Abstract
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer mortality worldwide. Various imaging modalities provide important information about HCC for its clinical management. Since positron-emission tomography (PET) or PET-computed tomography was introduced to the oncologic setting, it has played crucial roles in detecting, distinguishing, accurately staging, and evaluating local, residual, and recurrent HCC. PET imaging visualizes tissue metabolic information that is closely associated with treatment. Dynamic PET imaging and dual-tracer have emerged as complementary techniques that aid in various aspects of HCC diagnosis. The advent of new radiotracers and the development of immuno-PET and PET-magnetic resonance imaging have improved the ability to detect lesions and have made great progress in treatment surveillance. The current PET diagnostic capabilities for HCC and the supplementary techniques are reviewed herein.
Keywords: Hepatocellular carcinoma, Positron-emission tomography, Radiotracer, Immuno-positron emission tomography
Core tip: Positron-emission tomography (PET) is an effective and noninvasive modality for visualizing hepatocellular carcinoma (HCC). This paper reviews the clinical utility of PET for HCC, including the detection of intrahepatic or extrahepatic lesions, differential diagnosis, prediction of differentiation and prognosis, and evaluation of therapeutic response. Complementary technologies, such as dynamic blood flow and dual-phase and dual-tracer PET imaging, are also mentioned. Novel radiotracers and immuno-PET have shown great potential for PET imaging and have become the focus of current research, which may enhance the diagnostic capability of PET for HCC. An overview of the current PET diagnostic status of HCC is reviewed.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the second most common cause of death from cancer worldwide, with more than 740000 deaths each year[1]. Although modern management methodologies for HCC patients, such as surgical resection and comprehensive treatment (radiotherapy, chemotherapy, immunotherapy, in-terventional therapy, or combined) have been developed, the overall survival (OS) rate remains low. Liver transplantation (LT), partial liver resection, and ablation remain the main therapeutic tools for HCC and have a high rate of complete response. However, most patients are diagnosed at an advanced stage and are complicated with multiple lesions and liver cirrhosis; no more than 40% of HCC patients have the opportunity to undergo surgery[2].
Careful selection of candidates is vital for improving treatment outcomes. Evaluation of HCC should be referred to multidisciplinary teams that include surgeons, oncologists, hepatologists, and radiologists. Serum alpha-fetoprotein (AFP) levels have been widely used to diagnose HCC in the early stage; however, this test is limited due to its low sensitivity of approximately 25%[3]. Contrast-enhanced ultrasound (CEUS) imaging is useful for HCC diagnosis only when the tumor sites are identified by B-mode US[4]. Contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) are also available for HCC screening, and each has its own advantages, such as multiphase enhancement characteristics and easy acquisition, but their accuracy may be lower when lesions are smaller than 2 cm[5-7]. Digital angiography is another examination method that can diagnose HCC, but it is invasive and is usually only performed when transarterial treatment is necessary[4]. Although these examinations are well utilized by surgeons for preoperative staging, they often show only a part of the body and detect morphologic changes that can occur quite slowly in HCC. In addition, these traditional examination techniques cannot detect recurrent, residual, or metastatic lesions well.
Positron-emission tomography (PET) seems to be a more effective and noninvasive modality than traditional radiography techniques for scanning the whole body[8]. Although 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) PET has a low sensitivity, between 36% and 70%, in detecting HCC[9-11], the application of PET-CT for diagnosing HCC has made great progress in recent years. This review presents an overview of the current status and future prospects of PET for diagnosing HCC.
RADIOTRACERS
18F-FDG is the most widely used radiotracer for PET-CT; 18F has a long half-life (110 min), the best imaging spatial resolution, and favorable nuclear and chemical properties[12]. 18F-FDG is a radiolabeled glucose analog in which the positron emitter radioactive isotope 18F replaces the hydroxyl group at the C2 position in the glucose molecule. 18F-FDG is transported across the cell membrane by glucose transporters (GLUTs); in HCC, GLUT1, GLUT3, and, more recently, GLUT12, have been associated with the transport of this radiopharmaceutical into cancer cells. Multidrug resistance (MDR) is the ability of tumor cells to become resistant to different drugs and represents a major barrier to successful treatments. The overexpression of MDR proteins is thought to be a major obstacle to successful chemotherapy in various cancer types, including HCC. Studies have shown that cells that present increased MDR protein expression exhibit lower 18F-FDG accumulation[13]. In intracellular terms, 18F-FDG is phosphorylated by hexokinase II to 18F-FDG-6-phosphate, which cannot be metabolized in the glycolytic pathway and accumulates in metabolically active cells.
18F-labeled amino acids and peptides have potential application value for PET imaging in HCC or other tumors[14-18]. Sun et al[19] synthesized N-(2-18F-fluoro-propionyl)-L-glutamate (18F-FPGLU), and the radiochemical purity was higher than 95%, with a specific activity of 30-40 GBq/μmol. Although the novel tracer showed good tumor-to-background contrast and good stability in vitro, 18F-FPGLU was metabolically unstable in plasma, urine, and tumor tissues[20].
18F-fluorocholine is another radiotracer used in PET imaging that radiolabels phosphocholine, the major metabolite in cancer cells that is responsible for choline uptake and has a steady distribution that is available within 10 min, demonstrating high sensitivities of 89% for hepatic HCC and 100% for extrahepatic HCC[21,22]. Although the 18F-labeled metabolites are not able to be synthesized in every medical center, they still perform better than other radiolabels in diagnosing HCC.
Other promising radiopharmaceuticals currently used in PET-CT include 11C-labeled acetate (11C-ACT) and 11C-labeled choline (11C-CHOL)[23]. 11C-ACT is a radiopharmaceutical that is widely used in the imaging of HCC, primary brain tumors, carcinoid tumors, prostate adenocarcinoma, and transitional cell carcinoma[24]. As a substrate, 11C-ACT enters the Krebs cycle for β-oxidation in fatty acid synthase (FASN) and cholesterol synthesis. Fatty acid synthesis is thought to be the key factor for the uptake of 11C-ACT by liver neoplasms. Increased 11C-ACT uptake is often considered to reflect the increased de novo lipogenesis rate and to be associated with increased FASN expression[25,26]. Unlike 18F-FDG, 11C-ACT mainly reflects the growth activity of tumor cells and may provide a complementary role to conventional radiotracers[27].
11C-CHOL is a precursor for phospholipid synthesis of the cell membrane. 11C-CHOL has a high PET signal in liver tumor cells due to the increased activities of choline transporter and choline kinase. In addition, HCC foci gained a better tumor-to-background contrast with CHOL[28,29]. Nevertheless, 11C has a short half-life of approximately 20 min, and the use of 11C-labeled tracers is limited based on access to an on-site cyclotron, whereas 18F has a longer half-life than 11C[28].
Another alternative tracer is the 68Ga-labeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) conjugate of serum albumin or peptides. With an appropriate physical half-life (68 min) and good blood clearance, 68Ga-DOTA may be a potential radiotracer for use in imaging HCC[30,31]. Studies have shown that 68Ga-DOTA has a higher sensitivity than 18F-DOTA, as 68Ga-DOTA had a greater PET uptake than 18F-FDG in low-grade neuroendocrine tumors[29]. Gao et al[32] demonstrated that 68Ga-asparagine-glycine-arginine uptake was higher than 18F-FDG uptake for imaging well-differentiated HCC xenografts. However, limited data using 68Ga for HCC are now emerging, and its potential clinical utility is unclear.
64Cu radionuclide has a half-life of 12.7 h and is a novel biomarker for molecular imaging of HCC. 64CuCl2 PET-CT was able to detect early intracranial and other extrahepatic metastases located in areas with low physiological uptake, such as musculoskeletal tissues, which is important for determining the stage and prognosis of patients with HCC. This radionucleotide also plays an important role in treatment method selection. However, 64Cu has an abundant physiological distribution in the liver, which will decrease the tumor-to-background contrast and make the lesions unrecognizable, which will limit its value of evaluation for HCC. Furthermore, altered copper metabolism is expected to be a target for radionuclide therapy of HCC using therapeutic copper radionuclides[33]. Another promising radiotracer, 89Zr, will be reviewed in relation to immuno-PET in detail later in the article. The important findings about these radiotracers are summarized in Tables 1 and 2.
Table 1.
Positron emission tomography for intrahepatic or extrahepatic hepatocellular carcinoma
| Isotopes | Half-life | Radiotr-acer | Year | First author | No. of patients | Study design |
Intrahepatic |
Extrahepatic |
Related notable findings | ||
| Sensiti-vity | Specifi-city | Sensiti-vity | Specifi-city | ||||||||
| 18F | 110 min | 18F-FDG | 2019 | Lee et al[11] | - | Review | 0.36-0.70 | NA | NA | NA | 18F-FDG PET has demonstr-ated a higher sensitivity for detecting extrahepa-tic metastasis compared to primary HCC |
| 2012 | Hossein Jadvar[29] | Review | NA | 0.91 | NA | NA | |||||
| 2012 | Lin et al[89] | Meta-analysis | NA | NA | 0.77 | 0.98 | |||||
| 18F-FCH | 2014 | Bieze et al[21] | 30 | Prospective; single-center | 0.88 | 1.0 | 1.0 | 1.0 | 18F-FCH shows additional value in the assessment of intra- and extrahepa-tic diseases | ||
| 11C | 20 min | 11C-ACT | 2009 | Hwang et al[27] | 13 | Prospective | 0.83 | NA | 0.77 | NA | 11C increases the sensitivity in the detection of HCC lesions of more than 10 mm |
| 11C-CHOL | 2016 | Castilla-Lièvre et al[71] | 28 | Prospective; single-center | 0.67 | NA | NA | NA | Combining 18F-FDG with 11C-CHOL could be useful for clinicians in the manage-ment of HCC patients | ||
HCC: Hepatocellular carcinoma; NA: Not available;
F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; 18F-FCH: 18F-fluorocholine;
C-ACT: 11C-acetate; 11C-CHOL: 11C-choline.
Table 2.
Positron emission tomography in animal experiments of hepatocellular carcinoma
| Isotopes | Half-life | Radiotracer | Year | First author | Animal models | Related notable findings | Limitations |
| 18F | 110 min | 18F-FPGLU | 2017 | Sun et al[19] | Tumor-bearing mice (HCC SMCC-7721) | Radiochemical purity is higher than 95% with a specific activity of 30-40 GBq/μmol | Unstable in plasma, tumor, and urine |
| Stable in vitro | |||||||
| High uptake and retention in tumor | |||||||
| 64Cu | 12.7 h | 64CuCl2 | 2011 | Lièvre et al[71] | Athymic mice bearing extrahepatic HCC xenografts | Increased 64Cu radioactivity is well visualized | Abundant physiological distribution in liver |
| Useful for detection of intracranial HCC metastasis | |||||||
| 68Ga | 68 min | 68Ga-NGR | 2017 | Gao et al[32] | Tumor-bearing mice (HCC SMCC-7721) | 68Ga-NGR could visualize CD13-positive tumors | The uptake performance of 68Ga-NGR for poorly differentiated HCC needs further investigation |
| 68Ga-NGR uptake is significantly higher than that of 18F-FDG in well-differentiated HCC xenografts | |||||||
| 89Zr | 78.4 h | 89Zr-αGPC3 | 2014 | Sham et al[79] | HepG2 tumor-bearing mice | Excellent specificity | Long half-life in the blood, leading to suboptimal imaging pharmacokinetics, poorer tumor penetration, and increased immunogenicity due to relatively large size and intact Fc regions |
| Even smaller tumors (<1 mm) are able to be identified | |||||||
| 89Zr-αGPC3-F(ab′)2 | 2014 | Sham et al[80] | HepG2 tumor-bearing mice | Significantly reduces blood circulation time | Potential risk of fragment concentration in the kidneys, leading to organ dysfunction | ||
| Lower background liver uptake allows for early imaging | |||||||
| 89Zr-DFO-1G12 | 2014 | Yang et al[81] | HepG2 tumor-bearing mice | Specifically taken up by GPC3-positive HCC xenografts regardless of GPC3 expression levels | This probe should be further validated using a humanized anti-GPC3 antibody | ||
| High tumor-to-liver ratio | |||||||
| 89Zr-Df-YY146-ZW800 | 2016 | Hernandez et al[82] | HepG2 tumor-bearing mice | Excellent CD146-affinity, specificity, and stability | Bone-displaying PET signal is not matched by NIRF | ||
| Both PET and NIRF imaging are achieved |
HCC: Hepatocellular carcinoma; PET: Positron emission tomography;
F-FPGLU: N-(2-18F-fluoropropionyl)-L-glutamate;
Ga-NGR: 68Ga-labeled asparagine-glycine-arginine;
Zr-αGPC3: 89Zr-anti glypican-3; 89Zr-αGPC3-F(ab’)2: 89Zr-anti glypican-3-F(ab’)2; 89Zr-DFO-1G12: 89Zr-desferrioxamine-1G12; 89Zr-Df-YY146-ZW800: 89Zr- deferoxamine-YY146-ZW800; NIRF: Near-infrared fluorescence.
18F-FDG PET
Detection of intrahepatic or extrahepatic lesions
Although HCC is the only solid tumor that can be diagnosed by the characteristics of “arterial phase hyperenhancement” and “washout” on CT or MRI after contrast medium injection[5], there are still limitations in the biology of HCC that CT or MRI cannot show but that can be presented by the metabolic information from PET-CT[34]; however, none of these examinations can present the local tumor extent or detect distant metastases in the same examination. 18F-FDG PET-CT can also enhance the detection capacity for synchronous neoplasms in patients with HCC, which may be misdiagnosed as primary lesions or metastasis[35].
18F-FDG PET gives hepatologists complementary imaging details about primary HCC lesions and extrahepatic metastases, and this additional information is associated with treatment selection[36]. 18F-FDG PET-CT is usually a complementary method for routine examinations because the accumulation of FDG in HCC varies. According to the current European Association for the Study of the Liver Clinical Practice Guidelines for the management of HCC, 18F-FDG uptake was observed in less than 40% of HCC patients[37]. Studies have demonstrated that low 18F-FDG uptake is correlated with high FDG-6-phosphatase activity, high expression of P-glycoprotein, and low expression of GLUT1 or GLUT2 in moderately and well-differentiated HCC[38]. 18F-FDG is transported into cells and phosphorylated to FDG-6-phosphate, which is trapped within cells. However, high levels of FDG-6-phosphatase hydrolyzes FDG-6-phosphate to FDG, which is then transported outside the cells, and high expression of P-glycoprotein acts as an efflux pump to also transport FDG out of the cell, and low expression of GLUT1 or GLUT2 reduces the uptake of FDG. These reasons contribute to lower FDG accumulation in tumors[11,39,40].
PET scanning has a high sensitivity for detecting extrahepatic metastases but a low sensitivity for primary HCC[41]. The reason is that normal liver tissue has a relatively high FDG uptake, which reduces the tumor-to-liver standardized uptake value (SUV) ratio (TLR) and makes it difficult to visualize tumor lesions[42]. However, extrahepatic metastases usually have a low FDG uptake background to visualize. Based on the Barcelona Clinic Liver Cancer staging classification, patients with HCC have lymph node metastasis that usually indicates an advanced stage. Metastasis is a fairly common sequela in HCC, occurring in more than 50% of patients; most of these metastases frequently affect the lungs (18%-53.8%), lymph nodes (26.7%-53%), and diaphragm and skeleton (5.8%-38.5%)[43,44]. Among them, lymph node metastasis most frequently occurs, with an incidence of more than 50%. Retroperitoneal lymph node metastasis is more frequent than porta hepatis lymph node metastasis[45]. 18F-FDG PET-CT has a higher sensitivity to detect lymph node metastasis, which is a poor prognostic factor for HCC[29].
In view of the potential value of PET-CT for extrahepatic lesions, PET-CT should be considered for initial HCC staging work-ups to formulate a plan for patients who are candidates for hepatic resection (HR) or LT[44]. Although CT, MRI, and bone scintigraphy are recommended for preoperative HCC staging, HCC metastasis to uncommon sites, such as the oral cavity, jaw, thyroid, and adrenal glands, may be detected only by 18F-FDG PET-CT[43,46,47] and easily missed by conventional CT and MRI. Overall, 18F-FDG PET-CT has additional value for HCC staging.
Differential diagnosis
There are few studies on the differential diagnosis of HCC, and the utility of PET-CT for differential diagnosis is limited. Several case reports[48-50] have shown that PET-CT is a useful tool to differentiate primary or secondary neoplasms, but these studies did not systematically summarize the signs of differential diagnosis from HCC. Malignant lesions may increase radiotracer uptake, and PET-CT is of value for the following reasons.
18F-FDG PET-CT is very helpful to assess the malignant potential of hepatic lesions of unknown origin through simultaneous visualization of the liver and extrahepatic tissue and for further confirmation of a clinically suspected extrahepatic metastasis of known HCC[51]. 18F-FDG PET-CT has the potential value to distinguish malignant thrombus from a bland thrombus of the portal vein in patients with HCC, which is of great clinical significance for determining the therapeutic approach, predicting survival, and assessing candidates for LT[52]. However, no studies have shown that PET-CT has higher value to diagnose bland thrombus than CT, MRI, or even fine needle biopsy. 18F-FDG PET-CT may play an important role in differentiating malignant lymph metastasis from lymphoproliferative diseases[53]. However, there is no denying that these conclusions are drawn from case reports, and more evidence is needed to support these topics in further studies.
Additionally, there is a lack of literature to differentiate between HCC and intrahepatic cholangiocellular carcinoma using 18F-FDG PET alone. Conventional CT and MRI, especially with contrast enhancement, are useful[54], and developing new specific radiotracers can be a desirable alternative for enhancing the ability of differential diagnosis.
Prediction of differentiation and prognosis
18F-FDG PET is expected to describe tumor aggressiveness of HCC, and high accu-mulation of FDG is associated with biological malignancy[55]. Moderately and well-differentiated HCC may show low glucose metabolism, whereas 18F-FDG uptake by poorly differentiated HCC may be visualized as a hot spot on a PET scan[56]. The main reason is the high FDG-6-phosphatase activity in well-differentiated HCC, which resembles normal liver tissue, thus reducing FDG accumulation in the lesions[34,39].
Pretreatment 18F-FDG PET has incremental prognostic value for OS in both intrahepatic and extrahepatic diseases. In addition, for patients with intermediate-to-advanced stage HCC confined to the liver, TLR is an independent prognostic factor for progression-free survival (PFS) and OS[57,58]. A TLR of 1.2 or more has a statistically significant association with microvascular invasion (MVI)[59,60]. Patients with MVI and those with poorly differentiated grade show significantly higher recurrence rates[55]. Kobayashi et al[61] suggested that the combination of an SUVmax of 3.2 or greater and an AFP-L3 level of 19% or greater are useful for selecting small numbers of HCC patients for HR or LT.
18F-FDG PET-CT can also predict the prognosis of patients with HCC after treatment. 18F-FDG PET-CT is sensitive to detecting recurrent extrahepatic lesions of HCC after hepatectomy or radiofrequency ablation, which has a diagnostic sensitivity of 90–100% for recurrent or metastatic hepatic tumors[62]. 18F-FDG PET-CT is a valid prognostic tool in patients with HCC who are candidates for orthotopic liver transplantation (OLT); positivity on PET is the only factor related to early recurrence of HCC after OLT, and the combination of findings on PET and the AFP levels provides even more decisive results[63].
Evaluation of therapeutic response
18F-FDG uptake is closely related to therapeutic response in HCC and can offer additional information on the risk of HCC recurrence after surgery. PET status may be a significant and independent risk factor for posttreatment recurrence of HCC after LT[55]. 18F-FDG PET-CT scans reflect tissue metabolism, while the size changes do not serve as a predictor of tumor control.
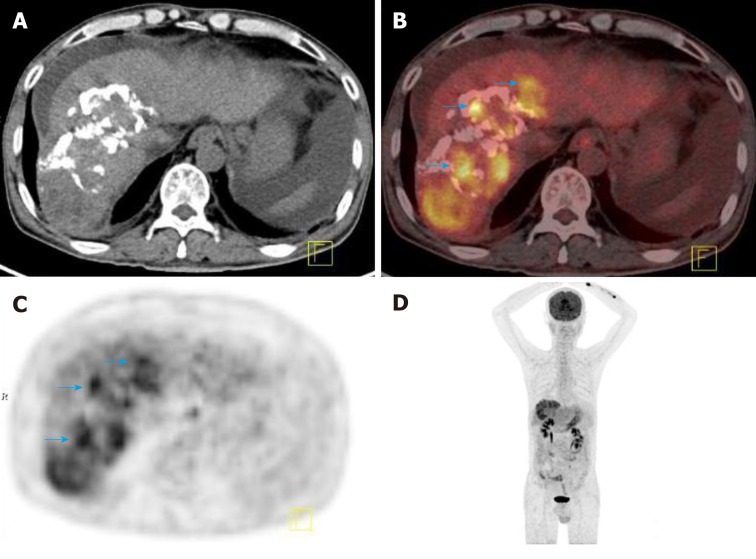
The SUV ratio is an important factor affecting treatment response, and a decreased SUV ratio after external beam radiotherapy is associated with the degree of tumor necrosis on the histological examination[64]. Kim et al[65] indicated that the maximum tumor-to-background ratio calculated by the inferior vena cava (TBRIVCmax) and the uptake-volume product measured by margin thresholds of the TBRIVC exhibit higher predictive power for patients after transplantation than other indices. PET-CT was also performed 1 month after interventional treatment to evaluate the therapeutic response. Song et al[66] revealed that 18F-FDG PET-CT was efficient in assessing the viability of HCC after transcatheter arterial chemoembolization (TACE) and was superior to CECT in grades I and II and similar in grade III; moreover, nonattenuation-corrected PET data may be helpful for avoiding false-positive results of tracer uptake induced by lipiodol deposition (Figure 1).
Figure 1.
2-Deoxy-2-(18F)fluoro-D-glucose positron-emission tomography-computed tomography detected tumor recurrence after intervention therapy in a 58-year-old male patient with hepatocellular carcinoma. A: Cross-sectional computed tomography (CT) image showing a large sheet of lipiodol deposition in the right lobe of live after HCC intervention therapy; B: Cross-sectional positron-emission tomography (PET-CT) fusion image showing increased 18F-FDG uptake in and around the area of lipiodol deposition (blue arrow); the size of the lesion was 5.8 × 13.3 cm; C: Cross-sectional PET image showing increased 18F-FDG uptake in the right lobe of the liver; D: Maximum intensity projection image showing increased 18F-FDG uptake in the right lobe of the liver. 18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; CT: Computed tomography; PET: Positron-emission tomography.
TLR only represents the point of the highest metabolic activity of the tumor and does not account for the tumor extent, while metabolic tumor volume (MTV) is a better parameter that represents the extent of abnormally increased FDG uptake by tumor tissue beyond the intensity of FDG uptake in normal tissue. MTV may be an independent prognostic factor for PFS and OS in patients with HCC after TACE[67].
Apart from HCC, 18F-FDG PET-CT is also a good predictive tool to assess treatment outcomes of HCC metastasis and for the early identification of treatment failure, especially when additional treatments remain a possibility. One study showed that preradiotherapy SUV ratios and a decline in postradiotherapy SUV ratios were identified as independent predictive factors for bone metastasis, and when combined, these factors predicted outcomes much more effectively than other methods[68].
DUAL-TRACER PET-CT
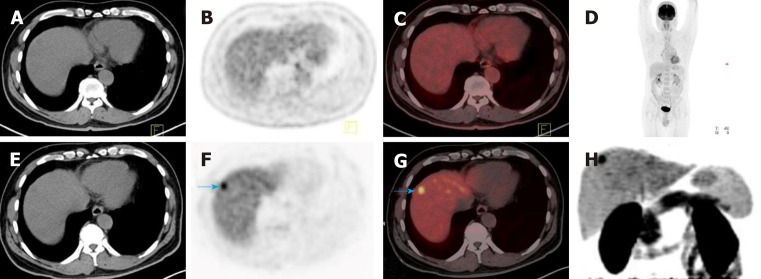
Dual-tracer PET-CT with 11C-ACT and 18F-FDG are biochemical probes of HCC and are complementary to each other. 11C-ACT uptake is related to well-differentiated HCC and a less aggressive cancer or a fair prognosis[69]. The regenerative nodules are avid for either of the two tracers, and they can be distinguished from HCC[70]. Combining 11C-CHOL and 18F-FDG PET-CT may also be a valuable option to detect HCC in the background of chronic liver disease. 11C-CHOL improves the detection rate for HCC in cases that are negative on conventional 18F-FDG PET-CT (Figure 2), and the combination of two tracers can increase the sensitivity of PET-CT to approximately 93%[71]. Dual-tracer PET-CT enhances its capacity to identify lesions and benefits the management of HCC patients.
Figure 2.
11C-choline positron-emission tomography-computed tomography detected a tumor that was missed on conventional 2-deoxy-2-(18F)fluoro-D-glucose positron-emission tomography-computed tomography in a 58-year-old patient with hepatocellular carcinoma. A-D: 2-deoxy-2-(18F)fluoro-D-glucose positron-emission tomography-computed tomography (18F-FDG PET-CT) showed that there was no increased 18F-FDG uptake in the liver; E-H: 11C-choline (11C-CHOL) PET-CT showed focal increased 11C-CHOL uptake in the upper segment of the anterior lobe of the liver, and the size of the lesion was 1.2 × 1.3 cm (blue arrow in F and G). Pathological examination confirmed well-differentiated hepatocellular carcinoma. 18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; CT: Computed tomography; PET: Positron-emission tomography; 11C-CHOL: 11C-choline.
DYNAMIC BLOOD FLOW AND DUAL-PHASE PET IMAGING
To obtain a better diagnostic result, additional emerging technologies can be performed, including early dynamic PET imaging and dual-phase PET-CT. Dynamic blood flow PET-CT is centered on the liver, beginning with the injection of 18F-FDG, to reveal information about both the spatial distribution and temporal kinetics of the tumor, and enables quantitative interpretation of PET data[72].
Time-activity curves are generated from the mean activities for each volume of interest; three blood flow parameters related to the first-pass delivery of FDG are acquired, including peak intensity, time to peak (TTP), and hepatic perfusion index (HPI), which are derived for the HCC lesion and the background liver parenchyma[42]. Studies show that TTP and HPI demonstrate significantly better performances than SUVmax for the discrimination between HCC and background liver tissue, and HPI represents the percentage of arterial supply of the total blood flow in the liver. Early dynamic 18F-FDG PET-CT generates significantly greater contrast during the arterial phase of imaging and similar contrast during the venous phase compared to CECT[73].
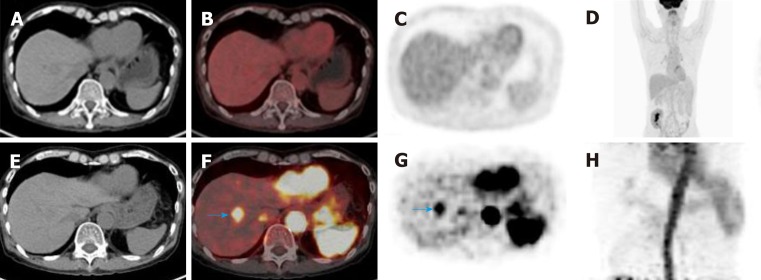
Of course, a conventional (standard static) PET-CT scan of the torso is routinely performed 60 min after FDG injection. Early dynamic 18F-FDG PET-CT allows for the improvement of the detection rate for HCC in cases that are negative for HCC on conventional 18F-FDG PET-CT (Figure 3), and the combination of early dynamic 18F-FDG PET-CT and conventional PET-CT improves the detection ability for HCC[74]. These results indicate that dynamic PET-CT may serve as an alternative where CECT or other conventional contrast-enhanced modalities are contraindicated or un-available. However, early dynamic 18F-FDG PET-CT shows perfusion parameters, and 18F-FDG can be absorbed into cells and become involved in intracellular metabolism at the same time[74]; therefore, more precise algorithms are needed to separate these parameters from those necessary for image analyses. In addition, formal and prospective clinical studies with a larger number of subjects are needed to explore the value of early dynamic 18F-FDG PET-CT in HCC diagnosis.
Figure 3.
Early dynamic 2-deoxy-2-(18F)fluoro-D-glucose positron-emission tomography-computed tomography detected a tumor that was missed on conventional 2-deoxy-2-(18F)fluoro-D-glucose positron-emission tomography-computed tomography in a 64-year-old patient with hepatocellular carcinoma. A-D: 2-deoxy-2-(18F)fluoro-D-glucose positron-emission tomography-computed tomography (18F-FDG PET-CT) showed that there was no increased 18F-FDG uptake in the lesion on conventional 18F-FDG PET-CT; E-H: Early dynamic 18F-FDG PET-CT showed focal 18F-FDG hyperperfusion in the upper segment of the anterior lobe of the liver, and the size of the lesion was 1.7 × 1.9 cm (blue arrow in F and G). 18F-FDG: 2-deoxy-2-(18F)fluoro-D-glucose; CT: Computed tomography; PET: Positron-emission tomography.
Dual-phase PET begins with whole-body scanning 60 min after the injection of 18F-FDG, and delayed scanning begins approximately 2 h after 18F-FDG injection. The tumor may be more clearly visualized by delayed PET-CT imaging of 18F-FDG, and the uptake may be higher, thus supporting the diagnosis of HCC[75]. Dual-phase imaging of 11C-ACT PET acquires early scan immediately after tracer administration and conventional scan in 11-18 min, and it seems to be an available method for the differential diagnosis of focal nodular hyperplasia (FNH) and small hemangioma from malignant lesions[76,77]. Both FNH and small hemangioma are hypervascularized lesions and exhibit positive tracer uptake ratios in early-phase imaging, followed by an immediate decline in the late phase[76]. However, well-differentiated HCC demonstrates an increasing tracer uptake over time. Furthermore, compared to CT, MRI, or ultrasound, 11C-ACT PET images are not easy to acquire because 11C has a short half-life (20 min), which limits its utility in clinical work.
IMMUNO-PET
To date, immuno-PET has emerged as a targeted molecular imaging method that represents a promising approach for diagnosing HCC. Glypican-3 (GPC3) is a cell surface protein that is highly expressed in HCC, melanoma, and clear cell carcinoma of the ovary. GPC3 has been proposed as an immunohistochemistry marker to differentiate HCC from benign hepatocyte nodules[78].
89Zr possesses a better half-life (78.4 h) than other radiotracers and performs well, especially when conjugated with monoclonal antibodies (mAbs). The 89Zr-conjugated mAb against GPC3 (89Zr-αGPC3) demonstrates antibody-dependent and antigen-specific tumor binding; the αGPC3 protein was created using the human GPC3 protein and demonstrated high-affinity binding. 89Zr-αGPC3 PET has shown a great capacity for identifying primary liver malignancies, even smaller lesions (< 1 mm), and has demonstrated the capacity to overcome background liver activity[79].
However, mAbs have long half-lives in the blood, leading to suboptimal imaging pharmacokinetics, poor tumor penetration, and increased immunogenicity due to their relatively large size and intact Fc regions. Smaller targeting moieties, such as F(ab′)2 fragments, have emerged as an alternative to mAbs. The half-life of 89Zr-αGPC3-F(ab′)2 in the blood is approximately 11 h and enables clear tumor visualization on PET 4 h after administration and has an excellent signal-to-noise ratio at an early time point[80].
Yang et al[81] synthesized a new PET probe, 89Zr-DFO-1G12, by bioconjugating and radiolabeling anti-GPC3 mAbs (clone 1G12) with 89Zr; this probe can be specifically taken up only by GPC3-expressing cells and achieved a high TLR. 89Zr-DFO-1G12 detected all GPC3-positive orthotopic HCC xenografts regardless of the level of GPC3 expression, highlighting its clinical value in the diagnosis of all GPC3-expressing HCC.
Another attractive molecular target is CD146, which is overexpressed in multiple cancers and associates with a high histological grade in HCC, but not in normal liver tissue. YY146 is an anti-CD146 mAb; when conjugated, the zwitterionic near-infrared fluorescence (NIRF) dye ZW800-1 and the chelator deferoxamine (Df) enable the labeling of Df-YY146-ZW800 with 89Zr and its subsequent detection with PET and NIRF imaging[82]. 89Zr-Df-YY146-ZW800 showed excellent properties as a dual-modality imaging agent and exhibited good stability.
The dual-modality imaging capacity not only provides diagnostic information but also may guide surgical resection. All radiotracers for immuno-PET benefit from the long half-life of 89Zr; hence, these radiotracers showed better properties for PET imaging. In addition, GPC3 is proposed to be an immunohistochemistry marker that can differentiate HCC from benign hepatocyte nodules[79]. However, most studies regarding immuno-PET were performed in animals, and further studies are needed to transfer this technique to clinical use.
PET-MRI
Combined PET and MRI utilize the advantages of MRI, including increased soft tissue contrast, multiple sequences, lack of ionizing radiation exposure, and use of MRI-specific contrast agents[83]. Diffusion-weighted imaging is widely used in tumor evaluation, and the apparent diffusion coefficient can quantify the Brownian motion of water molecules in tissue, which changes during a pathophysiological state. Kong et al[84] indicated that there may exist a negative correlation between increased FDG accumulation and water diffusion in hepatic tumors. PET-MRI is available in HCC staging and follow-up after treatment[85].
Although PET-MRI is promising in many aspects, PET-MRI systems are still relatively rare, and there are several hurdles that prevent its clinical application. Interpretation of PET-MRI requires technician knowledge on both nuclear medicine and MRI, and the examination may take more time. On the other hand, PET-MRI has limited evaluation of pulmonary parenchyma, and patients with metal implants in their bodies cannot undergo MRI. Further studies of PET-MRI utility in clinical applications are needed[83].
CONCLUSION
Although 18F-FDG PET-CT has emerged as an important noninvasive diagnostic tool in HCC, especially in staging and detecting metastatic lesions, the low sensitivity of 18F-FDG PET-CT limits its clinical use, especially for routine surveillance[86]. To improve the sensitivity of PET-CT for HCC diagnosis, many new techniques have been carried out, and several methods have been applied. The advent of novel radiotracers and dual-tracer PET-CT increases the sensitivity and enables the visualization of other metabolic processes apart from glucose metabolism. The new modalities require technical expertise and on-site cyclotron facilities; in addition, these radiotracers are expensive and inconvenient for patients[87]. Dynamic or dual-phase PET imaging is an alternative modality when other CE modalities cannot be utilized; dual-phase PET imaging provides multiple parameters for quantitative analysis and reflects the blood perfusion in detail. Although false-positive findings might sometimes occur in dynamic PET imaging, it has certain value in diagnosis and differential diagnosis[73].
Radiotracer synthesis and targeted molecular imaging modalities, such as immuno-PET, have already become the focus of current research, and their diagnostic capacity for smaller HCC lesions is encouraging. However, most research about radiotracers and immuno-PET is performed on experimental animals, and immuno-PET also shows the overexpression of molecular targets in many non-liver malignancies[79,88]. Further evaluation of their immune reactivity is needed, and clinical translation requires more evidence. Additionally, a useful surrogate marker of MVI in small HCC has not yet been established[61]. It is imperative to develop new molecular targets and new probes that can specifically bind to each other. The development of new probes will not only improve diagnostic sensitivity and specificity but also be adapted for the targeted delivery of therapeutic agents[81].
ACKNOWLEDGEMENTS
We thank our colleagues at the PET-CT Center of the First People’s Hospital of Yunnan Province for searching the literature and offering valuable comments.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All the authors have no conflict of interest related to the manuscript.
Peer-review started: April 12, 2019
First decision: May 16, 2019
Article in press: July 19, 2019
P-Reviewer: Aykan NF, Boin IFSF, Boteon YL, Kahraman A, Pompili M, Rodríguez-Perálvarez M, Sandhu DS S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Ren-Cai Lu, PET-CT Center, the First People’s Hospital of Yunnan Province, Kunming 650032, Yunnan Province, China.
Bo She, PET-CT Center, the First People’s Hospital of Yunnan Province, Kunming 650032, Yunnan Province, China.
Wen-Tao Gao, PET-CT Center, the First People’s Hospital of Yunnan Province, Kunming 650032, Yunnan Province, China.
Yun-Hai Ji, PET-CT Center, the First People’s Hospital of Yunnan Province, Kunming 650032, Yunnan Province, China.
Dong-Dong Xu, PET-CT Center, the First People’s Hospital of Yunnan Province, Kunming 650032, Yunnan Province, China.
Quan-Shi Wang, Nanfang PET Center, Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong Province, China.
Shao-Bo Wang, PET-CT Center, the First People’s Hospital of Yunnan Province, Kunming 650032, Yunnan Province, China; Yunnan Key Laboratory of Primate Biomedical Research, Institute of Primate Translational Medicine, Kunming University of Science and Technology, Kunming 650093, Yunnan Province, China. wshbo_98@126.com.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Han X, Yu X, Xu Z, Yang G, Liu B, Xiu P. Clinical applications of liquid biopsy as prognostic and predictive biomarkers in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. J Exp Clin Cancer Res. 2018;37:213. doi: 10.1186/s13046-018-0893-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M. Diagnostic imaging of hepatocellular carcinoma: Recent progress. Oncology. 2011;81 Suppl 1:73–85. doi: 10.1159/000333265. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M, Gabriel H, Miller FH. Role of Imaging in Surveillance and Diagnosis of Hepatocellular Carcinoma. Gastroenterol Clin North Am. 2018;47:585–602. doi: 10.1016/j.gtc.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 6.An C, Kim DY, Choi JY, Han KH, Roh YH, Kim MJ. Noncontrast magnetic resonance imaging versus ultrasonography for hepatocellular carcinoma surveillance (MIRACLE-HCC): Study protocol for a prospective randomized trial. BMC Cancer. 2018;18:915. doi: 10.1186/s12885-018-4827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennedige T, Venkatesh SK. Advances in computed tomography and magnetic resonance imaging of hepatocellular carcinoma. World J Gastroenterol. 2016;22:205–220. doi: 10.3748/wjg.v22.i1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao X, Wei J, Li Y, Zhong J, Liu Z, Liao S, Li Q, Wei C. 18F-FDG PET with or without CT in the diagnosis of extrahepatic metastases or local residual/recurrent hepatocellular carcinoma. Medicine (Baltimore) 2018;97:e11970. doi: 10.1097/MD.0000000000011970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JY, Lee YN, Kim YS, Kim SG, Jin SJ, Park JM, Choi GS, Chung JC, Lee MH, Cho YH, Choi MH, Kim DC, Choi HJ, Moon JH, Lee SH, Jeong SW, Jang JY, Kim HS, Kim BS. Predictability of preoperative 18F-FDG PET for histopathological differentiation and early recurrence of primary malignant intrahepatic tumors. Nucl Med Commun. 2015;36:319–327. doi: 10.1097/MNM.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 10.Jiang HY, Chen J, Xia CC, Cao LK, Duan T, Song B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J Gastroenterol. 2018;24:2348–2362. doi: 10.3748/wjg.v24.i22.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SM, Kim HS, Lee S, Lee JW. Emerging role of 18F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma. World J Gastroenterol. 2019;25:1289–1306. doi: 10.3748/wjg.v25.i11.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 13.Brito AF, Mendes M, Abrantes AM, Tralhão JG, Botelho MF. Positron emission tomography diagnostic imaging in multidrug-resistant hepatocellular carcinoma: Focus on 2-deoxy-2-(18F)Fluoro-D-Glucose. Mol Diagn Ther. 2014;18:495–504. doi: 10.1007/s40291-014-0106-3. [DOI] [PubMed] [Google Scholar]
- 14.Chiotellis A, Muller A, Mu L, Keller C, Schibli R, Krämer SD, Ametamey SM. Synthesis and biological evaluation of (18)F-labeled Fluoroethoxy tryptophan analogues as potential PET tumor imaging agents. Mol Pharm. 2014;11:3839–3851. doi: 10.1021/mp500312t. [DOI] [PubMed] [Google Scholar]
- 15.Krasikova RN, Kuznetsova OF, Fedorova OS, Belokon YN, Maleev VI, Mu L, Ametamey S, Schubiger PA, Friebe M, Berndt M, Koglin N, Mueller A, Graham K, Lehmann L, Dinkelborg LM. 4-[18F]fluoroglutamic acid (BAY 85-8050), a new amino acid radiotracer for PET imaging of tumors: Synthesis and in vitro characterization. J Med Chem. 2011;54:406–410. doi: 10.1021/jm101068q. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Zhang D, Shi Y, Guo Z, Wu X, Ren JL, Zhang X, Wu H. Syntheses and preliminary evaluation of [(18) F]AlF-NOTA-G-TMTP1 for PET imaging of high aggressive hepatocellular carcinoma. Contrast Media Mol Imaging. 2016;11:262–271. doi: 10.1002/cmmi.1688. [DOI] [PubMed] [Google Scholar]
- 17.Kavanaugh G, Williams J, Morris AS, Nickels ML, Walker R, Koglin N, Stephens AW, Washington MK, Geevarghese SK, Liu Q, Ayers D, Shyr Y, Manning HC. Utility of [18F]FSPG PET to Image Hepatocellular Carcinoma: First Clinical Evaluation in a US Population. Mol Imaging Biol. 2016;18:924–934. doi: 10.1007/s11307-016-1007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He S, Tang G, Hu K, Wang H, Wang S, Huang T, Liang X, Tang X. Radiosynthesis and biological evaluation of 5-(3-[18F]fluoropropyloxy)-L-tryptophan for tumor PET imaging. Nucl Med Biol. 2013;40:801–807. doi: 10.1016/j.nucmedbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Sun A, Liu S, Tang X, Nie D, Tang G, Zhang Z, Wen F, Wang X. Simple and rapid radiosynthesis of N-18F-labeled glutamic acid as a hepatocellular carcinoma PET tracer. Nucl Med Biol. 2017;49:38–43. doi: 10.1016/j.nucmedbio.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Hu K, Du K, Tang G, Yao S, Wang H, Liang X, Yao B, Huang T, Zang L. Radiosynthesis and biological evaluation of N-[18F]labeled glutamic acid as a tumor metabolic imaging tracer. PLoS One. 2014;9:e93262. doi: 10.1371/journal.pone.0093262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bieze M, Klümpen HJ, Verheij J, Beuers U, Phoa SS, van Gulik TM, Bennink RJ. Diagnostic accuracy of (18) F-methylcholine positron emission tomography/computed tomography for intra- and extrahepatic hepatocellular carcinoma. Hepatology. 2014;59:996–1006. doi: 10.1002/hep.26781. [DOI] [PubMed] [Google Scholar]
- 22.Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, Huchet V, Ancel D, Grange JD, Rosmorduc O. Detection of hepatocellular carcinoma with PET/CT: A prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51:1699–1706. doi: 10.2967/jnumed.110.075507. [DOI] [PubMed] [Google Scholar]
- 23.Menzel MI, Farrell EV, Janich MA, Khegai O, Wiesinger F, Nekolla S, Otto AM, Haase A, Schulte RF, Schwaiger M. Multimodal assessment of in vivo metabolism with hyperpolarized [1-13C]MR spectroscopy and 18F-FDG PET imaging in hepatocellular carcinoma tumor-bearing rats. J Nucl Med. 2013;54:1113–1119. doi: 10.2967/jnumed.112.110825. [DOI] [PubMed] [Google Scholar]
- 24.Song WS, Nielson BR, Banks KP, Bradley YC. Normal organ standard uptake values in carbon-11 acetate PET imaging. Nucl Med Commun. 2009;30:462–465. doi: 10.1097/MNM.0b013e32832aa7ce. [DOI] [PubMed] [Google Scholar]
- 25.Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912–1921. doi: 10.2967/jnumed.108.055087. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Che L, Wang C, Blecha JE, Li X, VanBrocklin HF, Calvisi DF, Puchowicz M, Chen X, Seo Y. [(11)C]acetate PET Imaging is not Always Associated with Increased Lipogenesis in Hepatocellular Carcinoma in Mice. Mol Imaging Biol. 2016;18:360–367. doi: 10.1007/s11307-015-0915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang KH, Choi DJ, Lee SY, Lee MK, Choe W. Evaluation of patients with hepatocellular carcinomas using [(11)C]acetate and [(18)F]FDG PET/CT: A preliminary study. Appl Radiat Isot. 2009;67:1195–1198. doi: 10.1016/j.apradiso.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Kolthammer JA, Corn DJ, Tenley N, Wu C, Tian H, Wang Y, Lee Z. PET imaging of hepatocellular carcinoma with 18F-fluoroethylcholine and 11C-choline. Eur J Nucl Med Mol Imaging. 2011;38:1248–1256. doi: 10.1007/s00259-011-1743-y. [DOI] [PubMed] [Google Scholar]
- 29.Jadvar H. Hepatocellular carcinoma and gastroenteropancreatic neuroendocrine tumors: Potential role of other positron emission tomography radiotracers. Semin Nucl Med. 2012;42:247–254. doi: 10.1053/j.semnuclmed.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Hoffend J, Mier W, Schuhmacher J, Schmidt K, Dimitrakopoulou-Strauss A, Strauss LG, Eisenhut M, Kinscherf R, Haberkorn U. Gallium-68-DOTA-albumin as a PET blood-pool marker: Experimental evaluation in vivo. Nucl Med Biol. 2005;32:287–292. doi: 10.1016/j.nucmedbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Haubner R, Finkenstedt A, Stegmayr A, Rangger C, Decristoforo C, Zoller H, Virgolini IJ. [(68)Ga]NODAGA-RGD - Metabolic stability, biodistribution, and dosimetry data from patients with hepatocellular carcinoma and liver cirrhosis. Eur J Nucl Med Mol Imaging. 2016;43:2005–2013. doi: 10.1007/s00259-016-3396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Wang Z, Ma X, Ma W, Zhao M, Fu T, Li G, Wang S, Wang Z, Yang W, Kang F, Wang J. The uptake exploration of 68Ga-labeled NGR in well-differentiated hepatocellular carcinoma xenografts: Indication for the new clinical translational of a tracer based on NGR. Oncol Rep. 2017;38:2859–2866. doi: 10.3892/or.2017.5933. [DOI] [PubMed] [Google Scholar]
- 33.Wachsmann J, Peng F. Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World J Gastroenterol. 2016;22:221–231. doi: 10.3748/wjg.v22.i1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornberg A, Küpper B, Tannapfel A, Büchler P, Krause B, Witt U, Gottschild D, Friess H. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl. 2012;18:53–61. doi: 10.1002/lt.22416. [DOI] [PubMed] [Google Scholar]
- 35.Hiraoka A, Hirooka M, Ochi H, Koizumi Y, Shimizu Y, Shiraishi A, Yamago H, Tanihira T, Miyata H, Ninomiya T, Kawasaki H, Ishimaru Y, Sogabe I, Inoue T, Abe M, Hiasa Y, Matsuura B, Onji M, Michitaka K. Importance of screening for synchronous malignant neoplasms in patients with hepatocellular carcinoma: Impact of FDG PET/CT. Liver Int. 2013;33:1085–1091. doi: 10.1111/liv.12161. [DOI] [PubMed] [Google Scholar]
- 36.Kawamura E, Shiomi S, Kotani K, Kawabe J, Hagihara A, Fujii H, Uchida-Kobayashi S, Iwai S, Morikawa H, Enomoto M, Murakami Y, Tamori A, Kawada N. Positioning of 18F-fluorodeoxyglucose-positron emission tomography imaging in the management algorithm of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:1722–1727. doi: 10.1111/jgh.12611. [DOI] [PubMed] [Google Scholar]
- 37.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Li YC, Yang CS, Zhou WL, Li HS, Han YJ, Wang QS, Wu HB. Low glucose metabolism in hepatocellular carcinoma with GPC3 expression. World J Gastroenterol. 2018;24:494–503. doi: 10.3748/wjg.v24.i4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu HB, Wang QS, Li BY, Li HS, Zhou WL, Wang QY. F-18 FDG in conjunction with 11C-choline PET/CT in the diagnosis of hepatocellular carcinoma. Clin Nucl Med. 2011;36:1092–1097. doi: 10.1097/RLU.0b013e3182335df4. [DOI] [PubMed] [Google Scholar]
- 40.Seo S, Hatano E, Higashi T, Nakajima A, Nakamoto Y, Tada M, Tamaki N, Iwaisako K, Kitamura K, Ikai I, Uemoto S. P-glycoprotein expression affects 18F-fluorodeoxyglucose accumulation in hepatocellular carcinoma in vivo and in vitro. Int J Oncol. 2009;34:1303–1312. [PubMed] [Google Scholar]
- 41.Anis M, Irshad A. Imaging of hepatocellular carcinoma: Practical guide to differential diagnosis. Clin Liver Dis. 2011;15:335–352, vii-vix. doi: 10.1016/j.cld.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Bernstine H, Braun M, Yefremov N, Lamash Y, Carmi R, Stern D, Steinmetz A, Sosna J, Groshar D. FDG PET/CT early dynamic blood flow and late standardized uptake value determination in hepatocellular carcinoma. Radiology. 2011;260:503–510. doi: 10.1148/radiol.11102350. [DOI] [PubMed] [Google Scholar]
- 43.Klasser GD, Echandi L, Shannon M. Hepatocellular carcinoma metastasis to the condyle: A case report and review of the literature. J Am Dent Assoc. 2014;145:1063–1067. doi: 10.14219/jada.2014.70. [DOI] [PubMed] [Google Scholar]
- 44.Cho Y, Lee DH, Lee YB, Lee M, Yoo JJ, Choi WM, Cho YY, Paeng JC, Kang KW, Chung JK, Yu SJ, Lee JH, Yoon JH, Lee HS, Kim YJ. Does 18F-FDG positron emission tomography-computed tomography have a role in initial staging of hepatocellular carcinoma? PLoS One. 2014;9:e105679. doi: 10.1371/journal.pone.0105679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia F, Wu L, Lau WY, Li G, Huan H, Qian C, Ma K, Bie P. Positive lymph node metastasis has a marked impact on the long-term survival of patients with hepatocellular carcinoma with extrahepatic metastasis. PLoS One. 2014;9:e95889. doi: 10.1371/journal.pone.0095889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Y, Jeng LB, Wang HY, Tsai SC, Lin WY, Kao CH. Clinical Value of 18F-FDG PET/CT in Detecting Adrenal Metastasis in Patients with Hepatocellular Carcinoma. Technol Cancer Res Treat. 2015;14:593–599. doi: 10.7785/tcrt.2012.500438. [DOI] [PubMed] [Google Scholar]
- 47.Sung TY, Lee SR, Lee JH, Lee YM, Lee AL, Hwang DW, Yoon JH, Gong G, Hong SJ, Park KM. Hepatocellular carcinoma eats medullary thyroid carcinoma, a case of tumor-in-tumor metastasis. Endocr J. 2011;58:801–805. doi: 10.1507/endocrj.ej11-0052. [DOI] [PubMed] [Google Scholar]
- 48.Pan JH, Dong MJ, Ouyang XB. A hepatoid adenocarcinoma of the stomach with liver metastasis mimicking hepatocellular carcinoma detected by F-18 FDG PET/CT imaging. Clin Nucl Med. 2011;36:1137–1139. doi: 10.1097/RLU.0b013e3182335ef9. [DOI] [PubMed] [Google Scholar]
- 49.Lee JH, Lee KG, Park HK, Song SY, Kim JY, Kim YH, Choi YY, Jang KS, Park MH. Inflammatory pseudotumor of the kidney mimicking malignancy on 18F-FDG PET/CT in a patient with diabetes and hepatocellular carcinoma. Clin Nucl Med. 2012;37:699–701. doi: 10.1097/RLU.0b013e3182443e4d. [DOI] [PubMed] [Google Scholar]
- 50.Dong A, Zhong X, Wang Y. Pedunculated Hepatocellular Carcinoma Mimicking Right Adrenal Tumor on FDG PET/CT. Clin Nucl Med. 2018;43:e242–e244. doi: 10.1097/RLU.0000000000002096. [DOI] [PubMed] [Google Scholar]
- 51.Tsurusaki M, Okada M, Kuroda H, Matsuki M, Ishii K, Murakami T. Clinical application of 18F-fluorodeoxyglucose positron emission tomography for assessment and evaluation after therapy for malignant hepatic tumor. J Gastroenterol. 2014;49:46–56. doi: 10.1007/s00535-013-0790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal KK, Shah D, Shah N, Mayank M. Differentiation of Malignant Thrombus From Bland Thrombus of the Portal Vein in Patient With Hepatocellular Carcinoma on 18F-FDG PET CT. Clin Nucl Med. 2017;42:e472–e474. doi: 10.1097/RLU.0000000000001840. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y, Liu Y, Shi H. Castleman disease in the hilum liver mimicking the lymph node metastasis of hepatocellular carcinoma on 18F-FDG PET/CT: A case report. Medicine (Baltimore) 2018;97:e9939. doi: 10.1097/MD.0000000000009939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang L, Tan H, Panje CM, Yu H, Xiu Y, Shi H. Role of 18F-FDG PET/CT Imaging in Intrahepatic Cholangiocarcinoma. Clin Nucl Med. 2016;41:1–7. doi: 10.1097/RLU.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 55.Takada Y, Kaido T, Shirabe K, Nagano H, Egawa H, Sugawara Y, Taketomi A, Takahara T, Wakabayashi G, Nakanishi C, Kawagishi N, Kenjo A, Gotoh M, Toyoki Y, Hakamada K, Ohtsuka M, Akamatsu N, Kokudo N, Takeda K, Endo I, Takamura H, Okajima H, Wada H, Kubo S, Kuramitsu K, Ku Y, Ishiyama K, Ohdan H, Ito E, Maehara Y, Honda M, Inomata Y, Furukawa H, Uemoto S, Yamaue H, Miyazaki M, Takada T LTx-PET study group of the Japanese Society of Hepato-Biliary-Pancreatic Surgery and the Japanese Liver Transplantation Society. Significance of preoperative fluorodeoxyglucose-positron emission tomography in prediction of tumor recurrence after liver transplantation for hepatocellular carcinoma patients: A Japanese multicenter study. J Hepatobiliary Pancreat Sci. 2017;24:49–57. doi: 10.1002/jhbp.412. [DOI] [PubMed] [Google Scholar]
- 56.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. AJR Am J Roentgenol. 2011;197:W260–W265. doi: 10.2214/AJR.11.6995. [DOI] [PubMed] [Google Scholar]
- 57.Na SJ, Oh JK, Hyun SH, Lee JW, Hong IK, Song BI, Kim TS, Eo JS, Lee SW, Yoo IR, Chung YA, Yun M. 18F-FDG PET/CT Can Predict Survival of Advanced Hepatocellular Carcinoma Patients: A Multicenter Retrospective Cohort Study. J Nucl Med. 2017;58:730–736. doi: 10.2967/jnumed.116.182022. [DOI] [PubMed] [Google Scholar]
- 58.Sposito C, Di Sandro S, Brunero F, Buscemi V, Battiston C, Lauterio A, Bongini M, De Carlis L, Mazzaferro V. Development of a prognostic scoring system for resectable hepatocellular carcinoma. World J Gastroenterol. 2016;22:8194–8202. doi: 10.3748/wjg.v22.i36.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, Han JK, Choi BI. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015;40:843–851. doi: 10.1007/s00261-014-0256-0. [DOI] [PubMed] [Google Scholar]
- 60.Ünal E, İdilman İS, Akata D, Özmen MN, Karçaaltıncaba M. Microvascular invasion in hepatocellular carcinoma. Diagn Interv Radiol. 2016;22:125–132. doi: 10.5152/dir.2015.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi T, Aikata H, Honda F, Nakano N, Nakamura Y, Hatooka M, Morio K, Morio R, Fukuhara T, Masaki K, Nagaoki Y, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Ohdan H, Awai K, Chayama K. Preoperative Fluorine 18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Prediction of Microvascular Invasion in Small Hepatocellular Carcinoma. J Comput Assist Tomogr. 2016;40:524–530. doi: 10.1097/RCT.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 62.Wang XY, Chen D, Zhang XS, Chen ZF, Hu AB. Value of ¹⁸F-FDG-PET/CT in the detection of recurrent hepatocellular carcinoma after hepatectomy or radiofrequency ablation: A comparative study with contrast-enhanced ultrasound. J Dig Dis. 2013;14:433–438. doi: 10.1111/1751-2980.12064. [DOI] [PubMed] [Google Scholar]
- 63.Cascales-Campos PA, Romero PR, Schneider MA, Lopez-Lopez V, Navarro JL, Frutos L, Pons Miñano JA, Paricio PP. Positron emission tomography/computed tomography in patients with hepatocellular carcinoma undergoing liver transplantation. Useful, necessary or irrelevant? Eur J Radiol. 2017;91:155–159. doi: 10.1016/j.ejrad.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Kim JW, Seong J, Yun M, Lee IJ, Yoon HI, Cho HJ, Han KH. Usefulness of positron emission tomography with fluorine-18-fluorodeoxyglucose in predicting treatment response in unresectable hepatocellular carcinoma patients treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1172–1178. doi: 10.1016/j.ijrobp.2010.11.076. [DOI] [PubMed] [Google Scholar]
- 65.Kim YI, Paeng JC, Cheon GJ, Suh KS, Lee DS, Chung JK, Kang KW. Prediction of Posttransplantation Recurrence of Hepatocellular Carcinoma Using Metabolic and Volumetric Indices of 18F-FDG PET/CT. J Nucl Med. 2016;57:1045–1051. doi: 10.2967/jnumed.115.170076. [DOI] [PubMed] [Google Scholar]
- 66.Song HJ, Cheng JY, Hu SL, Zhang GY, Fu Y, Zhang YJ. Value of 18F-FDG PET/CT in detecting viable tumour and predicting prognosis of hepatocellular carcinoma after TACE. Clin Radiol. 2015;70:128–137. doi: 10.1016/j.crad.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Lee JW, Yun M, Cho A, Han KH, Kim DY, Lee SM, Lee JD. The predictive value of metabolic tumor volume on FDG PET/CT for transarterial chemoembolization and transarterial chemotherapy infusion in hepatocellular carcinoma patients without extrahepatic metastasis. Ann Nucl Med. 2015;29:400–408. doi: 10.1007/s12149-015-0956-8. [DOI] [PubMed] [Google Scholar]
- 68.Choi SH, Chang JS, Jeong YH, Lee Y, Yun M, Seong J. FDG-PET predicts outcomes of treated bone metastasis following palliative radiotherapy in patients with hepatocellular carcinoma. Liver Int. 2014;34:1118–1125. doi: 10.1111/liv.12487. [DOI] [PubMed] [Google Scholar]
- 69.Park S, Kim TS, Kang SH, Kim HB, Park JW, Kim SK. 11C-acetate and 18F-fluorodeoxyglucose positron emission tomography/computed tomography dual imaging for the prediction of response and prognosis after transarterial chemoembolization. Medicine (Baltimore) 2018;97:e12311. doi: 10.1097/MD.0000000000012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, Fung JY, Yan Chan AC, Sharr W, Yau T, Poon RT, Fan ST. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: Surgeon's perspective. J Nucl Med. 2013;54:192–200. doi: 10.2967/jnumed.112.107516. [DOI] [PubMed] [Google Scholar]
- 71.Castilla-Lièvre MA, Franco D, Gervais P, Kuhnast B, Agostini H, Marthey L, Désarnaud S, Helal BO. Diagnostic value of combining ¹¹C-choline and ¹⁸F-FDG PET/CT in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2016;43:852–859. doi: 10.1007/s00259-015-3241-0. [DOI] [PubMed] [Google Scholar]
- 72.Dutta J, Leahy RM, Li Q. Non-local means denoising of dynamic PET images. PLoS One. 2013;8:e81390. doi: 10.1371/journal.pone.0081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freesmeyer M, Winkens T, Schierz JH. Contrast between hypervascularized liver lesions and hepatic parenchyma: Early dynamic PET versus contrast-enhanced CT. Ann Nucl Med. 2014;28:664–668. doi: 10.1007/s12149-014-0862-5. [DOI] [PubMed] [Google Scholar]
- 74.Wang SB, Wu HB, Wang QS, Zhou WL, Tian Y, Li HS, Ji YH, Lv L. Combined early dynamic (18)F-FDG PET/CT and conventional whole-body (18)F-FDG PET/CT provide one-stop imaging for detecting hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2015;39:324–330. doi: 10.1016/j.clinre.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Wu B, Zhao Y, Zhang Y, Tan H, Shi H. Does dual-time-point 18F-FDG PET/CT scan add in the diagnosis of hepatocellular carcinoma? Hell J Nucl Med. 2017;20:79–82. doi: 10.1967/s002449910511. [DOI] [PubMed] [Google Scholar]
- 76.Huo L, Dang Y, Lv J, Xing H, Li F. Application of dual phase imaging of 11C-acetate positron emission tomography on differential diagnosis of small hepatic lesions. PLoS One. 2014;9:e96517. doi: 10.1371/journal.pone.0096517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huo L, Wu Z, Zhuang H, Fu Z, Dang Y. Dual time point C-11 acetate PET imaging can potentially distinguish focal nodular hyperplasia from primary hepatocellular carcinoma. Clin Nucl Med. 2009;34:874–877. doi: 10.1097/RLU.0b013e3181bed06e. [DOI] [PubMed] [Google Scholar]
- 78.Ho M, Kim H. Glypican-3: A new target for cancer immunotherapy. Eur J Cancer. 2011;47:333–338. doi: 10.1016/j.ejca.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sham JG, Kievit FM, Grierson JR, Miyaoka RS, Yeh MM, Zhang M, Yeung RS, Minoshima S, Park JO. Glypican-3-targeted 89Zr PET imaging of hepatocellular carcinoma. J Nucl Med. 2014;55:799–804. doi: 10.2967/jnumed.113.132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sham JG, Kievit FM, Grierson JR, Chiarelli PA, Miyaoka RS, Zhang M, Yeung RS, Minoshima S, Park JO. Glypican-3-targeting F(ab')2 for 89Zr PET of hepatocellular carcinoma. J Nucl Med. 2014;55:2032–2037. doi: 10.2967/jnumed.114.145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X, Liu H, Sun CK, Natarajan A, Hu X, Wang X, Allegretta M, Guttmann RD, Gambhir SS, Chua MS, Cheng Z, So SK. Imaging of hepatocellular carcinoma patient-derived xenografts using ⁸⁹Zr-labeled anti-glypican-3 monoclonal antibody. Biomaterials. 2014;35:6964–6971. doi: 10.1016/j.biomaterials.2014.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hernandez R, Sun H, England CG, Valdovinos HF, Ehlerding EB, Barnhart TE, Yang Y, Cai W. CD146-targeted immunoPET and NIRF Imaging of Hepatocellular Carcinoma with a Dual-Labeled Monoclonal Antibody. Theranostics. 2016;6:1918–1933. doi: 10.7150/thno.15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ehman EC, Johnson GB, Villanueva-Meyer JE, Cha S, Leynes AP, Larson PEZ, Hope TA. PET/MRI: Where might it replace PET/CT? J Magn Reson Imaging. 2017;46:1247–1262. doi: 10.1002/jmri.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong E, Chun KA, Cho IH. Quantitative assessment of simultaneous F-18 FDG PET/MRI in patients with various types of hepatic tumors: Correlation between glucose metabolism and apparent diffusion coefficient. PLoS One. 2017;12:e0180184. doi: 10.1371/journal.pone.0180184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nielsen K, Scheffer HJ, Pieters IC, van Tilborg AA, van Waesberghe JH, Oprea-Lager DE, Meijerink MR, Kazemier G, Hoekstra OS, Schreurs HW, Sietses C, Meijer S, Comans EF, van den Tol PM. The use of PET-MRI in the follow-up after radiofrequency- and microwave ablation of colorectal liver metastases. BMC Med Imaging. 2014;14:27. doi: 10.1186/1471-2342-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayakawa N, Nakamoto Y, Nakatani K, Hatano E, Seo S, Higashi T, Saga T, Uemoto S, Togashi K. Clinical utility and limitations of FDG PET in detecting recurrent hepatocellular carcinoma in postoperative patients. Int J Clin Oncol. 2014;19:1020–1028. doi: 10.1007/s10147-013-0653-3. [DOI] [PubMed] [Google Scholar]
- 87.Dubash SR, Idowu OA, Sharma R. The emerging role of positron emission tomography in hepatocellular carcinoma. Hepat Oncol. 2015;2:191–200. doi: 10.2217/hep.15.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marquez BV, Zheleznyak A, Lapi SE. Glypican-3-targeted 89Zr PET imaging of hepatocellular carcinoma: Where antibody imaging dares to tread. J Nucl Med. 2014;55:708–709. doi: 10.2967/jnumed.113.136234. [DOI] [PubMed] [Google Scholar]
- 89.Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: A systematic review and meta-analysis. Eur J Radiol. 2012;81:2417–2422. doi: 10.1016/j.ejrad.2011.08.004. [DOI] [PubMed] [Google Scholar]