Abstract
Background
Survivors of myocardial infarction (MI) are at high risk of new major adverse cardiovascular events (MACE). Coronary flow reserve (CFR) is a strong and independent predictor of MACE. Understanding the prevalence of impaired CFR in this patient group and identifying risk markers for impaired CFR are important steps in the development of personalized and targeted treatment for high-risk individuals with prior MI.
Methods
PROFLOW is a prospective, exploratory, cross-sectional open study. We used information from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry) to identify high-risk patients with a history of type-1 MI. We measured CFR non-invasively in a left anterior descending artery (LAD) using transthoracic Doppler echocardiography. Coronary flow velocity was measured at rest and at maximal flow after induction of hyperemia by intravenous infusion of adenosine (140 μg/kg/min). Independent predictors of CFR were assessed with multiple linear regression.
Results
We included 619 patients. The median age was 69 (IQR 65–73), and 114 (18.4%) were women. Almost one-half of the patients, 285 (46.0%) had the multi-vessel disease, and 147 (23.7%) were incompletely revascularized. The majority were on optimal standard treatment eg ASA (93.1%), statins (90.0%), ACEI/ARB (82.6%) and beta-blockers (80.8%). The majority, 547 (88.4%) had no angina pectoris, and 572 (92.2%) were in NYHA class I. Evaluation of CFR was possible in 611 (98.7%) patients. Mean CFR was 2.74 (±0.79 (mean ± SD)). A substantial number of patients (39.7%) had CFR ≤2.5. In a multiple linear regression model age, dyslipidemia, smoking, hypertension, body mass index, incomplete revascularization, and treatment with angiotensin receptor blockers were independent predictors of CFR.
Conclusion
In this high-risk group of patients with prior MI, the prevalence of impaired CFR was high. Further risk stratification with CFR in addition to traditional cardiovascular risk factors may improve predictive accuracy for future MACE in this patient population.
Keywords: coronary flow reserve, myocardial infarction, secondary prevention, Doppler echocardiography, prognosis
Introduction
Coronary artery disease is the leading cause of death globally. Survivors of myocardial infarction (MI) have up to 30% risk of additional events after the first year and an annual death rate of 5% which is six times that in individuals without coronary heart disease.1,2 Today, we need better methods to identify high-risk individuals as well as more effective treatments to lower the risk of major adverse cardiovascular events (MACE) including death, MI, and stroke in this high-risk population.
It is now recognized that myocardial ischemia, caused by flow-limiting atherosclerosis and microvascular dysfunction, is the main pathophysiological feature driving residual cardiovascular risk in patients with ischemic heart disease.3 Coronary flow reserve can capture both flow-limiting atherosclerotic lesion in the epicardial segments of coronary arteries as well as microvascular dysfunction, and thus may be able to serve as a composite risk marker for residual risk.4
We and others have shown that coronary flow reserve (CFR) is a strong and independent predictor of MACE both in individuals without epicardial coronary artery disease as well as in those with known coronary artery disease.5–8 CFR is the ratio between maximum coronary blood flow and flow at rest. This ratio mirrors the capacity of the coronary circulation to increase flow and thus, oxygen and substrate delivery in response to increased myocardial demand. Coronary flow regulation is mainly controlled at the microcirculatory level, where endothelium has a central regulatory role. However, other factors influence CFR including atherosclerotic narrowing of the vessel lumen, rheological properties of blood, inflammation, and coagulation.9
In this cross-sectional study, recognizing the potential of CFR as a powerful prognostic marker, our primary objective was to investigate the prevalence and the degree of impaired CFR and to identify independent predictors of impaired CFR in a high-risk population with a history of MI.
Methods
Definition of the study population
PROFLOW (PROspective Evaluation of Coronary FLOW Reserve and Molecular Biomarkers in Patients with Established Coronary Artery Disease) is a prospective, exploratory and open study in high-risk patients with verified coronary artery disease. We have used data from SCAAR (Swedish Coronary Angiography and Angioplasty Registry) to identify patients with high risk for future MACE. We screened patients with a history of spontaneous type-1 MI (>3 months and <5 years) with at least one of the following additional risk factors: age ≥65 years, angiographic evidence of multivessel coronary artery disease, diabetes mellitus, hypertension, two or more spontaneous MIs, chronic, non-end-stage renal dysfunction (creatinine clearance calculated by Cockcroft Gault equation <60 mL/min*m2), incomplete revascularization. The eligible patients who fulfilled inclusion and exclusion criteria (Table 1) were contacted and offered participation in the study. In this paper, we present cross-sectional data measured at the time of inclusion in the study.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria: | |
| |
| 2. A history of presumed spontaneous type 1 myocardial infarction >3 months and <5 years prior to randomisation. | |
| 3. Subjects should also have at least one of the following risk factors: | |
| ● age ≥65 years; | |
| ● Angiographic evidence of multivessel coronary artery disease (CAD) ● Diabetes mellitus | |
| ● Hypertension; | |
| ● One or more additional spontaneous myocardial infarction (>3 months ago) | |
| ● Chronic renal dysfunction (creatinine clearance calculated by Cockcroft Gault equation <60 mL/min*m2) | |
| ● Incomplete revascularisation based on coronary angiography | |
| Exclusion criteria: | |
| 1. Atrial fibrillation (chronic, persistent or paroxysmal). | |
| 2. AV blockage, degree 2–3. | |
| 3. Angina at entry CCS* ≥3 despite maximal medical therapy. | |
| 4. Acute coronary syndrome within the previous 3 months, including acute revascularisation. | |
| 5. Elective PCI within 3 months or CABG within the previous 12 months. | |
| 6. Stroke within the previous 6 months. | |
| 7. Previous stroke/TIA and ongoing treatment with Persantin or Asasantin. | |
| 8. A history of ventricular tachycardia requiring therapy for termination, or symptomatic sustained ventricular tachycardia. | |
| 9. Previously known LVEF <30%. | |
| 10. NYHA class III-IV heart failure at entry or hospitalisation for exacerbation of chronic heart failure within the previous 3 months. | |
| 11. Non-ischemic dilated or hypertrophic cardiomyopathy. | |
| 12. Known anaemia that requires treatment or capillary Hb <100 g/L. | |
| 13. Severe renal disease (CKD stage 4 or 5) or previously known creatinine clearance as calculated by Cockcroft Gault equation H). | |
| 14. Severe aortic or mitral valvular disease or valvular disease that is likely to require surgery within 3 years. | |
| 15. A life expectancy of less than 1 year for non-cardiac related disorders. | |
| 16. Inability to comply with the protocol. |
Note: *Canadian Cardiovascular Society grading of angina pectoris.
Sample size and power calculation
We calculated the sample-size based on the aim to achieve a 95% confidence interval with high precision (±0.04) around the prevalence of impaired CFR. If we assumed that 20% of the study population would have CFR ≤2, then 600 patients would be needed based on the variability of CFR observed in the previous study.8
Patient enrolment
We enrolled 619 patients who fulfilled inclusion criteria at two centers in Sweden (Department of Cardiology at Sahlgrenska University Hospital in Göteborg and the Department of Cardiology at Skåne University Hospital in Lund, Sweden). Written informed consent was obtained in accordance with the International Conference on Harmonization Good Clinical Practice guidelines. The ethics committee at Gothenburg University approved the study.
Echocardiography and assessment of CFR
A basic transthoracic echocardiography protocol was performed according to recommendations from the European Association of Echocardiography.10 We used the Sequoia C256 (Acuson Siemens, Mountain View, CA) ultrasound system with a 4‐MHz probe. Detailed protocol and validation of Doppler flow measurements in LAD have been previously described.11,12 Briefly, the mid to distal part of the LAD was identified using 3.5‐MHz color Doppler in the interventricular sulcus in a modified 2‐chamber view. Flow velocity signals at rest and during adenosine infusion (140 μg/min/kg) over 5 mins was recorded with pulsed Doppler. We monitored blood pressure and ECG during the whole procedure. All studies were digitally stored for off-line measurements. CFR was analyzed offline using the ultrasound software Image-Arena (Tomtec, Unterschlissheim, Germany). Mean diastolic flow velocity at baseline and during peak hyperemia was measured by manual tracing of the diastolic Doppler flow signals. CFR was calculated as the ratio between the hyperemic and baseline flow velocity values. All evaluation CFR was performed by one experienced sonographer at the core lab (Sahlgrenska University Hospital) blinded to the information about patients’ characteristics. We divided patients into two groups based on the cut-off value for CFR of 2.5. This cut-off value has previously been demonstrated to be associated with worse prognosis.13,14
Statistics
Continuous variables are presented as mean ± SD and categorical variables as frequencies. Normal distribution was tested by histograms and Shapiro-Wilks test. The dependent variable (CFR) was transformed with a square root to a normal distribution. Differences between the groups with normal and decreased CFR (CFR>2.5 and CFR≤2.5) in continuous variables were tested with the Student’s t-test and differences in categorical variables were tested by the chi-square test. We imputed missing data using multiple imputation chain-equation method15 with 20 data sets. Continuous variables were imputed by ordinary least squares multiple regression, whereas categorical variables were imputed using logistic regression and categorical variables by multinomial logistic. The imputation procedure and subsequent analyses were performed according to Rubin’s protocol16 under the assumption that missing data are missing at random. Independent predictors of CFR were assessed with multiple linear regression. The following covariates were entered into the model for prediction of CFR as dependent variable; age, gender, BMI, smoking history, hypertension, diabetes mellitus, hyperlipidemia, renal dysfunction, chronic obstructive pulmonary disease (COPD), peripheral artery disease (PAD), previous stroke, months after MI, additional MI, multivessel disease, incomplete revascularization, previous PCI, previous CABG, angina pectoris and NYHA-class. The goodness of fit (calibration) of the regression model was assessed by the coefficient of determination (R2). Multicollinearity between the variables in the model was assessed by calculation of variance inflation factor (VIF). The interaction was evaluated by including an interaction term in the regression model. Heteroscedasticity was tested with the Breusch-Pagan/Cook-Weisberg test. Regression specification-error test was performed with Ramsey’s test. We calculated the sample-size calculation to achieve a 95% confidence interval with high precision (±0.04) around the prevalence of impaired CFR. If more than 20% of the study population have CFR ≤2 (the primary hypothesis), then 600 evaluable patients will be needed based on the previous study.8 All statistical analyses were performed using Stata® software (version 14, StataCorp, College Station, Texas, USA). All tests were two-tailed, and a P-value of <0.05 was considered statistically significant.
Results
Study population
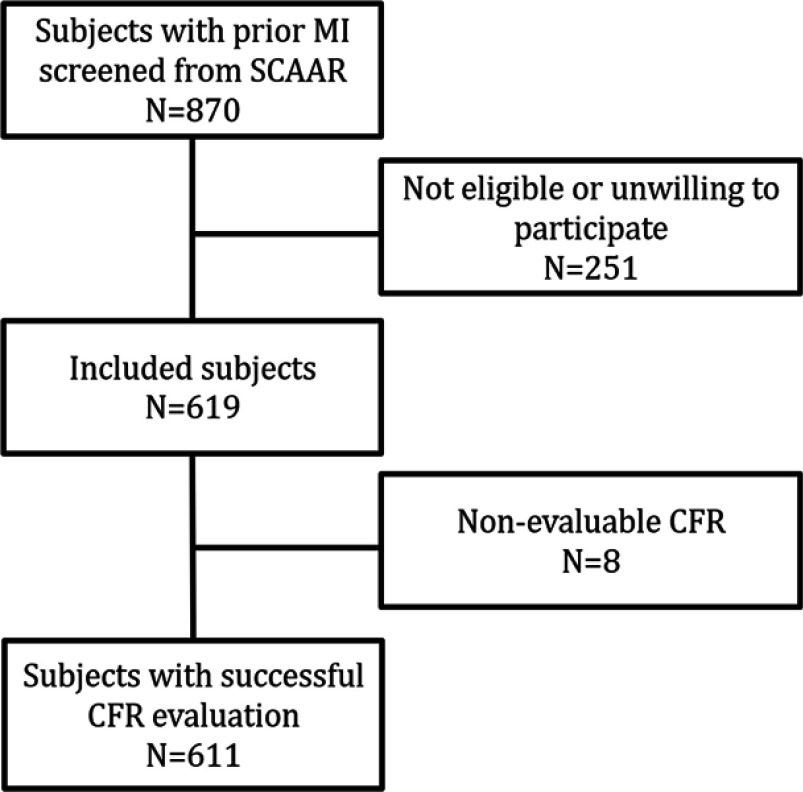
total of 619 patients were included in the study between July 12th 2013 and December 16th 2015 (Figure 1). Patient’s characteristics are presented in Table 2. The median age was 69.0 years (IQR 64.9–73.3), and 18.4% (114) were female. Mean BMI was 27.3 (±3.91). Approximately half of the study population (46.0%) had multivessel disease, and 12.3% (76) had experienced an additional MI. History of the previous PCI was reported by 95.2%, and 8.2% have had coronary artery by-pass surgery. Almost one quarter (23.7%) were incompletely revascularized. Two-thirds had hypertension (66.9%), while hyperlipidemia was present in 39.5% and 22.3% had diabetes mellitus. Just over a tenth (11.6%) reported exercise-induced angina pectoris at the time of inclusion in the study. Time from index MI was not an independent predictor of low CFR. Among predictors of CFR for patient characteristics used for risk-enrichment increasing age, the presence of hypertension, and incomplete revascularization were associated with decreased CFR (Table 3).
Figure 1.
Flow-chart for the inclusion of patients.
Table 2.
Patient’s characteristics
| All N=619 | CFR>2.5 N=365 | CFR≤2.5 N=246 | Missing | P-value | |
|---|---|---|---|---|---|
| Age median (IQR) | 69.0 (64.9–73.3) | 67.7 (63.8–72.0) | 69.7 (65.5–74.6) | 9 | 0.999 |
| Female gender n (%) | 114 (18.4) | 58 (15,9) | 56 (22.8) | 1 | 0.024 |
| BMI mean (± SD) | 27.3 (±3.91) | 27.5 (±3.91) | 26.97 (±3.899) | ||
| Tobacco use n (%) | 8 | 0.258 | |||
| Current smoker n (%) | 68 (11.0) | 38 (10.4) | 30 (12.2) | ||
| Previous smoker n (%) | 331 (50.0) | 191 (52.0) | 140 (56.9) | ||
| Non smoker n (%) | 212 (32.4) | 136 (37.3) | 76 (30.9) | ||
| Diabetes mellitus n (%) | 138 (22.3) | 75 (20.5) | 63 (25.6) | 0.142 | |
| Hypertension n (%) | 414 (66.9) | 238 (65.2) | 176 (71.5) | 0.100 | |
| Hyperlipidemia n (%) | 245 (39.5) | 158 (43.3) | 87 ((35.4) | 0.050 | |
| Renal dysfunction n (%) | 30 (4.8) | 18 (4.9) | 12 (4.9) | 0.976 | |
| Previous stroke n (%) | 32 (5.2) | 15 (4.10) | 17 (6.9) | 0.127 | |
| Previous PCI n (%) | 589 (95.2) | 354 (97.0) | 235 (95.6) | 0.343 | |
| Previous CABG n (%) | 51 (8.2) | 28 (7.7) | 23 (9.3) | 0.462 | |
| Previous PAD n (%) | 17 (2.7) | 9 (2.5) | 8 (3.3) | 0.562 | |
| COPD n (%) | 23 (3.7) | 12 (3.3) | 11 (4.5) | 0.451 | |
| Multivessel disease n (%) | 285 (46.0) | 183 (50.1) | 102 (41.4) | 0.063 | |
| Additional MI n (%) | 76 (12.3) | 53 (14.5) | 23 (9.3) | 0.071 | |
| Incomplete revascularization n (%) | 147 (23.7) | 71 (19.4) | 76 (30.9) | 0.001 | |
| Angina pectoris n (%) | 72 (11.6) | 44 (12.1) | 28 (11.4) | 0.800 | |
| NYHA | 0.889 | ||||
| Class I n (%) | 571 (92.2) | 343 (94.0) | 228 (92.7) | ||
| Class II n (%) | 40 (6.5) | 22 (6.0) | 18 (7.3) | ||
| Medication | |||||
| ASA n (%) | 576 (93.1) | 345 (94.5) | 231 (93.9) | 0.500 | |
| P2Y12 n (%) | 103 (16.6) | 75 (20.5) | 28 (11.4) | 0.004 | |
| No P2Y12 | 516 (83.4) | 298 (81.6) | 218 (88.6) | ||
| Prasugrel | 1 (0.2) | 0 (0) | 1 (0.4) | ||
| Clopidogrel | 23 (3.7) | 13 (3.6) | 10 (4.1) | ||
| Ticagrelor | 79 (12.8) | 62 (17.0) | 17 (6.9) | ||
| Statins n (%) | 557 (90.0) | 333 (91.5) | 224 (91.1) | 0.470 | |
| ACEI/ARB n (%) | 511 (82.6) | 295 (80.8) | 216 (87.8) | 0.005 | |
| Beta-receptor blocker n (%) | 500 (80.8) | 300 (82.2) | 200 (81.3) | 0.788 | |
| Diuretics n (%) | 114 (18.4) | 61 (16.7) | 53 (21.5) | 0.103 | |
| Insulin n (%) | 34 (5.5) | 18 (10.4) | 16 (6.5) | 0.370 | |
| Oral antidiabetics n (%) | 103 (16.6) | 55 (15.1) | 48 (7.8) | 0.119 | |
| Heart rate mean (±SD) | 60.5 (9.2) | 59.1 (8.9) | 62.5 (9.2) | <0.001 | |
| CFR LAD mean (±SD) | 2.74 (±0.79) | 3.24 (±0.58) | 2.04 (±0.42) | <0.001 |
Abbreviations: BMI, body mass index; PCI, percutaneous coronary intervention; CABG, coronary artery by-pass surgeryl; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; NYHA, New York Heart Association; ASA, acetylsalicylic acid; P2Y12, inhibitor of P2Y12 receptors; ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor inhibitor; CFR, coronary flow reserve; LAD, left anterior descending artery.
Table 3.
Predictors of CFR
| β-coefficients | 95% CI | P-value | |
|---|---|---|---|
| Age | −0.0000346 | −0.0000563, −0.0000129 | 0.002 |
| Gender | −0.0419 | −0.0920, 0.00821 | 0.101 |
| BMI | 0.00516 | −0.0000953, 0.0104 | 0.054 |
| Heart rate | −0.0129673 | −0.0200921, −0.0058426 | <0.000 |
| Diabetes mellitus | 0.0143 | −0.0724, 0.101 | 0.746 |
| Hypertension | −0.0339 | −0.0778, 0.0101 | 0.131 |
| Dyslipidemia | 0.0409 | 0.000646, 0.0812 | 0.046 |
| Smoking history | |||
| Current smoker | reference | ||
| Previous smoker | 0.0670 | 0.00310, 0.131 | 0.040 |
| Never smoker | 0.0937 | 0.0258, 0.162 | 0.007 |
| NYHA class | |||
| Class I | reference | ||
| Class II | −0.0258 | −0.109, 0.0572 | 0.541 |
| Angina pectoris | −0.0197 | −0.0819, 0.0424 | 0.533 |
| Previous CABG | −0.0410 | −0.116, 0.0337 | 0.282 |
| Previous PCI | 0.0129 | −0.0947, 0.120 | 0.814 |
| Peripheral artery disease | 0.0400 | −0.0761, 0.156 | 0.498 |
| Previous stroke | −0.0748 | −0.161, 0.0111 | 0.088 |
| Renal dysfunction | 0.00748 | −0.0835, 0.0984 | 0.872 |
| COPD | −0.0636 | −0.162, 0.0349 | 0.205 |
| Additional MI | 0.00166 | −0.0589, 0.0622 | 0.957 |
| Beta-receptor blocker | 0.0433 | −0.00655, 0.0932 | 0.089 |
| ACE inhibitor | −0.0408 | −0.0934, 0.0118 | 0.128 |
| ARB | −0.0800 | −0.138, −0.0218 | 0.007 |
| Statin | −0.0247 | −0.0927, 0.0433 | 0.476 |
| P2Y12 receptor blocker | |||
| No P2Y12 | reference | ||
| Prasugrel | −0.401 | −0.853, 0.0501 | 0.081 |
| Clopidogrel | 0.0131 | −0.0911, 0.117 | 0.805 |
| Ticagrelor | 0.0545 | −0.0127, 0.122 | 0.111 |
| ASA | 0.0634 | −0.0223, 0.149 | 0.147 |
| Diuretics | −0.0203 | −0.0723, 0.0317 | 0.443 |
| CCB | 0.0202 | −0.0285, 0.0689 | 0.416 |
| Insulin | −0.00924 | −0.105, 0.0867 | 0.850 |
| Oral antidiabetics | −0.0608 | −0.151, 0.0295 | 0.187 |
| Multivessel disease | 0.0402 | −0.00207, 0.0826 | 0.062 |
| Incomplete revascularization | −0.108 | −0.156, −0.0599 | <0.000 |
| Time after MI (months) | 0.000633 | −0.000842, 0.00211 | 0.400 |
Abbreviations: BMI, body mass index; NYHA, New York Heart Association; CABG, coronary artery by-pass surgery, PCI, percutaneous coronary intervention; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; P2Y12, inhibitor of P2Y12 receptors; ASA, acetylsalicylic acid; CCB, calcium channel blocker; ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor inhibitor; CFR, coronary flow reserve; LAD, left anterior descending artery.
Coronary flow reserve
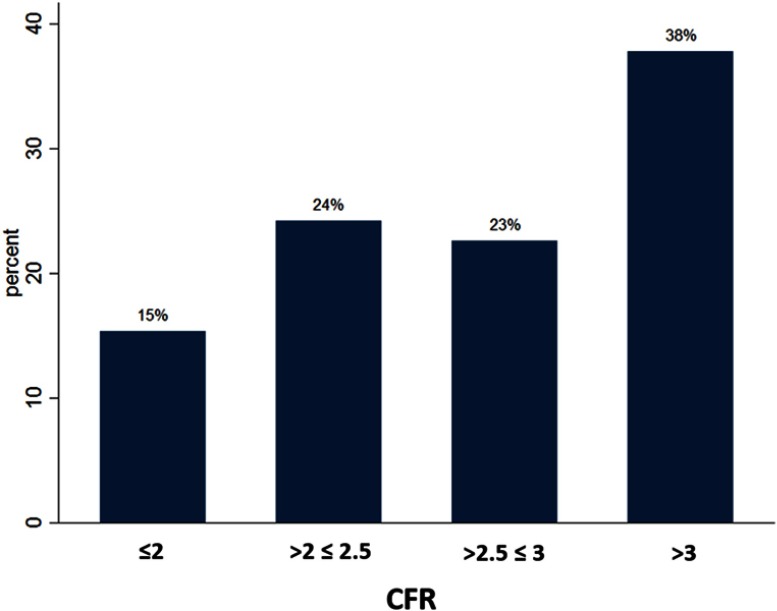
CFR in LAD was successfully evaluated in 611 (98.7%) patients (Figure 2). Mean CFR was 2.74±0.79. CFR ≤2.5 was found in 39.0%, and almost two-thirds (61.6%) had CFR ≤3.0 (Figure 3). In univariate linear regression analysis based on the variables we used for the risk enrichment, age >65, hypertension and incomplete revascularization were associated with significantly lower CFR values while multi-vessel disease, diabetes, previous MI and renal failure did not (Table 4). Patients who had CFR ≤2.5 were more likely to be females, had more often hyperlipidemia, and they were more often incompletely revascularized (Table 2). We found no differences in medical treatment between the patients with normal and low CFR except treatment with ACE/ARB, which were more common in the group with CFR ≤2.5. In the multiple linear regression model, independent predictors of CFR were age, dyslipidemia, smoking, hypertension, body mass index, incomplete revascularization, treatment with angiotensin receptor blockers and heart rate (Table 3). Gender, history of stroke, and treatment with beta-blockers showed a trend for statistical significance. There was no interaction between gender, age, history of diabetes, incomplete revascularization, multivessel disease. We found no evidence of multicollinearity as VIF was <10 for all the variables included in the model. Breusch-Pagan/Cook-Weisberg test did not show evidence of heteroscedasticity (P=0.141). We found no evidence of specification error in the model with Ramsey’s test (P=0.089).
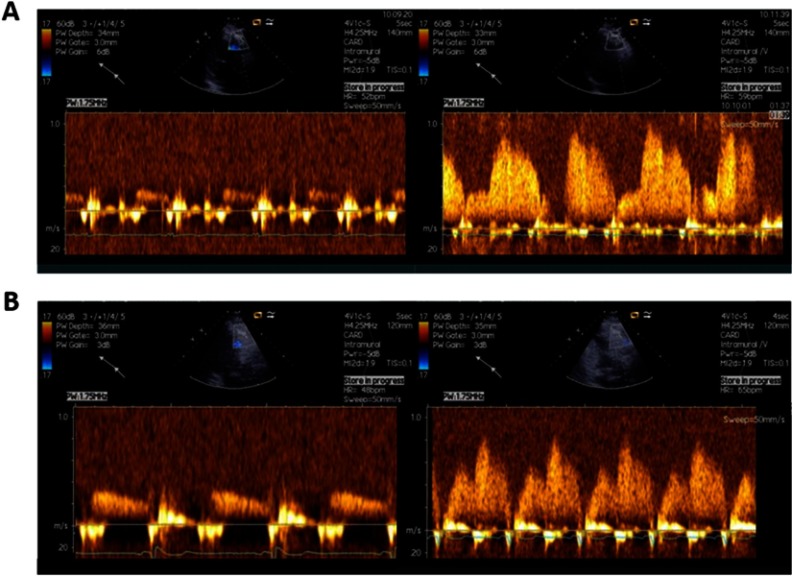
Figure 2.
Examples of CFR assessed by transthoracic Doppler of the mid-distal portion of LAD. Coronary flow reserve is calculated as the ratio of mean diastolic coronary flow velocity at hyperemia (induced by intravenous infusion of adenosine 140 µg/min/kg) to that in the resting condition. (A) Doppler flow recording in LAD from patients with normal CFR (4.6), (B) Doppler flow recording in LAD from patients with decreased CFR (1.7).
Figure 3.
The proportion of patients in different categories of CFR values. Almost two-thirds of the patients had CFR ≤3.0.
Table 4.
Predictors of CFR for patient characteristics used for risk-enrichment
| β-coefficients | 95% CI | P-value | |
|---|---|---|---|
| Age >65 years | −0.0581 | −0.102, −0.0145 | 0.009 |
| Multivessel disease | 0.0400 | −0.00185, 0.0818 | 0.061 |
| Diabetes | −0.0163 | −0.0624, 0.0298 | 0.488 |
| Hypertension | −0.0424 | −0.0832, −0.00158 | 0.042 |
| Additional MI | −0.00274 | −0.0622, 0.0567 | 0.928 |
| Renal failure | 0.0118 | −0.0813, 0.105 | 0.804 |
| Incomplete revascularization | −0.114 | −0.161, −0.0670 | <0.000 |
Abbreviation: MI, myocardial infarction.
Discussion
We evaluated CFR in 619 patients with high risk for future MACE. The most important finding of this study is that two-thirds of these patients with previous MI had evidence for decreased or abnormal CFR.
The heart can increase blood flow through coronary circulation three to six times in the settings of increased demand compared to the flow under resting conditions. Consequently, the cut-off at CFR >3 is regarded as the lower threshold for the normal value in the healthy human heart. An unexpectedly large proportion of patients in our study had evidence of disturbed CFR. Previous studies have demonstrated that traditional cardiovascular risk factors are associated with impaired CFR. It has been shown that impaired CFR is a strong predictor for MACE and therefore worse prognosis.6,7,9,17,18 Traditionally, reduced left the ventricular function, infarct size, age, and TIMI-flow are used for estimation of long-term prognosis in patients with known ischemic heart disease. But these markers are rather imprecise and frequently fail to identify individuals with increased risk even in a high-risk population. Several recent studies have shown that CFR is an independent factor that provides prognostic information in addition to traditional risk markers. In one of these studies, Murthy et al.7 performed rest/stress positron emission tomography (PET) and measured CFR in 2783 patients with previously known or suspected coronary artery disease. They found that patients in the lowest tercile (CFR<1.5) had a 5.6-fold increased risk of mortality compared to patients in the highest tercile (CFR>2.5). In a similar high-risk population consisting of 4313 patients, Cortigiani et al.6 performed stress echocardiography with an evaluation of CFR by transthoracic Doppler and found that the mortality at four-years was substantially higher in subjects with CFR <2 than in those with CFR >2.4 This finding was consistent both in the patients with and without ischemia at stress echocardiography. In these two studies, CFR provided additive value for risk stratification independently of the prevalence of ischemia measured by positron emission tomography and stress-echocardiography. Furthermore, it has been demonstrated that there is a direct negative linear relationship between CFR and increased risk of adverse cardiovascular events, ie, the lower the CFR, the higher the risk.5,8 The role of CFR as an independent predictor of cardiac mortality has been studied in other patient groups with increased risk for major adverse cardiovascular events. Murthy et al found that diabetic patients without known CAD but with impaired CFR had a similar rate of cardiac death as nondiabetics with known CAD. On the other hand, patients with diabetes without known CAD and preserved CFR had event rates comparable to non-diabetics.5 These findings have been confirmed by others.19
An important finding in our study is that incomplete revascularization was a strong independent predictor of impaired CFR. This is a particularly interesting observation in the light of the results from the recent clinical trials addressing the significance of completeness of revascularization in patients with STEMI who have the multi-vessel coronary disease. Until now, several studies have demonstrated that patients in whom infarct-related artery, as well as non-infarct related arteries (with significant stenosis), were successfully treated with coronary stents had less MACE compared to the strategy in which only the infarct-related artery was treated. The COMPARE ACUTE was the largest study so far in which these two strategies were compared and showed that patients with STEMI randomized to complete revascularization based on fractionally flow reserve-guided PCI, had a 65% reduced risk of MACCE (death from any cause, nonfatal myocardial infarction, revascularization, and cerebrovascular events) at one year (HR 0.35; 95% CI, 0.21–0.58; P<0.001).20
CFR is affected by coronary microvascular function as well as by significant epicardial stenosis. Epicardial coronaries constitute only approximately 15% of the coronary vasculature. The remaining part of the coronary circulation consists of pre-arterioles, arterioles, and capillaries, which are referred to as the coronary microcirculation. In the absence of significant coronary obstruction, impaired CFR reflects disturbed microvascular function.3–6
One of the possible mechanisms behind the long-term protective effects of complete revascularization in patients with STEMI with the multi-vessel coronary disease could be mediated by improved CFR in all three coronary arteries compared to the only infarct-related artery. Indeed, we have demonstrated that the presence of a significant lesion in one coronary artery predicts decreased CFR in another.12 One explanation for this association might be that coronary artery disease, being a progressive and systemic phenomenon, affects biological properties of the vessel wall in epicardial segments of coronary arteries but at the same time also the biology of microcirculation. When the advanced atherosclerotic lesions are present in one coronary territory, the regulation of coronary flow may be disturbed in the whole heart and not only in the most diseased vessel. The regulation of coronary blood flow is the result of a complex interaction between many factors with endothelium as regulatory center.4–6 Since normal coronary flow depends on the interplay between many known and possibly yet unknown physiological systems, CFR may contain integrated information about these complex biophysical and biochemical arrangements. Nevertheless, even when complete revascularization is achieved, CFR can still be reduced under the normal physiological level. In our study, 69% of patients with CFR ≤2.5 were completely revascularized at the time of the index procedure.
Physicians prescribe evidence-based pharmacological treatment to patients after MI for secondary prevention with ASA, statins, ACE/ARB, and beta blockade. Indeed, the majority of patients in our study were asymptomatic and had adequate pharmacological therapy. But despite the adequate therapy, the majority of patients had impaired CFR. Impaired CFR is associated with a higher prevalence of vulnerable plaques and greater plaque burden with higher levels of high-sensitivity C-reactive protein,21 indicating a pro-inflammatory and pro-thrombotic condition prone to future adverse cardiovascular events. Success in treatment of conventional risk factors improves CFR (normalization of high blood pressure, normalization of glucose levels in diabetes, normalization of atherogenic lipoproteins in hyperlipidemia, smoking cessation, increased physical activity, and reduced mental stress). Based on our results, we propose that apart from achieving full revascularization and adequate evidence-based secondary prevention, the high-risk individuals would benefit from pharmacological (and other) interventions with the specific aim to increase CFR. Individualizing length and extent of antithrombotic therapy could be one way to achieve this while novel pharmacological agents that interfere with inflammatory and coagulation pathways in the blood and vessels wall could be another. Perhaps coronary interventions in this patient population should combine mechanical revascularization with local and long-term administration of drugs specifically designed to improve CFR.
In conclusion, a majority of patients with previous MI and additional risk factors have decreased CFR despite adequate secondary preventive intervention long after the index event. Incomplete revascularization is the strongest independent predictor of CFR. CFR could be used for prognostics stratification in a high-risk population with previous MI. CFR could be used as a target in future studies with pharmacological intervention to decrease major adverse cardiovascular events.
Disclosure
Elmir Omerovic and David Erlinge have received institutional grants from AstraZeneca. Li-Ming Gan is an emplyee of AstraZeneca. The authors report no other conflicts of interest in this work.
References
- 1.Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord. 2017;17:53. doi: 10.1186/s12872-017-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 3.Task Force M; Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- 4.Brainin P, Frestad D, Prescott E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2018;254:1–9. doi: 10.1016/j.ijcard.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 5.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortigiani L, Rigo F, Gherardi S, et al. Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. JACC. 2012;5:1079–1085. doi: 10.1016/j.jcmg.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 7.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan LM, Svedlund S, Wittfeldt A, et al. Incremental value of transthoracic doppler echocardiography-assessed coronary flow reserve in patients with suspected myocardial ischemia undergoing myocardial perfusion scintigraphy. J Am Heart Assoc. 2017;6:e004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan LM, Wikstrom J, Fritsche-Danielson R. Coronary flow reserve from mouse to man–from mechanistic understanding to future interventions. J Cardiovasc Transl Res. 2013;6:715–728. doi: 10.1007/s12265-013-9497-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evangelista A, Flachskampf F, Lancellotti P, et al.. European association of echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur J Echocardiogr. 2008;9:438–448. doi: 10.1093/ejechocard/jen174 [DOI] [PubMed] [Google Scholar]
- 11.Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, et al. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol. 2013;61:723–727. doi: 10.1016/j.jacc.2012.11.032 [DOI] [PubMed] [Google Scholar]
- 12.Haraldsson I, Gan LM, Svedlund S, et al. Non-invasive evaluation of coronary flow reserve with transthoracic Doppler echocardiography predicts the presence of significant stenosis in coronary arteries. Int J Cardiol. 2014;176:294–297. doi: 10.1016/j.ijcard.2014.06.076 [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Saito N, Kirigaya H, et al. Impairment of coronary flow reserve evaluated by phase contrast cine-magnetic resonance imaging in patients with heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5:e002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawata T, Daimon M, Hasegawa R, et al. Prognostic value of coronary flow reserve assessed by transthoracic Doppler echocardiography on long-term outcome in asymptomatic patients with type 2 diabetes without overt coronary artery disease. Cardiovasc Diabetol. 2013;12:121. doi: 10.1186/1475-2840-12-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Inference and Missing Data. Biometrika. 1976;63:581–590. doi: 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 17.Nakanishi K, Fukuda S, Shimada K, et al. Impaired coronary flow reserve as a marker of microvascular dysfunction to predict long-term cardiovascular outcomes, acute coronary syndrome and the development of heart failure. Circ J. 2012;76:1958–1964. [DOI] [PubMed] [Google Scholar]
- 18.Serruys PW, Di Mario C, Piek J, et al. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE study (Doppler endpoints balloon angioplasty trial Europe). Circulation. 1997;96:3369–3377. doi: 10.1161/01.cir.96.10.3369 [DOI] [PubMed] [Google Scholar]
- 19.Cortigiani L, Rigo F, Gherardi S, et al. Additional prognostic value of coronary flow reserve in diabetic and nondiabetic patients with negative dipyridamole stress echocardiography by wall motion criteria. J Am Coll Cardiol. 2007;50:1354–1361. doi: 10.1016/j.jacc.2007.06.027 [DOI] [PubMed] [Google Scholar]
- 20.Smits PC, Abdel-Wahab M, Neumann F-J, et al. Fractional flow reserve–guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. doi: 10.1056/NEJMoa1701067 [DOI] [PubMed] [Google Scholar]
- 21.Dhawan SS, Corban MT, Nanjundappa RA, et al. Coronary microvascular dysfunction is associated with higher frequency of thin-cap fibroatheroma. Atherosclerosis. 2012;223:384–388. doi: 10.1016/j.atherosclerosis.2012.05.034 [DOI] [PubMed] [Google Scholar]