Abstract
Aim
This study aimed to estimate the prevalence of latent autoimmune diabetes of adults (LADA) and classic type 1 diabetes mellitus (T1DM) in newly diagnosed adult diabetes in China.
Method
This cross-sectional study involved 17,349 newly diagnosed diabetes in adults aged ≥30 years from 46 hospitals within 31 months. Demographic characteristics, clinical features, and medical history were collected by trained researchers. T1DM as a whole was comprised of classic T1DM and LADA. Classic T1DM was identified based on the clinical phenotype of insulin-dependency, and LADA was differentiated from patients with initially an undefined diabetes type with standardized glutamic acid decarboxylase autoantibody testing at the core laboratory. The age and sex distributions from a large national survey of diabetes in China conducted in 2010 were used to standardize the prevalence of classic T1DM and LADA.
Results
Among 17,349 adult patients, the prevalence of T1DM was 5.49% (95% CI: 4.90–6.08%) (5.14% [95% CI: 4.36–5.92%] in males and 6.16% [95% CI: 5.30–7.02%] in females), with 65% of these having LADA. The prevalence of classic T1DM decreased with increasing age (p<0.05), while that of LADA was stable (p>0.05). The prevalence of T1DM in overweight or obese patients was 3.42% (95% CI: 3.20–3.64%) and 2.42% (95% CI: 1.83–3.01%), respectively, and LADA accounted for 76.5% and 79.2% in these two groups.
Conclusion
We draw the conclusion that T1DM, especially LADA, was prevalent in newly diagnosed adult-onset diabetes in China, which highlights the importance of routine islet autoantibodies testing in clinical practice.
Keywords: diabetes, autoimmune, metabolism, differentiation
Introduction
Type 1 diabetes mellitus (T1DM) occurs when autoimmune T cells specifically attack pancreatic β-cells, resulting in lifelong insulin-dependency. Although China remains among the countries with the lowest incidence of T1DM in the world, the incidence has witnessed a rapid increase in children (eg, from 0.51 per 10,0000 person years in the 1984–1994 period to 1.93 per 10,0000 person years in the 2010–2013 period).1,2 However, the rising number of adults affected by diabetes has also garnered recent attention. In this regard, The T1D China Project reported that about 65% of the new onset T1DM cases occurred in adults as opposed to children.2
It is worth noting that the aforementioned survey was confined to the classic T1DM subtype (insulin-dependent) patients and few studies have been conducted to date to estimate the prevalence of T1DM in adults, likely due to the difficulties associated with differentiation of classic T1DM, latent autoimmune diabetes of adults (LADA), and type 2 diabetes mellitus (T2DM), the latter of which is the most common type of diabetes diagnosed in adults.
LADA is classified as a subtype of T1DM because of its autoimmune etiology, but it is more likely to affect adults and shares many clinical and immunogenetic characteristics with T2DM.3 Timely initiation of insulin therapy is essential to maintain good glycemic control and consequently, to reduce patients’ risk of micro- and macrovascular complications in LADA patients. Therefore, it is critically important to know the prevalence of T1DM and in particular, LADA in Chinese adults with newly diagnosed diabetes, to differentiate LADA from classic T1DM and to systematically investigate the determinants of LADA in China. To our knowledge, no such studies have been conducted to date. To address this, we carried out a nationwide, multicenter, and clinic-based study of adult-onset diabetes.
Materials and methods
Research setting and participants
In a cross-sectional study, a survey was administered enumerating patients ≥30 years of age with newly diagnosed diabetes. Tertiary care centers are the only institutions equipped with adequate instruments and staff to perform key tests such as islet autoantibodies and C-peptide, which are essential for the precise classification of diabetes; hence, we invited 46 tertiary care hospitals from 20 provincial administration areas and four cities directly under the administration of the central government to participate in this survey. The 46 participating hospitals were distributed across all of the seven geographic regions of China (4 Northeast, 8 North, 3 Northwest, 9 Central, 3 Southwest, 7 South, and 12 East) and thereby, represent the diversity in climates, cultures, and ethnicities of the Chinese population. The inclusion criteria were as follows: 1) diagnosis of diabetes at ≥30 years of age; 2) diabetes duration being <1 year; and 3) outpatients attending clinics within the department of endocrinology of the participating hospitals. The exclusion criteria were as follows: 1) being pregnant at the time of diagnosis of diabetes or having gestational diabetes mellitus (GDM) and 2) co-existing acute diseases that could influence the glucose metabolism, such as infectious diseases and acute myocardial infarction.
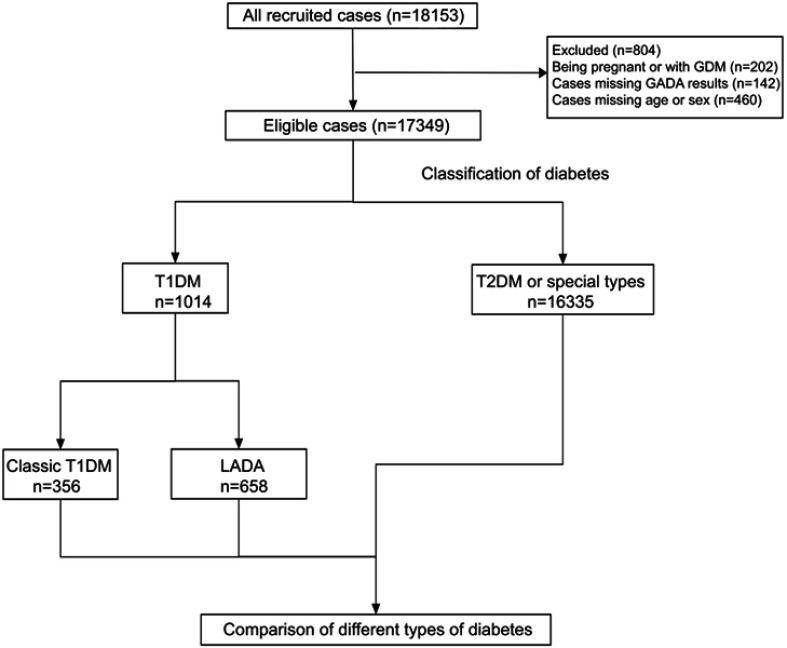
From April 2015 to October 2017, research nurses consecutively recruited 18,153 eligible outpatients from the clinics of the 46 tertiary care hospitals with an overall response rate of 95.4%. After exclusion of 460 patients with missing age or sex, 142 patients who did not have a glutamic acid decarboxylase autoantibody (GADA) result, and 202 patients who were pregnant or diagnosed with GDM, the remaining 17,349 patients with diabetes were included in this analysis (Figure 1). The ethics review committee/institutional review board of each of the participating hospitals approved the study protocol. Written informed consent was obtained from all the participants before data collection.
Figure 1.
Flow diagram and classification of 18,153 newly diagnosed patients with diabetes at 30 years of age or older in China.
Abbreviations: GDM, gestational diabetes mellitus; GADA, glutamic acid decarboxylase autoantibody; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; LADA, latent autoimmune diabetes of adults.
Classification of T1DM, T2DM, LADA, and other types of diabetes
The diagnosis of diabetes was based on the 1999 World Health Organization (WHO) criteria.4 Clinical characteristics and diabetes-related biochemical measurement results, including fasting and 2 hrs postprandial blood glucose and C-peptide, blood lipids, HbA1c, and GADA serum levels, were used to classify diabetes into T1DM, T2DM, and other specific types of diabetes. T1DM was further divided into classic T1DM and LADA. Classic T1DM was defined according to the classification of diabetes by the American Diabetes Association and WHO, as acute onset insulin-dependent diabetes due to pancreatic islet β-cell destruction and prone to ketoacidosis.4 Classic T1DM includes autoimmune type 1 (type 1A) diabetes and idiopathic (type 1B) diabetes determined according to the status of islet autoantibodies. LADA was defined as GADA positivity in patients with non-insulin requiring diabetes for at least the first six months. Autoimmune diabetes was considered as the combination of type 1A diabetes and LADA. Specific types of diabetes due to other causes (eg, monogenic diabetes, such as maturity-onset diabetes of the young), diseases of the exocrine pancreas (eg, cystic fibrosis), and drug- or chemical-induced diabetes (eg, in the treatment of HIV/AIDS or after organ transplantation) were collectively considered non-T1DM. This analysis classified diabetes as T1DM including classic T1DM and LADA, and non-T1DM.
Clinical measurements and data collection
Research nurses at each of the 46 participating hospitals participated in a series of training programs to standardize all procedures and methods of data collection. Patients self-reported demographic characteristics (ie, age, race, sex), clinical features, medical history, and lifestyle risk factors (ie, exercise habits, diet, smoking, alcohol consumption). Patient height, weight, waist circumference, hip circumference, and blood pressure (BP) were measured by research nurses using standard procedures. Drug use information was retrieved from medical notes. Completed questionnaires were checked by a second trained nurse for errors and missing values, and data were double-entered by a specially assigned staff member. The completed databases were uploaded onto a centralized database periodically.
Definitions of clinical and biochemical characteristics
Hyperglycemia, high BP, and dyslipidemia were defined using the ADA’s treatment targets (ie, HbA1c ≥7.0% (53 mmol/mol), BP ≥130/80 mmHg, low-density lipoprotein cholesterol (LDL-C) ≥2.6 mmol/L, triglycerides ≥1.7 mmol/L, and high-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L in men and <1.3 mmol/L in women).5 Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters. Chinese Diabetes Association’s criteria were used to define obesity (BMI ≥28 kg/m2) and overweight (BMI ≥24–<28 kg/m2) as well as central obesity (waist circumference ≥90 cm in males and 85 cm in females).6 Metabolic syndrome was defined according to advised National Cholesterol Education Program-Adult Treatment Panal III criteria.7Preserved β-cell function is defined as fasting C-peptide >0.2 nmol/L or postprandial C-peptide>0.4 nmol/L. GADA titers of 18 U/mL or higher were defined as positive and confirmed by a repeated assay. North and South of China were divided by the line formed by the Huai River and Qinling Mountains. Intensive insulin is defined as long-acting insulin associated with short-acting insulin therapy, premixed insulin is defined as premixed insulin therapy, basal insulin is defined as long-acting insulin treatment including Neutral Protamine Hagedorn insulin therapy.
Laboratory assays
Serum glucose and C-peptide levels, as well as plasma HbA1c, were assayed at the study sites by standard methods.
Serum samples for GADA assays were shipped with transportation on ice within 1 day. Serum samples were stored at −80°C before analysis. GADA was assayed from serum at the core laboratory (Central South University) by a standardized radioligand assay as previously reported.8 Intra- and inter-assay coefficients of variation were 8.9% and 11.2%, respectively. The sensitivity and specificity were 82% and 98%, respectively. The assay was validated by Islet Autoantibody Standardization Program 2012 sponsored by the Immunology of Diabetes Society.
Statistical analysis
Data were analyzed using the Statistical Analysis System Release 9.4, (SAS Institute, Cary, NC), unless specified. Q–Q plots were used to check the normality of all the continuous variables, which were expressed as mean (standard deviation, SD) or median (interquartile range) where appropriate. Log-transformation was performed if normal distribution was rejected. Categorical variables were expressed as percentages (number, n). Frequency differences were compared using Chi-square test. Differences among groups were compared using analysis of variance (ANOVA), and Dunnett’s test with LADA as the reference group was used to perform multiple comparisons. For analysis of categorical variables, z-test was used to compare differences between any two groups. General linear model was used to conduct ANOVA. Trend differences were compared using the Mantel-Haenszel Chi-square test. Sex differences were compared using logistic regression.
We calculated the sex-specific prevalence of LADA, classic T1DM, and total T1DM by age groups and then, standardized the rates using the age and sex distributions of newly diagnosed diabetes in China in 2010 (Table S1).9 The generalized logit model was used to obtain odds ratios (OR) and their 95% confidence intervals (CIs) of clinical and biochemical factors for LADA and classic T1DM in a simultaneous manner. First, we performed univariable analysis to obtain unadjusted ORs of clinical and biochemical factors for LADA and classic T1DM. Then, we entered all the significant clinical and biochemical factors either for LADA or for classic T1DM in multivariable analysis to obtain the multivariable adjusted ORs. Because of the nature of a cross-sectional survey, our study could not be used to address the effect of drug treatments, but it may be adequate to adjust for confounding effects of certain drugs.10 Therefore, we included antihypertensive drugs, lipid-lowering drugs, insulin, alpha-glucosidase inhibitor, metformin, sulphonylurea, and glucagon-like peptide-1-based drugs in the multivariable analysis. A p-value <0.05 was considered to be statistically significant.
Table S1.
Age and sex distribution of the population census data of the general population in China in 2010
| Age, years | Male | Female |
|---|---|---|
| n | n | |
| 30–34 | 135 | 94 |
| 35–39 | 227 | 181 |
| 40–44 | 340 | 299 |
| 45–49 | 450 | 384 |
| 50–54 | 412 | 396 |
| 55–59 | 419 | 472 |
| 60–64 | 362 | 391 |
| 65–69 | 255 | 259 |
| ≥70 | 389 | 467 |
| Total number | 2989 | 2943 |
Results
Characteristics of surveyed patients
The mean age of the 17,349 patients was 52.6 (SD: ±11.5) years, and 10,342 (59.6%) of them were male. 2650 (15.3%) and 6687 (38.5%) of enrolled patients were obese or overweight, respectively. As expected, patients with LADA had older age, higher BMI, higher rates of overweight and obesity, higher waist circumference and higher rates of central obesity, higher BP and SBP/DBP ≥130/80 mmHg than those patients with classic T1DM while all these variables or rates were lower than those patients with non-T1DM (Table 1). As expected, patients with LADA were more likely to have good glycemic control and preserved β-cell function than T1DM while these variables were poorer than in those patients with non-T1DM. Lifestyle factors like diet treatment and physical activity were more frequent in non-T1DM and less frequent in classic T1DM than in LADA while current smoking was similar in three groups. Patients with LADA were more likely to use antihypertensive drugs, metformin, and sulfonylurea than patients with classic T1DM while these LADA patients were less likely to use intensive and basal insulin treatment (Table 1).
Table 1.
Clinical characteristics of the study patients
| n | LADA | Classic T1DM | Non-T1DM | p-Value |
|---|---|---|---|---|
| 658 | 356 | 16,335 | ||
| Age, years | 50.84±11.74†,‡ | 45.44±11.23† | 52.82±11.47‡ | <0.0001 |
| Age at diagnosis, years | 50.75±11.79†,‡ | 45.34±11.28† | 52.82±11.47‡ | <0.0001 |
| Male, % | 368(55.9) | 215(60.4) | 9759(59.7) | 0.141 |
| BMI | 22.87±3.66†,‡ | 21.38±3.47† | 24.80±3.49‡ | <0.0001 |
| Overweight | 175(27.8)†,‡ | 54(16.2)† | 6458(41.3)‡ | <0.0001 |
| Obesity | 50(7.9) ‡ | 14(4.2) | 2586(16.5)‡ | <0.0001 |
| Waist circumference, cm | 83.64±11.11†,‡ | 80.76±10.09† | 88.35±10.40‡ | <0.0001 |
| Central obesity, % | 191(33.5)†,‡ | 73(23.2)† | 7496(52.6)‡ | <0.0001 |
| Family history of diabetes | 149(23.2)‡ | 79(22.9) | 4414(28.1)‡ | <0.0001 |
| Systolic BP | 123.86±15.89†,‡ | 119.76±16.08† | 128.20±16.40‡ | <0.0001 |
| Diastolic BP | 78.17±10.42†,‡ | 75.56±11.11† | 80.29±10.48‡ | <0.0001 |
| SBP/DBP≥130/80 mmHg | 233(37.4) ‡ | 112(33.0) | 7651(49.9)‡ | <0.0001 |
| LDL-C, mmol/L | 2.83±1.00† | 2.64±1.06† | 2.87±1.00 | <0.0001 |
| LDL-C≥2.6 mmol/L | 349(56.1)† | 160(47.9)† | 9115(59.7) | <0.0001 |
| Triglyceride, mmol/L | 1.73±1.44‡ | 1.54±1.36 | 2.25±1.69 ‡ | <0.0001 |
| Triglyceride≥1.70 mmol/L | 206(33.2) ‡ | 93(27.7) | 7810(51.3)‡ | <0.0001 |
| HDL-C, mmol/L | 1.26±0.41‡ | 1.24±0.42 | 1.18±0.38‡ | <0.0001 |
| HDL-C≤1.0 mmol/L in male or ≤1.3 mmol in female | 277(44.7)‡ | 141(42.2) | 7790(51.5)‡ | <0.0001 |
| Abnormal lipid profile | 389(62.3)‡ | 203(60.2) | 11,907(76.9)‡ | <0.0001 |
| HbA1c, % | 10.09±2.94†,‡ | 11.17±3.04† | 9.27±2.70‡ | <0.0001 |
| HbA1c <7.0% (53 mmol/mol) | 103(16.5)†,‡ | 36(10.5)† | 3750(24.3)‡ | <0.0001 |
| HbA1c ≥7.0% (53 mmol/mol) | 521(83.5)†,‡ | 308(89.5)† | 11,710(75.7)‡ | <0.0001 |
| Fasting C-peptide, nmol/L | 0.37(0.19–0.60)†,‡ | 0.09(0.03–0.18)† | 0.57(0.37–0.82)‡ | <0.0001 |
| Postprandial C-peptide, nmol/L | 0.80(0.39–1.62)†,‡ | 0.16(0.05–0.34)† | 1.46(0.90–2.28)‡ | <0.0001 |
| Preserved β-cell function | 497(81.2)†,‡ | 88(25.5)† | 14,628(96.4)‡ | <0.0001 |
| Metabolic syndrome | 280(64.2)†,‡ | 117(51.3)† | 10,260(85.5)‡ | <0.0001 |
| Lifestyle | ||||
| Current smoking | 191(29.8) | 116(33.6) | 4872(30.7) | 0.454 |
| Current drinking | 87(13.7)‡ | 57(16.6) | 2918(18.5)‡ | 0.006 |
| Diet treatment | 302(56.4)†,‡ | 171(53.4)† | 7174(61.2)‡ | 0.002 |
| Physical activity | 253(47.3)†,‡ | 142(44.4)† | 6075(51.8)‡ | 0.005 |
| Location of residence | ||||
| South vs North | 440(66.9) vs 218(33.1) | 243(68.3) vs 113(31.7) | 11,120(68.1) vs 5215(31.9) | 0.806 |
| Rural vs Urban | 115(24.2) vs 361(75.8) | 70(27.7) vs 183(72.3) | 2798(23.9) vs 8899(76.1) | 0.384 |
| Use of medications | ||||
| Antihypertensive drugs | 94(14.4)†,‡ | 24(6.8)† | 3594(22.1) ‡ | <0.0001 |
| Lipid lowering drugs | 42(6.4)‡ | 28(7.9) | 1759(10.8)‡ | <0.0001 |
| Insulin | 231(35.3)†,‡ | 235(66.4)† | 3457(21.2)‡ | <0.0001 |
| Intensive | 99(15.1)†,‡ | 164(46.3)† | 1100(6.8)‡ | <0.0001 |
| Premixed | 90(13.7)‡ | 52(14.7) | 1293(7.9)‡ | <0.0001 |
| Basal | 148(22.6)†,‡ | 191(54.0)† | 2240(13.8)‡ | <0.0001 |
| Metformin | 211(32.2)† | 66(18.6)† | 5313(32.7) | <0.0001 |
| Sulphonylurea | 89(13.6)† | 17(4.8)† | 2105(12.9) | <0.0001 |
| Alpha-glucosidase inhibitor | 143(21.8)‡ | 61(17.2) | 2643(16.2)‡ | 0.001 |
| GLP-1 based drugs | 5(0.8) | 1(0.3) | 148(0.9) | 0.434 |
Notes: Data were presented as median and their interquartile ranges or means and standard deviations or % and their numbers where appropriate; Obesity is defined as BMI≥28.0 kg/m2 and overweight defined as BMI<28.0 kg/m2 but ≥24 kg/m2; Central obesity is defined as waist circumference ≥85 cm in female and 90 cm in male; Preserved β-cell function is defined as fasting C-peptide >0.2 nmol/L or postprandial C-peptide>0.4 nmol/L; Intensive insulin is defined as long-acting insulin associated with short-acting insulin therapy; Premixed insulin is defined as premixed insulin therapy; Basal insulin is defined as long-acting insulin treatment including Neutral Protamine Hagedorn (NPH) insulin therapy; p-values were derived from Kruskal–Wallis test (or Chi-square test) or analysis of variance; For analysis of continuous variables, Dennett’s test with LADA as the reference group was used to perform multiple comparisons with identical marks (†,‡) indicating statistically significant differences between two means. For analysis of categorical variables, z-test was used to compare differences between any two groups.
Abbreviations: LADA, latent autoimmune diabetes of adults; T1DM, type 1 diabetes mellitus; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BP, blood pressure; TC, total cholesterol; GLP, glucagon-like peptide.
Distribution of diabetes types in newly diagnosed patients
The rates of classic T1DM tended to decrease with increased age in both sexes, from 7.10% in the 30–34 years group, to 4.13% in the 35–39 years group, to 2.04% in the 40–44 years group, and further to 1.33% in the ≥45 years group, while the rate of LADA was relatively stable in all age groups, from 4.90% in patients at 30–34 years of age to 3.72% in patients >70 years of age (Table 2).
Table 2.
Age-standardized sex-specific prevalence of T1DM among those with newly diagnosed diabetes
| Age, y | Male | Female | LADA | Classic T1DM | T1DM | |||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
| n | n | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | |
| 30–34 | 885 | 298 | 38(4.29) | 20(6.71) | 56(6.33) | 28(9.40) | 94(10.62) | 48(16.11) |
| 35–39 | 1106 | 394 | 46(4.16) | 28(7.11) | 39(3.53) | 23(5.84) | 85(7.69) | 51(12.94) |
| 40–44 | 1429 | 576 | 70(4.90) | 23(3.99) | 23(1.61) | 18(3.13) | 93(6.51) | 41(7.12) |
| 45–49 | 1607 | 955 | 57(3.55) | 46(4.82) | 32(1.99) | 15(1.57) | 89(5.54) | 61(6.39) |
| 50–54 | 1705 | 1442 | 53(3.11) | 53(3.68) | 27(1.58) | 22(1.53) | 80(4.69) | 75(5.20) |
| 55–59 | 1227 | 1076 | 31(2.53) | 41(3.81) | 20(1.63) | 14(1.30) | 51(4.16) | 55(5.11) |
| 60–64 | 1052 | 1055 | 33(3.14) | 35(3.32) | 6(0.57) | 11(1.04) | 39(3.71) | 46(4.36) |
| 65–69 | 675 | 657 | 20(2.96) | 19(2.89) | 9(1.33) | 4(0.61) | 29(4.30) | 23(3.50) |
| ≥70 | 656 | 554 | 20(3.05) | 25(4.51) | 3(0.46) | 6(1.08) | 23(3.51) | 31(5.60) |
| p for trend | 0.0020 | 0.0019 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Crude rate | 10,342 | 7007 | 368(3.56) | 290(4.14) | 215(2.08) | 141(2.01) | 583(5.64) | 431(6.15) |
| Standardized† | 3.40(2.75–4.05) | 4.20(3.47–4.93) | 1.72(1.25–2.19) | 1.96(1.45–2.47) | 5.14(4.36–5.92) | 6.16(5.30–7.02) | ||
| p for sex difference | 0.0039 | 0.0520 | 0.0004 | |||||
Notes: Data are expressed as n (%). Standardized prevalence was expressed as p (95% CI). p for trend was derived from the Mantel-Haenszel Chi-square test and p for sex difference was derived from logistic regression; †The prevalence was standardized to the patient population with newly diagnosed diabetes in China National Survey of Diabetes in 2010 and only those aged 30 years and above were included in the standardization, so the standardized prevalence was only for those aged 30 years and above.
Abbreviations: LADA, latent autoimmune diabetes in adults and including latent autoimmune diabetes in youth; T1DM, type 1 diabetes, including LADA and classic type 1 diabetes.
The age-standardized prevalence of classic T1DM was 1.72% (95% CI: 1.25%, 2.19%) in males, 1.96% (95% CI: 1.45%, 2.47%) in females (p=0.0520 for difference by sex), and 1.76% (95% CI: 1.43%, 2.09%) in both sexes combined. The age-standardized prevalence of LADA was 3.40% (95% CI: 2.75%, 4.05%) in males, 4.20% (95% CI: 3.47%, 4.93%) in females (p=0.0039 for difference by sex) and 3.72% (95% CI: 3.23%, 4.21%) in both sexes combined. Altogether, the age-standardized prevalence of T1DM was 5.14% (95% CI: 4.36%, 5.92%) in males, 6.16% (95% CI: 5.30%, 7.02%) in females (p=0.0004 for difference by sex) and 5.49% (95% CI: 4.90%, 6.08%) in both sexes (Table 2). Classic T1A, classic T1B and LADA accounted for 22.4% (n=227), 12.7% (n=129) and 64.9% (n=658) of the total T1DM cases, respectively. Etiologically, the proportion of autoimmunity-related cases in T1DM was 87.3%.
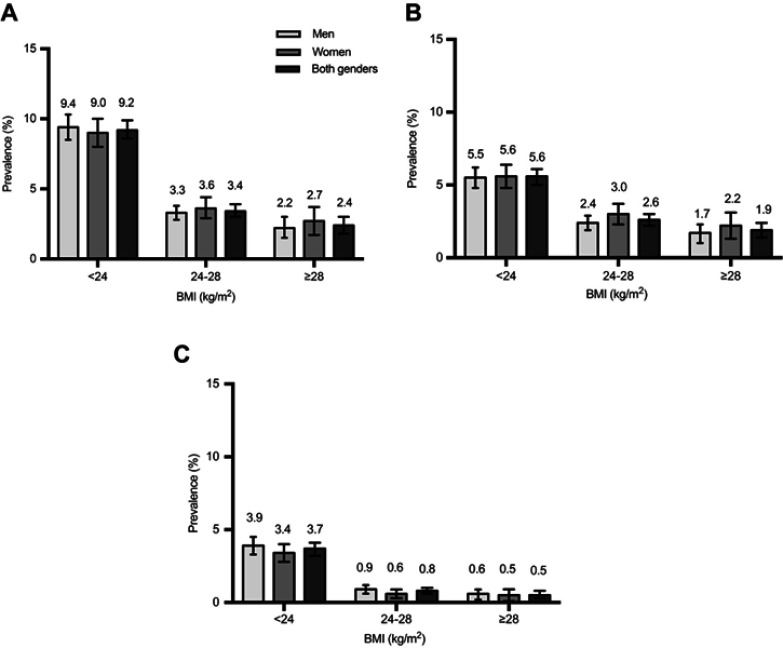
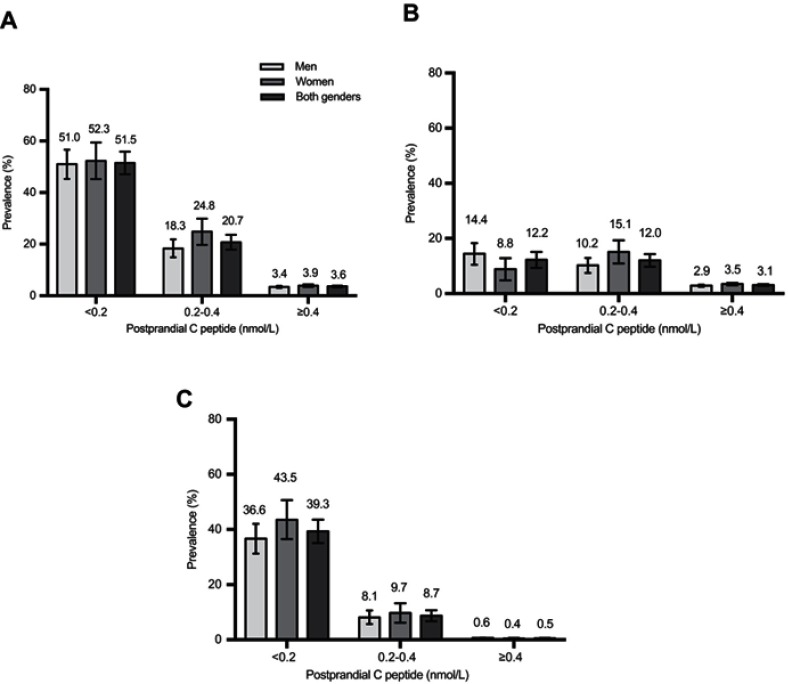
In overweight or obese patients, the prevalence of total T1DM was 3.4% and 2.4%, respectively, with 76.5% and 79.2% of these having LADA, which was lower than that in normal BMI patients (Figure 2). The same tendency of a lower prevalence of T1DM in patients with higher C-peptide levels was also shown. (Figure 3).
Figure 2.
Prevalence of total T1DM (A), LADA (B), Classic T1DM (C) among Chinese adults 30 years of age or older, according to BMI. The prevalence of total type 1 diabetes (A), LADA (B), Classic T1DM (C) among men, women, and both genders is shown, according to BMI. Total T1DM includes both classic T1DM and LADA. Bars indicate 95% confidence intervals.
Abbreviations: T1DM, type 1 diabetes mellitus; LADA, latent autoimmune diabetes of adults.
Figure 3.
Prevalence of total T1DM (A), LADA (B), Classic T1DM (C) among Chinese adults 30 years of age or older, according to postprandial C-peptide level. The prevalence of total T1DM (A), LADA (B), Classic T1DM (C) among men, women, and both genders is shown, according to postprandial C-peptide level. Total T1DM includes both classic T1DM and LADA. Bars indicate 95% confidence intervals.
Abbreviations: T1DM, type 1 diabetes mellitus; LADA, latent autoimmune diabetes of adults.
Determinants of classic T1DM and LADA
In univariable analyses, age, overweight/obesity, central obesity, family history of diabetes, BP, lipid analytes (LDL-C, HDL-C, and triglycerides), HbA1c, fasting C-peptide, postprandial C-peptide, current alcohol consumption, and use of medications were associated with LADA or classic T1DM (Table 3).
Table 3.
Univariable odds ratio of clinical factors for LADA and classic type 1 diabetes in Chinese patients with newly diagnosed diabetes
| LADA | Classic T1DM | |||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Age, years | <0.0001* | <0.0001* | ||
| 30–39 vs ≥50 | 1.60(1.31–1.97) | 4.80(3.76–6.13) | ||
| 40–49 vs ≥50 | 1.34(1.12–1.60) | 1.63(1.23–2.14) | ||
| Male vs female | 0.86(0.73–1.00) | 0.0507 | 1.03(0.83–1.27) | 0.8045 |
| Education | 0.1294 | 0.2150 | ||
| Tertiary education | 1.17(0.94–1.45) | 1.23(0.93–1.63) | ||
| Senior high school | 1.11(0.91–1.36) | 0.94(0.72–1.24) | ||
| Junior high school or lower | 1.0 | 1.0 | ||
| BMI | <0.0001* | <0.0001* | ||
| Overweight | 0.45(0.38–0.54) | 0.21(0.15–0.28) | ||
| Obesity | 0.32(0.24–0.43) | 0.12(0.07–0.21) | ||
| Normal weight | 1.0 | 1.0 | ||
| Central obesity | 0.45(0.38–0.54) | <0.0001 | 0.27(0.21–0.35) | <0.0001 |
| Family history of diabetes | 0.76(0.64–0.93) | 0.0074 | 0.76(059–0.98) | 0.0353 |
| BP <130/80 mmHg | 1.37(1.14–1.64) | 0.0009 | 2.70(1.99–3.66) | <0.0001 |
| LDL-C <2.6 mmol/L | 1.16(0.99–1.36) | 0.0725 | 1.60(1.29–1.99) | <0.0001 |
| TG <1.7 mmol/L | 2.13(1.80–2.53) | <0.0001 | 2.78(2.19–3.54) | <0.0001 |
| HDL-C >1.0 mmol/L in male or 1.3 mmol/L in female | 1.32(1.12–1.55) | 0.0009 | 1.39(1.11–1.73) | <0.0001 |
| HbA1c <7.0% (53 mmol/mol) | 0.62(0.50–0.77) | <0.0001 | 0.37(0.26–0.52) | <0.0001 |
| Fasting C-peptide | <0.0001* | <0.0001* | ||
| Upper quartile | 0.21(0.16–0.27) | 0.01(0.00–0.02) | ||
| Mid-high quartile | 0.29(0.23–0.37) | 0.02(0.01–0.04) | ||
| Mid-low quartile | 0.42(0.35–0.52) | 0.04(0.02–0.06) | ||
| Bottom quartile | 1.0 | 1.0 | ||
| Postprandial C-peptide | <0.0001* | <0.0001* | ||
| Upper quartile | 0.22(0.17–0.28) | 0.01(0.00–0.03) | ||
| Mid-high quartile | 0.24(0.19–0.30) | 0.01(0.00–0.03) | ||
| Mid-low quartile | 0.31(0.25–0.39) | 0.04(0.02–0.07) | ||
| Bottom quartile | 1.0 | 1.0 | ||
| Lifestyle | ||||
| Current smoking | 0.96(0.81–1.14) | 0.6262 | 1.14(0.91–1.43) | 0.2531 |
| Current drinking | 0.70(0.55–0.88) | 0.0020 | 0.88(0.66–1.17) | 0.3714 |
| Diet treatment | 1.08(0.93–1.27) | 0.3162 | 1.18(0.96–1.46) | 0.1221 |
| Physical activity | 1.06(0.90–1.24) | 0.5123 | 1.12(0.90–1.39) | 0.2979 |
| Location of residence | ||||
| South vs North | 0.95(0.80–1.12) | 0.5157 | 1.01(0.81–1.26) | 0.9414 |
| Rural vs Urban | 1.01(0.82–1.26) | 0.9046 | 1.22(0.92–1.61) | 0.1679 |
| Use of medicines | ||||
| Antihypertensive drugs | 0.59(0.47–0.74) | <0.0001 | 0.26(0.17–0.39) | <0.0001 |
| Lipid lowering drugs | 0.57(0.41–0.78) | 0.0004 | 0.71(0.48–1.05) | 0.0828 |
| Insulin | 2.19(1.75–2.75) | <0.0001 | 6.83(5.43–8.59) | <0.0001 |
| Alpha-glucosidase inhibitor | 1.44(1.19–1.74) | 0.0002 | 1.07(0.81–1.42) | 0.0618 |
| Metformin | 0.98(0.83–1.16) | 0.8149 | 0.47(0.36–0.62) | <0.0001 |
| Sulphonylurea | 1.06(0.84–1.33) | 0.6265 | 0.34(0.21–0.55) | <0.0001 |
| GLP-1 based drugs | 0.84(0.34–2.05) | 0.6987 | 0.31(0.04–2.21) | 0.2427 |
Note: *p for trend.
Abbreviations: LADA, latent autoimmune diabetes of adults; T1DM, type 1 diabetes mellitus; OR, odds ratio; GLP-1, glucagon-like peptide-1; Tertiary education is defined as college level or above.
In a multivariable analysis with all these factors included in the model (Table 4), young age was associated with increased risk of classic T1DM but to a lesser extent, was also associated with increased risk of LADA (ORs of 30–39, 40–49 years vs 50 and more years: 1.32, 95% CI: 1.02–1.71; 1.16, 0.93–1.44, p for trend=0.0219). In the same vein, overweight and obesity were associated with decreased risks of classic T1DM but to a lesser degree, tended to be associated with decreased risks of LADA (ORs of overweight and obesity vs normal weight: 0.74, 95% CI: 0.59–0.94; 0.66, 95% CI: 0.45–0.98, p for trend=0.0069). However, central obesity was not associated with decreased risks of LADA or classic T1DM. Alcohol use (OR: 0.75, 95% CI: 0.57–0.98) and family history of diabetes (OR: 0.80, 0.65–1.00) were associated with decreased risk of LADA but not with classic T1DM.
Table 4.
Multivariable odds ratio of clinical factors for LADA and classic T1DM in Chinese patients with newly diagnosed diabetes
| LADA | Classic T1DM | |||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Age, years | 0.0219* | <0.0001* | ||
| 30–39 vs ≥50 | 1.32(1.02–1.71) | 3.51(2.53–4.89) | ||
| 40–49 vs ≥50 | 1.16(0.93–1.44) | 1.35(0.95–1.90) | ||
| BMI | 0.0069* | 0.0007* | ||
| Overweight | 0.74(0.59–0.94) | 0.52(0.36–0.79) | ||
| Obesity | 0.66(0.45–0.98) | 0.40(0.19–0.88) | ||
| Normal weight | 1.0 | 1.0 | ||
| Central obesity | 0.84(0.66–1.05) | 0.1239 | 1.10(0.76–1.60) | 0.6025 |
| Family history of diabetes | 0.80(0.65–1.00) | 0.0463 | 0.74(0.54–1.03) | 0.0709 |
| BP <130/80 mmHg | 1.32(1.09–1.60) | 0.0045 | 1.16(0.88–1.54) | 0.2849 |
| LDL-C <2.6 mmol/L | 1.07(0.88–1.29) | 0.4978 | 1.35(1.02–1.78) | 0.0341 |
| TG <1.7 mmol/L | 1.48(1.21–1.81) | 0.0002 | 1.26(0.92–1.73) | 0.1462 |
| HDL-C >1.0 mmol/L in male or 1.3 mmol/L in female | 1.15(0.95–1.40) | 0.1555 | 0.94(0.70–1.25) | 0.6586 |
| HbA1c <7.0% (53 mmol/mol) | 0.82(0.63–1.07) | 0.1375 | 1.09(0.70–1.71) | 0.6919 |
| Fasting C-peptide | 0.0010* | <0.0001* | ||
| Upper quartile | 0.66(0.52–0.85) | 0.10(0.05–0.18) | ||
| Mid-high quartile | 0.68(0.50–0.93) | 0.11(0.04–0.28) | ||
| Mid-low quartile | 0.57(0.39–0.85) | 0.09(0.02–0.43) | ||
| Bottom quartile | 1.0 | 1.0 | ||
| Postprandial C-peptide | <0.0001* | <0.0001* | ||
| Upper quartile | 0.42(0.32–0.54) | 0.13(0.08–0.23) | ||
| Mid-high quartile | 0.38(0.28–0.52) | 0.03(0.01–0.13) | ||
| Mid-low quartile | 0.45(0.31–0.66) | 0.09(0.02–0.35) | ||
| Bottom quartile | 1.0 | 1.0 | ||
| Lifestyle | ||||
| Current drinking | 0.75(0.57–0.98) | 0.0336 | 0.94(0.65–1.35) | 0.7187 |
| Use of drugs | ||||
| Antihypertensive drugs | 0.91(0.70–1.19) | 0.4916 | 0.68(0.41–1.14) | 0.1432 |
| Lipid lowering drugs | 0.63(0.43–0.91) | 0.0131 | 0.90(0.54–1.53) | 0.7026 |
| Insulin | 1.79(1.38–2.32) | <0.0001 | 3.60(2.65–4.89) | <0.0001 |
| Alpha-glucosidase inhibitor | 1.41(1.13–1.76) | 0.0025 | 1.21(0.85–1.73) | 0.2933 |
| Metformin | 0.99(0.81–1.21) | 0.9285 | 0.58(0.41–0.83) | 0.0025 |
| Sulphonylurea | 1.12(0.86–1.47) | 0.4035 | 0.57(0.32–1.03) | 0.0613 |
Note: *p for trend.
Abbreviations: LADA, latent autoimmune diabetes of adults; T1DM, type 1 diabetes mellitus; OR, odds ratio; GLP-1, glucagon-like peptide-1.
LADA patients were more likely to achieve the BP target (1.32, 95% CI: 1.09–1.60), and the triglyceride target (1.48, 95% CI: 1.21–1.81). In contrast, classic T1DM patients were only more likely to achieve the LDL-C target, and the likelihood of reaching the targets for BP, triglycerides, HDL-C and HbA1c in classic T1DM were similar to non-T1DM patients.
Discussion
Our study is the first to assess the prevalence and proportion of LADA and classic T1DM in newly diagnosed adult diabetes in China. It had long been assumed that T1DM accounted for 5% of all four types of diabetes (ie, T1DM, T2DM, monogenic forms of diabetes, and GDM); although, solid data were not available. In this study in newly diagnosed diabetes in China, we found that the age-standardized prevalence of LADA in adults aged≥30 years to be as high as 3.40% in males and 4.20% in females with newly diagnosed diabetes in China, accounting for 65% of all adult-onset T1DM cases. In total, the prevalence of all T1DM in newly diagnosed adult diabetes was 5.8%. Although the prevalence of classic T1DM in patients older than 60 years old was lower than 1.0%, the total T1DM was still 4.1% due to the consistent prevalence of LADA across all age groups. Surprisingly, in overweight or obese patients, the prevalence of T1DM was 3.4% and 2.4%, respectively, but 76.5% or 79.2% of these subjects were characterized as having LADA, suggesting a more slowly progressive autoimmune process in older and obese patients.
Several population-based studies have reported the rate of LADA in patients initially diagnosed as having T2DM. An early study from Japan reported that the prevalence of GADA in adults with apparent T2DM was 3.8%.11 Similarly, the prevalence of LADA was 4.4% in Korean and Italian populations.12,13 Diabetes Outcomes Progression Trial reported that GADA positivity was 4.2% in North America and 3.7% in Southern Europe among individuals with T2DM, whereas in Northern Europe, the prevalence of LADA in patients with T2DM was estimated to be 7–10%.14–17 A prevalence of LADA at 3.8% in newly diagnosed diabetes in our Chinese cohort is similar to the rates reported in Eastern Asian populations and also consistent with higher rates in European and North American populations, although European and North American countries have a much higher rate of T1DM than in China.18 Our previous LADA-China study reported the rate of LADA to be 5.9% in a small sample of 4880 subjects with T2DM.8 In the large sample reported herein covering both T1DM and T2DM, our survey has updated the prevalence of LADA among all newly diagnosed diabetes cases and among T1DM cases in the adult population in China.
LADA is classified as slowly progressive T1DM in pathogenesis and differential diagnosis from T2DM is usually relied on GADA testing instead of on clinical features. Our study found that younger age and non-obesity were associated with increased risk of LADA. Our findings are inconsistent with those of other studies that older age and overweight/obesity were associated with a higher risk of LADA but this could be due to differences in the underlying research question.19,20 Indeed, other studies sought to identify LADA from the general population while our objective was to identify LADA from newly diagnosed diabetes, which is more relevant to clinical practice. Interestingly, alcohol consumption was associated with a lower risk of LADA but not associated with classic T1DM, and family history of diabetes was associated with a lower risk of LADA, the latter suggesting that the genetic predisposition of LADA may be weaker than T2DM. However, LADA patients had similar odds of achieving good glycemic control as compared to non-T1DM, presumably due to their increased likelihood of using insulin.
Our study showed that LADA, classic T1DM, and T2DM shared many clinical characteristics, which pose an even greater challenge for clinicians to distinguish the three conditions. Indeed, in our surveyed patients, those with LADA had a similar likelihood of using oral antidiabetic medications such as metformin and sulfonylurea, suggesting that they were likely to be treated as if they had T2DM. In this regard, few studies have shown that LADA patients on insulin treatment could benefit from the use of metformin and sulphonylurea.21–23 It has been established that the potential best therapeutic option for LADA patients should aim not only to obtain good metabolic control but also, to allow better preservation of residual β-cell function. Identification of LADA in newly diagnosed diabetes is a critically important step to preserve the β-cell function of patients with LADA. However, universal testing of antibodies for LADA in T2DM remains a debatable question that depends on the effectiveness and cost-effectiveness of early initiation of LADA treatment regimen. In this regard, Fourlanos et al, developed a screening tool for LADA using common clinical parameters.24 Indeed, instead of universal testing of autoantibody, a two-step procedure, ie, universal screening plus antibody testing, may be a more cost-effective practice although its effectiveness and cost-effectiveness need further studies.
Our study had limitations. First, this survey was not a random-sampling survey although we had made great efforts to recruit patients from extensive geographic locations in China. To minimize over- or under-estimation of the prevalence of LADA, we used a representative sample of Chinese adults with newly diagnosed diabetes to standardize these rates.9 Second, some socio-economic indicators such as income and wealth were not collected in our survey. Third, this analysis only reported data of those patients aged ≥30 years; however, this is the most relevant population for the current study with the adolescent onset of T2DM being rare in the Chinese population. We know there is a challenge to deal with the issue of “true LADA” and “false or low GADA titer patients “. In one way, the false-positive rate is likely very low given the high specificity of the assay and the enrichment of the cohort by selecting patients with non-insulin requiring adult-onset diabetes; in the other, we did the GADA titer distribution analysis in all age groups, and tried to figure out whether there was a constant level across all ages. It looked like that percentage is about 1% and if we defined “true LADA” strictly and removed those with low GADA titer, the prevalence of total T1DM would be 4.27%, with 58% of them belonged to LADA (Table S2).
Table S2.
Age-standardized sex-specific prevalence of LADA and type 1 diabetes after removal of low GADA titer LADA patients
| Age, years | Male | Female | LADA after removal of low GADA titer patients | Total T1DM (defined as Classic T1DM and LADA after removal of low GADA titer patients | ||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| n | n | n (%) | n (%) | n (%) | n (%) | |
| 30–34 | 885 | 298 | 30 (3.39) | 14 (4.7) | 86 (9.72) | 42 (14.09) |
| 35–39 | 1106 | 394 | 30 (2.71) | 19 (4.82) | 69 (6.24) | 42 (10.66) |
| 40–44 | 1429 | 576 | 52 (3.64) | 14 (2.43) | 75 (5.25) | 32 (5.56) |
| 45–49 | 1607 | 955 | 40 (2.49) | 30 (3.14) | 72 (4.48) | 45 (4.71) |
| 50–54 | 1705 | 1442 | 37 (2.17) | 36 (2.5) | 64 (3.75) | 58 (4.02) |
| 55–59 | 1227 | 1076 | 21 (1.71) | 27 (2.51) | 41 (3.34) | 41 (3.81) |
| 60–64 | 1052 | 1055 | 23 (2.19) | 19 (1.8) | 29 (2.76) | 30 (2.84) |
| 65–69 | 675 | 657 | 8 (1.19) | 12 (1.83) | 17 (2.52) | 16 (2.44) |
| ≥70 | 656 | 554 | 12 (1.83) | 20 (3.61) | 15 (2.29) | 26 (4.69) |
| Crude rate | 10,342 | 7007 | 253 (2.45) | 191 (2.73) | 468 (4.53) | 332 (4.74) |
| Standardized† | 2.29 (1.75-2.83) | 2.82 (2.22–3.42) | 4.02 (3.32–4.72) | 4.77 (4.00–5.54) | ||
Notes: Data are expressed as n (%). Standardized prevalence was expressed as p (95% CI). By analyzing the GADA titer distribution in all age groups, we defined the Low GADA titer as lower than 80 U/mL and removed this part of LADA for possible false-positive rate.
Abbreviations: LADA, latent autoimmune diabetes of adults; GADA, glutamic acid decarboxylase autoantibody; T1DM, type 1 diabetes mellitus.
Our findings have important clinical and public health implications. It is estimated that diabetes affected 113.9 million Chinese adults, with 79.6 million being newly diagnosed diabetes.9 If our rates of LADA and classic T1DM in newly diagnosed diabetes are also applicable to other newly diagnosed diabetes in China, it can be estimated that China had 4.3 million adults with LADA and 2.4 million with classic T1DM, that is there are 6.7 million patients with T1DM in total. Presumably, a majority of LADA patients are treated as though they have T2DM. If these patients were identified to have LADA and proper treatments were in place, their blood glucose regulation would be maintained and clinical comorbidities, such as microvascular and macrovascular complications, would be greatly reduced. Considering the large population of LADA in newly diagnosed diabetes in China, further studies are warranted to test the effectiveness and cost-effectiveness of universal screening and early initiation of insulin therapy needs further studies.
Supplementary materials
Acknowledgments
The authors thank all of the patients, nurses, doctors, investigators, and technicians involved at the 46 participating centers of National Clinical Research Center for Metabolic Diseases for their efforts in data and sample collection. The members of National Clinical Research Center for Metabolic Diseases (investigators and hospitals); Xueyao Han, Ling Chen, Xiaoling Chen, Peking University People’s Hospital; Lixin Guo, Xiaofan Jia, Shan Ding, Beijing Hospital; Xinhua Xiao, Cuijuan Qi, Xiaojing Wang, Peking Union Medical College Hospital; Zhongyan Shan, Yaxin Lai, Zhuo Zhang, The First Hospital of China Medical University; Yu Liu, Yan Cheng, Hanqing Cai, The Second Hospital of Jilin University; Yadong Sun, Yan Ma, Haiying Wang, People’s Hospital of Jilin Province; Yiming Li, Chaoyun Zhang, Shuo Zhang, Hua Shan Hospital, Fudan University; Tao Yang, Hao Dai, Mei Zhang, The First Affiliated Hospital with Nanjing Medical University; Liyong Yang, Peiwen Wu, Xiaofang Yan, The First Affiliated Hospital of Fujian Medical University; Yangang Wang, Fang Wang, Hong Chen, The Affiliated Hospital of Qingdao University; Qifu Li, Rong Li, The First Affiliated Hospital of Chongqing Medical University; Li Wang, Xiangyang Liu, Xijing Hospital, Fourth Military Medical University; Suhong Wei, Gansu Provincial Hospital; Yun Zhu, Rui Ma, The First Affiliated Hospital of Xinjiang Medical University; Gebo Wen, Xinhua Xiao, Jianping Qin, The First Affiliated Hospital of University of South China; Jian Kuang, Yan Lin, Guangdong General Hospital; Shaoda Lin, Kun Lin, the First Affiliated Hospital of Shantou University Medical College; Li Li, Heji Hospital Affiliated to Changzhi Medical College; Gan Huang, Shuoming Luo, The Second Xiangya Hospital of Central South University; Huibiao Quan, Leweihua Lin, Hainan General Hospital; Hongyu Kuang, Weihua Wu, The First Affiliated Hospital of Harbin Medical University; Yuling He, The First Affiliated Hospital of Guangxi Medical University; Xiaoyan Chen, Yuyu Tan, The First Affiliated Hospital of Guangzhou Medical University; Ling He, Guangzhou First People’s Hospital; Chao Zheng, The Second Affiliated Hospital of Wenzhou Medical University; Jianying Liu, Zhifang Yang, The First Affiliated Hospital of Nanchang University; Xiaoyang Lai, The Second Affiliated Hospital of Nanchang University; Ling Hu, Yan Zhu, Ying Hu, The Third Affiliated Hospital of Nanchang University; Xuqing Li, Henan Provincial People’s Hospital; Hong Li, Yushan Xu, The First Affiliated Hospital of Kunming Medical University; Heng Su, Yang Ou, The First People’s Hospital of Yunnan Province; Jianping Wang, The Second Hospital University of South China; Changqing Luo, Xiaoyue Wang, The First People’s Hospital of Yueyang; Zhiming Deng, Shenglian Gan, The First People’s Hospital of Changde City; Zhaohui Mo, Ping Jin, Honghui He, The Third Xiangya Hospital of Central South University; Qiuxia Huang, Dongguan People’s Hospital; Fang Wang, Heping Hospital Affiliated to Changzhi Medical College; Yi Zhang, Zhenzhen Hong, First Hospital of Quanzhou Affiliated to Fujian Medical University; Yuezhong Ren, Pengfei Shan, The Second Affiliated Hospital of Zhejiang University School of Medicine; Caifeng Yan, Hui Zhang, Northern Jiangsu People’s Hospital; Zhiwen Liu, Shanghai Xuhui District Central Hospital; Meibiao Zhang, The First People’s Hospital of Huaihua; Ming Liu, Heting Wang, Tianjin Medical University General Hospital; Hongwei Jiang, Liujun Fu, The First Affiliated Hospital of the Henan University of Science and Technology; Hui Fang, Tangshan Gongren Hospital; Hui Sun, The Affiliated Hospital of Inner Mongolia Medical University.
We also thank Prof. Guang Ning for his kind help in providing the population census data of the general population in China in 2010. The authors also thank Dr. Amanda Posgai and Dr. Mark Atkinson, from The University of Florida (USA), Dr. David Leslie from University of London, Dr. Zhenqi Liu from University of Virginia for their review and editorial assistance of this work. This work was supported by the National Science and Technology Infrastructure Program (2013BAI09B12 and 2015BAI12B13), the National Key R&D Program of China (2016YFC1305000 and 2017YFC1309604).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yang Z, Wang K, Li T, et al. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes Care. 1998;21:525–529. doi: 10.2337/diacare.21.4.525 [DOI] [PubMed] [Google Scholar]
- 2.Weng J, Zhou Z, Guo L, et al. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ. 2018;360:j5295. doi: 10.1136/bmj.j5295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer JP, Hampe CS, Chiu H, Goel A, Brooks-Worrell BM. Is latent autoimmune diabetes in adults distinct from type 1 diabetes or just type 1 diabetes at an older age? Diabetes. 2005;54(Suppl 2):S62–S67. doi: 10.2337/diabetes.54.suppl_2.s62 [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM; American Diabetes Association. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27 Suppl 1:S68–S71. doi: 10.2337/diacare.27.2007.s68 [DOI] [PubMed] [Google Scholar]
- 6.Chinese Diabetes Society. China guideline for type 2 diabetes. Chin J Diabetes Mellitus. 2014;6:447–498. [Google Scholar]
- 7.National Cholesterol Education Program (NCEP) Expert Panal on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panal III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panal on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panal III) final report. Circulation. 2002;106:3143-3421. [PubMed] [Google Scholar]
- 8.Zhou Z, Xiang Y, Ji L, et al. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes. 2013;62:543–550. doi: 10.2337/db12-0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Wang L, He J, et al.; China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118 [DOI] [PubMed] [Google Scholar]
- 10.Yang XL, Ma RC, So WY, Kong AP, Xu G, Chan JC. Addressing different biases in analysing drug use on cancer risk in diabetes in non-clinical trial settings–what, why and how? Diabetes Obes Metab. 2012;14:579–585. doi: 10.1111/j.1463-1326.2011.01551.x [DOI] [PubMed] [Google Scholar]
- 11.Takeda H, Kawasaki E, Shimizu I, et al. Clinical, autoimmune, and genetic characteristics of adult-onset diabetic patients with GAD autoantibodies in Japan (Ehime Study). Diabetes Care. 2002;25:995–1001. doi: 10.2337/diacare.25.6.995 [DOI] [PubMed] [Google Scholar]
- 12.Park Y, Hong S, Park L, et al. LADA prevalence estimation and insulin dependency during follow-up. Diabetes Metab Res Rev. 2011;27:975–979. doi: 10.1002/dmrr.1278 [DOI] [PubMed] [Google Scholar]
- 13.Guglielmi C, Palermo A, Pozzilli P. Latent autoimmune diabetes in the adults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes Metab Res Rev. 2012;28(Suppl 2):40–46. doi: 10.1002/dmrr.2345 [DOI] [PubMed] [Google Scholar]
- 14.Zinman B, Kahn SE, Haffner SM, et al. Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes. 2004;53:3193–3200. doi: 10.2337/diabetes.53.12.3193 [DOI] [PubMed] [Google Scholar]
- 15.Turner R, Stratton I, Horton V, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350:1288–1293. doi: 10.1016/s0140-6736(97)03062-6 [DOI] [PubMed] [Google Scholar]
- 16.Tuomi T, Carlsson A, Li H, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48:150–157. doi: 10.2337/diabetes.48.1.150 [DOI] [PubMed] [Google Scholar]
- 17.Hawa MI, Buchan AP, Ola T, et al. LADA and CARDS: a prospective study of clinical outcome in established adult-onset autoimmune diabetes. Diabetes Care. 2014;37:1643–1649. doi: 10.2337/dc13-2383 [DOI] [PubMed] [Google Scholar]
- 18.International Diabetes Federation. IDF Diabetic Atlas 8th edition; 2017. Available from: http://www.diabetesatlas.org/resources/2017-atlas.html/. [Accessed May22, 2018].
- 19.Carlsson S, Midthjell K, Tesfamarian MY, Grill V. Age, overweight and physical inactivity increase the risk of latent autoimmune diabetes in adults: results from the Nord-Trondelag health study. Diabetologia. 2007;50:55–58. doi: 10.1007/s00125-006-0518-7 [DOI] [PubMed] [Google Scholar]
- 20.Hjort R, Ahlqvist E, Carlsson PO, et al. Overweight, obesity and the risk of LADA: results from a Swedish case-control study and the Norwegian HUNT Study. Diabetologia. 2018;61:1333–1343. doi: 10.1007/s00125-018-4596-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton J, Cummings E, Zdravkovic V, Finegood D, Daneman D. Metformin as an adjunct therapy in adolescents with type 1 diabetes and insulin resistance: a randomized controlled trial. Diabetes Care. 2003;26:138–143. doi: 10.2337/diacare.26.1.138 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Nakanishi K, Murase T, Kosaka K. Small doses of subcutaneous insulin as a strategy for preventing slowly progressive beta-cell failure in islet cell antibody-positive patients with clinical features of NIDDM. Diabetes. 1996;45:622–626. doi: 10.2337/diab.45.5.622 [DOI] [PubMed] [Google Scholar]
- 23.Maruyama T, Tanaka S, Shimada A, et al. Insulin intervention in slowly progressive insulin-dependent (type 1) diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2115–2121. doi: 10.1210/jc.2007-2267 [DOI] [PubMed] [Google Scholar]
- 24.Fourlanos S, Perry C, Stein MS, Stankovich J, Harrison LC, Colman PG. A clinical screening tool identifies autoimmune diabetes in adults. Diabetes Care. 2006;29:970–975. doi: 10.2337/diacare.295970 [DOI] [PubMed] [Google Scholar]