Abstract
BACKGROUND
Liver cancer is the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide. Socioeconomic development, indicated by the Human Development Index (HDI), is closely interconnected with public health. But the manner in which social development and medical advances influenced liver cancer patients in the past decade is still unknown.
AIM
To investigate the influence of HDI on clinical outcomes for patients with existing liver cancer from 2008 to 2018.
METHODS
The HDI values were obtained from the United Nations Development Programme, the age-standardized incidence and mortality rates of liver cancer were obtained from the GLOBOCAN database to calculate the mortality-to-incidence ratio, and the estimated 5-year net survival of patients with liver cancer was provided by the CONCORD-3 program. We then explored the association of mortality-to-incidence ratio and survival with HDI, with a focus on geographic variability across countries as well as temporal heterogeneity over the past decade.
RESULTS
From 2008 to 2018, the epidemiology of liver cancer had changed across countries. Liver cancer mortality-to-incidence ratios were negatively correlated and showed good fit with a modified “dose-to-inhibition response” pattern with HDI (r = -0.548, P < 0.0001 for 2018; r = -0.617, P < 0.0001 for 2008). Cancer survival was positively associated with HDI (r = 0.408, P < 0.01) and negatively associated with mortality-to-incidence ratio (r = -0.346, P < 0.05), solidly confirming the interrelation among liver cancer outcome indicators and socioeconomic factors. Notably, in the past decade, the HDI values in most countries have increased alongside a decreasing tendency of liver cancer mortality-to-incidence ratios (P < 0.0001), and survival outcomes have simultaneously improved (P < 0.001), with significant disparities across countries.
CONCLUSION
Socioeconomic factors have a significant influence on cancer outcomes. HDI values have increased along with improved cancer outcomes, with significant disparities among countries.
Keywords: Liver cancer, Human Development Index, Incidence, Mortality, Survival
Core tip: This study identified the interrelation of area-specific development and liver cancer outcomes. We verified that the Human Development Index (HDI) correlated negatively with mortality-to-incidence ratio and positively with survival rates of liver cancer in a “dose-to-inhibition response” pattern. Analyzing epidemiological data of liver cancer from 2008 to 2018, it was found that HDI in most countries has increased, and the liver cancer outcomes have improved. Our findings provide strong evidence of healthcare disparities related to socioeconomic factors, and we provide a substantial summary of the development of liver cancer health care in the last decade.
INTRODUCTION
Liver cancer is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide, which has drawn widespread attention because of its high complexity, heterogeneity, mortality, and disability; it is therefore regarded as the devil of cancer[1,2]. Apart from the traditional approaches to treatment, such as surgical resection, transarterial chemoembolization, and targeted agents, treatment strategies against liver cancer have developed rapidly in recent years. However, given its high malignant potential, frequent drug resistance, and systemic side effects, liver cancer still lacks an ideal radical therapeutic regimen. The prognosis of liver cancer lags far behind that of other types of carcinoma, such as mutation-positive lung cancer and breast cancer[3-5].
Socioeconomic development is closely interconnected with public health[6]. The rapid development of socioeconomic condition and cancer health care would absolutely influence the clinical outcomes of liver cancer, but neither the manner in which social development and medical advances have benefited liver cancer patients nor the degree of the impact through the past decade has been clarified yet. The Human Development Index (HDI) is a composite index focusing on three basic dimensions of socioeconomic development: Life expectancy, years of schooling, and gross national income per capita[7]. The liver cancer outcomes such as incidence and mortality could be influenced by a series of risk factors such as hepatitis virus infection (hepatitis B virus, hepatitis C virus, or hepatitis D virus) and its vaccine control, aflatoxin exposure, as well as alcohol abuse. However, the clinical outcomes of existing liver cancer patients, interpreted as the mortality-to-incidence ratio (MIR) or 5-year net survival, might have a strong relationship with socioeconomic factors. Multiple studies have demonstrated that global cancer disparities are related to socioeconomic status according to the HDI[8-10].
Several databases have provided reliable estimates of cancer outcomes. The GLOBOCAN 2018 database contains high-quality registry data of cancer incidence and mortality at the national level[1]. The CONCORD-3 program, with the largest and most up-to-date cancer survival database, established cumulative surveillance for cancer survival for 37.5 million patients during the 2000-2014 period[11]. These databases provide a fundamental basis for studies of liver cancer outcomes. In this study, we aimed to determine the influence of HDI values on liver cancer outcomes, including MIR and survival, with a focus on the global transition of HDI values and liver cancer outcomes from 2008 to 2018.
MATERIALS AND METHODS
Data sources
HDI data for United Nations members in the 2008-2018 period were available in the United Nations Development Programme (UNDP) database (http://hdr.undp.org/ en/statistics). The UNDP divided countries into four subgroups according to HDI levels[7].
National incidence and mortality estimates for liver cancer in 186 countries in 2018 were originally obtained from the GLOBOCAN 2018 database (http://gco.iarc.fr)[12] and were maintained by the International Agency for Research on Cancer, and data of 184 countries in 2008 were originally collected from GLOBOCAN 2008[8]. Estimates for both rates were calculated according to the world standard population, presented as age-standardized rates per 100000 person-years[1,13].
The estimated 5-year net survival for patients with liver cancer in the 2000-2014 period was collected from the CONCORD-3 report[11]. The net survival represents the cumulative probability of surviving for up to a given length of time since diagnosis (e.g., 5 years) after corrections are made for other causes of death. The survival estimates were age-standardized with the International Cancer Survival Standard weights[11].
Statistical analysis
Cancer MIRs were calculated from the obtained incidence and mortality rates. Extreme values (0, 1, or > 1) were considered abnormal and were excluded from the analysis. Then, MIR values were analyzed with their corresponding HDIs in 2008 and 2018 via correlation analysis and nonlinear regression. Correlation was established with a significant P-value in the nonparametric Spearman correlation test. Nonlinear regression was based on a modified “dose-to-response” model using the formula:
MIR = 1/[1 + 10(HDI50-HDI) × Slope]
Where HDI50 refers to the half-maximal controlled HDI (equivalent to the HDI value at half-maximal MIR) and slope is a parameter indicating the steepness of the fitted curve[8].
MIRs within the four HDI groups were compared via one-way ANOVA followed by Tukey-Kramer post hoc tests. The correlation analysis was also applied to the HDI-to-survival and survival-to-MIR patterns. The extremely unreliable net survival values (e.g., 0% of Malta and 40.0% of Jordan in 2018; 27.1% of South Africa, 39.0% of Costa Rica, and 71.5% of Jordan in 2008) were excluded. Comparisons of the age-standardized incidence, mortality, calculated MIR, and survival between 2008 and 2018 were based on weighted Chi-square test. A P-value less than 0.05 was considered statistically significant. Statistical analysis and plotting were performed using Prism 7 (GraphPad, San Diego, CA, United States) and SPSS 25 (IBM Corporation, Armonk, New York, NY, United States).
Geographical maps showing the gradient distribution of HDI values, incidence, mortality, calculated MIR, and survival were edited using TileMill (a GitHub project maintained by MapBox, Washington, DC, United States), with map data sources from the Natural Earth database rendered by the Mapnik Library (https://mapnik.org/).
RESULTS
Global distribution of HDI values and liver cancer epidemiology
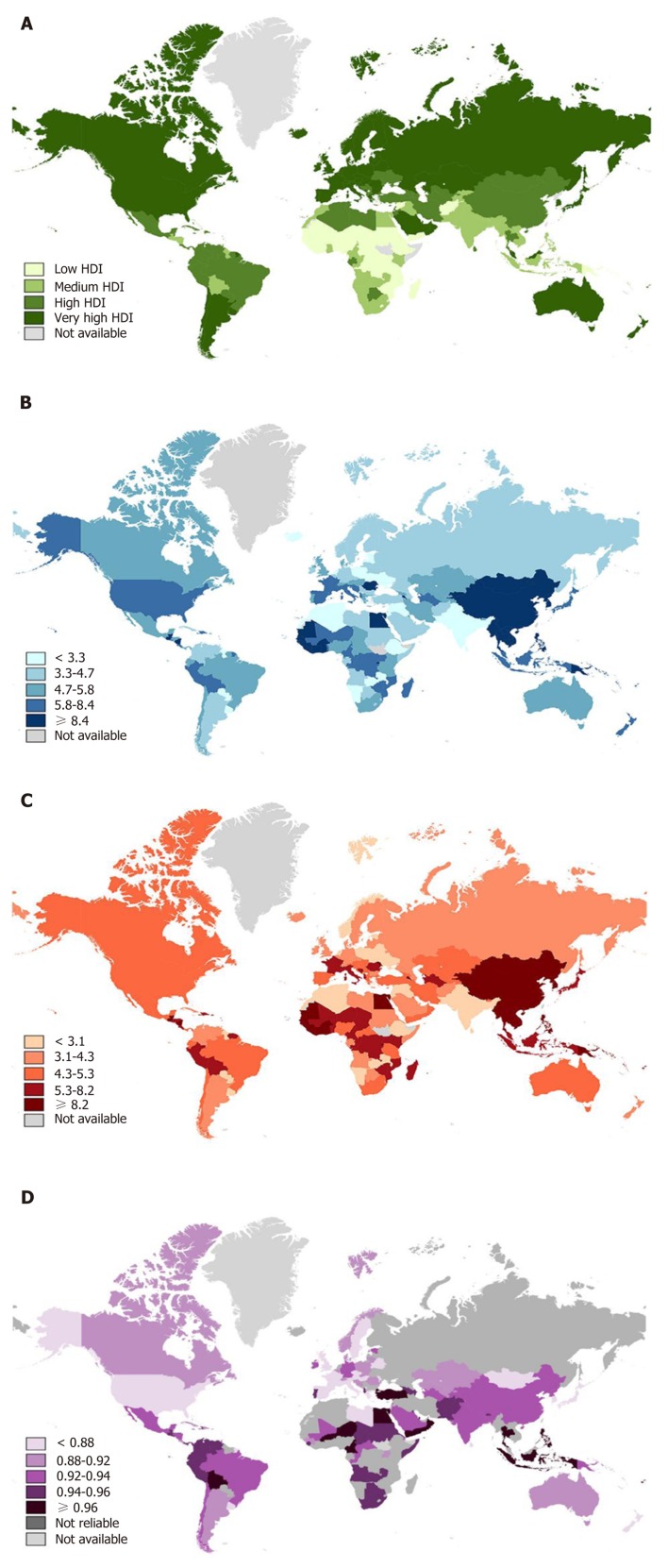
In the analysis, we included 174 countries with data available in both the 2018 GLOBOCAN database (186 countries) and HDI values (189 countries). Countries were classified into groups according to the four HDI tiers (very high, high, medium, and low) reported by the UNDP (Figure 1A). The distributions of national liver cancer incidence and mortality, reported by GOLOBOCAN 2018, were portrayed in the form of world maps (Figure 1B and C). In 2018, liver cancer caused annual age-standardized incidence and mortality rates of, respectively, 9.3 and 8.5 per 100000 population[1]. Overall, the incidence rates of liver cancer were 2-fold greater in developed countries[1]. However, unlike in other sites, the highest rates were observed mainly in lower-HDI settings, especially in Africa and Southeast Asia (Figure 1B). The top 10 countries with the highest incidence of liver cancer were Mongolia, Egypt, Gambia, Vietnam, Lao PDR, Cambodia, Guinea, Thailand, China, and Korea. Notably, the incidence in Mongolia far exceeded that of other countries, with an estimated rate of 4-fold those in China and Korea.
Figure 1.
Worldwide distribution of Human Development Index and liver cancer burden in 2018. A total of 174 countries were classified into four tiers according to different levels of the following: A: Human Development Index (green); B: Age-standardized incidence (blue); C: Age-standardized mortality (red) rates per 100000 population, indicated in a gradient color scale; D: Calculated mortality-to-incidence ratio results, indicated in purple-gradient colors. Countries with data unavailable (light gray) or unreliable (dark gray) are denoted. HDI: Human Development Index.
Sixty-four countries had data available on the incidence, mortality, and MIR in both 2008 and 2018 for time-dependent comparisons. The incidence rates have declined substantially in the majority of countries with a high liver cancer burden[1,14,15]. The top 10 countries with the greatest decrease in incidence were Thailand, Honduras, China, Korea, South Africa, Japan, Guinea, Greece, and Qatar. Interestingly, the incidence increased in many high-HDI countries considered to be at low risk for liver cancer[1,15,16], such as Portugal, the United States, New Zealand, Australia, Singapore, the United Kingdom, Turkey, and France (Table 1). Age-standardized mortality rates showed similar distributions and temporal transitions to incidence rates (Figure 1C, Table 1). Due to disparities in the epidemiological changes in liver cancer, com-parisons of the global incidence or mortality between 2008 and 2018 did not show any evident variance (Supplementary Figure S1A and S1B).
Table 1.
Comparisons of Human Development Index and age-standardized incidence rates, mortality rates, and mortality-to-incidence values for liver cancer between 2008 and 2018
|
2008 |
2018 |
Transitions in a decade |
||||||||||
| Country | HDI | ASI | ASM | MIR | HDI | ASI | ASM | MIR | ΔHDI | ΔASI | ΔASM | ΔMIR |
| Argentina | 0.786 | 3.4 | 3.2 | 0.958 | 0.825 | 3.6 | 3.2 | 0.889 | 0.039 | 0.24 | -0.02 | -0.069 |
| Australia | 0.924 | 3.5 | 2.7 | 0.764 | 0.939 | 5.7 | 4.8 | 0.842 | 0.015 | 2.23 | 2.15 | 0.078 |
| Austria | 0.876 | 5.1 | 4.4 | 0.856 | 0.908 | 5.4 | 4.3 | 0.796 | 0.032 | 0.32 | -0.05 | -0.060 |
| Bahrain | 0.806 | 4.7 | 4.7 | 0.994 | 0.846 | 3.4 | 3.3 | 0.971 | 0.040 | -1.29 | -1.36 | -0.023 |
| Bangladesh | 0.484 | 3.8 | 3.8 | 0.992 | 0.608 | 2.2 | 2 | 0.909 | 0.124 | -1.6 | -1.77 | -0.083 |
| Botswana | 0.624 | 2.8 | 2.7 | 0.964 | 0.717 | 3.9 | 3.8 | 0.974 | 0.093 | 1.12 | 1.12 | 0.010 |
| Brazil | 0.705 | 4.9 | 4.8 | 0.973 | 0.759 | 4.7 | 4.4 | 0.936 | 0.054 | -0.19 | -0.36 | -0.037 |
| Canada | 0.903 | 3.3 | 2.9 | 0.870 | 0.926 | 5.3 | 4.5 | 0.849 | 0.023 | 1.98 | 1.61 | -0.021 |
| Chile | 0.796 | 4.5 | 4.4 | 0.962 | 0.843 | 5.4 | 4.9 | 0.907 | 0.047 | 0.88 | 0.55 | -0.055 |
| China | 0.665 | 25.7 | 23.7 | 0.922 | 0.752 | 18.3 | 17.1 | 0.934 | 0.087 | -7.37 | -6.58 | 0.012 |
| Costa Rica | 0.737 | 5.4 | 5.2 | 0.972 | 0.794 | 6.3 | 5.6 | 0.889 | 0.057 | 0.92 | 0.37 | -0.083 |
| Cuba | 0.767 | 4.2 | 4.1 | 0.969 | 0.777 | 3.8 | 3.3 | 0.868 | 0.010 | -0.38 | -0.75 | -0.100 |
| Czech Republic | 0.864 | 4.3 | 3.9 | 0.915 | 0.888 | 4.2 | 3.2 | 0.762 | 0.024 | -0.06 | -0.7 | -0.154 |
| Denmark | 0.891 | 3.1 | 2.8 | 0.907 | 0.929 | 4.9 | 4.2 | 0.857 | 0.038 | 1.77 | 1.36 | -0.050 |
| Dominican Republic | 0.677 | 8.7 | 8.2 | 0.948 | 0.736 | 6.5 | 5.8 | 0.892 | 0.059 | -2.18 | -2.43 | -0.056 |
| Egypt | 0.633 | 9.3 | 9.1 | 0.981 | 0.696 | 32.2 | 31.8 | 0.988 | 0.063 | 22.95 | 22.73 | 0.007 |
| El Salvador | 0.668 | 5.2 | 5.2 | 0.992 | 0.674 | 6.7 | 6.5 | 0.970 | 0.006 | 1.47 | 1.31 | -0.022 |
| Fiji | 0.684 | 6.9 | 6.7 | 0.965 | 0.741 | 8.3 | 8.2 | 0.988 | 0.057 | 1.4 | 1.54 | 0.023 |
| France (metropolitan) | 0.879 | 6.0 | 5.8 | 0.962 | 0.901 | 7.8 | 6.3 | 0.808 | 0.022 | 1.81 | 0.54 | -0.154 |
| Georgia | 0.722 | 5.8 | 5.5 | 0.958 | 0.780 | 5.4 | 5.2 | 0.963 | 0.058 | -0.37 | -0.33 | 0.005 |
| Germany | 0.902 | 4.0 | 3.4 | 0.831 | 0.936 | 4.2 | 3.8 | 0.905 | 0.034 | 0.17 | 0.45 | 0.073 |
| Greece | 0.862 | 8.4 | 5.7 | 0.684 | 0.870 | 5.7 | 4.5 | 0.789 | 0.008 | -2.65 | -1.21 | 0.106 |
| Guatemala | 0.568 | 15.7 | 15.6 | 0.991 | 0.650 | 14.9 | 14.5 | 0.973 | 0.082 | -0.84 | -1.1 | -0.018 |
| Guinea | 0.34 | 25.3 | 25.1 | 0.992 | 0.459 | 21.8 | 19.5 | 0.894 | 0.119 | -3.49 | -5.58 | -0.097 |
| Haiti | 0.445 | 7.1 | 7.0 | 0.976 | 0.498 | 8.1 | 7.8 | 0.963 | 0.053 | 0.98 | 0.85 | -0.013 |
| Honduras | 0.619 | 14.3 | 14.2 | 0.994 | 0.617 | 5.7 | 5.4 | 0.947 | -0.002 | -8.6 | -8.81 | -0.046 |
| India | 0.527 | 2.2 | 2.0 | 0.927 | 0.640 | 2.2 | 2 | 0.909 | 0.113 | 0.01 | -0.03 | -0.018 |
| Indonesia | 0.598 | 6.7 | 6.6 | 0.976 | 0.694 | 7.6 | 7.5 | 0.987 | 0.096 | 0.89 | 0.95 | 0.011 |
| Italy | 0.871 | 8.6 | 6.6 | 0.772 | 0.880 | 7.9 | 5.7 | 0.722 | 0.009 | -0.69 | -0.93 | -0.050 |
| Japan | 0.896 | 11.2 | 9.2 | 0.815 | 0.909 | 7.6 | 5.4 | 0.711 | 0.013 | -3.64 | -3.76 | -0.104 |
| Kazakhstan | 0.729 | 7.3 | 7.3 | 0.999 | 0.800 | 5.6 | 5 | 0.893 | 0.071 | -1.73 | -2.32 | -0.106 |
| Korea, Republic of | 0.886 | 23.5 | 17.0 | 0.726 | 0.903 | 17.3 | 11.8 | 0.682 | 0.017 | -6.17 | -5.23 | -0.044 |
| Kyrgyzstan | 0.611 | 8.2 | 8.2 | 0.993 | 0.672 | 9.5 | 8.7 | 0.916 | 0.061 | 1.27 | 0.53 | -0.077 |
| Libya | 0.759 | 3.4 | 3.3 | 0.976 | 0.706 | 4.1 | 3.3 | 0.805 | -0.053 | 0.75 | 0.0 | -0.171 |
| Lithuania | 0.806 | 2.5 | 2.4 | 0.955 | 0.858 | 3.8 | 3.3 | 0.868 | 0.052 | 1.3 | 0.94 | -0.087 |
| Luxembourg | 0.867 | 6.6 | 5.8 | 0.875 | 0.904 | 6.5 | 4.4 | 0.677 | 0.037 | -0.1 | -1.4 | -0.198 |
| Mexico | 0.761 | 6.3 | 6.2 | 0.998 | 0.774 | 5.4 | 5.1 | 0.944 | 0.013 | -0.85 | -1.14 | -0.054 |
| Mongolia | 0.641 | 94.4 | 79.9 | 0.847 | 0.741 | 93.7 | 75.4 | 0.805 | 0.100 | -0.7 | -4.51 | -0.042 |
| Nepal | 0.443 | 1.4 | 1.4 | 0.964 | 0.574 | 1.1 | 1 | 0.909 | 0.131 | -0.3 | -0.35 | -0.055 |
| New Zealand | 0.904 | 3.5 | 2.6 | 0.733 | 0.917 | 5.8 | 3.9 | 0.672 | 0.013 | 2.32 | 1.35 | -0.060 |
| Nicaragua | 0.583 | 8.9 | 8.9 | 0.994 | 0.658 | 10.5 | 9.9 | 0.943 | 0.075 | 1.58 | 1.03 | -0.052 |
| Norway | 0.941 | 1.8 | 1.3 | 0.714 | 0.953 | 3.4 | 2.9 | 0.853 | 0.012 | 1.58 | 1.6 | 0.139 |
| Pakistan | 0.495 | 2.5 | 2.5 | 0.988 | 0.562 | 3.1 | 3 | 0.968 | 0.067 | 0.6 | 0.53 | -0.020 |
| Panama | 0.758 | 4.0 | 4.0 | 0.988 | 0.789 | 4.8 | 4.6 | 0.958 | 0.031 | 0.76 | 0.61 | -0.029 |
| Peru | 0.712 | 7.2 | 7.2 | 0.990 | 0.750 | 6.6 | 6.3 | 0.955 | 0.038 | -0.63 | -0.86 | -0.036 |
| Philippines | 0.635 | 10.6 | 10.0 | 0.940 | 0.699 | 11.5 | 11.4 | 0.991 | 0.064 | 0.91 | 1.45 | 0.052 |
| Portugal | 0.802 | 2.2 | 2.1 | 0.955 | 0.847 | 5.4 | 5.2 | 0.963 | 0.045 | 3.2 | 3.1 | 0.008 |
| Qatar | 0.825 | 6.5 | 6.5 | 0.994 | 0.856 | 4.1 | 4 | 0.976 | 0.031 | -2.42 | -2.48 | -0.018 |
| Saudi Arabia | 0.76 | 3.6 | 3.6 | 0.975 | 0.853 | 4.5 | 4.2 | 0.933 | 0.093 | 0.86 | 0.65 | -0.042 |
| Singapore | 0.855 | 10.2 | 9.0 | 0.890 | 0.932 | 12.3 | 11.4 | 0.927 | 0.077 | 2.15 | 2.37 | 0.037 |
| Slovenia | 0.875 | 4.7 | 4.6 | 0.972 | 0.896 | 5.8 | 5 | 0.862 | 0.021 | 1.08 | 0.41 | -0.110 |
| South African Republic | 0.608 | 9.5 | 9.4 | 0.986 | 0.699 | 5 | 4.8 | 0.960 | 0.091 | -4.54 | -4.61 | -0.026 |
| Spain | 0.871 | 5.8 | 4.6 | 0.797 | 0.891 | 6.5 | 4.7 | 0.723 | 0.020 | 0.68 | 0.06 | -0.074 |
| Sri Lanka | 0.676 | 1.6 | 1.5 | 0.956 | 0.770 | 2.7 | 2.2 | 0.815 | 0.094 | 1.12 | 0.69 | -0.141 |
| Sudan | 0.399 | 3.9 | 3.9 | 0.992 | 0.502 | 3.8 | 3.7 | 0.974 | 0.103 | -0.12 | -0.19 | -0.019 |
| Switzerland | 0.892 | 4.9 | 3.8 | 0.784 | 0.944 | 4.8 | 4.1 | 0.854 | 0.052 | -0.05 | 0.3 | 0.071 |
| Tajikistan | 0.597 | 4.9 | 4.9 | 0.992 | 0.650 | 4.7 | 4.4 | 0.936 | 0.053 | -0.22 | -0.48 | -0.056 |
| Thailand | 0.672 | 29.7 | 25.4 | 0.856 | 0.755 | 21 | 20.9 | 0.995 | 0.083 | -8.67 | -4.51 | 0.139 |
| Turkey | 0.691 | 2.5 | 2.4 | 0.968 | 0.791 | 4.5 | 4.4 | 0.978 | 0.100 | 2.01 | 1.99 | 0.010 |
| Turkmenistan | 0.671 | 7.4 | 7.3 | 0.980 | 0.706 | 6 | 5.6 | 0.933 | 0.035 | -1.43 | -1.68 | -0.046 |
| United Kingdom | 0.86 | 3.0 | 2.6 | 0.882 | 0.922 | 5.1 | 4 | 0.784 | 0.062 | 2.14 | 1.39 | -0.097 |
| United States of America | 0.907 | 4.5 | 3.6 | 0.799 | 0.924 | 6.8 | 4.9 | 0.721 | 0.017 | 2.33 | 1.33 | -0.078 |
| Uzbekistan | 0.624 | 4.8 | 4.7 | 0.992 | 0.710 | 5.6 | 4.7 | 0.839 | 0.086 | 0.82 | -0.04 | -0.152 |
| Venezuela | 0.73 | 3.7 | 3.7 | 0.992 | 0.761 | 3.6 | 3.5 | 0.972 | 0.031 | -0.11 | -0.18 | -0.020 |
A total of 64 countries with age-standardized incidence rate, age-standardized mortality rate, and age-standardized mortality-to-incidence ratio available in both years. HDI: Human Development Index; ASI: Age-standardized incidence rate; ASM: Age-standardized mortality rate; MIR: Age-standardized mortality-to-incidence ratio.
Association between national HDI values and liver cancer MIRs
Similar to results in 2008, the liver cancer MIRs varied across countries in 2018. The global MIR in 2018 was 0.914. The available MIRs ranged from 0.490 (Samoa) to 0.995 (Thailand) after the exclusion of extreme values in 2018 (62 countries were out of limit: 0, 1, or > 1) (Figure 1D). The lowest MIRs were achieved mainly in highly developed regions, such as New Zealand, Luxembourg, Korea, Japan, and the United States (Table 1).
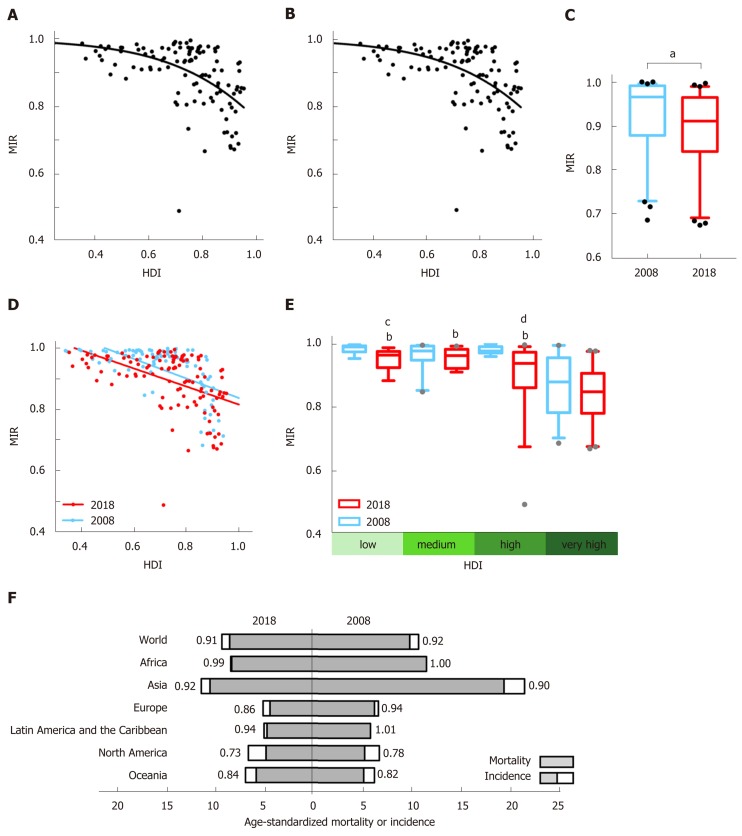
As the level of national HDI increased, the corresponding liver cancer MIR was relatively lower. The country-specific MIR of liver cancer was negatively correlated with national HDI in both 2018 and 2008 (r = -0.548, P < 0.0001 for 2018; r = -0.617, P < 0.0001 for 2008). The nonlinear regression analysis verified a “dose-to-response” inhibitory effect between HDI values and MIRs (Figure 2A and B).
Figure 2.
Correlation between Human Development Index and mortality-to-incidence ratio and its transition from 2008 to 2018. The correlation of liver cancer mortality-to-incidence ratios (MIRs) with national Human Development Indexes (HDIs) with the best-fit lines by modified nonlinear regression (“dose-to-response” model) in A: 2018 (slope = -1.868, HDI50 = 1.27, R2 = 0.261); B: 2008 (slope = -4.989, HDI50 = 1.03, R2 = 0.592); C: Significant reduction in overall MIRs in 64 overlapping countries from 2008 (orange) to 2018 (red). aP < 0.0001, Weighted Chi-square test; D: Transition of the correlation patterns of liver cancer MIR with national HDI from 2008 (orange, r = -0.617, P < 0.0001) to 2018 (red, r = -0.548, P < 0.0001), showing a declining tendency of MIRs within the decade; E: MIRs of liver cancer in the four HDI groups, with significant differences among the very high, high, medium, and low groups (indicated in green-gradient colors) and a decreasing tendency in each specific group between 2008 (orange) and 2018 (red). bP < 0.001, vs very-high-HDI countries in 2018, one-way ANOVA followed by Tukey-Kramer post hoc test. The statistical significance among countries in 2008 is not indicated. cP < 0.001, 2008 vs 2018 in the low-HDI group; dP < 0.0001, 2008 vs 2018 in the high-HDI group, paired t-test; F: Regional age-standardized mortality (gray) and incidence (white plus gray) rates per 100000 individuals for liver cancer in 2018 (on the left) and 2008 (on the right), with MIR values denoted. HDI: Human Development Index; MIR: Mortality-to-incidence ratio.
We then classified the included countries into four-tier HDI subgroups, and liver cancer MIRs differed among the groups (P < 0.0001, one-way ANOVA). For the 2018 data, the mean MIR in very-high-HDI countries (0.835) was significantly lower than that in high- (0.906), medium- (0.954), or low- (0.949) HDI countries (P < 0.001, Tukey's post hoc test; Figure 2E). For the 2008 data, similar results were obtained (P < 0.001, very-high-HDI vs other subgroups, Tukey's test; Figure 2E), indicating persistent disparities associated with HDI levels.
Transition of MIR from 2008 to 2018
From 2008 to 2018, the HDIs for majority countries have increased more than 1%. The global MIR of liver cancer declined significantly in the last decade (Figure 2C). Among 64 countries with usable data in both years, the MIR increased in 16 countries and decreased in the other 48 countries (Table 1). Liver cancer MIRs in five countries (Luxembourg, Libya, France, Czech Republic, and Uzbekistan), all of which were in high- or very-high-HDI areas, decreased more than 15% (Table 1). In contrast, no countries showed an increase of more than 15%. To illustrate the MIR transition, national HDIs and MIRs in 2018 and 2008 are plotted together, showing similar distributions. Notably, there is an obvious difference in that the linear regression line has shifted to the lower-left direction from 2008 to 2018, implying that the integral HDI values increased along with the decline in MIRs (Figure 2D). Furthermore, within each specific HDI level, the MIRs in 2018 had an obvious decreasing tendency in comparison to the 2008 data, though not significant for all HDI categories (P = 0.007 for the low-, P = 0.09 for the medium-, P < 0.001 for the high-, and P = 0.18 for the very-high-HDI group, paired t-test; Figure 2E).
Differences and transitions in regional cancer epidemiology
The incidence and mortality rates, as well as the MIRs of liver cancer, also varied among different continents. Asia was continuously top-ranked in susceptibility to liver cancer and mortality rates. Although the incidence and mortality rates in Asia nearly halved in 10 years (incidence: 11.4 in 2018, 21.6 in 2008; mortality: 10.5 in 2018, 19.5 in 2008), the continental MIRs had no obvious fluctuation. Latin America and the Caribbean had the lowest liver cancer burden (incidence: 5.0 in 2018, 5.8 in 2008; mortality: 4.7 in 2018, 5.9 in 2008). Africa had the highest MIRs (0.99 in 2018, 1.01 in 2008) of liver cancer worldwide. Oceania and North America had MIR results significantly lower than those in other regions (0.84 for Oceania and 0.73 for North America). The change in MIR over 10 years was most evident in Europe, which had an 8% reduction (MIR = 0.86 in 2018; MIR = 0.94 in 2008). Among the top five countries with the greatest MIR declines in the world (Table 1), three (Luxemburg, France, and Czech Republic) were located in Europe (Figure 2F).
The transition of 5-year survival for patients with liver cancer
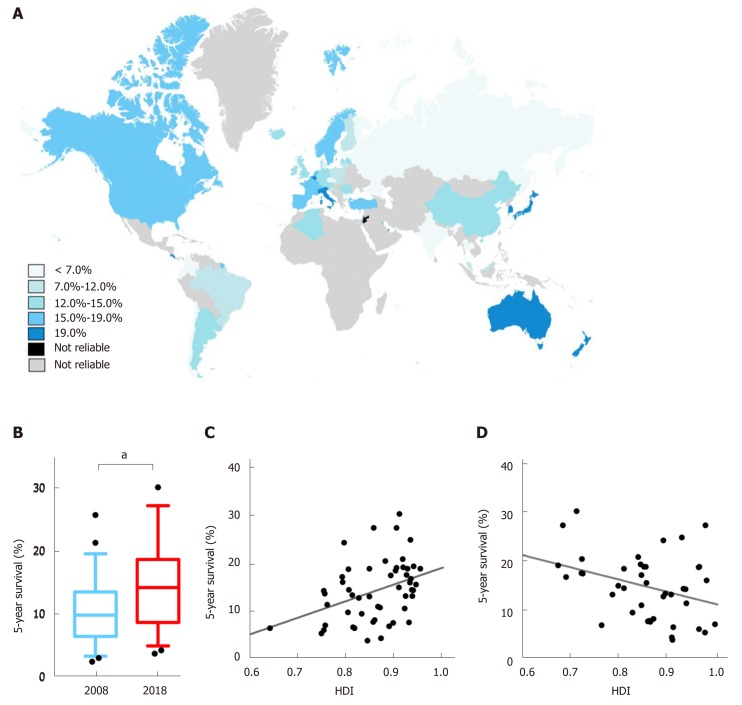
The 5-year net survivals for those with liver cancer were available from 61 countries, with 52 countries remaining after the exclusion of extreme values and the establishment of matches with available HDI values (Figure 3A). For patients diagnosed with liver cancer during 2010-2014, the best survival occurred in Southeast Asia. The top three countries with the best survival were Japan (30.1%), Korea (27.2%), and Singapore (27.2%). In contrast, poor survival was observed in Chile (3.7%) and Estonia (4.2%). Forty-eight countries had data available both in 2008 and 2018 (Table 2). The survival rates generally increased (P < 0.001) (Figure 3B). Survival outcomes increased by over 10% during the 10-year period in five countries, namely, Qatar (+20.1%), Korea (+11.9%), Singapore (+11.5%), Norway (+10.8%), and Iceland (+10.4%) (Table 2).
Figure 3.
Distributions of liver cancer survival and its correlation with Human Development Index values and mortality-to-incidence ratio. A: Distribution of regional estimated 5-year net survival for patients with liver cancer in 2018, indicated in blue-gradient colors; B: Comparisons of worldwide liver cancer survival between 2008 and 2018. aP < 0.001, 2018 (sky blue) vs 2008 (dark blue); C: A positive correlation pattern between the survival of the patients diagnosed in 2010-2014 and the Human Development Index value in 2018 (r = 0.408, P = 0.0027); D: Correlation between national mortality-to-incidence ratio and liver cancer survival in 2018 (r = -0.346, P = 0.0278). HDI: Human Development Index; MIR: Mortality-to-incidence ratio.
Table 2.
Distribution of Human Development Index and 5-year net survival values for patients with liver cancer from 2008 to 2018
|
2008 |
2018 |
Transitions in a decade |
|||||
| Country | HDI | Survival | HDI | Survival | ΔHDI | Δsurvival | |
| Algeria | 0.686 | 6.2 | 0.754 | 13.5 | 0.068 | 7.3 | |
| Argentina | 0.786 | 14.1 | 0.825 | 12.6 | 0.039 | -1.5 | |
| Australia | 0.924 | 14.2 | 0.939 | 19.2 | 0.015 | 5 | |
| Austria | 0.876 | 11.2 | 0.908 | 14.8 | 0.032 | 3.6 | |
| Belgium | 0.882 | 21.3 | 0.916 | 20.7 | 0.034 | -0.6 | |
| Brazil | 0.705 | 15.4 | 0.759 | 11.2 | 0.054 | -4.2 | |
| Bulgaria | 0.765 | 4 | 0.813 | 6.5 | 0.048 | 2.5 | |
| Canada | 0.903 | 17.4 | 0.926 | 18.7 | 0.023 | 1.3 | |
| Chile | 0.796 | 3.6 | 0.843 | 3.7 | 0.047 | 0.1 | |
| China | 0.665 | 11.7 | 0.752 | 14.1 | 0.087 | 2.4 | |
| Colombia | 0.697 | 4.8 | 0.747 | 5.2 | 0.050 | 0.4 | |
| Croatia | 0.795 | 9.2 | 0.831 | 9.3 | 0.036 | 0.1 | |
| Cyprus | 0.827 | 8.4 | 0.869 | 10.6 | 0.042 | 2.2 | |
| Czech Republic | 0.864 | 3 | 0.888 | 6.7 | 0.024 | 3.7 | |
| Denmark | 0.891 | 4.4 | 0.929 | 7.5 | 0.038 | 3.1 | |
| Ecuador | 0.714 | 8.1 | 0.752 | 5.9 | 0.038 | -2.2 | |
| Estonia | 0.832 | 5.5 | 0.871 | 4.2 | 0.039 | -1.3 | |
| Finland | 0.883 | 7.1 | 0.920 | 10.4 | 0.037 | 3.3 | |
| France (metropolitan) | 0.879 | 14 | 0.901 | 18.3 | 0.022 | 4.3 | |
| Germany | 0.902 | 12.5 | 0.936 | 13 | 0.034 | 0.5 | |
| Iceland | 0.895 | 3.9 | 0.935 | 14.3 | 0.040 | 10.4 | |
| India | 0.527 | 2.4 | 0.640 | 6.3 | 0.113 | 3.9 | |
| Ireland | 0.909 | 11.6 | 0.938 | 14.2 | 0.029 | 2.6 | |
| Israel | 0.882 | 15.1 | 0.903 | 18.9 | 0.021 | 3.8 | |
| Italy | 0.871 | 15.9 | 0.880 | 20.3 | 0.009 | 4.4 | |
| Japan | 0.896 | 25.7 | 0.909 | 30.1 | 0.013 | 4.4 | |
| Korea, Republic of | 0.886 | 15.3 | 0.903 | 27.2 | 0.017 | 11.9 | |
| Kuwait | 0.757 | 11.4 | 0.803 | 18.6 | 0.046 | 7.2 | |
| Latvia | 0.803 | 12 | 0.847 | 12.9 | 0.044 | 0.9 | |
| Lithuania | 0.806 | 7.9 | 0.858 | 8 | 0.052 | 0.1 | |
| Malta | 0.829 | 4.2 | 0.802 | 9.6 | -0.027 | 5.4 | |
| New Zealand | 0.904 | 12.4 | 0.917 | 19 | 0.013 | 6.6 | |
| Norway | 0.941 | 7.9 | 0.953 | 18.7 | 0.012 | 10.8 | |
| Poland | 0.804 | 9.5 | 0.865 | 10.8 | 0.061 | 1.3 | |
| Portugal | 0.802 | 13.6 | 0.847 | 18.7 | 0.045 | 5.1 | |
| Qatar | 0.825 | 7.1 | 0.856 | 27.2 | 0.031 | 20.1 | |
| Russian Federation | 0.748 | 7.4 | 0.816 | 6.3 | 0.068 | -1.1 | |
| Singapore | 0.855 | 13.2 | 0.932 | 24.7 | 0.077 | 11.5 | |
| Slovakia | 0.831 | 5.6 | 0.855 | 7.6 | 0.024 | 2 | |
| Slovenia | 0.875 | 3.8 | 0.896 | 7.4 | 0.021 | 3.6 | |
| Spain | 0.871 | 14.4 | 0.891 | 17.3 | 0.020 | 2.9 | |
| Sweden | 0.9 | 7.8 | 0.933 | 16.6 | 0.033 | 8.8 | |
| Switzerland | 0.892 | 12.2 | 0.944 | 15.4 | 0.052 | 3.2 | |
| Thailand | 0.672 | 7.7 | 0.755 | 6.9 | 0.083 | -0.8 | |
| The Netherlands | 0.904 | 10.1 | 0.931 | 15.8 | 0.027 | 5.7 | |
| Turkey | 0.691 | 20.1 | 0.791 | 15.9 | 0.100 | -4.2 | |
| United Kingdom | 0.86 | 7.1 | 0.922 | 13 | 0.062 | 5.9 | |
| United States of America | 0.907 | 12.5 | 0.924 | 17.4 | 0.017 | 4.9 | |
A total of 48 countries with survival rates available in both years. HDI: Human Development Index.
We also investigated the association between survival rates and HDI. There was a positive correlation between the survival, as estimated according to patients diagnosed in 2010-2014, and HDI values in 2018 (r = 0.408, P = 0.0027; Figure 3C). Furthermore, the MIR and survival were also negatively correlated (r = -0.346, P = 0.0278) (Figure 3D).
DISCUSSION
HDI is regarded as the gold standard for the comparison of development, quantified by the composite measures of health, education, and economy[7]. HDI has been demonstrated by multiple studies as an important parameter interconnected with public health[8-10]. Being reported by UNDP every year, HDI is also easy to obtain for measuring and comparing. MIR and 5-year net survival both represent clinical outcomes of existing liver cancer. MIR may serve as an indicator of cancer health care efficacy by indirectly measuring true biological differences in attributes such as screening, diagnostic modality, treatment, and follow-up[8,10,14]. Five-year net survival is a more direct indicator of the effectiveness of cancer treatment and has been labeled with more importance, since the cancer patients who survive for a considerable time span can, in a way, be considered cured[11,17]. Our present study showed that the HDI values increased along with the corresponding decline in MIRs and improvement in survival, with significant disparities among countries.
At the very beginning, we took a quick look at the incidence of liver cancer and found that it distributed unequally. An interesting and predictable finding is that liver cancer incidence decreased in high-risk countries but increased in low-risk areas from 2008 to 2018. First, there are distinct predisposing factors in geographically heterogeneous regions. In high-risk areas such as China and Western and Eastern sub-Saharan Africa, the key determinants are chronic hepatitis B infection and aflatoxin exposure, whereas in Japan and Egypt, hepatitis C infection is likely the predominant cause[1,15]. In Mongolia, dual and triple hepatitis infections are highly prevalent, with heavy alcohol consumption also contributing to the incidence[18-22]. Second, the development of preventive strategies against liver cancer contributes to the reduction in incidence in high-risk countries with specific risk factors. The popularization of the hepatitis vaccine has significantly lowered the incidence in countries at high risk for hepatitis-associated liver cancer, which are mostly located in Southeast Asia and sub-Saharan Africa[1,23], and this benefit continues to grow. Recent developments in antivirus medication also effectively prevent the occurrence of a considerable number of liver cancer cases[24]. Approaches against other carcinogenesis-related factors, such as reductions in aflatoxin exposure and the prevention of unsafe transfusions and contaminated needle use, have also been available in high-risk countries, with a few exceptions, such as Egypt[1,15,16]. Third, the cohort effect of high hepatitis C exposure in the 1970s and the rising prevalence of obesity, type 2 diabetes, and nonalcoholic fatty liver disease, contribute to the observed growing incidence in some high-HDI, low-risk countries[25]. A recent study aiming to project future liver cancer epidemiology showed results consistent with our conclusions, predicting incidence increases in Norway, the United States, Canada, Switzerland, and Germany and significant decreases in China, Japan, Singapore, and parts of Europe until 2030[26]. Currently, available approaches include continuous control of aflatoxin exposure, complete rollout of the hepatitis B vaccination, blood supply protection, safe medical injection practices, discouragement of heavy alcohol intake, and new primary prevention strategies for obesity.
A universally high fatality leads to smaller differences in mortality related to liver cancer between developed and developing economies. To clarify the socioeconomic-associated treatment efficacies and clinical outcomes, we further examined forms of cancer MIR and survival. Based on our previous study, we demonstrated an inverse correlation of MIR[8] and a positive association between survival and HDI. The correlation is primarily caused by national inequalities in health care, which has been regarded as a major contributing factor to treatment effectiveness. A lethal disease such as liver cancer requires more advanced health care, with higher-quality care more readily available in highly developed areas. Other factors, such as economic status, diet and tobacco use, and awareness of one’s own health and disease prevention, all of which are influenced by the HDI, also contribute to the country-specific cancer MIR and survival. Taken together, patients in countries with higher HDIs have better cancer outcomes. Certain countries, such as New Zealand, Japan, and Korea, have achieved high survival and low MIR with comprehensive interventional approaches over the last decade[27-29]. Effective approaches include the promotion of aggressive clinical guidelines, the development of sophisticated technology, the continual improvement of socioeconomic status, and an advanced system for practice and communication[27-29].
Analyzing time-dependent trends allows for the identification of successful strategies as well as concerning patterns. Scientific advances have resulted in rapidly growing medical technology and treatment strategies for liver cancer. The rapid development of laparoscopic and robotic surgery, especially the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, United States), has offered a less invasive approach while ensuring oncological remission and expected survival[30]. Combination therapy of the classic targeted agents, such as sorafenib, with transarterial che-moembolization[31], percutaneous radiofrequency, or microwave ablation[32-34] has shown promising therapeutic effects[35]. Meanwhile, novel approaches have also emerged as powerful methods against tumor progression, relapse, and metastasis; these approaches include the following: Nucleotide-based regulation via nanoparticle-mediated drug delivery; various immune checkpoint blockades targeting cytotoxic T-lymphocyte-associated protein 4, programmed cell death protein 1 (PD-1) or its ligand PD-L1; and even cancer vaccines[36-39]. Comprehensive integration has promoted heightened awareness, preventive measures, earlier detection, and better patient care. However, there is still no radical therapeutic regimen for liver cancer. Heterogeneity in the pathogenesis and biological behavior of liver cancer has led to drawbacks of new systemic agents[40]. Furthermore, advances in immunotherapy and robotic surgery, though promising, are not feasible or affordable for generalized application in settings with limited health care resources[39]. Although issues concerning health care disparities have aroused much public interest in reducing the gaps among populations, several developed countries have even tried to eliminate health inequalities; however, the effect is limited, and lower-HDI countries have not yet been able to close the gap between their cancer outcomes and those of other countries[41-43]. The current availability of essential technology and resources for prevention, early detection, effective treatment, and palliative care is still sparse[9,44,45].
There are some limitations to our study. A few countries with the highest transitions in survival and MIR had contradictory values for these indicators (e.g., Δsurvival = +10.8%, ΔMIR = +13.9% in Norway), indicating the potential for conflicts in the estimated data from the GLOBOCAN and CONCORD programs. First, there was a discrepancy between countries with available MIR data or survival rates. Second, the MIR estimates provide a valuable assessment of cancer outcomes but are not intended to serve as a substitute for continuous approaches to authoritative data recording, such as high-quality population-based cancer registries and vital registration systems[1,15]. Third, the present lack of high-quality local data in many transitioning countries directly influenced the robustness of the included estimates. For example, in survival data from Jordan, linkage with the national death index has been insufficient because only approximately 70% of deaths are certified[11]. Furthermore, as described by CONCORD-3, estimates of liver cancer are flagged as less reliable than those of other cancers because of the exclusion of higher proportions of death-certificate-only registrations[11].
In conclusion, HDI values are significantly correlated with liver cancer outcomes. Even with successful prevention strategy measures, cases of liver cancer are likely to increase over the next several decades owing to population aging and growth. Such disparities in cancer health care should compel us to exert greater effort in improving socioeconomic conditions in less developed countries and territories. Given the dismal prognosis of liver cancer, research on new and available approaches against liver cancer is urgently needed.
ARTICLE HIGHLIGHTS
Research background
Liver cancer is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide. Novel treatment strategies against liver cancer have been developed rapidly recent years. Given its high malignant potential, liver cancer burden is still a serious public health problem over the past decade.
Research motivation
Socioeconomic development is closely interconnected with public health. However, little is known about healthcare disparities in individuals suffering from liver cancer. Furthermore, the manner in which social development and medical advances influenced liver cancer patients in the past decade is waiting to be clarified.
Research objectives
To investigate the influence of national Human Development Index (HDI) on mortality-to-incidence ratio (MIR) and 5-year net survival of patients with existing liver cancer, and transition in liver cancer epidemiology from 2008 to 2018.
Research methods
We explored the association of MIR and survival of liver cancer with corresponding HDI via correlation analysis and nonlinear regression. Specifically, nonlinear regression was based on a modified “dose-to-inhibition response” model. We further investigated cancer geographic variability across continents and countries, among which MIRs within the four-tier HDI groups were compared via one-way ANOVA followed by Tukey-Kramer post hoc tests. Then we focused on temporal heterogeneity over the past decade, and comparisons of the incidence, mortality, calculated MIR, and survival between 2008 and 2018 were based on Weighted Chi-square test. A P-value less than 0.05 was considered statistically significant.
Research results
Liver cancer MIRs were negatively correlated and showed good fit with a modified “dose-to-inhibition response” pattern with HDI (P < 0.0001). Cancer survival was positively associated with HDI (P < 0.01) and negatively associated with MIR (P < 0.05), solidly confirming the interrelation among liver cancer outcome indicators and socioeconomic factors. Also, liver cancer MIRs of 4-tier HDI subgroups differed from each other. Notably, in the past decade, as the levels of national HDI in most countries have increased, the corresponding liver cancer MIR was relatively lower (P < 0.0001) and survival has simultaneously improved (P < 0.001), with long-existing region- or country-specific disparities.
Research conclusions
This study is the first to explore the exact relationship between the epidemiology of liver cancer and socioeconomic development in a long time scale. We showed the association between MIR and survival of liver cancer and area-specific HDI. Moreover, HDI values have increased along with improved liver cancer outcomes over the last decade, with significant disparities among countries. Our findings provide strong evidence of healthcare disparities related to socioeconomic factors, and we provide a substantial summary of the development of liver cancer health care in the last decade.
Research perspectives
The great disparities in cancer health care should compel us to exert greater effort in improving socioeconomic conditions in less developed countries and territories. Even the clinical outcomes of liver cancer have improved in the past decade, it still lacks an ideal radical therapeutic regimen. Given the dismal prognosis of liver cancer, research on new and available approaches against liver cancer is urgently needed in the future.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study did not involve any human or animal subjects. We have conducted this secondary analysis based on existing public data. Our major data resources are (1) GLOBOCAN database 2018 and 2008; (2) HDI: UNDP database; and (3) The CONCORD-3 report. Approval file of Institutional Review Board is not applicable.
Informed consent statement: Informed consent statement is not applicable.
Conflict-of-interest statement: All the authors have no conflict of interest related to the manuscript.
Data sharing statement: No additional data are available.
STROBE statement: The guidelines of the STROBE Statement have been adopted for this manuscript.
Peer-review started: May 6, 2019
First decision: June 16, 2019
Article in press: July 19, 2019
P-Reviewer: Hoyos S, Vradelis S, Yu JS S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Shi-Yi Shao, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Qi-Da Hu, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Meng Wang, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Xin-Yu Zhao, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Wang-Teng Wu, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Jun-Ming Huang, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Ting-Bo Liang, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China; Zhejiang Innovation Center for the Study of Pancreatic Diseases (ICSPD-ZJ), Hangzhou 310003, Zhejiang Province, China. liangtingbo@zju.edu.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hochnadel I, Kossatz-Boehlert U, Jedicke N, Lenzen H, Manns MP, Yevsa T. Cancer vaccines and immunotherapeutic approaches in hepatobiliary and pancreatic cancers. Hum Vaccin Immunother. 2017;13:2931–2952. doi: 10.1080/21645515.2017.1359362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet. 2011;378:1812–1823. doi: 10.1016/S0140-6736(11)61539-0. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr, Wu YL, Paz-Ares L. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 5.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock T. Health, human development and the community ecosystem: three ecological models. Health Promot Int. 1993;8:41–47. [Google Scholar]
- 7.United Nations Development Programme. 2018. Human Development Reports. Human Development Indices and Indicators; p. Statistical Update. 2018. Available from: http://hdr.undp.org/en/2018-update. [Google Scholar]
- 8.Hu QD, Zhang Q, Chen W, Bai XL, Liang TB. Human development index is associated with mortality-to-incidence ratios of gastrointestinal cancers. World J Gastroenterol. 2013;19:5261–5270. doi: 10.3748/wjg.v19.i32.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidler MM, Bray F. Global Cancer Inequalities. Front Oncol. 2018;8:293. doi: 10.3389/fonc.2018.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AR, Prasad SM, Shih YC, Eggener SE. The association of the human development index with global kidney cancer incidence and mortality. J Urol. 2012;187:1978–1983. doi: 10.1016/j.juro.2012.01.121. [DOI] [PubMed] [Google Scholar]
- 11.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. 2018. International Agency for Research on Cancer. Global Cancer Observatory. Available from: http://gco.iarc.fr/ [Google Scholar]
- 13.Schneiderman MA. New York: Springer-Verlag; 1966. Cancer Incidence in Five Continents: A Technical Report. Doll R, Payne P, Waterhouse J, editors; pp. 804–804. [Google Scholar]
- 14.Hébert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA, Puett R, Burch JB, Steck SE, Bolick-Aldrich SW. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115:2539–2552. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thursz M, Fontanet A. HCV transmission in industrialized countries and resource-constrained areas. Nat Rev Gastroenterol Hepatol. 2014;11:28–35. doi: 10.1038/nrgastro.2013.179. [DOI] [PubMed] [Google Scholar]
- 17.Berkson J, Gage RP. Survival Curve for Cancer Patients Following Treatment. J Am Stat Assoc. 1952;47:501–515. [Google Scholar]
- 18.Chimed T, Sandagdorj T, Znaor A, Laversanne M, Tseveen B, Genden P, Bray F. Cancer incidence and cancer control in Mongolia: Results from the National Cancer Registry 2008-12. Int J Cancer. 2017;140:302–309. doi: 10.1002/ijc.30463. [DOI] [PubMed] [Google Scholar]
- 19.Dashtseren B, Bungert A, Bat-Ulzii P, Enkhbat M, Lkhagva-Ochir O, Jargalsaikhan G, Enkhbat A, Oidovsambuu O, Klemen J, Dashdorj N, Dashdorj N, Genden Z, Yagaanbuyant D. Endemic prevalence of hepatitis B and C in Mongolia: A nationwide survey amongst Mongolian adults. J Viral Hepat. 2017;24:759–767. doi: 10.1111/jvh.12697. [DOI] [PubMed] [Google Scholar]
- 20.Tsatsralt Od B. Epidemiology of Viral Hepatitis and Liver Diseases in Mongolia. Euroasian J Hepatogastroenterol. 2015;5:37–39. doi: 10.5005/jp-journals-10018-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Znaor A, Chimed T, Laversanne M, Tudev U, Sanjaajamts E, Sandagdorj T, Bray F. The public health challenge of liver cancer in Mongolia. Lancet Gastroenterol Hepatol. 2018;3:660–662. doi: 10.1016/S2468-1253(18)30243-7. [DOI] [PubMed] [Google Scholar]
- 22.Baatarkhuu O, Uugantsetseg G, Munkh-Orshikh D, Naranzul N, Badamjav S, Tserendagva D, Amarsanaa J, Do Young K. Viral Hepatitis and Liver Diseases in Mongolia. Euroasian J Hepatogastroenterol. 2017;7:68–72. doi: 10.5005/jp-journals-10018-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Geneva: World Health Organization; 2017. Global Health Observatory (GHO) data: Hepatitis B 3rd Dose (HepB3) Immunization Coverage. Available from: http://www.who.int/gho/immunization/hepatitis/en/ [Google Scholar]
- 24.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med. 2016;67:103–117. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- 26.Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600–611. doi: 10.1002/hep.29498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16:465–522. doi: 10.3348/kjr.2015.16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765–770. doi: 10.1159/000439101. [DOI] [PubMed] [Google Scholar]
- 29.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y Liver Cancer Study Group of Japan. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014;87 Suppl 1:7–21. doi: 10.1159/000368141. [DOI] [PubMed] [Google Scholar]
- 30.Levi Sandri GB, de Werra E, Mascianà G, Colasanti M, Santoro R, D'Andrea V, Ettorre GM. Laparoscopic and robotic approach for hepatocellular carcinoma-state of the art. Hepatobiliary Surg Nutr. 2016;5:478–484. doi: 10.21037/hbsn.2016.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin PP, Shao SY, Wu WT, Zhao XY, Huang BF, Fu QH, Que RS, Hu QD. Combination of transarterial chemoembolization and sorafenib improves outcomes of unresectable hepatocellular carcinoma: An updated systematic review and meta-analysis. Jpn J Clin Oncol. 2018;48:1058–1069. doi: 10.1093/jjco/hyy138. [DOI] [PubMed] [Google Scholar]
- 32.Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Chen M, Qian G, Kuang M. Advanced Recurrent Hepatocellular Carcinoma: Treatment with Sorafenib Alone or in Combination with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;287:705–714. doi: 10.1148/radiol.2018171541. [DOI] [PubMed] [Google Scholar]
- 33.Zhu K, Huang J, Lai L, Huang W, Cai M, Zhou J, Guo Y, Chen J. Medium or Large Hepatocellular Carcinoma: Sorafenib Combined with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;288:300–307. doi: 10.1148/radiol.2018172028. [DOI] [PubMed] [Google Scholar]
- 34.Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68:783–797. doi: 10.1016/j.jhep.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Allaire M, Nault JC. Advances in management of hepatocellular carcinoma. Curr Opin Oncol. 2017;29:288–295. doi: 10.1097/CCO.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 36.Flynn MJ, Sayed AA, Sharma R, Siddique A, Pinato DJ. Challenges and Opportunities in the Clinical Development of Immune Checkpoint Inhibitors for Hepatocellular Carcinoma. Hepatology. 2019;69:2258–2270. doi: 10.1002/hep.30337. [DOI] [PubMed] [Google Scholar]
- 37.Kim HY, Park JW. Current immunotherapeutic strategies in hepatocellular carcinoma: Recent advances and future directions. Therap Adv Gastroenterol. 2017;10:805–814. doi: 10.1177/1756283X17722061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 39.Dutta R, Mahato RI. Recent advances in hepatocellular carcinoma therapy. Pharmacol Ther. 2017;173:106–117. doi: 10.1016/j.pharmthera.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 41.Marmot MG, Bell R. Action on health disparities in the United States: Commission on social determinants of health. JAMA. 2009;301:1169–1171. doi: 10.1001/jama.2009.363. [DOI] [PubMed] [Google Scholar]
- 42.Mackenbach JP. Can we reduce health inequalities? An analysis of the English strategy (1997-2010) J Epidemiol Community Health. 2011;65:568–575. doi: 10.1136/jech.2010.128280. [DOI] [PubMed] [Google Scholar]
- 43.Alcorn T. New Zealand's bold strategy for reducing health disparities. Lancet. 2011;378:1689–1690. doi: 10.1016/s0140-6736(11)61726-1. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, Balch C, Brennan MF, Dare A, D'Cruz A, Eggermont AM, Fleming K, Gueye SM, Hagander L, Herrera CA, Holmer H, Ilbawi AM, Jarnheimer A, Ji JF, Kingham TP, Liberman J, Leather AJ, Meara JG, Mukhopadhyay S, Murthy SS, Omar S, Parham GP, Pramesh CS, Riviello R, Rodin D, Santini L, Shrikhande SV, Shrime M, Thomas R, Tsunoda AT, van de Velde C, Veronesi U, Vijaykumar DK, Watters D, Wang S, Wu YL, Zeiton M, Purushotham A. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16:1193–1224. doi: 10.1016/S1470-2045(15)00223-5. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. 2015. Medicines for Treatment of the Following Cancers-Review-EML and EMLc. Available from: http://www.who.int/selection_medicines/committees/expert/20/applications/cancer/en/ [Google Scholar]