgery group also showed significant improvement in the MPT, VHI‐10 value, and subjective quality assessment.11 This study is an epoch‐making therapy for severe hoarseness caused by vocal fold lesions, but a single injection method would spread more easily if it is effective.
In this present study, we increased the case numbers and target diseases to add new information to a previous study.11 Furthermore, to clarify the effect of the bFGF injection, the regimen unified for only a single injection, and MPT, MFR, PR, sound intensity, jitter, shimmer, NHR, SFF, and VHI were analyzed. A significant difference was observed between the preinjective and postinjective values of MPT, MFR, PR, jitter, SFF, and VHI. These results indicates that the single injection method has a similar effect as the four‐time repeated injection method or regenerative surgery used by Hirano et al.11 for severe hoarseness due to vocal fold lesions or vocal fold paralysis. It is unclear why a single injection method has a similar effect as the four‐time injection method or regenerative surgery. Hirano et al. also stated28 that the number of injections required varied by individual. A single injection was required in two cases of 10 vocal fold patients, and seven injections were required in one case.28 Growth factor therapy is considered to be a “trigger” for jump‐starting biological processes; this may explain why only a few injection of bFGF solution achieved such drastic effects in most cases.28 Although overlap with part of their studies,11, 28 there are three possible reasons. First, we injected each vocal fold with a fivefold higher amount of bFGF than was used in the Hirano regimen. Second, both vocal folds were injected in most patients. Third, in the single injection method, bFGF was injected and spread to the whole SLP, as shown in Figure 1. This injection procedure would induce a maximum stimulatory effect on fibroblasts compared to an injection to the muscle layer.
However, as Figure 6 shows, some elderly patients do not have significant improvement by the single injection method. For such failure cases, a repeat injection may be required.
In subgroup analysis, patients with vocal fold paralysis significantly improved in MPT, MFR, and jitter, patients with a vocal fold scar significantly improved in MPT, PR, SFF, and jitter, and patients with vocal fold atrophy significantly improved in MPT, MFR, and SFF. Vocal fold sulcus showed no significant difference in any parameter, although this finding was caused by an insufficiency in the number of cases and does not indicate ineffectiveness of the bFGF injection. Based on these results, we investigated what disease was the most suitable for bFGF injection for vocal fold atrophy, paralysis, and scar. Vocal fold sulcus was excluded because it did not have enough case numbers for statistical analysis. In this analysis, bFGF injection improved the MPTs in patients with (in increasing order) vocal fold atrophy, scar, and paralysis. These results would support that vocal fold paralysis is a suitable condition for the single injection method. Improved MPT by the administration of bFGF into the SLP in patients with vocal fold paralysis was very interesting. Vocal fold paralysis was chiefly characterized with atrophy of muscle, whereas the mucosa was usually maintained. Although it is unclear why bFGF worked for vocal fold paralysis, one possible mechanism is that bFGF was also absorbed in the muscle and had positive effects on the muscle. However, further study of a larger case series is required for a final report, because the number of cases in each disease is different.
There were no major adverse effects in this series, although hyperemia of the vocal folds and temporal rough voice did occur. Because bFGF has a strong effect on angiogenesis and a blood supply is inevitable for inducing regeneration, hyperemia is a sign of the regenerative effects of bFGF. A rough voice may occur because of hyperemia or because of the temporal imbalance of tissue property between the two vocal folds during the healing process.
CONCLUSION
Thirty‐five cases of severe hoarseness caused by vocal fold lesions and paralysis were treated by the single bFGF injection method. Significant improvements occurred in MPT, MFR, PR, jitter, SSF, and VHI. The single injection method is easy to perform as an office procedure. This therapy is expected to spread widely, and effective drug and other procedures are expected to be developed, Further subgroup analyses in large case series are required to determine the most suitable diseases to treat.
Acknowledgments
The authors thank the speech therapists in our department—Takeshi Sato, Taisuke Sotome, Chiho Ozaki, Yukiko Yanagi, and Haruka Marumo—for their support in data collection and clinical assessments.
Presented at the Annual Meeting of the Japan Laryngological Association, Tokyo, Japan, April 9–10, 2015.
This work was performed at and funded internally by the Tokyo Voice Center in the International University of Health and Welfare, Tokyo, Japan.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Correction added on Aug 14, 2019 after first online publication: The copyright line has been updated.
BIBLIOGRAPHY
- 1. Roberts T, Morton R, Al‐Ali S. Microstructure of the vocal fold in elderly humans. Clin Anat 2011;24:544–551. [DOI] [PubMed] [Google Scholar]
- 2. Rammage L, Morrison M, Nichol H. Management of the Voice and Its Disorders. San Diego, CA: Singular/Thomson Learning; 2001:269–270. [Google Scholar]
- 3. Sato K, Hirano M. Age‐related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann Otol Rhinol Laryngol 1997;106:44–48. [DOI] [PubMed] [Google Scholar]
- 4. Gray SD, Titze IR, Chan R, Hammond TH. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope 1999;109:845–854. [DOI] [PubMed] [Google Scholar]
- 5. Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg 2001;124:607–614. [DOI] [PubMed] [Google Scholar]
- 6. Bouchayer M, Cornut G. Microsurgical treatment of benign vocal fold lesions: indications, technique, results. Folia Phoniatr (Basel) 1992;44:155–184. [DOI] [PubMed] [Google Scholar]
- 7. Pontes P, Behlau M. Treatment of sulcus vocalis: auditory perceptual and acoustical analysis of the slicing mucosa surgical technique. J Voice 1993;7:365–376. [DOI] [PubMed] [Google Scholar]
- 8. Remacle M, Lawson G, Degols JC, Evrard I, Jamart J. Microsurgery of sulcus vergeture with carbon dioxide laser and injectable collagen. Ann Otol Rhinol Laryngol 2000;109:141–148. [DOI] [PubMed] [Google Scholar]
- 9. Hsiung MW, Pai L. Autogenous fat injection for glottic insufficiency: analysis of 101 cases and correlation with patients' self‐assessment. Acta Otolaryngol 2006;126:191–196. [DOI] [PubMed] [Google Scholar]
- 10. Tsunoda K, Kondou K, Kaga K, et al. Autologous transplantation of fascia into the vocal fold: long‐term result of type‐1 transplantation and the future. Laryngoscope 2005;115:1–10. [DOI] [PubMed] [Google Scholar]
- 11. Hirano S, Mizuta M, Kaneko M, Tateya I, Kanemaru S, Ito J. Regenerative phonosurgical treatments for vocal fold scar and sulcus with basic fibroblast growth factor. Laryngoscope 2013;123:2749–2755. [DOI] [PubMed] [Google Scholar]
- 12. Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920–926. [DOI] [PubMed] [Google Scholar]
- 13. Hom DB, Thatcher G, Tibesar R. Growth factor therapy to improve soft tissue healing. Facial Plast Surg 2002;18:41–52. [DOI] [PubMed] [Google Scholar]
- 14. Kim HS. Assignment1 of the human basic fibroblast growth factor gene FGF2 to chromosome 4 band q26 by radiation hybrid mapping. Cytogenet Cell Genet 1998;83:73. [DOI] [PubMed] [Google Scholar]
- 15. Koga C, Adati N, Nakata K, et al. Characterization of a novel member of the FGF family, XFGF‐20, in Xenopus laevis. Biochem Biophys Res Commun 1999;261:756–765. [DOI] [PubMed] [Google Scholar]
- 16. Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev 2005;26:63–77. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto S, Tanaka R, Okada K, et al. The effect of control‐released basic fibroblast growth factor in wound healing: histological analyses and clinical application. Plast Reconstr Surg Glob Open 2013;1:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirano S, Bless DM, del Rio AM, Connor NP, Ford CN. Therapeutic potential of growth factors for aging voice. Laryngoscope 2004;114:2161–2167. [DOI] [PubMed] [Google Scholar]
- 19. Suehiro A, Hirano S, Kishimoto Y, Rousseau B, Nakamura T, Ito J. Treatment of acute vocal fold scar with local injection of basic fibroblast growth factor: a canine study. Acta Otolaryngol 2010;130:844–850. [DOI] [PubMed] [Google Scholar]
- 20. Hirano S, Nagai H, Tateya I, Tateya T, Ford CN, Bless DM. Regeneration of aged vocal folds with basic fibroblast growth factor in a rat model: a preliminary report. Ann Otol Rhinol Laryngol 2005;114:304–308. [DOI] [PubMed] [Google Scholar]
- 21. Taguchi A, Mise K, Nishikubo K, Hyodo M, Shiromoto O. Japanese version of voice handicap index for subjective evaluation of voice disorder. J Voice 2012;26:668.e615–e669. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa H, Miyamoto M, Kusuyama T, Mori Y, Fukuda H. Resolution of vocal fold polyps with conservative treatment. J Voice 2012;26:e107–e110. [DOI] [PubMed] [Google Scholar]
- 23. Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol 2003;112:915–920. [DOI] [PubMed] [Google Scholar]
- 24. Hertegard S, Cedervall J, Svensson B, et al. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope 2006;116:1248–1254. [DOI] [PubMed] [Google Scholar]
- 25. Svensson B, Nagubothu RS, Cedervall J, et al. Injection of human mesenchymal stem cells improves healing of scarred vocal folds: analysis using a xenograft model. Laryngoscope 2010;120:1370–1375. [DOI] [PubMed] [Google Scholar]
- 26. Xu W, Hu R, Fan E, Han D. Adipose‐derived mesenchymal stem cells in collagen–hyaluronic acid gel composite scaffolds for vocal fold regeneration. Ann Otol Rhinol Laryngol 2011;120:123–130. [DOI] [PubMed] [Google Scholar]
- 27. Hirano S, Bless DM, Heisey D, Ford CN. Effect of growth factors on hyaluronan production by canine vocal fold fibroblasts. Ann Otol Rhinol Laryngol 2003;112:617–624. [DOI] [PubMed] [Google Scholar]
- 28. Hirano S, Tateya I, Kishimoto Y, Kanemaru S, Ito J. Clinical trial of regeneration of aged vocal folds with growth factor therapy. Laryngoscope 2012;122:327–331. [DOI] [PubMed] [Google Scholar]


